Abstract
Context
Inflammatory cytokines or cytokine inducers can alter basal ganglia activity, including reducing responsiveness to rewarding stimuli that may be mediated by cytokine effects on dopamine function.
Objectives
To determine whether long-term administration of the inflammatory cytokine interferon alfa reduces the basal ganglia response to reward and whether such changes are associated with decreased presynaptic striatal dopamine function and altered behavior.
Design
Cross-sectional and longitudinal studies.
Setting
Outpatient research unit and neuroimaging facilities at Emory University, Atlanta, Georgia.
Patients
Medically stable adults with chronic hepatitis C virus (HCV) infection eligible for interferon alfa treatment.
Main Outcome Measures
Neural activity in the ventral striatum during a hedonic reward task as measured by functional magnetic resonance imaging, uptake and turnover of radiolabeled fluorodopa F 18 (18F-dopa) in caudate and putamen using positron emission tomography, and interferon alfa–induced depression, anhedonia, fatigue, and neurotoxicity.
Results
Patients with HCV receiving interferon alfa for 4 to 6 weeks (n=14) exhibited significantly reduced bilateral activation of the ventral striatum in the win vs lose condition of a gambling task compared with patients with HCV awaiting interferon alfa treatment (n=14). Reduced activation of the ventral striatum was, in turn, significantly correlated with anhedonia, depression, and fatigue. In a separate longitudinal study, patients with HCV treated with interferon alfa for 4 to 6 weeks (n=12) exhibited significantly increased 18F-dopa uptake and decreased 18F-dopa turnover in caudate and putamen and in the same ventral striatal regions identified in the functional magnetic resonance imaging study. Baseline and percentage change in 18F-dopa uptake and turnover were correlated with behavioral alterations, including depression, fatigue, and neurotoxicity, during interferon alfa administration.
Conclusions
These data replicate and extend findings that inflammatory stimuli, including inflammatory cytokines, such as interferon alfa, alter basal ganglia activity and behavior in association with significant changes in presynaptic striatal dopamine function consistent with decreased dopamine synthesis or release.
Recent data suggest that inflammation and release of inflammatory cytokines may be involved in the pathogenesis of neuropsychiatric disorders, including major depression.1 To further understand mechanisms by which cytokines affect the brain and behavior, investigators have used neuroimaging strategies to examine neurocircuits that may be associated with neuropsychiatric symptoms during inflammatory exposure.
One brain region that has consistently been implicated in the impact of cytokines on the brain is the basal ganglia. Using the inflammatory cytokine interferon alfa and positron emission tomography (PET), early studies2–4 demonstrated that interferon alfa administration was associated with significant increases in the metabolism of fluorodeoxyglucose F 18 throughout basal ganglia nuclei in patients with malignant melanoma and patients with hepatitis C, consistent with findings in Parkinson disease (PD). Interferon alfa–induced increases in basal ganglia glucose metabolism were, in turn, associated with fatigue.2 Studies using functional magnetic resonance imaging (fMRI) have also shown altered activation patterns in basal ganglia after administration of inflammatory stimuli, including typhoid vaccination and endotoxin. For example, during a task of low-level visual stimulation, healthy volunteers vaccinated with Salmonella typhi exhibited increased neural reactivity in substantia nigra, which was correlated with psychomotor slowing and increased plasma concentrations of interleukin 6.5 In addition, administration of endotoxin to healthy volunteers was shown to reduce basal ganglia (ventral striatum) activation during a monetary reward task.6 Moreover, endotoxin-induced increases in depressed mood were mediated by between-group decreases in ventral striatal responses to monetary reward cues.6
These alterations in basal ganglia function may be secondary to the impact of inflammatory stimuli on the function of relevant monoamine neurotransmitters, including dopamine. Dopamine has a fundamental role in basal ganglia circuitry, including a primary role in stimulus-reward-response associations and motor activity.7,8 Chronic administration of interferon alfa to rhesus monkeys was shown to lead to depressive-like huddling behavior that was associated with significant reductions in the dopamine metabolite homovanillic acid.9 Huddling behavior was first described in rhesus monkeys that were chronically administered the monoamine depleting drug reserpine.10 Clinical observations in patients receiving interferon alfa for chronic hepatitis C have also documented Parkinson-like symptoms, which were relieved by treatment with the dopamine precursor levodopa, providing further evidence of the impact of interferon alfa on dopamine function.11,12
Based on these findings, the aim of the present study was to further examine the mechanisms by which inflammatory stimuli affect basal ganglia activity, with special emphasis on the impact of inflammatory cytokines on dopamine function. Accordingly, we studied patients with hepatitis C virus (HCV) undergoing long-term treatment with interferon alfa. As noted, interferon alfa has been shown to alter basal ganglia activity and reduce dopamine function in humans and nonhuman primates and has been associated with significant behavioral alterations, including depression, fatigue, and motor slowing, in numerous studies.9,13–20 Moreover, interferon alfa has been shown to access the brain and decrease monoamine metabolism through activation of a central inflammatory response that includes increased cerebrospinal fluid concentrations of interleukin 6.9,21 To establish interferon alfa effects on basal ganglia activity, we first undertook an fMRI study to examine the basal ganglia response to hedonic reward during a gambling task previously shown to activate relevant basal ganglia nuclei (ventral striatum).22 To determine the potential role of presynaptic striatal dopamine function in the observed effects, we also conducted a PET study to examine uptake and turnover of radiolabeled fluorodopa F 18 (18F-dopa). Together, these complementary neuroimaging strategies provided a comprehensive assessment of basal ganglia activity and dopamine function in the context of long-term cytokine exposure and allowed testing of the hypothesis that interferon alfa would reduce hedonic tone and decrease presynaptic striatal dopamine function in association with interferon alfa–induced behavioral changes.
METHODS
PATIENTS
Thirty-five men and women with HCV were included in the fMRI and PET studies. To qualify, patients were required to be serum positive for anti-HCV antibodies or HCV-RNA positive by reverse transcription–polymerase chain reaction. The exclusion criteria included unstable cardiovascular, endocrine, hematologic, renal, or neurologic disease (determined by physical examination and laboratory test results); human immunodeficiency virus infection (reported by patients’ treating physicians); decompensated liver disease; liver disease from any cause other than HCV; a history of schizophrenia or bipolar disorder or a diagnosis of major depression within 6 months of study enrollment (as determined by the Structured Clinical Interview for DSM-IV)23; and a score of less than 28 on the Mini-Mental State Examination,24 indicating cognitive impairment. All the patients had discontinued using antidepressant, anti-psychotic, or mood stabilizer medications for at least 4 weeks before brain imaging, except for 1 patient in the 18F-dopa study who required escitalopram, 20 mg/d, for depression during interferon alfa treatment. Participants were allowed to take zolpidem tartrate for sleep (n=5), but this use was discontinued at least 24 hours before imaging. No patients discontinued antidepressant medication for the purpose of study participation. In addition, patients with a history of alcohol or psycho-active substance abuse or dependence in the past year as determined by the Structured Clinical Interview for DSM-IV were excluded from the study. Urine drug screenings were conducted before all the brain imaging to rule out active substance abuse. Participants provided written informed consent, and study procedures were approved by the Emory University institutional review board.
ASSESSMENT OF BASAL GANGLIA RESPONSE TO A HEDONIC STIMULUS USING fMRI
Twenty-eight right-handed patients with HCV as described previously herein participated in the fMRI study. Participants included 14 patients with HCV treated with a combination of interferon alfa plus ribavirin for a mean (SEM) of 36.4 (2.9) days (treatment group) vs 14 patients with HCV awaiting combination therapy of interferon alfa and ribavirin (controls). Patients in the interferon alfa treatment group received either pegylated interferon alfa-2b (Pegintron; Schering-Plough), 1.5 μg/kg weekly (n=2), or pegylated interferon alfa-2a (Pegasys; Roche) (n=12), 180 μg weekly, both administered subcutaneously. Interferon alfa–treated patients also received oral ribavirin (800–1400 mg/d). Participation in the treatment group vs the control group was determined by patients and their treating physicians and was not based on standardized criteria or controlled by study protocol. The most common reason for delaying interferon alfa treatment was scheduling preference and was unrelated to issues involving time since sustained abstinence from drug abuse or history of psychiatric disease. No significant differences were noted between the interferon alfa–treated and control groups for mean (SD) age (43.6 [9.5] vs 48.2 [10.9] years, P=.25), race (9 black and 5 white vs 5 black, 1 Hispanic, and 8 white; P=.24), sex distribution (6 women and 8 men in each group), history of depression (1 control and 3 treated with interferon alfa), or history of substance abuse (8 controls [including 6 stimulant abusers, 1 of whom used intravenous stimulants] and 8 treated with interferon alfa [including 7 stimulant abusers, none of whom used intravenous stimulants]).
fMRI Task
A previously published gambling task that proved effective in eliciting specific activation of basal ganglia structures was adapted for the study.22 In the task, participants had to guess which of 2 cards was “red” (hearts or diamonds) by pressing 1 of 2 buttons on an MRI response box held in their right hand. Two seconds into the trial, the selected card was turned over, and, depending on its color, the participant either won (red) or lost (black) $1. Unbeknownst to the patient, the sequence of wins and losses was temporally arranged as a noisy sinusoid with a slow linear trend that favored wins over time. This procedure allowed for experimental control of the task while masking the deterministic nature of the game from the participant, thereby eliciting a realistic feeling of gambling. At the beginning of the game, each participant started with a credit of $16 and ended with a total win sum of $23, which the volunteer believed was contingent on his or her specific gambling choice but was, in fact, fixed for all participants.
Clinical Assessments
Clinical assessments included measurements of depression, anhedonia, fatigue, and symptoms of neurotoxicity, including sickness, cognitive dysfunction, and motor symptoms. Depression was assessed using the observer-rated Montgomery-Asberg Depression Rating Scale, a 10-item scale that includes measures of sadness, inner tension, concentration difficulties, inability to feel, pessimistic thoughts, suicidal thoughts, reduced sleep, reduced appetite, and lassitude.25 Anhedonia was assessed using the 14-item self-rating Snaith-Hamilton Pleasure Scale, with higher values corresponding to more severe anhedonia.26 Fatigue was assessed using the Multidimensional Fatigue Inventory, a 20-item self-rating scale yielding specific subscales for general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue, with the total score of each subscale ranging from 4 (best) to 20 (worst).27 Finally, interferon alfa–induced neurotoxicity was assessed using the Neurotoxicity Rating Scale,28,29 a 39-item self-report questionnaire with questions rated from 0 (not present) to 4 (extremely severe) that has been used to measure symptoms experienced by patients exposed to chronic inflammatory stimuli, including interferon alfa. For data analysis, Neurotoxicity Rating Scale symptoms were grouped into 3 symptom domains: sickness (tiredness/fatigue, fever, sick feeling, body aches, joint/ muscle pain, and headaches), cognitive symptoms (difficulty making decisions, distractibility, episodes of confusion, word-finding problems, and memory problems), and motor symptoms (motor slowing, walking problems, and tremor/shakiness), as described elsewhere.29,30
fMRI Processing and Analysis
All the MRI data were collected using a 3-T scanner (Siemens Trio; Siemens Medical Solutions) using 3 functional runs (gradient echo-planar imaging; repetition time, 2.35 seconds; echo time, 28 milliseconds; 135 volumes; voxel size, 3-mm isotropic, 64×64 voxel matrix; 35 sections) and a high-resolution T1-weighted anatomical scan (MPRAGE; repetition time, 2.3 seconds; echo time, 3.02 milliseconds; 1-mm isotropic voxels; whole-brain coverage) (eAppendix 1; http://www.archgenpsychiatry.com). All fMRI processing was performed using the AFNI (Analysis of Functional NeuroImages) software package.31
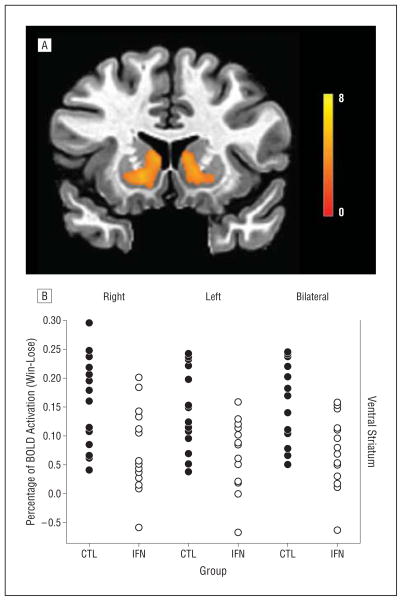
To select relevant regions of interest (ROIs), a whole-brain voxelwise t test was performed comparing the mean value for the win-lose contrast across all the patients to 0. The statistical threshold for significance was set at a single-voxel P<.005 and cluster size k≥19 voxels, as determined by a Monte Carlo simulation of cluster size distribution under the null hypothesis (using the AFNI routine 3dClustSim) to yield an experiment-wise (corrected) α <.05.32 Using this procedure, 20 clusters were identified. Two significant clusters in the basal ganglia corresponding to the right and left ventral striatal regions were selected as ROIs (Figure 1A). These clusters were 146 and 119 voxels in size, corresponding to 146×27 mm3 and 119×27 mm3, respectively (voxel size=3×3×3 mm). The stereotactic coordinates of the peak voxels in these clusters were x=13.5, y=4.5, z=−3.5 and x=−10.5, y=4.5, z=−3.5, respectively. The ventral striatum was defined as the inferior aspect of the corpus striatum where the caudate and the putamen join.
Figure 1.
Group differences in striatal responses to a hedonic reward task. Neural activation in the ventral striatum was assessed by blood oxygen level–dependent (BOLD) responses in the win-lose condition of a gambling task using functional magnetic resonance imaging. A, Right and left regions of interest (ROIs) (depicted here) reflect maximal activation in the groups combined, and the average percentage of BOLD signal change corresponding to the win-lose contrast across all ROI voxels (right, left, or bilateral) was used for group comparisons and correlations with behavioral end points. B, The interferon alfa treatment group (IFN) had significantly decreased activation in the right, left, and bilateral ventral striatal regions compared with the untreated control group (CTL) (P<.01 for all comparisons). The color bar ranges from t=0 to t=8, with a group activation threshold of P<.005 uncorrected and a cluster size k≥19 voxels (P<.05 corrected).
Statistical Analysis
Evoked activity in the ventral striatum (right, left, and bilateral) was determined by averaging the estimated percentage of blood oxygen level–dependent signal change corresponding to the win-lose contrast across all ROI voxels. Results were compared between interferon alfa–treated and untreated patients with HCV by a 2-sample t test. Neuropsychiatric scores were also compared between groups using the same analytic strategy. Relationships between evoked ventral striatal activity and neuropsychiatric symptoms were assessed using Bravais-Pearson correlations.
ASSESSMENT OF PRESYNAPTIC STRIATAL DOPAMINE FUNCTION USING PET
This study enrolled 12 patients with HCV (10 right-handed and 2 left-handed, including 5 individuals who also participated as interferon alfa–treated patients in the fMRI study) who fit the inclusion and exclusion criteria described previously herein (mean [SD] age, 47.4 [5.7] years; 6 women and 6 men) and were eligible to receive treatment with either pegylated interferon alfa-2b (Pegintron) (n=5), 1.5 μg/kg/wk, or pegylated interferon alfa-2a (Pegasys) (n=7), 180 μg/wk, plus oral ribavirin, 800 to 1200 mg/d, for 48 weeks. Two patients had a history of depression and 9 patients had a history of substance abuse, including 6 with a history of stimulant abuse, 1 of whom had intravenous stimulant abuse.
PET Scanning and Image Preprocessing
Presynaptic dopamine function was assessed by 18F-dopa PET at baseline (before initiation of interferon alfa; visit 1) and after a mean (SEM) of 41.2 (5.2) days of interferon alfa administration (visit 2). Time taking interferon alfa did not differ from that in the fMRI study (P=.43). All the patients were required to fast for 4 hours before PET to stabilize neutral amino acid levels. One hour before injection of 18F-dopa, patients were given a combination of oral carbidopa, 150 mg (decarboxylase inhibitor), and oral entacapone, 400 mg (catechol-O-methyltransferase inhibitor), to prevent early decarboxylation and breakdown of 18F-dopa. Before scanning, participants had an intravenous catheter inserted into an antecubital vein for tracer injection.
After a transmission scan for attenuation correction and injection of 5.0 mCi of 18F-dopa, patients underwent 94-minute-long PET (four 1-minute frames, three 2-minute frames, three 3-minute frames, and fifteen 5-minute frames) (eAppendix 2). The ROIs for the left and right putamen and the caudate nucleus along with the occipital cortex were identified by the LONI probabilistic brain atlas after conversion to Montreal Neurological Institute sterotactic space.33 Time activity curves were computed from the scans after spatial normalization to Montreal Neurological Institute space using the software package SPM5 by computing a weighted average of each frame’s voxel intensities according to probability values encoded in the atlas ROIs.
Estimation of F-Dopa Uptake Rate
Using the Patlak graphical method,34,35 Ki, the F-dopa uptake rate constant, was determined based on values starting from 24 minutes to the end of scanning (except for 1 patient where the interval for graphical fitting was restricted from 24 to 64 minutes due to excessive motion at the end of scanning).
Estimation of Effective Dopamine Turnover
Effective dopamine turnover values were computed for ROIs using the occipital cortex as reference tissue, limiting the graphical fit to the interval 24 to 94 minutes (again except for 1 patient: 24–64 minutes due to excessive motion).36 Note that the Patlak method, which was used to derive the uptake rate constants, assumes that 18F-dopa is irreversibly bound, whereas the reference tissue approach described by Sossi et al36 assumes some degree of loss. To verify that uptake results using the Patlak approach were similar when loss was factored in, uptake rate constants were computed using the full reference tissue model.37 The effects of interferon alfa on uptake rate constants were still significant when derived using the full reference tissue model (data not shown).
Clinical Assessments
The clinical scales as described for the fMRI study were used to assess neuropsychiatric symptoms before and during interferon alfa administration.
Statistical Analysis
Changes in 18F-dopa uptake rates and turnover and neuropsychiatric symptoms between visit 1 (baseline) and visit 2 (study follow-up) were assessed using paired t tests. Relationships between 18F-dopa kinetics in specific ROIs and neuropsychiatric symptoms were assessed using Bravais-Pearson correlations. To examine potential vulnerability markers for the development of interferon alfa–induced neuropsychiatric symptoms, correlations were performed between baseline 18F-dopa uptake rates and turnover and longitudinal changes in neuropsychiatric symptoms (visit 2–visit 1). All the probabilities were 2-tailed, with an α level of P<.05.
RESULTS
INTERFERON ALFA–INDUCED ALTERATIONS IN VENTRAL STRIATAL RESPONSE AND RELATIONSHIP WITH NEUROPSYCHIATRIC SYMPTOMS
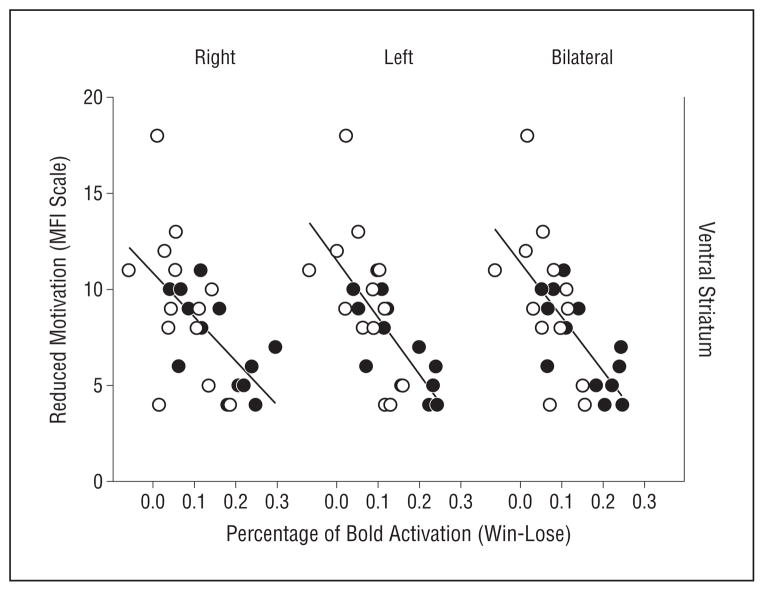
Patients undergoing interferon alfa therapy for 4 to 6 weeks exhibited significantly lower activation in the ventral striatum (right, left, and bilateral) during winning trials relative to losing trials compared with untreated patients with HCV (right side: 0.158 vs 0.075, P=.008; left side: 0.145 vs 0.071, P=.006; and bilateral: 0.151 vs 0.073, P=.005) (Figure 1B). Results were unchanged when controlling for history of depression, substance abuse, and stimulant abuse, alone or combined (P≤.01 for all). In addition, interferon alfa–treated patients exhibited higher neuropsychiatric scores than did untreated patients, with the greatest differences being in scales assessing depressive symptoms and anhedonia (Table 1). In all the participants, reduced evoked activity in the ventral striatum was associated with increased intensity of neuropsychiatric symptoms, with the highest correlations occurring in association with reduced motivation, reduced activity, and depression (Table 2 and Figure 2). Similar relationships were observed for ROIs in the right and left ventral striatal regions and in the combined, bilateral ROI. Significant correlations between ventral striatal activity (right, left, and bilateral) and Montgomery-Asberg Depression Rating Scale scores persisted after removing scale items related to anhedonia and fatigue. Finally, correlation coefficients between ventral striatal activity and symptoms were similar in interferon alfa–treated and control groups when examined separately, with no significant differences in slopes between groups (data not shown).
Table 1.
Neuropsychiatric Symptoms in 14 Interferon Alfa–Treated Patients With HCV Compared With 14 Untreated Controls With HCV
| Symptom Dimension | Scale Score, Mean (SD)
|
P Value | |
|---|---|---|---|
| Control Group | Interferon Alfa–Treated Group | ||
| Depression (MADRS) | 3.4 (3.1) | 11.1 (9.6) | <.01 |
| Anhedonia (SHAPS) | 2.6 (3.2) | 9.6 (8.1) | <.01 |
| Fatigue (MFI)a | |||
| General fatigue | 9.1 (4.7) | 11.6 (5.5) | .22 |
| Physical fatigue | 8.8 (2.7) | 10.4 (5.2) | .32 |
| Reduced activity | 7.8 (2.6) | 9.4 (3.6) | .21 |
| Reduced motivation | 7.2 (2.4) | 9.1 (3.9) | .16 |
| Mental fatigue | 8.3 (3.4) | 9.6 (4.1) | .40 |
| Neurotoxicity (NRS) | |||
| Sickness symptoms | 3.8 (3.3) | 6.3 (3.8) | .08 |
| Cognitive symptoms | 2.1 (2.2) | 2.8 (2.6) | .41 |
| Motor symptoms | 0.7 (1.3) | 1.1 (1.5) | .52 |
Abbreviations: HCV, hepatitis C virus; MADRS, Montgomery-Asberg Depression Rating Scale; MFI, Multidimensional Fatigue Inventory; NRS, Neurotoxicity Rating Scale; SHAPS, Snaith-Hamilton Pleasure Scale.
Data for the MFI scale were missing for 1 untreated patient with HCV.
Table 2.
Bravais-Pearson Correlations Between Evoked Activity in the Ventral Striatum and Neuropsychiatric Symptoms
| Symptom Dimension | Ventral Striatum
|
|||||
|---|---|---|---|---|---|---|
| Bilateral | P Value | Right | P Value | Left | P Value | |
| Depression (MADRS) | −0.525 | <.005a | −0.446 | <.05 | −0.561 | <.005a |
| Anhedonia (SHAPS) | −0.483 | <.01 | −0.437 | <.05 | −0.492 | <.01 |
| Fatigue (MFI) | ||||||
| General fatigue | −0.416 | <.05 | −0.319 | .10 | −0.476 | <.05 |
| Physical fatigue | −0.426 | <.05 | −0.327 | .09 | −0.488 | <.01 |
| Reduced activity | −0.570 | <.005a | −0.511 | <.01 | −0.585 | <.005a |
| Reduced motivation | −0.669 | <.001a | −0.604 | <.001a | −0.683 | <.001a |
| Mental fatigue | −0.382 | <.05 | −0.283 | .15 | −0.446 | <.05 |
| Neurotoxicity (NRS) | ||||||
| Sickness symptoms | −0.299 | .12 | −0.297 | .12 | −0.279 | .14 |
| Cognitive symptoms | −0.409 | <.05 | −0.356 | .06 | −0.431 | <.05 |
| Motor symptoms | −0.248 | .20 | −0.257 | .18 | −0.223 | .25 |
Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale; MFI, Multidimensional Fatigue Inventory; NRS, Neurotoxicity Rating Scale; SHAPS, Snaith-Hamilton Pleasure Scale.
Significant after controlling for multiple comparisons (Bonferroni) across symptom dimensions (0.05 / 10 = .005).
Figure 2.
Relationship between activation in the ventral striatum and reduced motivation. Neural activation in the ventral striatum was assessed by blood oxygen level–dependent (BOLD) responses in the win-lose condition of a gambling task using functional magnetic resonance imaging in patients with hepatitis C virus receiving interferon alfa for 4 to 6 weeks (white dots) and those awaiting interferon alfa treatment (black dots). Significant correlations were found between ventral striatal activation and reduced motivation as measured by the Multidimensional Fatigue Inventory (MFI) (P <.001 in all cases).
INTERFERON ALFA–INDUCED ALTERATIONS IN 18F-DOPA UPTAKE AND TURNOVER AND RELATIONSHIP WITH NEUROPSYCHIATRIC SYMPTOMS
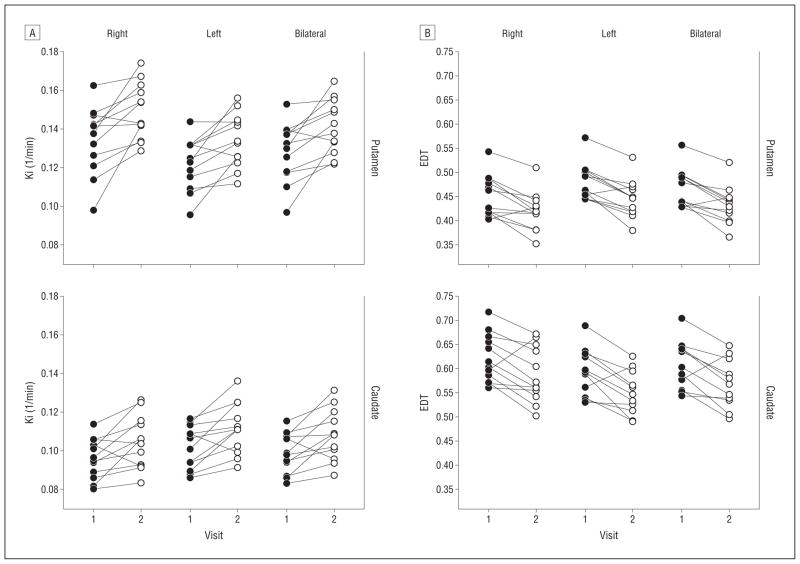
Figure 3 shows PET scans depicting mean uptake in the sample as a whole before and during interferon alfa treatment. Treatment with interferon alfa was associated with significant bilateral increases in 18F-dopa uptake in caudate and putamen (Table 3, PFigure 3, and Figure 4A). Dopamine turnover was also altered by interferon alfa administration, with a significant decrease in caudate and putamen (Table 3 and Figure 4B). No differences were found between patients with and without a history of depression, substance abuse, or stimulant abuse, alone or combined ( >.15 for all). To examine whether interferon alfa treatment altered 18F-dopa uptake and turnover in the same brain regions where activation differences were found in the fMRI study, 18F-dopa uptake and turnover were measured in the ventral striatal ROIs from the fMRI study. Significant increases in 18F-dopa uptake and decreases in 18F-dopa turnover were found in the right, left, and bilateral ventral striatal regions (P <.05 for all) (eFigure 1). Finally, regional differences in 18F-dopa uptake before vs during interferon alfa administration exhibited a striking overlap with the ventral striatal regions that showed a significant difference between interferon alfa–treated and control participants in the fMRI response to the gambling task (eFigure 2).
Figure 3.
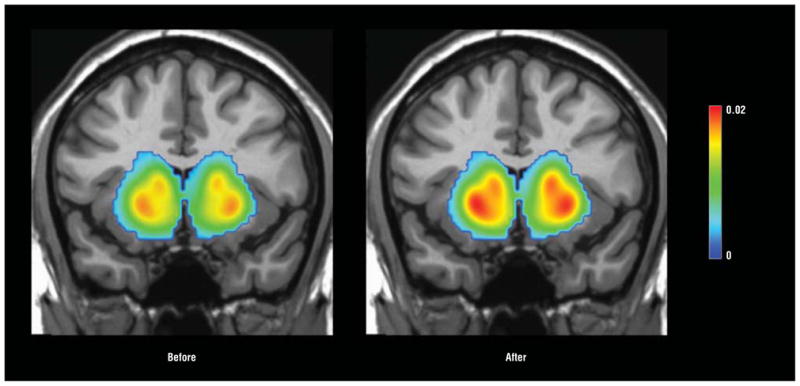
Mean fluorodopa F 18 (18F-dopa) uptake before and after interferon alfa therapy. Patients with chronic hepatitis C virus were administered 18F-dopa followed by positron emission tomography before and after 4 to 6 weeks of interferon alfa treatment. Mean images from the sample as a whole before and during interferon alfa administration indicate significantly higher uptake of 18F-dopa in caudate and putamen during interferon alfa treatment. The color bar represents the range of uptake values (Ki [1/min]) from 0 to 0.02.
Table 3.
Change in 18F-Dopa Uptake (Ki) and Turnover (EDT) During Interferon Alfa Administration
| Region |
18F-Dopa Turnover, Mean (SEM)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cluster Ki (k rate 1/min)
|
EDT
|
|||||||
| Baseline (Visit 1) | Treatment (Visit 2) | Delta | P Valuea | Baseline (Visit 1) | Treatment (Visit 2) | Delta | P Valuea | |
| Caudate | ||||||||
| Left | 0.0102 | 0.0111 | 0.00097 (0.000282) | <.01 | 0.594 | 0.550 | −0.0436 (0.0105) | <.01 |
| Right | 0.0096 | 0.0105 | 0.00086 (0.000307) | <.05 | 0.623 | 0.587 | −0.0358 (0.0116) | <.05 |
| Bilateral | 0.0099 | 0.0108 | 0.00092 (0.000290) | <.01 | 0.608 | 0.567 | −0.0401 (0.0108) | <.01 |
| Putamen | ||||||||
| Left | 0.0122 | 0.0134 | 0.00118 (0.000333) | <.005 | 0.481 | 0.445 | −0.0367 (0.00707) | <.005 |
| Right | 0.0135 | 0.0149 | 0.00150 (0.000402) | <.005 | 0.451 | 0.419 | −0.0326 (0.00821) | <.005 |
| Bilateral | 0.0128 | 0.0141 | 0.00133 (0.000360) | <.005 | 0.466 | 0.432 | −0.0345 (0.00744) | <.005 |
Abbreviations: EDT, effective dopamine turnover; 18F-dopa, fluorodopa F 18.
P values were obtained by paired t tests.
Figure 4.
Fluorodopa F 18 (18F-dopa) uptake and effective dopamine turnover before (black dots) and during (white dots) interferon alfa therapy. Patients with chronic hepatitis C virus were administered 18F-dopa followed by positron emission tomography before (visit 1) and after 4 to 6 weeks of interferon alfa treatment (visit 2). A, The 18F-dopa uptake (Ki [1/min]) was measured using the Patlak method (see the “Methods” section) in putamen and caudate. Significant increases in 18F-dopa uptake were found during interferon alfa administration compared with baseline (putamen: P <.005 for all; caudate: P <.05 for all) (Table 3), B, Effective dopamine turnover (EDT) was calculated by the method of Sossi et al36 (see the “Methods” section) in putamen and caudate. Significant decreases in EDT were found during interferon alfa administration compared with baseline (putamen: P <.005 for all; caudate: P <.05 for all) (Table 3).
BASELINE PRESYNAPTIC STRIATAL DOPAMINE FUNCTION AND INTERFERON ALFA–INDUCED BEHAVIORAL CHANGES
Presynaptic striatal dopamine function at baseline correlated significantly with the development of interferon alfa–induced neuropsychiatric symptoms (visit 2 – visit 1). Lower 18F-dopa uptake rates in caudate nucleus (bilateral) at baseline were associated with the development of fatigue, especially in dimensions of mental fatigue (R = −0.704, P = .01) and reduced motivation (R = −0.579, P = .048) as well as sickness symptoms (R = −0.611, P = .04) and cognitive symptoms (R = −0.636, P = .03). Similarly, lower baseline 18F-dopa uptake in putamen (bilateral) was associated with the development of mental fatigue (R = −0.0713, P = .009) and sickness symptoms (R = −0.696, P = .01) during interferon alfa administration. On the other hand, higher 18F-dopa turnover rates at baseline in the caudate and putamen were associated with the development of depressive symptoms (caudate: R = 0.699, P = .01; putamen: R = 0.626, P = .03), mental fatigue (caudate: R = 0.667, P = .02; putamen: R = 0.789, P = .002), and cognitive symptoms (caudate: R = 0.637, P = .03; putamen: R = 0.670, P = .02).
CHANGE IN 18F-DOPA UPTAKE AND TURNOVER AND INTERFERON ALFA–INDUCED BEHAVIORAL SYMPTOMS
Because of the significant relationship between dopamine activity at baseline and development of neuropsychiatric symptoms during interferon alfa therapy, the relationship between interferon alfa–induced changes in dopamine function and symptoms was assessed controlling for 18F-dopa baseline values (percentage change from baseline computed as [treatment − baseline / baseline] × 100). Results indicated that percentage change in 18F-dopa uptake in caudate and putamen from baseline was not associated with the development of neuropsychiatric symptoms during interferon alfa treatment (visit 2 – visit 1) but was predictive of symptom intensity at visit 2 during interferon alfa treatment. A greater percentage increase in 18F-dopa uptake in the caudate nucleus during interferon alfa therapy was associated with more intense symptoms of mental fatigue (R = 0.578, P = .049) and sickness (R = 0.587, P = .045) at 4 to 6 weeks of interferon alfa therapy. Similarly, a greater percentage increase in 18F-dopa uptake in the putamen during interferon alfa treatment was associated with more intense symptoms of sickness at visit 2 (R = 0.619, P = .03). No correlations were found between percentage change in 18F-dopa turnover and development of neuropsychiatric symptoms (visit 2 – visit 1) or symptoms at visit 2 during interferon alfa administration. Moreover, no significant correlations were found between change in 18F-dopa uptake or turnover (visit 2 – visit 1) and development of neuropsychiatric symptoms (visit 2 – visit 1).
COMMENT
Data reported herein replicate previous findings using fMRI that inflammatory stimuli are associated with decreased basal ganglia activity involving hedonic reward circuitry, which correlated with behavioral alterations, including anhedonia, depression, and fatigue.2,5,6 In addition, the present findings extend these data by demonstrating that presynaptic striatal dopamine function is directly affected by inflammatory cytokines, such as interferon alfa, as manifested by increased uptake and decreased turnover of the radiolabeled dopamine precursor 18F-dopa in ventral striatal regions that corresponded to those found to differ between interferon alfa–treated and control participants in the fMRI study. Together, these findings provide further evidence that inflammatory stimuli, including inflammatory cytokines, target basal ganglia and dopamine function to induce behavioral changes associated with inflammation in humans.
Despite overlap of interferon alfa–induced behavioral changes with PD and induction of frank Parkinson-like symptoms in some interferon alfa–treated patients, the findings of increased uptake of 18F-dopa after interferon alfa administration is in stark contrast to the decreased 18F-dopa uptake seen in the basal ganglia of patients with PD. Decreased 18F-dopa uptake in PD is believed to be a function of loss of dopaminergic neurons or their projections throughout the basal ganglia.38–40 Thus, increased 18F-dopa uptake after interferon alfa administration suggests that neuronal loss is absent or is in such an early stage that only compensatory metabolic changes are apparent. Indeed, in early-stage PD, increased 18F-dopa uptake has been reported in the frontal cortex, ostensibly reflecting compensation for neuronal loss in the basal ganglia.39,41 Also, in contrast to PD, interferon alfa administration was associated with decreased dopamine turnover. In PD, although uptake is low owing to loss of dopaminergic neurons, the neurons that are available to uptake dopamine exhibit rapid turnover, indicating that synthesis and release is intact (or even increased) in surviving cells.40,42 Nevertheless, although increased uptake was observed during interferon alfa treatment, there was no evidence of complementary increased turnover, suggesting impaired dopamine release or increased dopamine reuptake.
Given that 18F-dopa is capable of passive entry into relevant dopaminergic neurons (as opposed to requiring active uptake through the dopamine transporter), the increased uptake of 18F-dopa coupled with decreased turnover/release suggests that during interferon alfa administration, 18F-dopa is being synthesized into dopamine by aromatic amino acid decarboxylase (AADC) in dopaminergic nerve terminals but is then being trapped.40 The presence of enhanced 18F-dopa uptake provides evidence that AADC activity is intact or increased given that 18F-dopa uptake is severely impaired in AADC deficiency.43 An increase in synthetic activity of AADC is seen after dopamine depletion with reserpine44 and, therefore, may represent a compensatory response to an interferon alfa–induced disruption of upstream enzyme machinery responsible for production of dopa, including reduced activity of tyrosine hydroxylase, which converts tyrosine to dopa or phenylalanine hydroxylase, which converts phenylalanine to tyrosine. Tetrahydro-biopterin (BH4) is an essential cofactor for both of these enzymes while also being a cofactor for nitric oxide (NO) synthase, which converts arginine to NO.45 Of relevance to interferon alfa and inflammation, BH4 is very labile and highly sensitive to inflammation-induced oxidative stress and nonenzymatic oxidation, which leads to irreversible degradation of BH4 to dihydroxyanthopterin.45 In addition, BH4 can be consumed in the context of inflammation-induced production of NO.45 For example, intramuscular injection of interferon alfa to rats has been shown to decrease central nervous system concentrations of BH4 through stimulation of NO.46 Treatment with an inhibitor of NO synthase was found to reverse interferon alfa’s inhibitory effects on brain concentrations of BH4 and dopamine. Interleukin 6, which is increased in cerebrospinal fluid after interferon alfa administration,21 also has been shown to reduce BH4 content in sympathetic neurons.47
Another mechanism that may contribute to observed alterations in dopamine function during interferon alfa treatment, in particular decreased dopamine turnover, is impaired dopamine release. One factor particularly relevant in this regard is kynurenic acid. Kynurenic acid is synthesized in astrocytes from kynurenine, which is generated from the breakdown of tryptophan by the enzyme indoleamine 2,3-dioxygenase (IDO).48 Evidence of IDO activation as reflected by decreased peripheral blood tryptophan levels and increased kynurenine levels has been repeatedly associated with depressive symptoms in interferon alfa–treated patients,49,50 and a recent study51 has shown that a polymorphism in the promoter region of the IDO gene predicts depressive symptoms during interferon alfa therapy. Interferon alfa administration has been shown to lead to increased kynurenine and kynurenic acid concentrations in cerebrospinal fluid of interferon alfa–treated patients with HCV.52 Relevant to dopamine turnover, intrastriatal administration of kynurenic acid to rats has been shown to lead to marked reductions in extracellular dopamine that can be reversed by the allosteric alpha-7 nicotinic acetylcholine receptor agonist galantamine.53 These effects of kynurenic acid are believed to be related to inhibition of dopamine release through inhibition of glutamate release by blocking the alpha-7 nicotinic acetylcholine receptor.53 Glutamate is an important regulator of dopamine release.54
Another pathway that may be involved in decreased dopamine turnover during exposure to inflammatory stimuli, such as interferon alfa, is increased dopamine reuptake, thereby serving to concentrate radiolabeled dopamine in the nerve terminal. Data suggest that activation of cytokine signaling pathways, including components of the mitogen-activated protein kinase cascade, can lead to significant increases in activity and expression of monoamine transporters, including the dopamine transporter.55,56 For example, human dopamine transporter–expressing cells transfected with a constitutively activated mitogen-activated protein kinase kinase exhibit increased dopamine reuptake, whereas treatment of rat striatal synaptosomes with mitogen-activated protein kinase kinase inhibitors was associated with decreased dopamine reuptake in a concentration- and time-dependent manner.55
Regarding the relationship of basal ganglia changes with behavior, the correlation of reduced neural activation to hedonic reward with depression, anhedonia, and fatigue is not surprising given the importance of this neurocircuit in motivation and motor activity. However, reduced 18F-dopa uptake at baseline was predictive of interferon alfa–induced behavioral changes, indicating that dopaminergic tone may also serve as an important vulnerability factor regarding the impact of cytokines on behavior.
Several strengths and limitations warrant consideration. Regarding strengths, the study capitalized on administration of a standardized chronic inflammatory stimulus to a population of individuals who were free of psychotropic medications or unstable medical illness. Moreover, in the PET study, each patient served as his or her own control in a pre-post design, although lacking a test-retest control group limits the ability to assess potential effects of time and repeated exposure to the scanning environment. Finally, the consistency of results regarding the impact of interferon alfa on the basal ganglia across experimental paradigms (eg, fMRI and PET) in conjunction with the consistency of results with studies using other inflammatory stimuli (eg, typhoid vaccination and endotoxin) supports the generalizability of the findings. Regarding limitations, all the patients were infected with hepatitis C and were receiving ribavirin. In addition, several patients had a history of substance abuse, including a history of stimulant abuse, which could have long-term effects on dopamine function. Therefore, it is possible that these factors may have contributed to the observations reported herein. Nevertheless, in the fMRI study, the groups were balanced regarding representation of patients with a history of stimulant abuse, and similar results were obtained in both studies when history of stimulant abuse was considered in the statistical analyses. Moreover, note that interferon alfa administered in isolation to patients with cancer without infection (or ribavirin treatment or a history of substance or stimulant abuse) led to similar alterations in basal ganglia function as measured by PET.2 Also note that the sample sizes for these studies were modest and, therefore, only large effect sizes could be detected. Finally, we did not routinely obtain blood or cerebrospinal fluid samples from these patients, so it is impossible to correlate neuroimaging findings with measures of dopamine and its metabolites or cytokines.
In summary, the present studies replicate and extend previous work on the effects of inflammatory stimuli on basal ganglia by demonstrating involvement of presynaptic striatal dopamine function as an important target of basal ganglia cytokine effects. In addition, the findings suggest that effects of cytokines and inflammation on dopamine function may involve a variety of mechanisms, including impairment in dopamine synthesis, release, or reuptake. Finally, the data indicate that one mechanism by which cytokines may contribute to anhedonia, fatigue, and other behavioral changes seen during depression may be due to effects on dopamine function. Further understanding of the mechanisms of these effects of cytokines on dopamine function may, in turn, lead to new treatments for depression, especially in patients with increased inflammation.
Acknowledgments
Funding/Support: This work was supported by funds from the National Institute of Mental Health (MH067990 [Dr Miller]) and the DANA Foundation (Dr Capuron). In addition, the study was supported by Public Health Service grant UL1 RR025008 from the Clinical and Translational Science Award program and Public Health Service grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources.
Footnotes
Author Contributions: Drs Capuron and Pagnoni contributed equally to the article. Dr Miller takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study.
Financial Disclosure: None reported.
Online-Only Material: The eAppendixes and eFigures are available at http://www.archgenpsychiatry.com.
References
- 1.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32(11):2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 3.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152(4):383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- 4.Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S, Fahn S. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14(5):783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 5.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grace AA. Dopamine. In: Davis KL, Charney DS, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 119–132. [Google Scholar]
- 8.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 9.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a non-human primate model of cytokine-induced depression. Biol Psychiatry. 2007;62(11):1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKinney WT, Jr, Eising RG, Moran EC, Suomi SJ, Harlow HF. Effects of reserpine on the social behavior of rhesus monkeys. Dis Nerv Syst. 1971;32(11):735–741. [PubMed] [Google Scholar]
- 11.Mizoi Y, Kaneko H, Oharazawa A, Kuroiwa H. Parkinsonism in a patient receiving interferon alpha therapy for chronic hepatitis C [in Japanese] Rinsho Shinkeigaku. 1997;37(1):54–56. [PubMed] [Google Scholar]
- 12.Bersano A, Aghemo A, Rumi MG, Ballabio E, Candelise L, Colombo M. Recovery after L-DOPA treatment in peginterferon and ribavirin induced parkinsonism. Eur J Intern Med. 2008;19(5):370–371. doi: 10.1016/j.ejim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747(2):348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- 14.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19 (2):105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56(11):819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Kraus MR, Schäfer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64(6):708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 17.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22(6):870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maddock C, Landau S, Barry K, Maulayah P, Hotopf M, Cleare AJ, Norris S, Pariante CM. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10(4):332–333. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- 19.Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23(8):1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentine AD, Meyers CA. Neurobehavioral effects of interferon therapy. Curr Psychiatry Rep. 2005;7(5):391–395. doi: 10.1007/s11920-005-0042-3. [DOI] [PubMed] [Google Scholar]
- 21.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8(2):147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 24.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination [letter] Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 27.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 28.Valentine AD, Meyers CA, Talpaz M. Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest. 1995;13(6):561–566. doi: 10.3109/07357909509024923. [DOI] [PubMed] [Google Scholar]
- 29.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 30.Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70(2):175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 32.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 33.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J The International Consortium for Brain Mapping (ICBM) A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- 34.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data: generalizations. J Cereb Blood Flow Metab. 1985;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 35.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 36.Sossi V, Doudet DJ, Holden JE. A reversible tracer analysis approach to the study of effective dopamine turnover. J Cereb Blood Flow Metab. 2001;21(4):469–476. doi: 10.1097/00004647-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham VJ, Hume SP, Price GR, Ahier RG, Cremer JE, Jones AK. Compartmental analysis of diprenorphine binding to opiate receptors in the rat in vivo and its comparison with equilibrium data in vitro. J Cereb Blood Flow Metab. 1991;11(1):1–9. doi: 10.1038/jcbfm.1991.1. [DOI] [PubMed] [Google Scholar]
- 38.Leenders KL, Palmer AJ, Quinn N, Clark JC, Firnau G, Garnett ES, Nahmias C, Jones T, Marsden CD. Brain dopamine metabolism in patients with Parkinson’s disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry. 1986;49(8):853–860. doi: 10.1136/jnnp.49.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaasinen V, Nurmi E, Brück A, Eskola O, Bergman J, Solin O, Rinne JO. Increased frontal [(18)F]fluorodopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain. 2001;124(Pt 6):1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- 40.Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15 (6):635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- 41.Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson’s disease A 3D [(18)F]dopa-PET study. Brain. 1999;122(Pt 9):1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- 42.Kumakura Y, Gjedde A, Danielsen EH, Christensen S, Cumming P. Dopamine storage capacity in caudate and putamen of patients with early Parkinson’s disease: correlation with asymmetry of motor symptoms. J Cereb Blood Flow Metab. 2006;26(3):358–370. doi: 10.1038/sj.jcbfm.9600202. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh HJ, Lin SH, Liu HM. Visualisation of impaired dopamine biosynthesis in a case of aromatic L-amino acid decarboxylase deficiency by co-registered 18F-FDOPA PET and magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2005;32(4):517. doi: 10.1007/s00259-004-1618-6. [DOI] [PubMed] [Google Scholar]
- 44.Hadjiconstantinou M, Wemlinger TA, Sylvia CP, Hubble JP, Neff NH. Aromatic L-amino acid decarboxylase activity of mouse striatum is modulated via dopamine receptors. J Neurochem. 1993;60(6):2175–2180. doi: 10.1111/j.1471-4159.1993.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 45.Neurauter G, Schröcksnadel K, Scholl-Bürgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9(7):622–627. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- 46.Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978(1–2):104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;86(3):774–783. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- 48.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303(1):1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 49.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 51.Smith AK, Simon JS, Gustafson EL, Noviello S, Cubells JF, Epstein MP, Devlin DJ, Qiu P, Albrecht JK, Brass CA, Sulkowski MS, McHutchinson JG, Miller AH. Association of a polymorphism in the indoleamine- 2,3-dioxygenase gene and interferon-α-induced depression in patients with chronic hepatitis C [published online June 21, 2011] Mol Psychiatry. 2011 doi: 10.1038/mp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114 (1):33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 54.Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91(1):220–229. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- 55.Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23(24):8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1β and tumor necrosis factor-α activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]