Synopsis
Group B Streptococcus (GBS) requires capsular sialic acid (Sia) for virulence and partially modifies this sugar by O-acetylation. We describe serotype specific patterns of GBS Sia O-acetylation that can be manipulated by genetic and biochemical means. In vitro and in vivo assays demonstrate this subtle modification attenuates GBS Sia-mediated neutrophil suppression and animal virulence.
Keywords: sialic acid, O-acetylation, Streptococcus agalactiae, capsular polysaccharide, neutrophil
INTRODUCTION
Complex carbohydrates (glycans) on bacterial cell surfaces can sometimes elicit extraordinary phenotypes in biological systems, in particular, when they closely resemble host cell surface glycan motifs [1–3]. One prominent example of microbial carbohydrate mimicry involves the sialic acids (Sias). Sias are nine-carbon backbone acidic sugars displayed prominently on the surfaces of all vertebrate cells. In mammals, the most common sialic acid (Sia) is N-acetylneuraminic acid (Neu5Ac), a cell surface molecule with wide-ranging roles in mammalian physiology, participating in renal filtration, neuronal plasticity and suppression of innate and adaptive immune responses [4–9]. Neu5Ac is also expressed by several important human pathogens, and this molecular resemblance to the host facilitates infection by multiple mechanisms [10–14]. For example, Neu5Ac blocks cellular opsonization by the alternative pathway of complement [8, 10, 13, 15] by interacting with a key counter-regulator of alternative complement, Factor H [8]. Neu5Ac expression also allows bacteria to engage Siglecs, a family of 14 Sia-binding immunoglobin superfamily lectins expressed on human leukocytes [1, 4, 16–18], many of which possess intracellular tyrosine-based inhibitory motifs thought to restrict immune activation against “self” [4].
Genomic approaches have identified a growing number of mammalian pathogens that express Sias or related molecules, suggesting that this form of immune evasion may be more common than currently appreciated [19]. Chemical modifications of Sia structure have also been reported in a number of pathogens that engage in this form of molecular mimicry [20–25]. However, the impact of Sia structural variation on the potential for invasive bacterial infection has not been studied.
Group B Streptococcus (GBS) is a Gram-positive opportunistic pathogen and a model system for understanding the mechanisms and consequences of Sia molecular mimicry. A leading cause of bacterial sepsis and meningitis in newborns, GBS asymptomatically colonizes the lower gastrointestinal and vaginal mucosa in up to 1/3 of women sampled at a single time and 2/3 of women sampled at multiple times over a year [26, 27]. Invasive GBS disease can occur following ascending infection of placental membranes or aspiration by the neonate during the birthing process [28]. A critical virulence factor of GBS is its surface capsular polysaccharide (CPS), the outermost glycan layer surrounding the bacterium. While each serotype strain (nine in all) expresses an antigenically unique structure [29], all display terminal α2–3 linked Sia residues, which are identical to host Sia motifs and essential to GBS virulence [11]. During invasive GBS disease, Sias promote immune evasion and bacterial proliferation by suppressing the alternative complement pathway [10], impairing opsonophagocytosis, and by engaging the neutrophil receptor Siglec 9, to suppress the oxidative burst and release of granule proteases [1]. Currently available biochemical evidence suggests that CPS is the only GBS surface structure bearing Sias.
It was recently discovered that GBS partially O-acetylates terminal Sias at the 7-carbon position, a modification that spontaneously migrates to the 9-position (via the 8-position) in a pH-dependent and unidirectional manner [25]. The ultimate level of Sia O-acetylation is determined by the relative activities of a GBS Sia O-acetyltransferase, NeuD [30], and a Sia-specific O-acetylesterase. The GBS O-acetylesterase is linked by gene fusion to the C-terminus of a CMP-Sia synthetase essential for nucleotide activation of sialic acid prior to capsular assembly, together forming the dual activity enzyme, NeuA [31, 32]. Using non-polar gene deletion and site-directed mutagenesis to specifically manipulate GBS Sia O-acetyl esterase activity, we previously demonstrated that levels of surface Sia O-acetylation can be driven up or down as a single chemical variable [33]. Molecular analyses of these strains indicated that high levels of Sia O-acetylation disrupts interactions with human Siglec-9 but does not alter deposition of complement on the GBS surface [33]. Here we combine biochemical analyses with cellular and animal infection models to investigate the prevalence and patterns of Sia O-acetylation in native GBS populations and, for the first time, to define the physiological significance of bacterial Sia O-acetylation during invasive infection.
EXPERIMENTAL
Bacterial Strains and Growth Conditions
GBS strains with high (~75%) and low (<5%) levels O-acetylation were generated in the serotype III background by allelic variation of the NeuA Sia O-acetyl esterase and were defined previously by thorough biochemical analyses [31, 33]. These isogenic bacterial strains were grown in Todd-Hewitt Broth (THB; Difco, BD Diagnostics) containing 5 μg/ml of erythromycin (Erm). For infection studies, bacteria were cultivated at 37°C to mid-log phase and resuspended to an OD600 of 0.4, followed by serial dilution and enumeration of colony-forming units in each experimental inoculum. GBS isolates used in biochemical studies were from newborns who developed early-onset GBS disease (invasive strains) or newborns that were colonized but did not develop GBS disease (colonizing strains) from the National Institutes of Child Health and Development (NICHD) multi-center study [34, 35]. NICHD strains were cultivated overnight in THB without antibiotics. GBS strains engineered to express alternate serotype Ia or III capsule polymerase genes [36] were grown in the presence of 5 μg/ml chloramphenicol.
Biochemical Analysis of Sialic Acid O-Acetylation
Bacterial pellets from 1 ml of culture were washed and Sias were released by mild acid hydrolysis, isolated, derivatized with 1,2-diamino-4,5-methylene dioxybenzene, and a small aliquot analyzed by HPLC in parallel with Sia standards as previously described [25, 31]. Percent Sia O-acetylation was determined by automated integration of peak areas for 7- and 9-O-acetylated N-acetyl neuraminic acids versus areas for all Sias combined. In a subset of strains, 0.1M sodium hydroxide treatment [25] was used to verify the identity of peaks corresponding to O-acetyl esters; these data were very similar to results produced by the peak integration method. The Rank-sum (Mann Whitney) test was used to evaluate statistical differences in O-acetylation between serotypes.
Neutrophil Isolation
Normal human volunteers donated small blood samples for the isolation of neutrophils, with informed consent under protocols approved by the University of California, San Diego Human Subjects Institutional Review Board. Neutrophil isolation was performed using the Polymorphprep system (Axis-Shield, Oslo, Norway) and resuspended in HBSS without Ca2+ or Mg2+.
Neutrophil Granule Protease Release
Bacterial strains resuspended in HBSS with Ca2+ and Mg2+ (Hyclone) were added to neutrophils at a multiplicity of infection (MOI) of 10 or 25. After incubated at 37° C for 30 min with orbital rotation, tubes were centrifuged at 1000 g for 5 min and the supernatant collected into a 96-well microtiter plate. 0.5 μL of 20mM N-methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (Sigma) dissolved in Dimethyl sulfoxide was added to each well. After incubation at room temperature for 20 min, hydrolysis of the substrate was monitored spectrofluorometrically by change in absorbance at 405 nm. Assays were performed in triplicate and repeated at least three times. Paired two-tailed t-tests were used for statistical evaluation.
Neutrophil Oxidative Burst
Neutrophils were labeled with dichlorofluorescin diacetate (Sigma) at a final concentration of 20 μM in HBSS by incubation for 20 minutes at 37° C, then resuspended in 1ml HBSS. Approximately 106 neutrophils in 100 μl of HBSS were combined with bacteria (MOI 10–50) in 50 μl of HBSS with Ca2+/Mg2+. Cells were spun down together to initiate contact at 1000 × g for 5 min, then resuspended and incubated at 37°C for up to 45 min with orbital rotation. Aliquots (50 μl) were removed at 15 min intervals and oxidative burst was measured using a FACSCaliber flow cytometer (BD Biosciences). Neutrophils displaying positive oxidative burst were gated and mean fluorescence intensity calculated from this subpopulation using FlowJo software. Data shown are representative of experiments performed at least 5 times.
Bacterial Survival in Whole Blood
Blood was drawn from healthy volunteers into heparinized tubes with informed consent under protocols approved by the University of California, San Diego (UCSD) Human Subjects Institutional Review Board. Either 103 or 104 colony forming units of bacteria in 100 μl PBS were added to 300 μl fresh whole blood and incubated at 37° C with orbital rotation. 25 μl aliquots were removed and plated in serial dilutions for enumeration of surviving bacteria at various time points up to 2 h. Statistical significance was evaluated by paired two-tailed t-tests.
Mouse Infection Studies
All animal experiments were approved by the UCSD Committee on the Use and Care of Animals and performed using accepted veterinary standards. Outbred 9 week-old male CD-1 mice (Charles River Laboratories) were injected intraperitoneally with approximately 4 × 107 bacteria in a total volume of 150 μl mixed 1:2 with autoclaved 10% gastric mucin (MP Biomedicals) as previously described [37]. At 13 h post infection, blood was collected from the retro-orbital vein and bacterial titers were determined by serial dilution and plating. Statistical significance of bacterial blood titers was evaluated by paired two-tailed t-test. Survival studies were conducted using the same procedure and animals monitored for 10 days. Statistical comparisons of survival curves were performed using the Log-rank (Mantel-Cox) test.
RESULTS
Conserved serotype-specific patterns of GBS O-acetylation
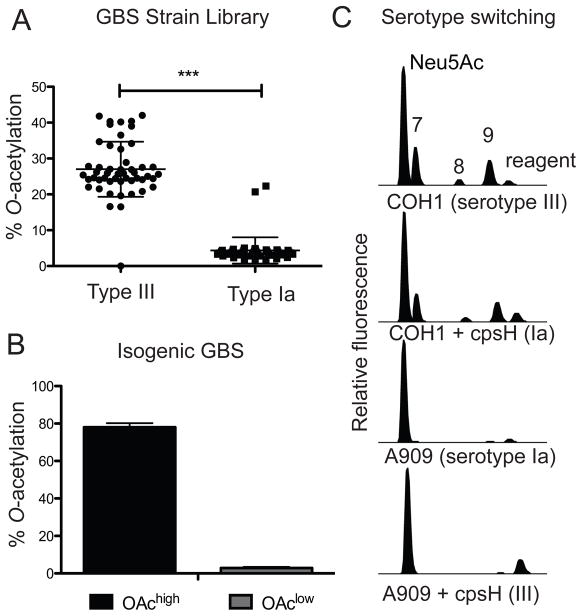
Previous studies have shown that all tested GBS strains (24 in all, representing 9 serotypes) have detectable, but highly variable levels of Sia O-acetylation [25, 31, 38]. It is unclear whether patterns of GBS O-acetylation are serotype-specific or whether different levels of this modification, regardless of serotype, may be associated with different clinical outcomes. To further clarify the prevalence and patterns of O-acetylation in natural GBS populations, we performed a quantitative analysis of Sia O-acetylation in serotypes Ia and III that cause disease in the United States. Structural differences in serotype Ia and III polysaccharides arise from distinct glycosyltransferases (24% identity at amino acid level) that are poised in the same position of the capsule operon, but which polymerize identical oligosaccharide repeating units in a slightly different manner [36]. Nearly 100 GBS isolates were evaluated in all (44 colonizing and 5 invasive type Ia strains, and 43 colonizing and 7 invasive type III strains).
Sia analyses were performed by acid hydrolysis and 1,2-diamino-4,5-methylene dioxybenzene-derivatization, followed by reverse phase HPLC resolution of O-acetylated and non-O-acetylated Sias as described previously [25]. This study revealed that the level of GBS O-acetylation was not associated with clinical outcome, but rather, a conserved feature of the particular serotype Ia or III capsular structure (Fig. 1A). Of the 49 serotype Ia strains evaluated in this study, 47 exhibited detectable, but very low levels of O-acetylation (1.7 – 5%). In contrast, 49/50 serotype III strains had significantly higher levels of O-acetylation (16.5 – 41.8% P < 0.0001).
Figure 1. Natural patterns and isogenic manipulation of O-acetylation phenotypes in GBS.
A. Native O-acetylation levels were evaluated by HPLC following 1,2-diamino-4,5-methylene dioxybenzene derivatization as described in the Experimental section, from 99 clinical and colonizing isolates. Data were analyzed using the Mann-Whitney test and indicate a significant correlation between GBS serotype and Sia O-acetylation phenotype, where type III strains have high levels of O-acetylation and type Ia strains have low levels (P<0.0001). B. Experimental variation of Sia O-acetylation as a single biochemical parameter was accomplished by isogenic manipulation of the NeuA Sia O-acetylesterase as previously described [33]. C. HPLC of fluorescently derivatized Sias from previously published strains where capsule serotype is switched between Ia and III, by overexpression of alternate capsule polymerase genes (cpsH). COH1 is a serotype III reference strain while A909 is a Ia reference strain; both were isolated from serious neonatal infections.
To determine whether differences in O-acetylation are due to restricted acceptor specificities of type Ia and III capsule polymerases, biochemical analyses compared type Ia and III reference strains to isogenic strains expressing the opposite CPS structure [produced by heterologous over-expression of capsule polymerase (cpsH) genes] [36]. Isogenic switching of CPS serotype (III to Ia and vise versa) did not result in a change in the overall level of O-acetylation. Strains maintained the level of O-acetylation present on the native CPS (Fig 1C). These results show that prevailing O-acetylation phenotypes are not related to the activities of CPS polymerase. The data further indicate that the architecture of the Ia or III polysaccharide does not per se constrain overall O-acetylation to a narrow range.
We previously demonstrated that polymorphism in the O-acetyl transferase (neuD) relates to differences in overall Sia O-acetylation, where Phe at amino acid position 88 (F88) appears to confer higher O-acetylation than C88. Here we extend these findings to show that highly O-acetylated outliers among the type Ia strains display polymorphisms in Sia biosynthetic genes that are identical to type III strains (Table 1). Consistent with other studies [34, 39, 40], these data that suggest GBS strains of different serotypes engage in horizontal gene exchange. Moreover, it appears that exchange of capsule biosynthetic genes from type III to type Ia strains can result in increased overall Sia O-acetylation independent of changes in capsule serotype (see Ia outliers in Fig 1A and Table 1).
Table 1.
Polymorphisms in Sia biosynthetic gene locus from strains in Fig 1A. Variations at amino acid positions 88 and 57 respectively of the NeuD O-acetyltransferase and NeuB Sia synthase sequences correlate with O-acetylation phenotypes. ND, not determined (this strain had little Neu5Ac expression).
| Serotype | Strain | Polymorphisms
|
%OAc | |
|---|---|---|---|---|
| NeuD-88 | NeuB-57 | |||
| III | COH1 | F | A | 40.5 |
| NICHD-1 | F | A | 27.1 | |
| NICHD-2 | F | A | 21.8 | |
| NICHD-3 | F | A | 38.5 | |
| NICHD-4 | F | A | 19.6 | |
| NICHD-5 | F | A | 36.6 | |
| NICHD-6 | F | A | 21.4 | |
| NICHD-7 | F | A | 20.7 | |
| NICHD-8 | F | A | 16.4 | |
| NICHD-9 | F | A | 20.1 | |
| NICHD-10 | F | A | 20 | |
| D136 | C | E | 3.7 | |
| NICHD-39 | ND | ND | 0 | |
| Serotype | Strain | Polymorphisms
|
%OAc | |
|---|---|---|---|---|
| NeuD-88 | NeuB-57 | |||
| Ia | A909 | C | E | 3.7 |
| NICHD-51 | C | E | 2.4 | |
| NICHD-52 | C | E also P176L | 4.5 | |
| NICHD-53 | C | E | 2.8 | |
| NICHD-54 | C | E | 1.8 | |
| NICHD-56 | C | E | 3.1 | |
| NICHD-57 | C | E | 3.4 | |
| NICHD-58 | C | E | 4.4 | |
| NICHD-59 | C | E | 3.3 | |
| NICHD-60 | C | E | 4.3 | |
| NICHD-61 | C | E | 3.8 | |
| NICHD-78 | F | A | 22.3 | |
| NICHD-90 | F | A | 20.7 | |
Sialic Acid O-Acetylation Reduces Sia-Mediated GBS Neutrophil Suppression
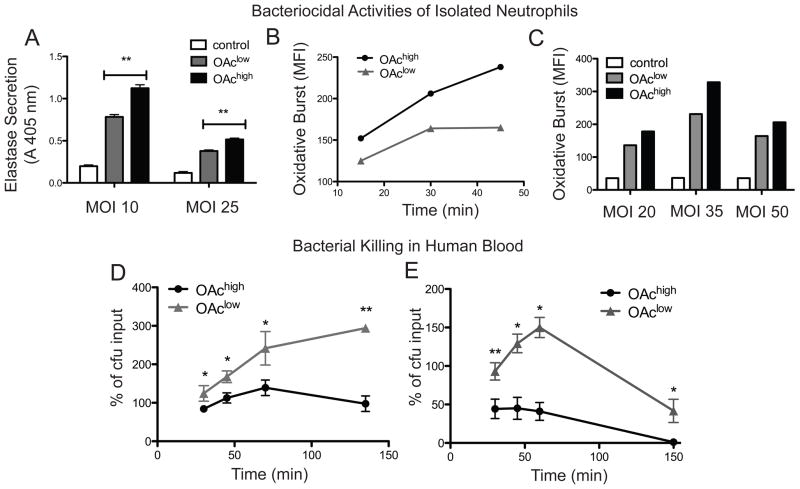
Sias on the surface of GBS contribute to immune evasion by engaging the Sia-binding receptor Siglec-9 on the surface of neutrophils. Blocking this interaction with a monoclonal antibody allowed increased neutrophil activation and increased bacterial killing [1]. We have previously shown that O-acetylation is a natural modification of Sias that also impairs GBS interactions with purified Siglec-9 [33]. Here we measured characteristic responses of freshly isolated human neutrophils to isogenic GBS strains to determine whether O-acetylation impairs Sia-mediated suppression of neutrophil functions. We use previously published GBS strains [33] that vary by a single inactivating amino acid substitution in the NeuA Sia O-acetylesterase, which allows variation of Sia O-acetylation as a single chemical parameter (i.e. without changing overall levels of capsule, sialic acids, or exposed galactose residues, summarized in Fig. 1B). These strains are hereafter referred to as OAchigh and OAclow GBS.
Neutrophils can kill bacteria in a variety of ways. Elastase is a broad-spectrum serine protease secreted by activated neutrophils that contributes to extracellular bacterial killing. Neutrophil elastase activity assays were performed on cell supernatants following incubation of OAchigh or OAclow bacteria. These experiments showed that the OAchigh strain induced significantly higher levels of granule protease secretion than the OAclow strain (P < .01) (Fig. 2A). In contrast, intracellular killing of bacteria can occur following phagocytosis and subsequent granule fusion to create a phagolysosome in which reactive oxygen species are produced. This process, referred to as oxidative burst, was used as a second measure of neutrophil activation to determine whether additional Sia-dependent neutrophil evasion mechanisms are altered by structural changes in GBS capsular Sias. Freshly isolated DCFH-DA labeled human neutrophils were incubated with GBS strains for up to 45 minutes. Neutrophil oxidative burst was measured at 15-min intervals by flow cytometry. Assays monitored over time (Fig. 2B) or with different multiplicity of infection (MOI) (Fig. 2C) show that the OAchigh strain stimulates a greater oxidative burst in neutrophils than the OAclow strain. These data further corroborate that GBS Sia O-acetylation interrupts bacterial suppression of neutrophil bactericidal activities.
Figure 2. Sia O-Acetylation stunts GBS suppression of human neutrophils and hampers bacterial killing in whole human blood.
A. Neutrophils were incubated with GBS at multiplicity of infection 10 or 25, and granule protease activity in the supernatant was measured using the substrate MeOSuc-Ala-Ala-Pro-Val-Nmec in a fluorescence liberation assay. The OAchigh strain stimulated greater secretion of elastase from isolated primary human neutrophils compared to the OAclow strain (**P < 0.01). B. and C. Oxidative burst was measured in dichlorofluorescin diacetate-labeled neutrophils by flow cytometry following incubation with GBS for 30 minutes at different MOI or at MOI 50 from 15–45 minutes. In all cases, the OAchigh strain stimulates a greater oxidative burst response as compared to the OAclow strain. D. Kinetics of bacterial proliferation (104 inocuclum) and E. bacterial killing (103 inocuclum) in 400 μl whole human blood indicate greater killing of the OAchigh strain (* P < 0.05 ** P < 0.005).
Sialic Acid O-Acetylation Alters the Kinetics of Bacterial Killing in Whole Human Blood
During systemic infection, interactions between GBS and neutrophils occur in the environment of the bloodstream, which contains many other sialylated entities, including serum proteins and additional immune and non-immune cells. To verify that GBS O-acetylation impacts bacterial survival in this more complex physiological milieu, the kinetics of bacterial proliferation and killing were examined in human whole blood. In experiments at higher inocula, the OAclow strain showed uncontrolled proliferation, while the OAchigh strain was maintained near the level of inoculation (Fig. 2D). At a lower initial inoculum where bacterial killing was observed, it took about five times longer to observe 50% killing of the OAclow strain compared to the OAchigh (Fig. 2E). Together, these results indicate that Sia O-acetylation imparts a marked fitness cost in the milieu of human whole blood.
Sia O-Acetylation Attenuates GBS Virulence in-vivo
To determine whether differences in bacterial fitness in vitro influence the outcome of invasive infection in vivo, OAchigh and OAclow strains were evaluated in a murine infection model. CD-1 mice were subjected to intraperitoneal (IP) injection of approximately 4 × 107 bacteria (n = 12 per group). At 13 hours post infection, blood drawn from mice infected with the OAclow strain had five fold higher levels of bacterial colony forming units than blood from mice infected with the OAchigh strain (P < 0.02) (Fig. 3A). Consistent with the differences observed in bacterial titers in blood, OAchigh GBS displayed attenuated virulence in the mouse model compared with the OAclow GBS (P < 0.0001). Whereas all mice injected with the OAclow strain succumbed to infection within 24 h, the majority of mice injected with the OAchigh strain cleared the infection (Fig. 3B). Taken together, these studies indicate that a subtle chemical modification of the GBS capsular polysaccharide structure has a profound effect on bacterial virulence.
Figure 3. Sia O-Acetylation attenuates strain virulence in vivo.
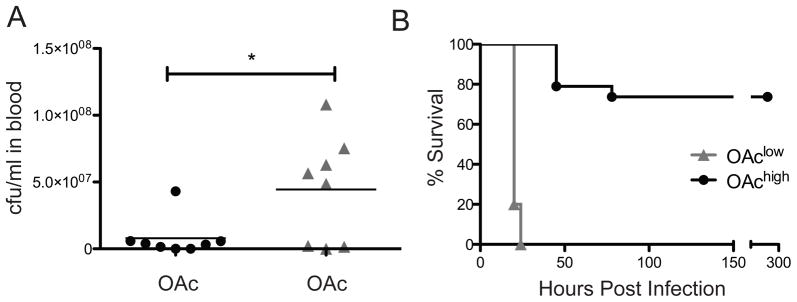
9 week old CD-1 male mice were injected intraperitonially with 4×107 colony forming units of OAchigh or OAclow GBS. A. Bacterial titers from blood collected at 13 hours post infection show a higher systemic bacterial load in mice infected with the OAclow strain compared to the OAchigh strain (P < 0.02) B. In a separate experiment, infected mice were monitored over the course of 10 days for mortality. Consistent with all other data the OAchigh strain is much less virulent than the OAclow strain (P < 0.0001).
DISCUSSION
We add this work to a growing body of literature demonstrating that bacterial cell surface carbohydrates and their modifications can have profound effects on host-microbe interactions [1, 2, 41–43]. Here we show for the first time, the physiological significance of bacterial capsular Sia O-acetylation (the most common structural modification of Sias) in the context of invasive GBS infection. GBS engages in an intracellular cycle of Sia O-acetylation (encoded by neuD) and de-O-acetylation (encoded by neuA) to arrive at a final level of the surface modification. Here we show that there are conserved serotype-specific patterns of Sia O-acetylation in GBS, which are related to a previously reported polymorphism in the NeuD O-acetyltransferase. Among serotype III strains, which express relatively high levels of this Sia modification, none of the 50 strains tested displayed more than 42% Sia O-acetylation. When these natural limits were overcome by genetic methods, increased levels of Sia O-acetylation impaired the ability of GBS Sias to suppress the bactericidal functions of isolated human neutrophils. Further investigations in the more complete milieu of whole human blood or infected animals revealed even more pronounced differences between OAclow and OAchigh GBS. These data show that Sia O-acetylation impairs GBS evasion of neutrophil killing mechanisms, likely mediated by reduced interaction with neutrophil receptor Siglec-9 or its functional murine counterpart, Siglec-E [1, 33]. However, the profound differences in bacterial proliferation in human blood and during murine infection suggest that the effect of O-acetylation in virulence may be multifactorial.
These studies clearly demonstrate that Sia O-acetylation is not a virulence factor in the conventional sense. However, the conserved partial O-acetylation of the serotype III capsule still requires explanation. We suggest that O-acetylation may be favored during bacterial colonization or persistence in the human vaginal or gastrointestinal tracts, environments where other bacteria express sialidases. In other contexts, sialidases are known to scavenge host Sia residues or reveal underlying structures for adhesion [44, 45]. Our previous studies lend credibility to this hypothesis, showing that O-acetylation protects GBS surface Sias from degradation by several sialidases of gastrointestinal bacteria [33]. Indeed, Sia removal from the GBS surface could be an effective form of niche competition, due to increased complement deposition, loss of immune suppression through Siglec engagement, enhanced innate immune recognition of underlying galactose residues, or enhanced adaptive immune recognition of immunogenic epitopes. This potential protective role of GBS O-acetylation in the context of microbial ecology during bacterial colonization may explain why serotype III GBS have highly conserved levels of this modification, despite the apparent cost of this modification during invasive infection.
Acknowledgments
We thank the UCSD GlycoTechnology Core Facility for assistance with HPLC analysis and Silpa Patel for GBS gene sequencing.
Funding
This work was supported by the UC President’s Postdoctoral Fellowship Program (AL), the Gianinni Family Foundation (AL), and National Institutes of Health grants R01-HD051796 (VN) and P01-HL057345 (AV).
Abbreviations
- CPS
capsular polysaccharide
- CFU
colony forming units
- GBS
Group B Streptococcus
- MOI
multiplicity of infection
- Neu5Ac
N-acetylneuraminic acid
- Sia
sialic acid
References
- 1.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 3.Yuki N. Carbohydrate mimicry: a new paradigm of autoimmune diseases. Curr Opin Immunol. 2005;17:577–582. doi: 10.1016/j.coi.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 5.Crespo HJ, Cabral MG, Teixeira AV, Lau JT, Trindade H, Videira PA. Effect of sialic acid loss on dendritic cell maturation. Immunology. 2009;128:e621–631. doi: 10.1111/j.1365-2567.2009.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Rauch U, Korpos E, Song J, Loser K, Crocker PR, Sorokin LM. Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J Immunol. 2009;182:6508–6516. doi: 10.4049/jimmunol.0804247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pangburn MK, Rawal N, Cortes C, Alam MN, Ferreira VP, Atkinson MA. Polyanion-induced self-association of complement factor H. J Immunol. 2009;182:1061–1068. doi: 10.4049/jimmunol.182.2.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 10.Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982;128:1278–1283. [PubMed] [Google Scholar]
- 11.Wessels MR, Rubens CE, Benedi VJ, Kasper DL. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci U S A. 1989;86:8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahler CM, Martin LE, Shih GC, Rahman MM, Carlson RW, Stephens DS. The (alpha2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–5947. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluschke G, Mayden J, Achtman M, Levine RP. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983;42:907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsch JA, Ram S. Factor H and Neisserial pathogenesis. Vaccine. 2008;26(Suppl 8):I40–45. doi: 10.1016/j.vaccine.2008.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlin AF, Lewis AL, Varki A, Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007;189:1231–1237. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avril T, Wagner ER, Willison HJ, Crocker PR. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun. 2006;74:4133–4141. doi: 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 2003;49:1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 19.Lewis AL, Desa N, Hansen EE, Knirel YA, Gordon JI, Gagneux P, Nizet V, Varki A. Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure. Proc Natl Acad Sci U S A. 2009;106:13552–13557. doi: 10.1073/pnas.0902431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orskov F, Orskov I, Sutton A, Schneerson R, Lin W, Egan W, Hoff GE, Robbins JB. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979;149:669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can J Biochem. 1976;54:1–8. doi: 10.1139/o76-001. [DOI] [PubMed] [Google Scholar]
- 22.Gamian A, Kenne L. Analysis of 7-substituted sialic acid in some enterobacterial lipopolysaccharides. J Bacteriol. 1993;175:1508–1513. doi: 10.1128/jb.175.5.1508-1513.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamian A, Romanowska E, Ulrich J, Defaye J. The structure of the sialic acid-containing Escherichia coli O104 O-specific polysaccharide and its linkage to the core region in lipopolysaccharide. Carbohydr Res. 1992;236:195–208. doi: 10.1016/0008-6215(92)85016-s. [DOI] [PubMed] [Google Scholar]
- 24.Glode MP, Lewin EB, Sutton A, Le CT, Gotschlich EC, Robbins JB. Comparative immunogenicity of vaccines prepared from capsular polysaccharides of group C Neisseria meningitidis O-acetyl-positive and O-acetyl-negative variants and Escherichia coli K92 in adult volunteers. J Infect Dis. 1979;139:52–59. doi: 10.1093/infdis/139.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Lewis AL, Nizet V, Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci U S A. 2004;101:11123–11128. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyn LA, Krohn MA, Hillier SL. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am J Obstet Gynecol. 2009;201:76 e71–77. doi: 10.1016/j.ajog.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol. 2000;96:498–503. doi: 10.1016/s0029-7844(00)00977-7. [DOI] [PubMed] [Google Scholar]
- 28.Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med. 2008;10:e27. doi: 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker CJ, Kasper DL. Group B streptococcal vaccines. Rev Infect Dis. 1985;7:458–467. doi: 10.1093/clinids/7.4.458. [DOI] [PubMed] [Google Scholar]
- 30.Lewis AL, Hensler ME, Varki A, Nizet V. The group B streptococcal sialic acid O-acetyltransferase is encoded by neuD, a conserved component of bacterial sialic acid biosynthetic gene clusters. J Biol Chem. 2006;281:11186–11192. doi: 10.1074/jbc.M513772200. [DOI] [PubMed] [Google Scholar]
- 31.Lewis AL, Cao H, Patel SK, Diaz S, Ryan W, Carlin AF, Thon V, Lewis WG, Varki A, Chen X, Nizet V. NeuA sialic acid O-acetylesterase activity modulates O-acetylation of capsular polysaccharide in group B Streptococcus. J Biol Chem. 2007;282:27562–27571. doi: 10.1074/jbc.M700340200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haft RF, Wessels MR. Characterization of CMP-N-acetylneuraminic acid synthetase of group B streptococci. J Bacteriol. 1994;176:7372–7374. doi: 10.1128/jb.176.23.7372-7374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiman S, Dahesh S, Carlin AF, Varki A, Nizet V, Lewis AL. Genetic and biochemical modulation of sialic acid O-acetylation on group B Streptococcus: phenotypic and functional impact. Glycobiology. 2009;19:1204–1213. doi: 10.1093/glycob/cwp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohnsack JF, Whiting A, Gottschalk M, Dunn DM, Weiss R, Azimi PH, Philips JB, 3rd, Weisman LE, Rhoads GG, Lin FY. Population structure of invasive and colonizing strains of Streptococcus agalactiae from neonates of six U.S. Academic Centers from 1995 to 1999. J Clin Microbiol. 2008;46:1285–1291. doi: 10.1128/JCM.02105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin FY, Whiting A, Adderson E, Takahashi S, Dunn DM, Weiss R, Azimi PH, Philips JB, 3rd, Weisman LE, Regan J, Clark P, Rhoads GG, Frasch CE, Troendle J, Moyer P, Bohnsack JF. Phylogenetic lineages of invasive and colonizing strains of serotype III group B Streptococci from neonates: a multicenter prospective study. J Clin Microbiol. 2006;44:1257–1261. doi: 10.1128/JCM.44.4.1257-1261.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaffin DO, Beres SB, Yim HH, Rubens CE. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J Bacteriol. 2000;182:4466–4477. doi: 10.1128/jb.182.16.4466-4477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming DO. Mucin model for group B type III streptococcal infection in mice. Infect Immun. 1980;27:449–454. doi: 10.1128/iai.27.2.449-454.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pannaraj PS, Edwards MS, Ewing KT, Lewis AL, Rench MA, Baker CJ. Group B streptococcal conjugate vaccines elicit functional antibodies independent of strain O-acetylation. Vaccine. 2009;27:4452–4456. doi: 10.1016/j.vaccine.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchaim D, Efrati S, Melamed R, Gortzak-Uzan L, Riesenberg K, Zaidenstein R, Schlaeffer F. Clonal variability of group B Streptococcus among different groups of carriers in southern Israel. Eur J Clin Microbiol Infect Dis. 2006;25:443–448. doi: 10.1007/s10096-006-0163-6. [DOI] [PubMed] [Google Scholar]
- 40.Jones N, Oliver KA, Barry J, Harding RM, Bisharat N, Spratt BG, Peto T, Crook DW. Enhanced invasiveness of bovine-derived neonatal sequence type 17 group B streptococcus is independent of capsular serotype. Clin Infect Dis. 2006;42:915–924. doi: 10.1086/500324. [DOI] [PubMed] [Google Scholar]
- 41.Vollmer W, Tomasz A. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect Immun. 2002;70:7176–7178. doi: 10.1128/IAI.70.12.7176-7178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, Prevost MC, Prochnicka-Chalufour A, Delepierre M, Tanguy M, Tang CM. Optimization of virulence functions through glucosylation of Shigella LPS. Science. 2005;307:1313–1317. doi: 10.1126/science.1108472. [DOI] [PubMed] [Google Scholar]
- 43.Liu CH, Lee SM, Vanlare JM, Kasper DL, Mazmanian SK. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc Natl Acad Sci U S A. 2008;105:3951–3956. doi: 10.1073/pnas.0709266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corfield T. Bacterial sialidases--roles in pathogenicity and nutrition. Glycobiology. 1992;2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- 45.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]