Abstract
A regioselective Pd-catalyzed cross-dehydrogenative coupling between uracils and alkenes is reported. This protocol provides easy access to a variety of 5-alkenyluracil structural motifs.
Functionalization of uracils at the C5-position is of interest for the purpose of labelling1 and the preparation of bioactive uracil derivatives.2 The C5-position of pyrimidines is the location of choice for structural modification because this site is not involved in Watson-Crick base pairing.3 5-Alkenyluracils have been shown to exhibit antitumor4 and potent antiviral activities.5 Structure-activity relationship (SAR) studies demonstrated that the antiviral activity was enhanced when the C5-position was equipped with an unsaturated, E-configured substituent bearing a hydrophobic electronegative functionality (such as an ester) in conjugation with the pyrimidine ring.6
C5-alkenylation of uracils has in the past typically involved transition metal-catalyzed cross-coupling reactions between preactivated uracils (e.g. 5-iodo7, 5-triflated8 uracils) and metallated alkenes (e.g. stannane1a or boron9). These methods rely on multistep reaction sequences, lack atom economy, and are therefore of low efficiency. In addition, toxic stannane impurities could be problematic for biological studies. Direct alkenylation of uracils in a dehydrogenative fashion would address all the above problems and provide an efficient method to synthesize 5-alkenylated uracils.10
Direct C–H functionalization chemistry has emerged as a powerful new tool to construct C–C bonds.11 Direct C–H arylation,12 acetoxylation,13 trifluoromethylation,14 and dimerization15 of uracils have been reported by Pd-catalysis. In contrast, C–H alkenylation of uracils has been typically achieved using stoichiometric amounts of Pd. So far only one report has appeared that describes two examples employing catalytic Pd loading, using tert-butyl perbenzoate as the reoxidant.16 We therefore decided to investigate the substrate scope of the Pd-catalyzed direct C5-alkenylation in more detail. Herein, we disclose the development of an efficient dehydrogenative alkenylation of unactivated uracils with various alkenes via Pd-catalysis.
We initiated our investigation with an extensive optimization of the alkenylation conditions (Table 1). 1,3-Dimethyluracil (1a) and tert-butyl acrylate (2a) were chosen as test substrates. Our previous alkenylation conditions, developed for structurally related cyclic enaminones,17 performed disappointingly (entry 1), and the Rh-based protocol failed to yield product, presumably due to the absence of a directing group (entry 2).18 Screening of additional Cu oxidants led to significantly reduced yields (entries 3–4), while organic oxidants only provided moderate yield improvement (entries 5–6). Among a set of Ag oxidants (entries 7–9), AgOAc was the most effective one. Addition of K2CO3 was detrimental to the reaction, while weak acids, pivalic acid (PivOH) in particular, increased the yields (entries 10–12). The effectiveness of the Pd/PivOH combination, employed in many recent C–H activation reactions,19 can be attributed to both lowered transition state energies and increased basicity of its conjugated base serving as a proton shuttle.20 However, the concerted-metallation-deprotonation (CMD) mechanism20c is less likely because the uracil C6-proton is more acidic than the C5-proton,21 which would predict C6-alkenylation if this mechanism was at work, but does not correlate with the observed exclusive C5-regioselectivity, although we cannot rule out the possibility of a Pd 1,2-migration. A solvent screen revealed a significant solvent dependence, where DMF turned out to be optimal (entries 12–14). Lastly, a reduced loading with Pd (OAc)2 (5 mol%) showed better catalytic efficiency than Pd (TFA)2 (entry 15). Fine-tuning of the stoichiometry and temperatures afforded a satisfactory yield (92%) at 60°C (entry 16). It is worth noting that this efficient transformation proceeds under ambient air. In comparison, alkenylation under N2 afforded a marginally lowered yield (entry 17), indicating that O2 might serve as a minor oxidant.
Table 1.
Optimization of C–H alkenylation conditionsa
 | |||||
| Entry | Catalyst | Oxidant | Additive | Solvent | Yield (%)b |
|---|---|---|---|---|---|
| 1 | Pd (OAc)2 | Cu (OAc)2 | KTFA | DMF | 33 |
| 2c | [Cp*RhCl2]2 | Cu (OAc)2 | AgSbF6 | DMF | 0 |
| 3 | Pd (TFA)2 | CuCl2 | — | DMF | 0 |
| 4 | Pd (TFA)2 | Cu (OTf)2 | — | DMF | 6 |
| 5 | Pd (TFA)2 | PhCO3tBu | — | DMF | 45 |
| 6 | Pd (TFA)2 | duroquinone | — | DMF | 20 |
| 7d | Pd (TFA)2 | Ag2CO3 | — | DMF | 20 |
| 8 | Pd (TFA)2 | AgOBz | — | DMF | 55 |
| 9 | Pd (TFA)2 | AgOAc | — | DMF | 68 |
| 10 | Pd (TFA)2 | AgOAc | K2CO3 | DMF | 19 |
| 11 | Pd (TFA)2 | AgOAc | AcOH | DMF | 70 |
| 12 | Pd (TFA)2 | AgOAc | PivOH | DMF | 77 |
| 13 | Pd (TFA)2 | AgOAc | PivOH | MeCN | 62 |
| 14 | Pd (TFA)2 | AgOAc | PivOH | NMP | 64 |
| 15e | Pd (OAc)2 | AgOAc | PivOH | DMF | 83 |
| 16e,f | Pd (OAc)2 | AgOAc | PivOH | DMF | 91 (92)g |
| 17e,f,h | Pd (OAc)2 | AgOAc | PivOH | DMF | 87 |
Conditions: uracil 1a (0.2 M), acrylate 2a (2.0 equiv.), catalyst (10 mol%), oxidant (2.0 equiv.), additive (2.0 equiv.), solvent (0.5 mL) under air at 80°C, 24 h.
NMR yields with Ph3SiMe (1.0 equiv.) as the internal standard.
5 mol% of [Cp*RhCl2]2 and 20 mol% of AgSbF6.
Ag2CO3 (1.0 equiv.).
Pd (OAc)2 (5 mol%).
AgOAc (3.0 equiv.) and PivOH (3.0 equiv.) at 60°C.
Isolated yield.
Under N2.
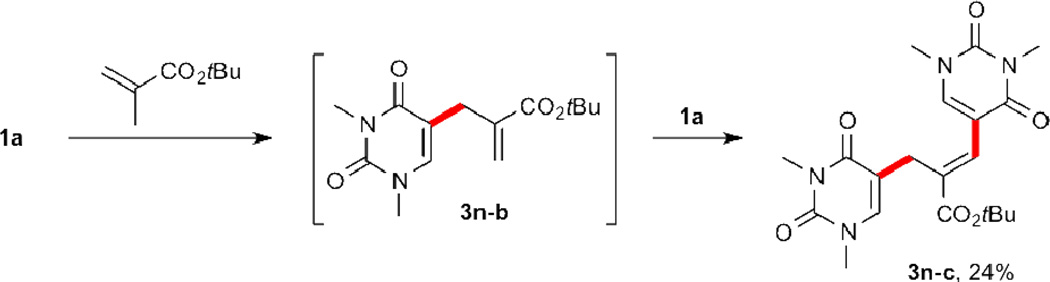
Utilizing these optimized catalytic conditions, a broad range of alkenes was found to undergo dehydrogenative coupling with uracil 1a in good to excellent yields (Table 2). The newly formed 5-alkenyluracils were obtained with absolute regio- and stereoselectivity (E-isomers), and a variety of functionalities were tolerated. Acrylate esters and styrene furnished excellent yields (3a–3e). Remarkably, quantitative conversion was observed when acrylamide was employed (3f). Acrolein, dimethyl vinylphosphonate, and methylvinylketone were also good alkenylating partners albeit showing slightly lowered yields (3g–3i). Notably, acrolein (2g) and acrolein diethyl acetal (2g’) furnished the same aldehyde 3g/3g’ with comparable yields, presumably due to in situ hydrolysis by PivOH of the acetal functional group. As for disubstituted alkenes, overall moderate yields were observed, possibly due to the steric repulsion exerted by the substituents (3j, 3k, 3m, 3n). As expected, when more than one β-hydrogen was present, final products 3l–3n showed double bond tautomerization with a preference for unconjugated (to uracil) 5-allyluracils 3m-b and 3n-b.17,22 However, allyl acetate 2l predominantly afforded the conjugated 5-alkenyluracil 3l-a. As documented previously,23 the coordination between O (from the acetyl) and Pd locks the conformation and subsequently favors Ha for syn-elimination (Scheme 1). In addition, double C–H activation was observed in by-product 3n-c to our surprise (Scheme 2), where 3n-b served as an alkene for subsequent coupling with uracil 1a to furnish 3n-c albeit in a low yield.
Table 2.
Scope of alkenes for dehydrogenative alkenylationa
 |
Conditions: uracil 1a (0.2 M), acrylate 2 (2.0 equiv.), Pd (OAc)2 (5 mol%), AgOAc (3.0 equiv.), PivOH (3.0 equiv.), DMF (0.5 mL) under air at 60°C, 24 h. Isolated yields.
Acrolein diethyl acetal (2.0 equiv.) was used as the alkene.
Ratio determined by 1H NMR.
Scheme 1.
Dehydrogenative alkenylation with allyl acetate.
Scheme 2.
Double C–H activation of 1a.
We next probed the scope of uracils24 under the optimized conditions (Table 3). In addition to N-methyl groups (3a), uracil can be protected with other electron-donating groups, such as benzyl (3o), methoxymethyl (MOM) (3p), and p-methoxybenzyl (PMB) (3r), and provide reaction products in good to excellent yields. The new protocol was also effectively applied to 1-benzyl-3-(3’,5’-dimethylbenzyl) uracil (1q), which has exhibited potent antiviral activity against the human immunodeficiency virus (HIV-1) and the human cytomegalovirus (HCMV).25 On the other hand, electronically-attenuated uracils (with electron-withdrawing groups or without protecting groups) were unreactive in the alkenylation process (3u–3w). Although poor solubility may play a role, we presume that decreased nucleophilicity of the uracils is the reason for the observed lack of reactivity. In further investigations we found that protected uridine and 2’-deoxyuridine were also good substrates (3s and 3t), demonstrating the applicability of our reaction protocol to uracil-based nucleosides.
Table 3.
Scope of uracils for dehydrogenative alkenylationa
 |
Conditions: uracil 1a (0.2 M), acrylate 2 (2.0 equiv.), Pd (OAc)2 (5 mol%), AgOAc (3.0 equiv.), PivOH (3.0 equiv.), DMF (0.5 mL) under air at 60°C, 24 h. Isolated yields.
In light of both the exclusive C5-regioselectivity and the lack of reactivity from electron-poor uracils, an electrophilic palladation pathway10b was envisaged. Similar to our previously investigated enaminone system,17 uracil’s nucleophilic C5-position is most likely first attacked by the Pd (II) species. Prior coordination of the Pd (II) to the carbonyl group or the double bond is a possibility as well.26 Deprotonation by the more basic pivalate ligand then forms a palladated uracil, which then undergoes migratory alkene insertion. Subsequent β-H elimination delivers the desired 5-alkenyluracil. Reductive elimination and reoxidation by Ag (I) regenerates Pd (II) to resume the catalytic cycle.
In summary, we have developed an efficient and highly atom-economical protocol for cross-dehydrogenative coupling of uracils and alkenes. The generality of this transformation provides a promisingly direct route to synthesize 5-alkenyluracils, which are of importance in medicinal chemistry.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM081267) and the University of Minnesota through the Vince and McKnight Endowed Chairs (to G.I.G).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures, detailed reaction optimization data, and spectroscopic data. See DOI: 10.1039/b000000x/
Notes and references
- 1.Recent examples: Wicke L, Engels JW. Bioconjugate Chem. 2012;23:627. doi: 10.1021/bc200659j. Srivatsan SG, Tor Y. Chem.–Asian J. 2009;4:419. doi: 10.1002/asia.200800370. Cahova H, Havran L, Brazdilova P, Pivonkova H, Pohl R, Fojta M, Hocek M. Angew. Chem. Int. Ed. 2008;47:2059. doi: 10.1002/anie.200705088.
- 2.Recent examples: Srivastav NC, Shakya N, Bhavanam S, Agrawal A, Tse C, Desroches N, Kunimoto DY, Kumar R. Bioorg. Med. Chem. Lett. 2012;22:1091. doi: 10.1016/j.bmcl.2011.11.114. Medda F, Russell RJM, Higgins M, McCarthy AR, Campbell J, Slawin AMZ, Lane DP, Lain S, Westwood NJ. J. Med. Chem. 2009;52:2673. doi: 10.1021/jm8014298. Chen C, Wu DP, Guo ZQ, Xie Q, Reinhart GJ, Madan A, Wen J, Chen TK, Huang CQ, Chen M, Chen YS, Tucci FC, Rowbottom M, Pontillo J, Zhu YF, Wade W, Saunders J, Bozigian H, Struthers RS. J. Med. Chem. 2008;51:7478. doi: 10.1021/jm8006454. Tsoukala E, Agelis G, Jan Dolinšek, Botić T, Cencič A, Komiotis D. Bioorg. Med. Chem. 2007;15:3241. doi: 10.1016/j.bmc.2007.02.031.
- 3.Watson JD, Crick FH. Nature. 1953;171:737. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 4.Theocharis DA, Coutsogeorgopoulos C. Biochemistry. 1992;31:5861. doi: 10.1021/bi00140a023. [DOI] [PubMed] [Google Scholar]
- 5.Recent examples: Gazivoda T, Raic-Malic S, Marjanovic M, Kralj M, Pavelic K, Balzarini J, De Clercq E, Mintas M. Bioorgan. Med. Chem. 2007;15:749. doi: 10.1016/j.bmc.2006.10.046. Kumar R, Nath M, Tyrrell DLJ. J. Med. Chem. 2002;45:2032. doi: 10.1021/jm010410d. Choi Y, Li L, Grill S, Gullen E, Lee CS, Gumina G, Tsujii E, Cheng YC, Chu CK. J. Med. Chem. 2000;43:2538. doi: 10.1021/jm990543n.
- 6.Goodchild J, Porter RA, Raper RH, Sim IS, Upton RM, Viney J, Wadsworth HJ. J. Med. Chem. 1983;26:1252. doi: 10.1021/jm00363a009. [DOI] [PubMed] [Google Scholar]
- 7.(a) Moa MJG, Besada P, Teran C. Synthesis. 2006:3973. [Google Scholar]; (b) Ding VL, Girardet JL, Hong Z, Shaw SZ, Yao NH. Heterocycles. 2006;68:521. [Google Scholar]
- 8.Crisp GT, Flynn BL. Tetrahedron Lett. 1990;31:1347. [Google Scholar]
- 9.Roh KR, Kim JY, Kim YH. Tetrahedron Lett. 1999;40:1903. [Google Scholar]
- 10.Recent reviews: Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. Le Bras J, Muzart J. Chem. Rev. 2011;111:1170. doi: 10.1021/cr100209d. Yoo WJ, Li CJ. In: C-H Activation. Yu JQ, Shi Z, editors. vol. 292. 2010. p. 281. Scheuermann CJ. Chem.–Asian J. 2010;5:436. doi: 10.1002/asia.200900487. Li CJ. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n.
- 11.Recent reviews: Song GY, Wang F, Li XW. Chem. Soc. Rev. 2012;41:3651. doi: 10.1039/c2cs15281a. Engle KM, Mei TS, Wasa M, Yu JQ. Acc. Chem. Res. 2012;45:788. doi: 10.1021/ar200185g. Wencel-Delord J, Droge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a. Ackermann L. Chem. Rev. 2011;111:1315. doi: 10.1021/cr100412j. Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e.
- 12.(a) Kim KH, Lee HS, Kim JN. Tetrahedron Lett. 2011;52:6228. [Google Scholar]; (b) Cernova M, Cerna I, Pohl R, Hocek M. J. Org. Chem. 2011;76:5309. doi: 10.1021/jo2006494. [DOI] [PubMed] [Google Scholar]; (c) Cernova M, Pohl R, Hocek M. European J. Org. Chem. 2009:3698. [Google Scholar]
- 13.Lee HS, Kim SH, Kim JN. B. Korean Chem. Soc. 2010;31:238. [Google Scholar]
- 14.(a) Nagib DA, MacMillan DWC. Nature. 2011;480:224. doi: 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ji YN, Brueckl T, Baxter RD, Fujiwara Y, Seiple IB, Su S, Blackmond DG, Baran PS. Proc. Natl. Acad. Sci. USA. 2011;108:14411. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Uraguchi D, Yamamoto K, Ohtsuka Y, Tokuhisa K, Yamakawa T. Appl. Catal. A–Gen. 2008;342:137. [Google Scholar]
- 15.Kim KH, Lee HS, Kim SH, Kim JN. Tetrahedron Lett. 2012;53:1323. [Google Scholar]
- 16.Hirota K, Isobe Y, Kitade Y, Maki Y. Synthesis. 1987:495. [Google Scholar]
- 17.Yu Y-Y, Niphakis MJ, Georg GI. Org. Lett. 2011;13:5932. doi: 10.1021/ol202677g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Colby DA, Tsai AS, Bergman RG, Ellman JA. Acc. Chem. Res. 2012;45:814. doi: 10.1021/ar200190g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recent examples: Moon Y, Hong S. Chem. Commun. 2012;48:7191. doi: 10.1039/c2cc33204c. Guchhait SK, Kandekar S, Kashyap M, Taxak N, Bharatam PV. J. Org. Chem. 2012;77:8321. doi: 10.1021/jo301065s. Gigant N, Gillaizeau I. Org. Lett. 2012;14:3304. doi: 10.1021/ol301249n.
- 20.(a) Lafrance M, Lapointe D, Fagnou K. Tetrahedron. 2008;64:6015. [Google Scholar]; (b) Lafrance M, Fagnou K. J. Am. Chem. Soc. 2006;128:16496. doi: 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]; (c) Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956. [DOI] [PubMed] [Google Scholar]
- 21.Kurinovich MA, Lee JK. J. Am. Soc. Mass Spectr. 2002;13:985. doi: 10.1016/S1044-0305(02)00410-5. [DOI] [PubMed] [Google Scholar]
- 22.Recent examples: Miyasaka M, Hirano K, Satoh T, Miura M. J. Org. Chem. 2010;75:5421. doi: 10.1021/jo101214y. García-Rubia A, Arrayás RG, Carretero JC. Angew. Chem. Int. Ed. 2009;48:6511. doi: 10.1002/anie.200902802.
- 23.Recent examples: Li Z, Zhang Y, Liu Z-Q. Org. Lett. 2012;14:74. doi: 10.1021/ol202859b. Lee HS, Kim KH, Kim SH, Kim JN. Adv. Synth. Catal. 2012;354:2419. Pan D, Yu M, Chen W, Jiao N. Chem.–Asian J. 2010;5:1090. doi: 10.1002/asia.200900558.
- 24.Preparation of protected uracils and uridines: LaFrate AL, Katzenellenbogen JA. J. Org. Chem. 2007;72:8573. doi: 10.1021/jo071255z. Frieden M, Giraud M, Reese CB, Song QL. J. Chem. Soc.–Perkin Trans. 1. 1998:2827. Ishikawa I, Itoh T, Takayanagi H, Oshima J, Kawahara N, Mizuno Y, Ogura H. Chem. Pharm. Bull. 1991;39:1922. Su TL, Huang JT, Burchenal JH, Watanabe KA, Fox JJ. J. Med. Chem. 1986;29:709. doi: 10.1021/jm00155a021. Kundu NG, Sikdar S, Hertzberg RP, Schmitz SA, Khatri SG. J. Chem. Soc.–Perkin Trans. 1. 1985:1295. Robins MJ, Wilson JS, Hansske F. J. Am. Chem. Soc. 1983;105:4059.
- 25.Maruyama T, Kozai S, Demizu Y, Witvrouw M, Pannecouque C, Balzarini J, Snoecks R, Andrei G, De Clercq E. Chem. Pharm. Bull. 2006;54:325. doi: 10.1248/cpb.54.325. [DOI] [PubMed] [Google Scholar]
- 26.(a) Wang D-H, Hao X-S, Wu D-F, Yu J-Q. Org. Lett. 2006;8:3387. doi: 10.1021/ol061384m. [DOI] [PubMed] [Google Scholar]; (b) Giri R, Yu J-Q. J. Am. Chem. Soc. 2008;130:14082. doi: 10.1021/ja8063827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.