Abstract
Objectives
Osteoarthritis (OA) is characterized by the failure of chondrocytes to respond to injury and perform the cartilage remodeling process. Human articular chondrocytes actively produce reactive oxygen and nitrogen species (ROS and RNS) capable of causing cellular dysfunction and death. A growing body of evidence indicates that mitochondrial dysfunction and mitochondrial DNA (mtDNA) damage play a causal role in disorders linked to excessive generation of oxygen free radicals. The aim of this study was to determine whether mtDNA damage was present in OA chondrocytes, and whether mtDNA repair capacity is compromised in OA chondrocytes following oxidative stress, leading to chondrocyte death.
Methods
Human articular cartilage was isolated from knee joints of cadavers available through the Anatomical Gifts Program at the University of South Alabama (normal donors) or OA patients undergoing total knee replacement surgeries (OA patients). Total DNA was isolated from either chondrocytes released following collagenase digestion, or from first passage chondrocytes grown in culture and exposed to ROS or RNS. Mitochondrial DNA integrity and repair capacity was analyzed by quantitative Southern blot analysis, using a mtDNA-specific radioactive probe. Cell viability was determined by the trypan blue exclusion method.
Results
Mitochondrial DNA damage was found in chondrocytes from OA patients compared to normal donors. It was accompanied with reduced mtDNA repair capacity, cell viability, and increased apoptosis in OA chondrocytes following exposure to ROS and RNS.
Conclusions
These results indicate that mtDNA damage and poor mtDNA repair capacity for removing damage caused by oxidative stress may contribute to the pathogenesis of osteoarthritis.
Introduction
A growing body of evidence suggests that reactive oxygen species (ROS) play a critical role in the regulation of normal chondrocyte function and contribute to the structural and functional cartilage damage observed in the pathogenesis of joint disease. ROS regulate some normal chondrocyte activities such as cell activation, proliferation and matrix remodeling (1–3). In response to partial oxygen pressure variations, mechanical stress, and immunomodulatory and inflammatory mediators, chondrocytes produce abnormal levels of ROS. When ROS production exceeds the antioxidant and repair capacities of the cell, oxidative stress occurs which leads to cartilage damage. Free-radical elicited oxidative stress can lead to DNA, protein, and other cellular macromolecule damage, which ultimately can result in cellular dysfunction and death.
Numerous reports have demonstrated that oxidative damage, due to the overproduction of nitric oxide (NO) and other ROS, may be involved in the pathogenesis of osteoarthritis (OA) (4–6). Lower antioxidative capacity and stronger staining for nitrotyrosine, a reaction product of ROS and NO, were observed in degenerating regions of OA cartilage, as compared with the intact regions from the same explants (7). Immunostaining for nitrotyrosine correlated with the severity of histological changes to OA cartilage, suggesting an association between oxidative damage and articular cartilage degeneration. Other studies have demonstrated that oxidative stress affects chondrocyte telomeric DNA, replicative lifespan, and cartilage matrix proteoglycan structure and composition (8–10). Additionally, OA patients exhibit a significant increase in DNA damage in peripheral blood leukocytes and erythrocyte lipid peroxydation, along with decreased antioxidant levels, including plasma glutathione, ascorbic acid, vitamin E and catalase (11, 12).
Oxidative stress-induced cellular alterations are observed during age-related degeneration of mitochondria resulting from leakage from the electron transport chains and significant oxidative damage to mitochondrial macromolecules (13). Despite the fact that disrupted mitochondrial respiration and mitochondrial damage have been found to expedite aging, and cause cellular dysfunction, degeneration, and death, the contribution of mitochondrial dysfunction to the pathogenesis of OA has not been fully elucidated. However, it has been shown that oxygen does diffuse into articular cartilage, and articular chondrocytes possess functional mitochondria. Mitochondrial oxidative phosphorylation (OXPHOS) may account for up to 25% of the ATP produced in cartilage (14). Respiratory chain activity and mitochondrial membrane potential have been found to be significantly reduced in cultured human chondrocytes from OA patients, when compared to normal donors (15). A key factor likely playing a role in oxidative stress-induced mitochondrial dysfunction is mitochondrial DNA (mtDNA). Each mitochondrion has multiple copies of its own genome. It is widely regarded that the mitochondrial genome is prone to oxidative damage, being between 10 to 100 fold more sensitive to this stress than nuclear DNA (16). Moreover, mutations and deletions in the mitochondrial genome have been linked to neurodegenerative disorders and other age-related diseases (17, 18). Depletion of mtDNA is known to suppress ATP synthesis and cause defects in cellular function (19). Additionally, there is evidence which suggests the involvement of mtDNA damage in the initiation of apoptosis (20). It remains unclear whether this is applicable to the chondrocyte, which has low oxygen consumption and, therefore, might be less prone to oxidative damage to its mtDNA. Therefore, the purpose of the current investigation was to evaluate whether mtDNA damage and mitochondrial DNA repair dysfunction are present during the development of osteoarthritis (OA).
Experimental Procedures
Cartilage specimens and establishment of primary cultures of chondrocytes
Two types of cartilage were used for the current investigation: cartilage obtained as surgical waste from total knee replacement surgery of 24 OA patients and cartilage from 13 age-matched normal donors obtained through the University of South Alabama Anatomical Gifts Program. Cartilage from OA patients was obtained from areas within the distal femur without exposed bone. Cartilage obtained from normal donors was used only if there was no gross evidence of cartilage abnormalities within the knee. No radiographs of normal donor knees were obtained prior to testing. OA patients ranged in age from 54 to 69 years, and normal donor ages ranged from 62 to 74 years. Primary chondrocyte cultures from normal donors and OA patients were generated by overnight digestion of minced cartilage samples with 5 mg/ml collagenase B (Roche) in DMEM/F12 media (Cellgro) supplemented with 10% fetal bovine serum (FBS; Hyclone). Cells were allowed to attach for 48 h before the first media change. Following this time, 90–95% of the cells were attached and these adherent cells were used for experiments. Part of the resulting chondrocyte yield was lysed for DNA isolation in order to perform comparative mtDNA damage studies between OA patients and normal donors. The remaining cells were plated in 100 mm dishes in culture medium containing DMEM/F12 with 50 ng/ml of gentamycin (Gibco BRL) and 10% FBS and used, after reaching confluence (7–10 days), for repair and viability experiments. Only first passage cells were used for experiments in order to preserve chondrocyte phenotype.
Drug preparation and exposure
For dose response and repair experiments with ROS or reactive nitrogen species (RNS) generators, primary chondrocyte cultures were exposed to 150, 300, and 600 µM of peroxynitrite (Cayman Chemicals) or 10, 20, and 40 mU of xanthine oxidase (XO)/with 0.5 mM hypoxanthine (Sigma) in serum-free DMEM/F12 medium. Control cultures were exposed to serum-free DMEM/F12 media only. Chondrocytes were exposed to these genotoxins for a duration of 30 min, after which cells were lysed immediately, or rinsed and placed in culture medium to allow time for recovery and repair.
Mitochondrial DNA damage and repair assay
Chondrocyte cultures or cell suspensions were lysed in a buffer containing 0.5% SDS, 10 mM Tris-HCl (pH 8.0), 20 mM EDTA (pH 8.0), 100 mM NaCl, and 300 µg/ml proteinase K (Roche) overnight in a 37C water bath. For repair experiments, cultures were lysed immediately following drug exposure, or replenished with culture media and left for repair for 6 h. Once the cells were lysed, DNA was isolated by a standard phenol/chloroform extraction method, precipitated with cold ethanol, and subjected to overnight digestion with BamHI (10 U/µg DNA). BamHI was selected because human mitochondrial DNA has a single restriction site for this enzyme, so that upon digestion it linearizes the mtDNA. Hybridization with the human mitochondrial gene-specific probe to cytochrome c oxidase subunit III recognizes the restriction band of 16569 bp, corresponding to the whole mitochondrial genome. Each DNA sample was precisely quantified with a Hoefer TKO100 mini-fluorometer using Hoechst 33258 dye. Prior to loading on an alkaline agarose gel for Southern blot analysis, each sample containing 5 µg of total DNA was incubated with 0.1 N NaOH to reveal single-strand breaks. After the gel electrophoresis was completed, DNA was stained with ethidium bromide and viewed under UV light to ensure loading accuracy. Next, DNA was transferred via vacuum to a nylon membrane and hybridized with a PCR-generated radioactive mtDNA-specific probe, which contained the sequence for part of the cytochrome c oxidase subunit III human mitochondrial gene. Autoradiograms were scanned for hybridization band intensity. Mitochondrial DNA damage was assessed as the diminished intensity in the 10 kb major restriction band, indicating that DNA breaks have caused smaller size fragments, which migrate further down in the gel and, thus, reduced the number of full size restriction fragments. For DNA repair studies, the break frequency was determined using the Poisson expression (s= −lnP0, where s is the number of breaks per fragment, and Po is the fraction of fragments that are free of breaks). The extent of repair was calculated as the value obtained from subtracting the breaks at time t from the breaks present at t = 0 h, and dividing by the breaks at t = 0 h. The resulting value was then converted to a percentage by multiplying by 100 (21).
Slot-blot analysis
This assay was used to determine whether there were any differences in mtDNA content between different DNA samples. DNA samples obtained from normal donors and OA patients were digested with Bam HI, precisely quantified, adjusted to the same concentration with H20, and treated with 0.3 M NaOH to denature the DNA. Fractions of 100, 50, and 20 ng were then blotted onto a nylon membrane (Nytran, Schleicher and Schuell, Keen, NH) using a slot blot apparatus (Minifold II, Schleicher and Schuell) and membranes were hybridized with a mtDNA-specific probe and washed according to the manufacturer’s suggestions.
Cell Viability and Apoptosis evaluation
For viability studies, cells were plated in 24-well plates and exposed to the same concentrations of peroxynitrite and xanthine oxidase used for the repair experiments. Following 30 min of exposure, the cells were replenished with normal growth media and allowed to recover for 24 h. After 24 h, trypan blue (Sigma) was added to the trypsinized cells to distinguish dead cells from live ones. Viable cells were counted in a hemocytometer using a light microscope and expressed as a percentage of the total number of cells. Apoptotic cells were identified using the DAPI (4',6-diamidino-2-phenylindole; Sigma) staining method. Briefly, 24 h following exposure to the ROS/RNS generators, cells were washed three times with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. The fixed cells were washed again with PBS and stained with 1 µg/ml DAPI, a fluorescent dye that binds to DNA. Stained cells were examined by fluorescent microscopy to identify apoptotic cells, which were defined morphologically by cytoplasmic and nuclear shrinkage, chromatin condensation or fragmentation. To evaluate the percentage of apoptotic cells, 6–7 fields from each experimental condition were viewed in a blinded fashion until a total of 500 cells were observed.
Statistical analysis
Statistical analyses were performed using either Student’s t-test, or one- or two-way analysis of variance (GraphPad Prism) where appropriate. A difference of P<0.05 was considered significant. The Bonferroni posthoc test was used to determine the source of difference.
Results
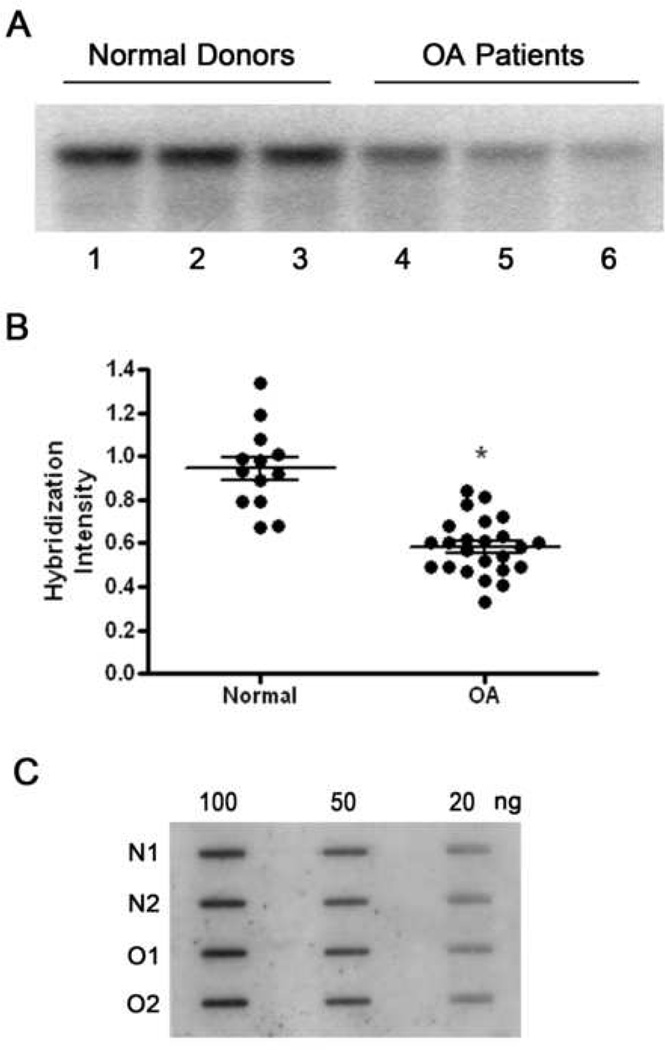
There are two components to the present study. The first is the investigation of the mtDNA damage which has accumulated in cartilage from OA patients compared to normal donors. The second is the study of mtDNA repair capacity and cell viability following experimentally induced oxidative stress in chondrocytes from OA patients and normal donors. To compare mtDNA integrity between normal donors and OA patients, DNA was isolated directly from chondrocyte suspensions after collagenase digestion of cartilage specimens, and Southern blot analysis was performed to reveal the extent of mtDNA damage (Fig. 1A). First, we wanted to make sure that differences in mtDNA integrity in normal donors compared to OA patients were not due to damage to mtDNA in OA chondrocytes caused by the collagenase digestion. Immediately following overnight digestion with collagenase, the resulting cells were evaluated for viability using trypan blue exclusion. In the normal chondrocyte cultures 87±5% of the cells viable, while in OA chondrocyte cultures, viability was 82±9%. These results show that the vast majority of cells in either group had intact plasma membranes. Thus, it is unlikely that any mtDNA damage seen in OA chondrocytes is the result of collagenase entering mitochondria and damaging their DNA.
Fig. 1. Mitochondrial DNA is damaged during the pathogenesis of OA.
Fig. 1A is a representative autoradiogram from Southern blot analyses of mtDNA from three normal donors (lanes 1–3) and three OA patients (line 4–6). Human chondrocytes were lysed for DNA extraction following overnight digestion with collagenase B. The reduced intensity of the hybridization bands indicates that mtDNA damage has increased. Fig.1B displays a comparison of the hybridization band intensities obtained from the Southern blot analysis of 13 normal donors and 24 OA patients. * indicates a significant difference (P<0.05) between normal donors and OA patients. Fig.1C is a representative autoradiogram from slot-blot analyses performed on the DNA from normal donors and OA patients and shows that there is no variation in the amount of mtDNA between normal donors and OA patients, as well as between separate DNA samples.
The results of this study indicated that mtDNA integrity was better maintained in normal donors compared to OA patients, as demonstrated by the greater intensity of the major restriction bands from normal donors compared to the lower intensity of bands obtained from OA patients (Fig. 1B). In other words, this finding indicated that mtDNA from OA patients sustained more damage than that from healthy controls. To ensure that differences between hybridization band intensities were due to mtDNA damage and not mtDNA content, slot-blot analysis of all samples used for Southern blot analysis was performed. This analysis revealed that there were no differences in the amount of mtDNA between DNA samples from normal donors and OA patients (Fig. 1C). The same gels were used to evaluate possible nuclear DNA damage. To perform this study, quantitative alkaline gel electrophoresis analysis was used. Following alkaline electrophoresis, gels were stained with ethidium bromide and total cellular DNA integrity was assessed using densitometric software (BioRad GS-250 molecular imager). This analysis revealed that any nuclear DNA damage present in the samples from either normal donors or OA patients was below the level of detection (data not shown).
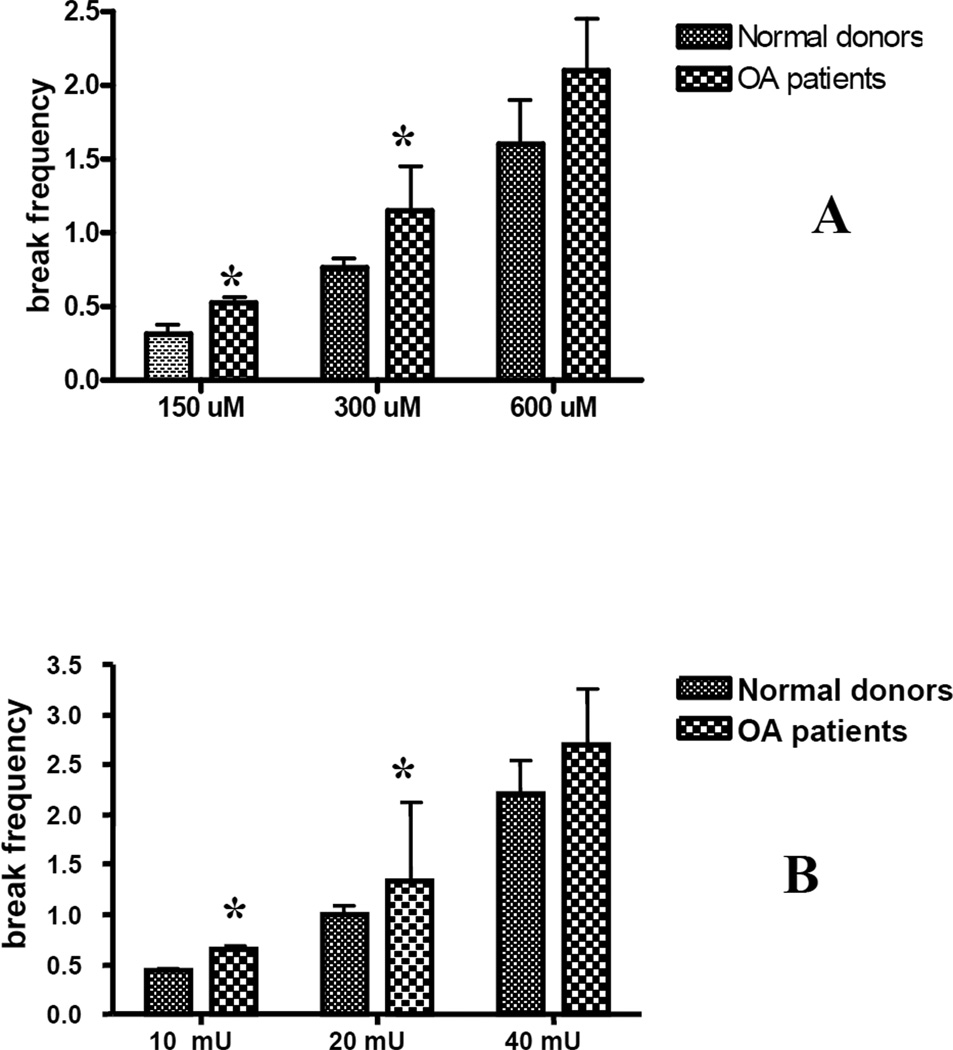
To evaluate the ability of chondrocytes to repair damage to mtDNA caused by ROS or RNS, chondrocytes in primary culture, from either OA patients or normal donors, were exposed to the ROS generator xanthine oxidase/hypoxanthine or the RNS generator peroxynitrirte. Initial dose-response experiments were carried out to determine the genotoxin concentration required to induce approximately 1 break per 10 kb of mtDNA. Cells were treated with different doses of XO/hypoxanthine or peroxynitrite for 30 min and lysed overnight. Total DNA was isolated, digested with BamHI, and subjected to quantitative Southern blot analysis. As can be seen in Fig. 2, chondrocytes treated with 300 µM of peroxynitrite or 20 mU of XO/0.5 mM hypoxanthine showed the desired levels of mtDNA damage, and these concentrations were used for repair experiments. Chondrocytes obtained from OA patients were more sensitive to both peroxynitrite and XO than normal donors (Fig. 2). These results indicate that mtDNA from normal donors and OA patients are vulnerable targets for stress caused by either ROS or RNS and that mtDNA from OA chondrocytes is more sensitve to either stress.
Fig. 2. Mitochondrial DNA in chondrocytes from OA patients is more sensitive to oxidative stress caused by exposure to ROS or RNS.
Chondrocytes were exposed to increased concentrations of peroxynitrite (panel A) or xanthine oxidase with hypoxanthine (panel B) for 30 min. Cells were lysed, DNA was isolated and subjected to Southern blot analysis. An Increase in the break frequency indicates that more mtDNA damage has accumulated. The results were obtained from a minimum of 8 independent experiments, and the values represent the mean break frequency ± SEM. * indicates a significant difference (P<0.05) in damage in mtDNA in chondrocytes from OA patients compared to normal donors.
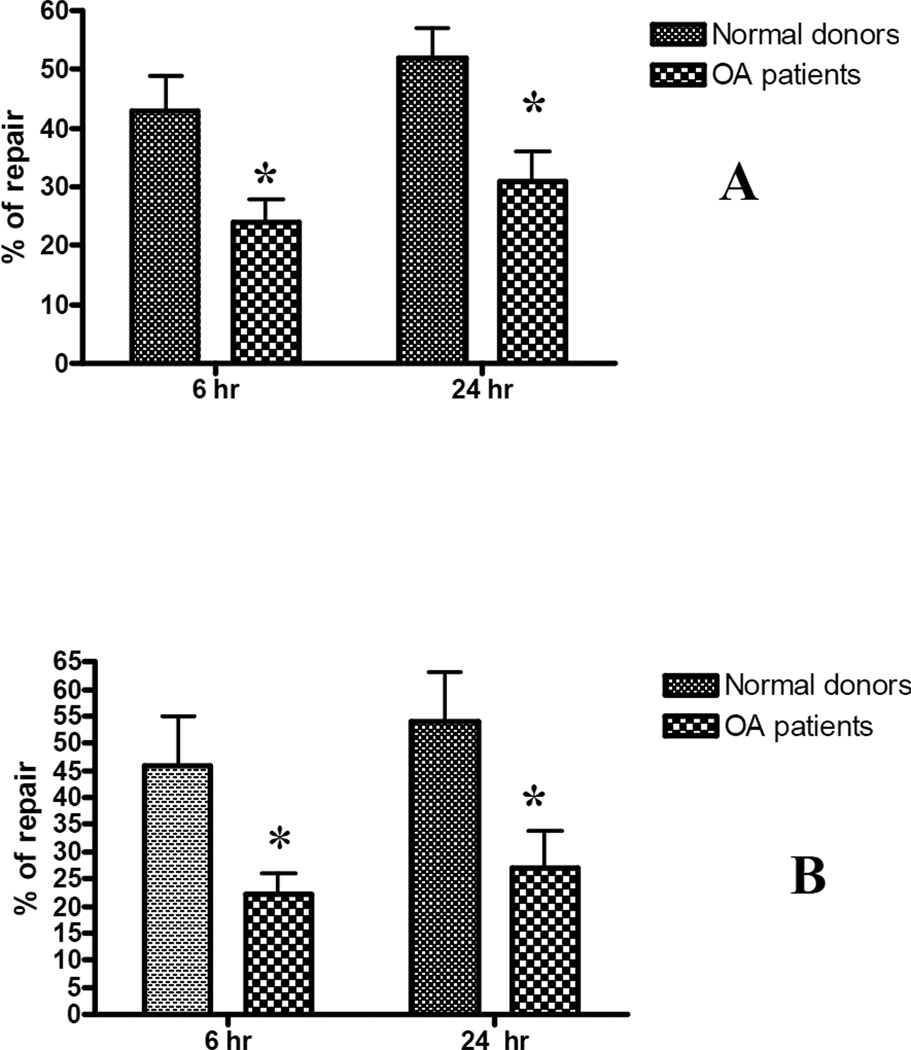
For repair experiments, following exposure to ROS or RNS, cultures were either lysed immediately, and DNA extracted for initial DNA damage evaluation, or replenished with normal culture medium and allowed to repair for 6 h and 24 h before cells were lysed and DNA isolated. Southern blot analysis was performed on the extracted DNA to determine the differences in the integrity of mtDNA immediately after the damage was introduced or following the two intervals for repair. Autoradiograms were scanned, break frequencies calculated based on densitometry measurements, and a percentage of repair was calculated. By 6 h after exposure to either the ROS or RNS, mtDNA had been repaired somewhat, as indicated by the decrease in the break frequency at 6 h following drug treatment compared to the break frequencies at 0 h. (Fig.3). After 24 h, there was not a significant increase in repair following exposure to either free radical. These data show that human chondrocytes have a limited capacity to repair oxidative damage. Additionally, OA chondrocytes had decreased mtDNA repair compared to chondrocytes from normal donors. These data indicate that the capacity to repair mtDNA is compromised during the progression of OA and potentially contributes to the progressive mitochondrial dysfunction.
Fig. 3. OA chondrocytes have a diminished capacity to repair oxidative damage.
Primary human chondrocytes from OA patients and normal donors were exposed to 300 µM of peroxynitrite (panel A) or 20 mU xanthine oxidase /0.5 mM hypoxanthine for 30 min and allowed to repair for 6 and 24 h. Cells were lysed, DNA was isolated and subjected to Southern blot analysis. The results were obtained from a minimum of 7 independent experiments, and the values displayed represent the mean break frequency ± SEM. * indicates a significant difference (P<0.05) between OA chondrocytes and those isolated from normal donors.
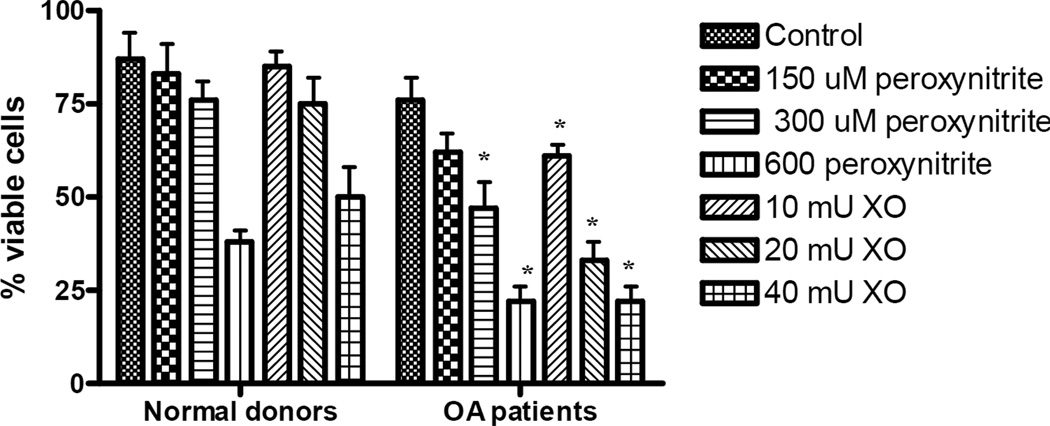
A similar experimental design to that used for the repair studies was utilized to evaluate viability and apoptosis of normal and OA chondrocytes following oxidative stress induced by peroxynitrite or xanthine oxidase and hypoxanthine. Cells were seeded in 24-well plates and after reaching confluence were treated for 30 min with 300 µM of peroxynitrite or 20 mU XO/0.5 mM hypoxanthine. Following 24 h of recovery, cells were trypsinized, incubated with trypan blue dye, and the percentages of viable (white) and dead (blue) cells were calculated. The results of these studies indicated that, after oxidative stress was introduced, more chondrocytes from normal donors were viable than chondrocytes from OA patients (Fig.4). Significant differences were observed between normal and OA chondrocytes after treatment with 300 and 600 µM peroxynitrite and all concentrations of xanthine oxidase with hypoxanthine following 24 h of recovery. These results show that OA chondrocyte function is already diminished by oxidative stress, which has accumulated during the progression of OA and this makes it difficult for these cells to recover and preserve their viability when additional oxidative stress is introduced.
Fig. 4. Chondrocyte viability following exposure to ROS or RNS.
Confluent cell cultures were exposed to increasing concentrations of xanthine oxidase or peroxynitrite for 30 min and left for recovery for 24 h. After 24h, cells were trypsinized away from the culture vessel, incubated with trypan blue and viewed by light microscopy to calculate the numbers of viable and dead cells. The results were obtained from a minimum of 6 independent experiments, and the values represent the mean percentage of viable cells ± SEM. * indicates a significant difference (P<0.05) in OA chondrocytes compared to those from normal donors. Note the diminished OA chondrocyte viability following exposure to both genotoxins.
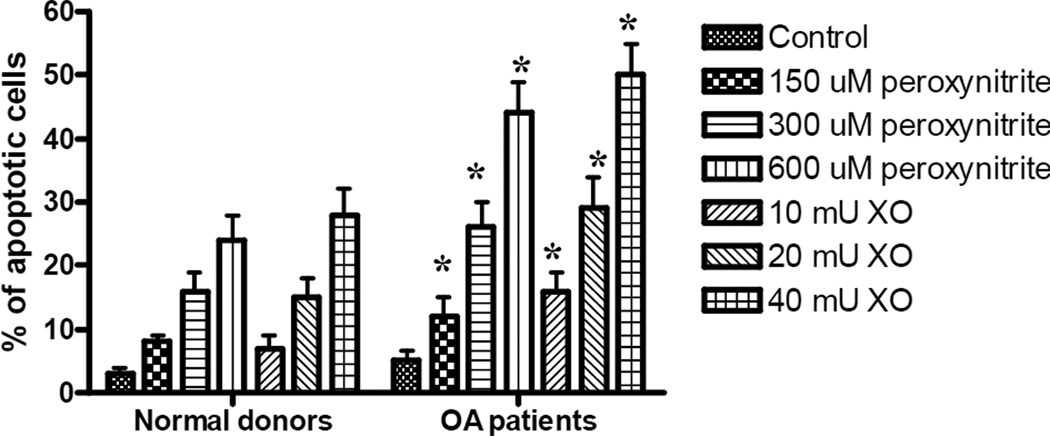
Studies were performed to evaluate the induction of apoptosis in both normal and OA chondrocytes following exposure to ROS or RNS. Primary cell cultures were exposed to the same doses of peroxynitrite or xanthiine oxidase as were used for the repair and viability studies. Following 24h of recovery, cells were fixed and stained with DAPI. Stained cells were examined by fluorescent microscopy to identify apoptotic cells, which were defined morphologically by cytoplasmic and nuclear shrinkage, and chromatin condensation or fragmentation. To evaluate the percentage of apoptotic cells, 6–7 fields from each experimental condition were viewed, in a blinded fashion, until a total of 500 cells were observed. Data obtained from these studies show that primary cultures of human chondrocytes, obtained from OA patients, contained significantly greater amounts of apoptotic cells following oxidative stress (Fig. 5).
Fig. 5. The accumulation of apoptotic chondrocytes in cultures obtained from OA patients and normal donors following exposure to ROS or RNS.
Twenty four hours following exposure to increasing concentrations of xanthine oxidase or peroxynitrite, cells were washed three times with PBS and fixed with 4% paraformaldehyde. The fixed cells were washed again with PBS and stained with 1 µg/ml DAPI. Stained cells were examined by fluorescent microscopy to identify apoptotic cells. To evaluate the percentage of apoptotic cells, 6–7 fields from each experimental condition were viewed in a blinded fashion until a total of 500 cells were observed. The results were obtained from a minimum of 6 independent experiments, and the values represent the mean percentage of apoptotic cells ± SEM. * indicates a significant difference (P<0.05) in the percentage of apoptotic cells in cultures from OA patients compared to normal donors. Note the increased in apoptosis in OA chondrocytes following exposure to both genotoxins.
DISCUSSION
The novel findings of this study are: (i) mtDNA damage accumulates in OA chondrocytes, indicating the presence of oxidative stress in chondrocyte mitochondria during disease progression; and (ii) OA chondrocytes have lower mtDNA repair capacity, which correlated with a decrease in viability and a resultant increase in the induction of apoptosis following experimentally induced oxidative stress. This indicates that these cells are less able to resist or recover from free radical-induced damage. Our study further proves that oxidative stress is a contributor to the chondrocyte dysfunction observed in OA. The present study may raise some concerns about the concentrations of genotoxins used to study differences in mtDNA repair capacity. Unfortunately, there is no way to correlate the amounts of ROS/RNS generated by peroxynitrite or xanthine oxidase used in our study with those present physiologically in OA cartilage, especially in the microenvironment around cartilage cells. Free radicals are reactive species with very short half-lives, usually measured in seconds. Therefore, ROS and RNS levels cannot be directly and accurately measured in cartilage samples in order to determine the extent to which they are elevated in aging or OA cartilage. However, by using indirect measures of ROS/RNS formation, such as measurements of oxidative damage to DNA and proteins and modulation of antioxidant capacity of cells, it has been shown that oxidative stress and increased NO production are present in OA cartilage (22, 23, 24, 25). The levels of ROS/RNS generators used in the present study are comparable to those which we have used to study oxidative stress in other cells and those used by other investigators (26, 27, 28). Furthermore, the levels used were not excessively high, as the majority of cartilage cells were able to repair the damage they caused in mtDNA. However, the repair was more proficient in normal cells.
The present findings indicate that mitochondrial DNA integrity and mtDNA repair are negatively affected during the progression of OA and point to the mitochondrial genome as a potential therapeutic target for amelioration of OA. Also, it needs to be emphasized that the maintenance of DNA integrity is a complex process, which includes not only sufficient DNA repair enzymes, but also many antioxidant defense systems. The analysis of the antioxidant capacity of OA chondrocytes was beyond the scope of the current investigation. However, this important issue will be actively pursued in future studies.
In mammalian cells, the maintenance of mtDNA requires an efficient DNA repair system to protect them from endogenous and exogenous oxidative stress. ROS or RNA resulting from exposure to cytokines or mechanical stimuli have been implicated as major factors in the regulation of chondrocyte metabolism during both normal conditions and the development of OA. These highly reactive molecules are known to regulate gene expression, transcription factor activation, cellular proliferation, and cause DNA damage and apoptosis. Because intracellular ROS are primarily generated by the mitochondrial electron transport chain, this mitochondrial network is a prime target for oxidative damage and an important player in aging and many degenerative processes. ROS production induces damage to lipids, proteins, and nucleic acids in mitochondria. Furthermore, ROS-induced mtDNA damage leads to mtDNA mutations which in turn can lead to the synthesis of functionally impaired respiratory chain subunits, causing respiratory chain dysfunction and augmented ROS production (21). This vicious cycle is proposed to cause an exponential increase in mtDNA mutations and damage over time, resulting in enhanced aging and degenerative diseases (22). We, and others have demonstrated that mtDNA is more sensitive than nuclear DNA to damage induced by ROS or RNS (23–25)). This is due to its close proximity to the inner mitochondrial membrane, where ROS are endogenously generated by the electron transport chain, and the high lipid content of the mitochondrial membrane that is potentially susceptible to lipid peroxidation chain reactions leading to the formation of toxic hydroperoxides and aldehydes. ROS and chronic inflammation have been shown to promote chronic oxidative stress in chondrocytes, which appears to have a significant functional impact in experimental OA (26–29). Although detailed information about mitochondria in human chondrocytes is scanty, there are a number of investigations supporting the notion that mitochondria may be involved in degeneration of cartilage and the development of OA. In one study, a 4977-bp deletion of mtDNA (also called the “common deletion”) clearly associated with degenerative abnormalities in knee cartilage retrieved from elderly patients with idiopathic OA (30). The frequency of occurrence of this deletion in the OA group was significantly higher than in the aged cartilage group. This deletion was found in only one specimen from subjects in a young control group. This finding indicates that the frequency of mtDNA mutations increases as cartilage ages and is further enhanced in OA. The results of our work support this notion and show that the accumulation of mtDNA mutations in chondrocytes during the progression of OA may be the result of an inability to appropriately repair oxidative damage in mtDNA.
A number of studies from our research group have shown that targeting DNA repair enzymes into mitochondria can not only enhance the repair of mtDNA lesions, but also increase the viability of the treated cells and protect them against the induction of apoptosis (30–33). We believe that mtDNA damage is involved in pathological changes in chondrocytes during the progression of OA and directly participates in the development of chondrocyte dysfunction and induction of apoptosis. Future studies in animal models, will involve the gene transfer of DNA repair enzymes into chondrocytes to protect against persistent mtDNA damage and enhance mtDNA repair. These studies should establish definitively the importance of mtDNA damage, as an early prerequisite step, for initiating the chondrocyte dysfunction and death seen in OA and suggest novel strategies for aborting this deleterious process.
Acknowledgment
This work was in part supported by the Orthopaedic Research and Education Foundation and NIH grant ES03456 to GLW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 2.Mazzetti I, Grigolo B, Pulsatelli L, Dolzani P, Silvestri T, Roseti L, et al. Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin Sci (Lond) 2001;101:593–599. [PubMed] [Google Scholar]
- 3.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:729–737. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 4.Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995;146:75–85. [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin AR, Di Cesare PE, Vyas P, Attur M, Tzeng E, Billiar TR, et al. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: Evidence for up-regulated neuronal nitric oxide synthase. J Exp Med. 1995;182:2097–2102. doi: 10.1084/jem.182.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeser RF, Carlson CS, Del Carlo M, Cole A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002;46:2349–2357. doi: 10.1002/art.10496. [DOI] [PubMed] [Google Scholar]
- 8.Yudoh K, Nguyen T, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–R391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 10.Cao M, Westerhausen-Larson A, Niyibizi C, Kavalkovich K, Georgescu HI, Rizzo CF, et al. Nitric oxide inhibits the synthesis of type-ii collagen without altering col2a1 mrna abundance: Prolyl hydroxylase as a possible target. Biochem J. 1997;324(Pt 1):305–310. doi: 10.1042/bj3240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27:339–344. doi: 10.1007/s00296-006-0247-8. [DOI] [PubMed] [Google Scholar]
- 12.Surapaneni KM, Venkataramana G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin e and antioxidant enzymes in patients with osteoarthritis. Indian J Med Sci. 2007;61:9–14. [PubMed] [Google Scholar]
- 13.Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 2007;232:592–606. [PubMed] [Google Scholar]
- 14.Lee RB, Urban JP. Evidence for a negative pasteur effect in articular cartilage. Biochem J. 1997;321(Pt 1):95–102. doi: 10.1042/bj3210095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maneiro E, Martin MA, de Andres MC, Lopez-Armada MJ, Fernandez-Sueiro JL, del Hoyo P, et al. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 16.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998;1366:211–223. doi: 10.1016/s0005-2728(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 19.Chandel NS, Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 1999;454:173–176. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- 20.Esteve JM, Mompo J, Garcia de la Asuncion J, Sastre J, Asensi M, Boix J, et al. Oxidative damage to mitochondrial DNA and glutathione oxidation in apoptosis: Studies in vivo and in vitro. Faseb J. 1999;13:1055–1064. doi: 10.1096/fasebj.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 21.Ledoux SP, Driggers WJ, Hollensworth BS, Wilson GL. Repair of alkylation and oxidative damage in mitochondrial DNA. Mutat Res. 1999 Jul 30;434(3):149–159. doi: 10.1016/s0921-8777(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 22.Olinski R, Zastawny T, Budzbon J, Skokowski J, Zegarski W, Dizdaroglu M. DNA base modifications in chromatin of human cancerous tissues. FEBS Lett. 1992;309:193–198. doi: 10.1016/0014-5793(92)81093-2. [DOI] [PubMed] [Google Scholar]
- 23.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- 24.Grishko VI, Druzhyna N, LeDoux SP, Wilson GL. Nitric oxide-induced damage to mtdna and its subsequent repair. Nucleic Acids Res. 1999;27:4510–4516. doi: 10.1093/nar/27.22.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1300–L1308. doi: 10.1152/ajplung.2001.280.6.L1300. [DOI] [PubMed] [Google Scholar]
- 26.Grishko V, Solomon M, Breit JF, Killilea DW, Ledoux SP, Wilson GL, et al. Hypoxia promotes oxidative base modifications in the pulmonary artery endothelial cell vegf gene. Faseb J. 2001;15:1267–1269. doi: 10.1096/fj.00-0755fje. [DOI] [PubMed] [Google Scholar]
- 27.Brooks P. Inflammation as an important feature of osteoarthritis. Bulletin of the World Health Organization. 2003;81:689–690. [PMC free article] [PubMed] [Google Scholar]
- 28.Buckwalter JA, Mow VC, Hunziker EB. Osteoarthritis: Diagnosis and medical/surgical management. In: Buckwalter JA, editor. Osteoarthritis: Diagnosis and medical / surgical management. Philadelphia: Saunders; 2001. pp. 101–114. [Google Scholar]
- 29.Bolton MC, Dudhia J, Bayliss MT. Age-related changes in the synthesis of link protein and aggrecan in human articular cartilage: Implications for aggregate stability. Biochem J. 1999;337(Pt 1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Chang MC, Hung SC, Chen WY, Chen TL, Lee CF, Lee HC, et al. Accumulation of mitochondrial DNA with 4977-bp deletion in knee cartilage--an association with idiopathic osteoarthritis. Osteoarthritis Cartilage. 2005;13:1004–1011. doi: 10.1016/j.joca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Rachek LI, Grishko VI, Alexeyev MF, Pastukh VV, LeDoux SP, Wilson GL. Endonuclease iii and endonuclease viii conditionally targeted into mitochondria enhance mitochondrial DNA repair and cell survival following oxidative stress. Nucleic Acids Res. 2004;32:3240–3247. doi: 10.1093/nar/gkh648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. Conditional targeting of the DNA repair enzyme hogg1 into mitochondria. J Biol Chem. 2002;277:44932–44937. doi: 10.1074/jbc.M208770200. [DOI] [PubMed] [Google Scholar]
- 33.Rachek LI, Grishko VI, Ledoux SP, Wilson GL. Role of nitric oxide-induced mtdna damage in mitochondrial dysfunction and apoptosis. Free Radic Biol Med. 2006;40:754–762. doi: 10.1016/j.freeradbiomed.2005.09.028. [DOI] [PubMed] [Google Scholar]