Abstract
Ciliary dysfunction has emerged as a common factor underlying the pathogenesis of both syndromic and isolated kidney cystic disease, an observation that has contributed to the unification of human genetic disorders of the cilium, the ciliopathies. Such grouping is underscored by two major observations: the fact that genes encoding ciliary proteins can contribute causal and modifying mutations across several clinically discrete ciliopathies, and the emerging realization that an understanding of the clinical pathology of one ciliopathy can provide valuable insight into the pathomechanism of renal cyst formation elsewhere in the ciliopathy spectrum. In this review, we discuss and attempt to stratify the different lines of proposed cilia-driven mechanisms for cystogenesis, ranging from mechano- and chemo-sensation, to cell shape and polarization, to the transduction of a variety of signaling cascades. We evaluate both common trends and differences across the models and discuss how each proposed mechanism can contribute to the development of novel therapeutic paradigms.
Keywords: Ciliopathies, Pleiotropic disorders, Disease modules, Paracrine signaling
Introduction
Cystic diseases of the kidney are a significant contributor to renal malformations and a common cause of end stage renal disease (ESRD). This classification encompasses a number of human disorders that range from conditions in which cyst formation is either the sole or the main clinical manifestation, to pleiotropic syndromes where cyst formation is but one of the observed pathologies, exhibits variable penetrance, and can sometimes be undetectable until later in life or upon necropsy (Table 1; [1]). Importantly, although the different cystic kidney disorders are clinically discrete entities, an extensive body of data fueled by a combination of mutation identification in humans and studies in animal models suggests a common thread, where virtually all known renal cystic disease-associated genes encode proteins necessary for aspects of ciliary function [2, 3]. Expanding from that observation, it is now becoming apparent that most—if not all—disorders of the cilium have a cystogenic component, which has in turn placed kidney cyst formation as a hallmark feature of the ciliopathies [4, 5].
Table 1.
Cystic diseases of the kidney: their causal genes, encoded proteins, localization, and proposed function
| Disease | Main features | Genes involved | Corresponding protein | Protein localization |
Postulated functions |
|---|---|---|---|---|---|
| Autosomal recessive PKD (ARPKD) | Renal cyst, enlarged kidneys, hepatic fibrosis | PKHD1 | Fibrocystin/polyductin (FPC) | Cilia and secreted | Calcium response; proliferation/differentiation |
| Autosomal dominant PKD (ADPKD) | Renal, hepatic, pancreatic and brain cysts | PKD1, PKD2 | Polycystin 1 (PC1) Polycystin 2 (PC2) | Cilia, Golgi apparatus, focal adhesions | Calcium response; proliferation/differentiation |
| Nephronophthisis (NPHP) and Senior Løken Syndrome (SNLS) | Renal fibrosis, renal cysts, tubular atrophy, retinal dystrophy (in SNLS) | NPHP1–NPHP11 | Nephrocystin-1, 2/inversin, 3, 4, 5, 6/CEP290, 7/GLIS2, 8/RPGRIP1L, 9/NEK8, 11/Meckelin | Cilia, basal bodies, centrosomes, focal adhesions. | Cell–cell and cell–matrix adhesion; actin cytoskeleton; cell division; Wnt and Shh signaling |
| Joubert syndrome (JS) | NPHP and cerebellar ataxia | AHI1, NPHP1, CEP290, JBTS6/TMEM67, RPGRIP1L, ARL13B, CC2D2A, INPP5E, JBTS2/TMEM216 | Jouberin, Nephrocystin, CEP290, Meckelin, RPGRIP1L, ARL13B, CC2D2A, INPP5E, TMEM216 | Cilia, basal bodies, centrosomes, cell junctions | Ciliogenesis, Sonic Hedgehog signaling |
| Bardet–Biedl syndrome (BBS) | Renal cysts, obesity, polydactyly, retinal dystrophy, mental retardation | BBS1-12, MKS1, CEP290, FRITZ, SDCCAG8 | BBS1-12, MKS1, CEP290, FRITZ, SDCCAG8 | Centrosomes, basal bodies | Pericentriolar organization, ciliogenesis, Wnt signaling |
| Meckel–Gruber syndrome (MKS) | Occipital meningoencephalocele, cystic kidneys, liver fibrosis, polydactyly | MKS1, MKS3/TMEM67, NPHP3, CEP290, RPGRIP1L, CC2D2A, MKS2/TMEM216 | MKS1, meckelin, nephrocystin 3, CEP290, RPGRIP1L, CC2D2A, TMEM216 | Centrosomes, cilia, plasma membrane | Basal body localization, ciliogenesis, Hedgehog signaling |
| Oral–facial–digital syndrome 1 (OFD1) | Malformations of face, oral cavity and digits, renal cysts, polydactyly | OFD1 | OFD1 | Cilia, basal bodies, centrosomes, nucleus | Ciliogenesis, L-R asymmetry, possibly gene regulation |
| Short-Rib Polydactily (incl. Jeune Asphyxiating Thoracic Dystrophy) | Renal cysts, shortened bones, polydactyly, situs inversus | DYN2CH1, IFT80 | Cytoplasmic dynein 2 heavy chain, IFT80 | Chondrocyte cilia, basal bodies | Intraflagelar transport, Hedgehog signaling |
| Uromodulin-associated kidney diseases (MCKD2, FJHN, GCKD) | Renal cysts, fibrosis, hypertension, hypoeruricemia, | UMOD, MCKD1, MCKD2 | Uromodulin | Cilia, basal bodies, centrosomes, secreted | Unknown ciliary role |
PKD, Polycystic kidney disease; MCKD2, medullary cystic kidney disease type 2; FJHN, familial juvenile hyperuricemic nephropathy; GCKD, glomerulocystic kidney disease
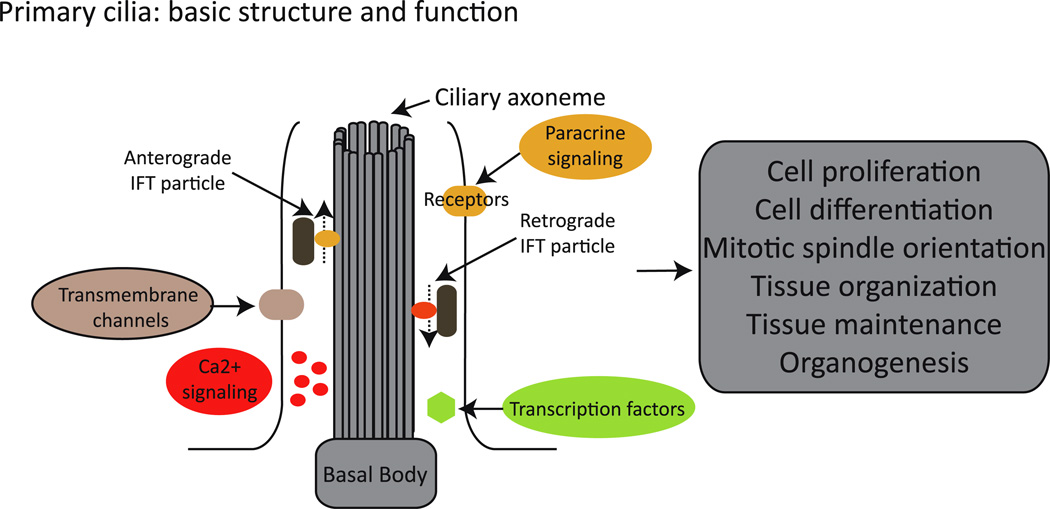
Primary cilia, motile cilia, and flagella are evolutionary conserved organelles that extend from the apical plasma membrane. Although cilia and flagella do demonstrate underappreciated structural and architectural diversity across phyla and tissue types [6], their fundamental organization is rigorously conserved and defined by a microtubule core (axoneme) that is organized from a basal body, which is a structure composed of nine microtubule triplets derived from the mother centriole of the centrosome. Along this microtubule core, multiprotein complexes and molecular motors are organized for transporting, both in (anterograde) and out (retrograde) of the cilium, the different components needed for the formation, maintenance, and function of this complex organelle (Fig. 1). This process is known as intraflagellar transport (IFT) and was described originally in the unicellular algae Chlamydomonas reinhardtii (for extensive reviews on the topic see, for example [7, 8]). Given its key role in maintaining ciliary function, it is therefore not surprising that alterations in different components of the IFT machinery have been shown to cause the renal phenotype observed in different human conditions and animal models of kidney cystic disease [4, 5, 9].
Fig. 1.
The cilium: basic structure and function. Cilia are formed by a microtubule core (axoneme) that is organized from a basal body. Along the axoneme, intraflagellar transport (IFT) particles are transported in (anterograde) and out (retrograde) of the cilium. The cilium concentrates and organizes a number of channels, receptors, and effectors, therefore playing a critical role in, for example, Ca2+ and paracrine signaling, ultimately regulating cellular, tissue, and organ homeostasis
A flurry of observations during the past decade have expanded our appreciation of cilia; transitioning from the classical models of motile cilia and their necessity for fluid movement or cellular propulsion, we now recognize that primary, sensory cilia are critical for cell–environment interactions. Discoveries driven primarily by phenotypic observations in both invertebrates and vertebrates (including humans) have shown that primary cilia are important for mechano- and chemo-sensation as well as for the signal transduction of short- and long-range paracrine signals, such as Wnt, Sonic Hedgehog (Shh), and platelet-derived growth factor receptor alpha (PDGFRα) [9–13]. Moreover, a number of additional receptors have been localized to subsets of both sensory and motile cilia, suggesting that additional signaling mechanisms will likely be assigned to these organelles. For example, it has been shown that the somatostatin receptor type 3 (Sstr3) and the melanin-concentrating hormone receptor 1 (Mchr1) do localize to cilia in neurons, thus providing an important insight into the feeding behavioral defects and obesity that characterize a number of ciliopathies [14]. Consequently, our deeper understanding of the complex nature of this organelle is contributing towards a better understanding of the mechanisms involved in the pathogenesis of ciliopathies, including the cystogenic process. In this review, we highlight how cilia have been linked to a number of renal cystic disorders and describe how our deeper understanding of the etiology of this group of disorders is impacting their genetic dissection. Finally, we discuss how such knowledge might help the development of successful therapeutic interventions, highlighted by some early successes and new possibilities.
Cystic diseases of the kidney and the link to primary cilia
Polycystic kidney disease
Polycystic kidney disease (PKD) is a heterogeneous group of disorders characterized by the abnormal proliferation and differentiation of epithelial cells in the kidney, resulting in the formation of fluid-filled cysts that can affect both the structure and function of the organ, often leading to ESRD [15]. PKD is divided in two major forms according to the pattern of inheritance and disease presentation. Autosomal dominant PKD (ADPKD) is the most common form of hereditary cystic kidney disease, with an estimated prevalence of between 1:400 and 1:1000 individuals irrespective of ethnicity, and is caused by mutations in PKD1 and PKD2, which encode polycystin 1 (PC1) and 2 (PC2), respectively [16–18]. Autosomal recessive PKD (ARPKD) presents in early childhood, is characterized by liver fibrosis and renal cysts, and affects 1:20,000 live births; it is caused by mutations in PKHD1, encoding the protein polyductin or fibrocystin [19, 20].
The first links between the pathogenesis of PKD and ciliary dysfunction were provided by the observation that lov-1, the closest Caenorhabditis elegans homolog of PKD1, localizes to primary cilia in sensory neurons in the nematode and by the characterization of the Oak Ridge polycystic kidney (orpk) mouse model of ARPKD, in which a hypomorphic mutation in Tg737 results in bilateral polycystic kidneys and liver lesions [21, 22]. A key observation arose when Tg737, which encodes the protein termed polaris, was found to encode the mouse ortholog of the Chlamydomonas IFT88, a protein that localizes to both basal bodies and cilia and which is necessary for ciliogenesis [23, 24]. In agreement with a postulated causal role of defective ciliary function in the tg737/Ift88 mutant, it was shown that both motile and primary cilia in orpk mice are shorter than normal. Furthermore, complete Tg737/Ift88 nulls lack cilia and present with a lethal phenotype that includes defective establishment of the left–right axis of symmetry, neural tube defects, and growth arrest during embryogenesis [25]. Importantly, all of these defects have been associated with other ciliary mutants (for extensive reviews, see [4–6, 9]). The protein product of PKHD1, polyductin/fibrocystin, localizes to primary cilia and basal bodies and has been postulated to be a receptor mediating the differentiation of collecting duct cells [20, 26]. Furthermore, it has been shown that polyductin/fibrocystin physically interacts with PC2 and is able to regulate renal tubular formation by affecting PC2 expression and function [27, 28]. More recently, it has been shown that mutant Pkhd1 mice present biliary duct cilia that are significantly shorter than those of the controls and that mutant animals present with liver and pancreatic cysts and renal tubular dilation [29].
The characterization of the proteins altered in ADPKD further support the central role of the cilium in the pathogenesis of PKD (reviewed in [30]). Briefly, PC1 is thought to be a G protein-coupled receptor (GPCR), while PC2 is a cation ion channel. These two proteins have been shown to interact, forming a Ca2+ channel that localizes to the primary cilium in renal epithelial cells [31–36]. Importantly, the ciliary localization of the polycystins appears to be necessary for their proposed role in Ca2+ signaling in response to mechanical cues in the lumen of the renal tubules (see section on Mechanosensation, shear stress and cystogenesis).
Nephronophthisis and Joubert syndrome
Nephronophthisis (NPHP) (OMIM 256100) is characterized by corticomedullary cysts and interstitial fibrosis and is the most frequent cause of ESRD in children and young adults. To date, ten genes (NPHP1–9, NPHP11/TMEM67) have been shown to cause NPHP, while mutations in XPNPEP3 cause an NPHP-like nephropathy ([37–39] and references within). Importantly, the characterization of the different NPHP proteins has been instrumental in developing the unifying theory of cystogenesis based on ciliary dysfunction. One of the first observations supporting this concept came from the characterization of nephrocystin 1 and inversin, the protein products of NPHP1 and NPHP2, respectively, in which the two proteins were shown to interact and colocalize with β-tubulin at the primary cilium of renal epithelial cells [40]. In all studies carried out since, the characterizations of all NPHP-related proteins have further supported the critical role of the cilium in the pathogenesis of this condition, providing important insight into the role of this organelle during cystogenesis (reviewed in [38]). For example, studies on inversin have not only made significant contributions to a unified concept of the ciliary-driven cystogenic mechanisms, but they have also provided some of the early clues that have linked ciliary function with paracrine signaling and, in particular, with the transduction of canonical and noncanonical (planar cell polarity) Wnt signaling, pathways that are critical for renal development and maintenance (see the Kidney morphogenesis/maintenance and cystogenesis: Wnt signaling section). Likewise, the characterization of NPHP7 implicated Shh signaling in the pathogenesis of cystic kidney disease, given that the gene encodes the transcription factor Gli-similar protein 2 (GLIS2) [41] (see the Hedgehog signaling in the pathogenesis of cystic kidney disease section).
A related disorder is Joubert syndrome (JS) (OMIM 213300), a recessive disease characterized by cerebellar vermis hypoplasia, hypotonia, mental retardation, irregular breathing, and eye movement abnormalities. In addition, JS patients might present additional clinical features, such as retinal dystrophy, cystic dysplasia, and nephronophthisis, encompassing what is known as Joubert syndrome and related disorders (JSRD). Nine genes have been causally linked to JS (INPP5E, AHI1, NPHP1, CEP290, TMEM67, RPGRIP1L, ARL13B, CC2D2A, and TMEM216), all of which encode basal body/ciliary proteins ([42–44] and references within).
Bardet–Biedl and Meckel–Gruber syndromes
Ciliary dysfunction can also cause highly pleiotropic syndromes where cystic kidney disease is a characteristic feature. Bardet–Biedl syndrome (BBS) (OMIM 209900) is characterized by obesity, polydactyly, retinal degeneration, mental retardation, and renal malformations that include the formation of cysts [45]. A total of 16 BBS genes have been identified to date (BBS1–12, MKS1, NPHP6/CEP290, FRITZ/C2ORF86, SDCCAG8) ([46–49] and references within). Importantly, the vast majority of BBS proteins tested to date localize to centrosomes and basal bodies, while some have also been observed in the cilium and play a role during ciliogenesis [50–56].
At the severe end of the phenotypic spectrum of the cystic diseases of the kidney lies Meckel–Gruber syndrome (MKS) (OMIM 249000), a perinatal lethal condition characterized by occipital encephalocoele, hepatic fibrosis, polydactyly, cleft palate, neural tube defects, and kidney cysts [57]. To date, seven MKS genes (MKS1, MKS3/TMEM67, CEP290, RPGRIP1L, MKS6/CC2D2A, NPHP3, and TMEM216/MKS2) have been identified [44, 58–63]. It has been shown that MKS1, MKS3/TMEM67, CEP290, and RPGRIP1L/MKS5 localize to the basal body of cilia [59, 64, 65]. MKS1 and MKS3/TMEM67 have been proposed to be important for correctly positioning the basal body underneath the cellular membrane—albeit this has been shown only in cell-based models—and are required for ciliogenesis both in vitro and in vivo [64, 66]. Also, mutations in MKS6/CC2D2A lead to the absence of cilia in fibroblasts from affected patients [62]. Interestingly, these observations in MKS, together with the lethality of the complete Tg737 nulls described earlier, support the notion that the complete lack of these organelles is incompatible with life, an observation that is not surprising given the plethora of biological functions in which they participate and the ubiquitous presence of these organelles in the vast majority of cell types of the human body (for a list of ciliated cell types, see http://www.bowserlab.org/primarycilia/ciliumpage2.htm).
A common cellular defect and a shared genetic basis
As described above, defects in different genes affecting the same cellular organelle can result in a spectrum of human conditions that share, to a different degree, a given set of phenotypes. Consequently, the same or similar phenotypes can be caused by mutations in more than one gene. At the level of individual syndromes, this phenomenon is observed in the form of genetic heterogeneity, a characteristic of most ciliopathies (Table 1), and is usually considered to be a major contributor of inter-familial phenotypic variability. Interestingly, not only can different genes cause the same phenotype, but mutations in different loci can also collaborate to modulate disease presentation. Importantly, several human disorders, including the ciliopathies, are also characterized by a significant intra-familial variability regarding disease presentation and progression. Although the impact of the environment and other stochastic factors cannot be ignored, oligogenicity and the interaction between different alleles are likely determinant factors of the final phenotypic outcome, thus further complicating our ability to predict disease presentation from genotype. In BBS, it has been demonstrated that although the disease segregates in the majority of families in a seemingly autosomal recessive fashion, in some cases mutations in more than one BBS gene, or second site modifiers, appear to collaborate to affect both the penetrance and expressivity of the disease [51, 67–69]. Furthermore, NPHP has also been found to behave as an oligogenic trait since mutations in more than one gene have been identified segregating in a given family [70].
Interestingly, the genetic interactions described above are not restricted to individual disorders but appear to span ciliopathies in general (Table 2). One example involves BBS and MKS, where it has been reported that mutations in BBS2, BBS4, and BBS6 were found in MKS-like fetuses [71]. More recently, it has been shown that mutations in three MKS genes, MKS1, MKS3/TMEM67, and CEP290 can either cause or modulate the severity of the BBS phenotype [47]. CEP290 has not only been identified as a causal gene in MKS, but it has also been shown to cause NPHP (NPHP6), JS, and BBS ([47, 58, 65, 72, 73]; for other examples, see Table 2 and [9, 74]). Moreover, mutations in the ciliary gene AHI1, which causes JS, can act as second site modifier to increase the risk of retinal degeneration in NPHP patients [75]. In addition, alleles in ciliary genes can have a modifier effect across a range of ciliopathies, further complicating the genetic dissection of this group of disorders. It was recently shown that a polymorphic A229T coding variant of RPGRIP1L, a gene mutated in MKS, is associated with photoreceptor loss and, hence, with the retinal phenotype in multiple ciliopathies [76].
Table 2.
Genetic interaction between cystic diseases of the kidney
| Mutatied gene | NPHP | MKS | JS | BBS | SLSN |
|---|---|---|---|---|---|
| AHl1 | ✓ | ✓ | |||
| NPHP1 | ✓ | ✓ | ✓ | ||
| NPHP3 | ✓ | ✓ | |||
| NPHP4 | ✓ | ✓ | |||
| NPHP5 | ✓ | ✓ | |||
| NPHP6 | ✓ | ✓ | ✓ | ✓ | |
| NPHP8 | ✓ | ✓ | ✓ | ||
| MKS1 | ✓ | ✓ | |||
| CC2D2A | ✓ | ✓ | |||
| TMEM67 | ✓ | ✓ | ✓ | ✓ | |
| TMEM216 | ✓ | ✓ |
Why can defects in a given gene result in more than one disease? In the case of BBS–MKS, important sources of insight have come from the in vivo analysis of the pathogenic effect of MKS mutations associated with BBS. Interestingly, it has been shown that these mutations are likely hypomorphs, whereas the mutations causing MKS are complete nulls [47]. Similarly, hypomorphic mutations in MKS3/TMEM67 can cause an NPHP-like phenotype and have been reported in NPHP patients [39, 63]. Therefore, it is not only the mutated gene, but also the number of genes and the combination of mutant alleles that appears to determine the final phenotype and, thus, its “classification” into one or other clinical entity. Importantly, dissecting these complex genetic interactions and evaluating the effect of the different mutant alleles that participate in them will likely be a necessary step towards generating accurate genotype–phenotype correlations that could be used for diagnostic and prognostic purposes in the clinic.
Cystogenic mechanisms
Primary cilia have been implicated in a number of biological processes that range from mechano- and chemo-sensation to the transduction of several paracrine signaling pathways. Therefore, the realization that ciliary dysfunction is intimately related to the pathogenesis of cystic kidney disease provides an entry point to understand the pathomechanisms underlying cyst formation. Driven by an improved understanding of the role(s) of cilia in the development and tissue maintenance, defects in several physiological processes have been put forth as plausible cystogenic candidates.
Mechanosensation, shear stress, and cystogenesis
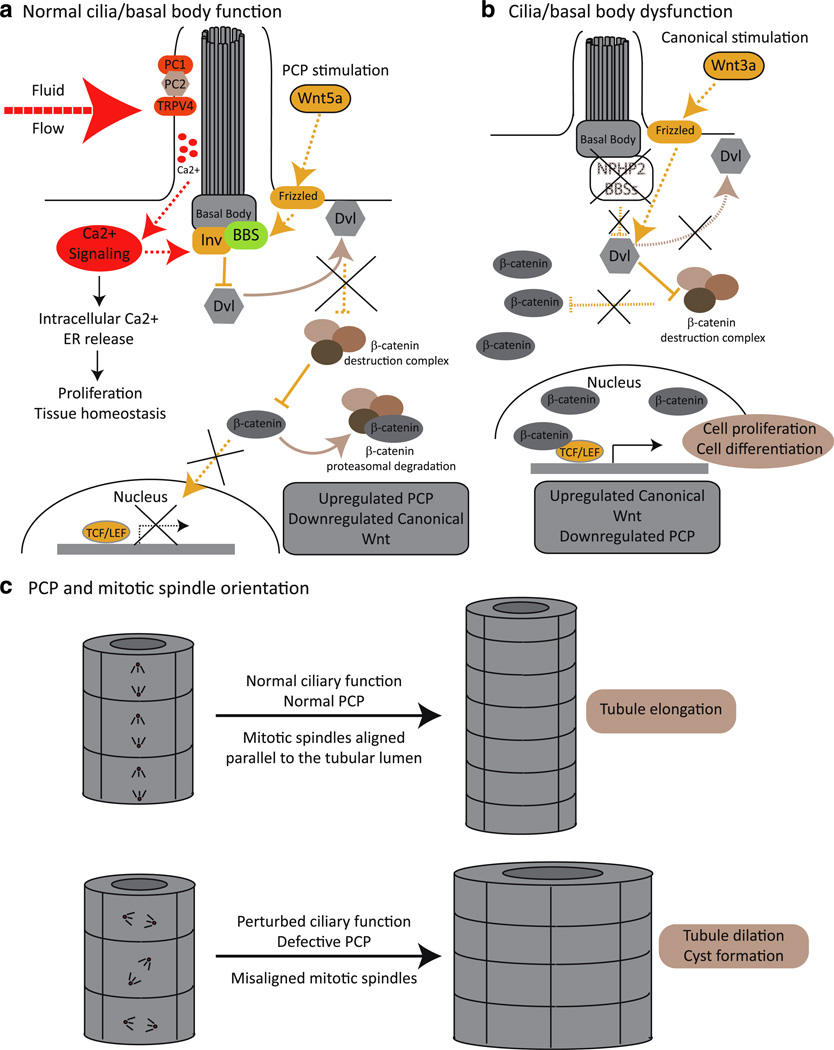
Primary cilia can act as mechano-sensory structures, sensing shear stress and signaling into the interior of the cell to regulate proliferation and differentiation through Ca2+ (Fig. 2a). It has been shown that the bending of primary cilia by an extracellular fluid flow causes an increase in the intracellular Ca2+ concentration through the activation of ion channels located on the axonemal membrane. The initial Ca2+ influx induces Ca2+ release from intracellular stores, and the signal is spread to adjacent cells through gap junctions [77]. Interestingly, PC1 and PC2, the proteins mutated in ADPKD, have been shown to heterodimerize and to be required in this process: the extracellular domain of PC1 has been postulated to sense mechanical forces, such as fluid flow, thus changing its conformation to activate PC2 and, thereby, allowing for Ca2+ entry [13, 32, 35]. Importantly, cilia-mediated Ca2+ signaling is not restricted to the renal epithelium. In the embryonic node, the left–right axis of symmetry is established through the activity of a group of ciliated cells generating a leftward fluid flow (nodal flow) that is sensed and transduced into an asymmetric Ca2+ signal in a process that requires PC2 [78, 79]. The flow hypothesis also links fluid flow sensing with the regulation of cilia-dependent signal transduction, given that inversin is upregulated by fluid flow, leading to the down-regulation of the canonical Wnt signaling and the upregulation of the non-canonical planar cell polarity pathway, thus favoring cell differentiation over proliferation, a balance clearly lost during cystogenesis [80] (Fig. 2a; see following section). However, a model based exclusively on fluid flow cannot fully reconcile different lines of evidence from both in vitro and in vivo experiments.
Fig. 2.
Primary cilia: mechano-sensors and Wnt transducers. a PC1, PC2, and TRPV4 form a Ca2+ channel that localizes to the primary cilium and which upon a mechanical stimulus, such as fluid flow, initiate a Ca2+ signaling cascade that regulates cell proliferation. Functional cilia are also needed to maintain the tight balance between canonical and noncanonical [planar cell polarity (PCP)] Wnt signaling. In the presence of a functional cilium, and upon PCP stimulation and Ca2+ signaling, basal bodies proteins, such as the BBSs and inversin (Inv)/NPHP2, mediate the degradation and/or relocalization of Disheveled (Dvl) to the cellular membrane. In this scenario, Dvl is no longer able to suppress the β-catenin destruction complex, and β-catenin is therefore degraded by the proteasome and is unable to act as a transcriptional activator. b When cilia and/or basal body function is compromised, canonical Wnt signals (for example, Wnt 3a) are not antagonized, Dvl is able to repress the β-catenin destruction complex, and β-catenin accumulates in the nucleus and drives the expression of TCF–LEF responsive genes. c The non-canonical PCP Wnt pathway is required for providing positional information to cells allowing the correct alignment of their mitotic spindles with respect to the lumen of the renal tubule. Upon ciliary dysfunction, the PCP signal is defective, and cell division orients randomly. Therefore, under normal ciliary function, cell division in the renal tubules results in tubular elongation, while ciliary dysfunction results in tubule dilation, facilitating cystogenesis
PC2 interacts not only with PC1 but also with TRPV4 and TRPC1, where the association of PC2 with TRPV4 appears to be required to form a mechano- and thermosensitive channel [35, 81–83]. Depletion of TRPV4 in mice does not lead to cystic kidney disease although it does abolish flow-induced Ca2+ signaling in renal epithelial cells, suggesting that flow sensing might not be sufficient to fully explain cystogenesis [81]. Likewise, cilia-independent factors might also be important during cystogenesis. For example, trans-epithelial pressure can result in Ca2+ release independently from the presence of cilia in Madin–Darby canine kidney (MDCK) cells [84]. Further levels of complexity regarding the physiology of the kidney and the role of the polycystins, both in normal tissue as well as during cystogenesis, has come from two independent studies demonstrating that cystogenesis is influenced significantly by the time of inactivation [85, 86]. Conditional mutants of Ift88, or of the anterograde IFT molecular motor protein Kif3A, in which the gene is ablated in adult animals, present with a mild cystic phenotype by 6 months after inactivation and progress to a more severe phenotype after 1 year. In contrast, ablation of these genes on embryonic day 17.5 (E17.5) results in cyst formation in the first 2 weeks of life [86]. Similarly, using a conditional Pkd1 mouse model, it has been shown that inactivation of the gene before postnatal day 13 (P13) results in rapid progression of the disease, with animals showing severe cystic kidneys within 3 weeks. However, ablating Pkd1 at P14 or later results in the formation of cysts only 5 months after the inactivation event [85]. Therefore, the renal tissue appears to have different requirements for the activity of the cilium during development and adulthood, and cilia-mediated sensing of fluid flow might not be the sole driver of cystogenesis [85, 86]. Interestingly, PC1 expression appears to accompany changes in proliferation rates in young versus older kidneys, with high levels of PC1 observed at around E15.5, subsequently decreasing by 2 weeks after birth; these changes accompany the rapid growth, high tubulogenesis rate, and ongoing tissue morphogenesis characteristic of developing kidneys [87].
Kidney morphogenesis/maintenance and cystogenesis: Wnt signaling
The Wnt signaling pathway plays an important role regulating both the development and maintenance of the kidney. During organogenesis, activated Wnt signaling is required for the induction of the metanephric mesenchyme to develop the proximal portion of the nephron, regulating cell proliferation and differentiation [88–91]. The Wnts are secreted proteins which, upon binding to the Frizzled receptor, can activate different signaling cascades with different consequences for cell homeostasis. The final outcome depends largely on the specific Wnt molecule but also on the activity of Disheveled (Dvl), a protein that represses the degradation of β-catenin, the major effector of the canonical Wnt pathway.
It has been shown that the cellular localization of Dvl is a major factor in determining which Wnt cascade is activated. To activate the canonical pathway, Dvl represses the β-catenin destruction complex (composed of GSK3β, axin, and APC), leading to the accumulation of nuclear β-catenin and allowing the expression of TCF–LEF1 responsive genes that control cell proliferation and differentiation [11, 92]. However, when Dvl is localized to the plasma membrane, it is no longer able to prevent β-catenin degradation, and noncanonical Wnt signaling pathways, particularly the planar cell polarity (PCP) pathway, can therefore be activated [11, 93]. PCP is required to provide positional information that allows cells to properly polarize, migrate, and orient themselves in the plane of the epithelium. Consequently, this signaling pathway plays an important role in maintaining the correct organization of tissues and organs [93].
NPHP2 encodes for the basal body protein inversin, which is mutated in the inv/inv mouse model of cystic kidney disease and has been shown to target Dvl for degradation [80, 94]. Therefore, in the absence of inversin, Dvl is free to repress the β-catenin destruction complex, thus leading to an upregulation of canonical Wnt signaling and also defective PCP [80]. Importantly, the different Wnt signaling cascades are tightly controlled and balanced between each other. Similarly, studies in BBS have shown that the depletion of different BBS proteins leads to defective PCP with the concomitant stabilization of β-catenin and thus upregulation of canonical signaling [95, 96], although in the context of that disorder, targeting of β-catenin to the proteasome has been proposed as a possible biochemical cause of this phenotype.
In a simplistic model, the canonical pathway can be visualized acting mostly in favor of cell proliferation, whereas the noncanonical cascades, and particularly PCP, appear to have the converse effect, leading to cell differentiation. During cystogenesis, there is a clear loss of balance between opposing, but non-equal forces. In this context, the presence of functional cilia appears to be necessary to suppress canonical Wnt signaling while favoring the PCP pathway (Fig. 2a). Mutations in Kif3a in both cells and mice lead to increased Wnt canonical signaling, while a kidney conditional mutation in Pkd2 resulted in a cystic phenotype and upregulated β-catenin [95, 97, 98]. Transgenic mice in which an oncogenic form of β-catenin is over-expressed develop kidney cysts in all parts of the nephron [99]. Interestingly, in vitro experiments using kidney cells in a chamber showed that the expression of inversin is upregulated by fluid flow, whereas β-catenin levels were mildly decreased [80]. It has been speculated that the beginning of fluid flow during early embryogenesis might trigger a switch in Wnt signaling in the kidney that both terminates the pro-proliferation canonical pathway and favors the prodifferentiation PCP cascade, thereby allowing terminal differentiation of epithelial cells and the correct morphogenesis of the organ [91]. However, this view of cystogenesis being driven by over-activation of canonical Wnt signaling is likely a simplification. For example, it has been shown recently that midgestation embryos and mouse embryonic fibroblasts bearing mutations in different components of the IFT machinery retain normal canonical Wnt activity and the ability to switch to noncanonical signaling [100], perhaps intimating that the requirement for cilia-mediated processes likely changes at different time-points during development and adult life. Additional evidence supporting this notion has come from studies by Lancaster and colleagues in mice bearing homozygous null mutations in Ahi1, a gene mutated in JS which encodes the protein Jouberin (Jbn). These authors showed that Jbn is a basal body/ciliary protein that regulates and promotes canonical Wnt signaling in the adult kidney by facilitating the nuclear translocation of β-catenin [101]. Furthermore, Ahi1−/− mice, which present with an NPHP-like phenotype, show impaired renal injury repair, a process that has been shown to trigger cystogenesis [102] and which relies on intact canonical Wnt signaling. Based on these data, the authors suggested that a tight regulation of Wnt signaling is needed for maintaining kidney homeostasis and the balance between proliferation and differentiation during tubular regeneration after injury. In this model, it is the loss of this fine regulation—rather than a simple over-activation of canonical Wnt signaling or a down-regulation of PCP—that leads to cyst formation [101].
In addition to misregulation of canonical Wnt signaling, the loss of PCP provides an attractive mechanistic explanation to cyst formation. During cell division, epithelial cells in the renal tubules have been shown to orient their mitotic spindles parallel to the axis of the tubule; this arrangement is lost in different models of PKD, such as kidney-specific HNF1β deficient mice and pck rats where Pkhd1 is mutated. According to this model, under normal PCP signaling, the net result of cell division is tubular elongation, whereas ciliary dysfunction and aberrant PCP result in tubular dilation (Fig. 2c; [103]). Supporting the role of PCP in this phenomenon, mouse mutants for the PCP gene Fat4 display neural tube defects and defective organization in the inner ear but also perturbed oriented cell divisions in the renal epithelium, resulting in dilated tubules and cystic kidney disease. Moreover, further perturbation of the pathway through the introduction of additional mutations in core PCP genes, such as Vangl2 and Fjx1, resulted in a more severe presentation of the cystic phenotype [104].
More recently, it has been shown that homozygous mice for a hypomorphic allele of Wnt9b, which is required for PCP in the collecting ducts and proximal tubules of the kidney, present with perturbed PCP, random mitotic spindle orientation, and tubule dilation [105]. Interestingly, an analysis of the cell shape in the renal tubules revealed that normally these epithelial cells appear elongated in the axis perpendicular to the lumen, suggesting that they are subjected to organized cell migration, such as convergent and extension, which is yet another PCP-dependent process [93, 105, 106]. It has also been shown recently that whereas conditional inactivation of Pkd1 or Pkd2 in the kidney does affect oriented cell division in dilated but not in pre-cystic tubules, mutations in Pkhd1 result in defective cell division orientation but no cyst formation. These data support the notion that although the loss of oriented cell division is altered during cyst formation, this event might not be sufficient or even required to initiate cystogenesis [107].
Hedgehog signaling in the pathogenesis of cystic kidney disease
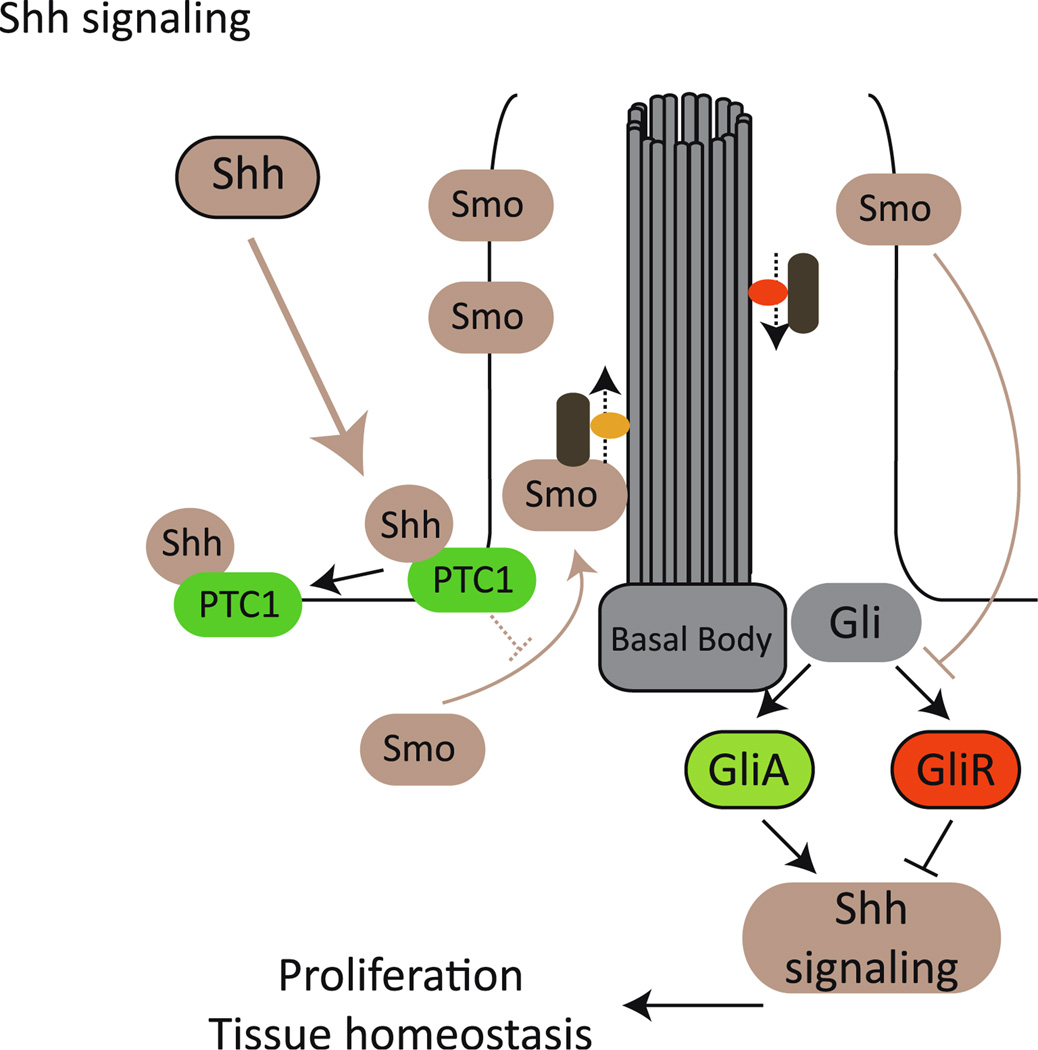
Another important signaling pathway that has been shown to depend heavily on a functioning cilium is sonic hedgehog (Shh) (see [108–113]). Shh binds and inactivates the Patched 1 receptor (Ptc1), which in turn releases Smoothened (Smo), enabling it to block the processing of the transcription factor Gli3 into its repressor form GliR. Consequently, Shh binding enables Gli3-mediated gene regulation (reviewed in [114]). It has been shown that ciliary localization of different cascade components is required for the correct processing of Gli. Shh activation leads to the relocation of Smo into the cilium, and this is required for its activity [108, 109]. Ptc1 also localizes to the ciliary membrane and appears to inhibit Smo by impeding its accumulation in the ciliary compartment, and Gli1, Gli2, and Gli3 also localize to the cilium (Fig. 3; [110, 113]).
Fig. 3.
Primary cilia and sonic hedgehog (Shh) transduction. Binding of Shh to Patched 1 receptor (PTC1) results in the re-localization of Smoothened (Smo) into the ciliary compartment, thus inhibiting the formation of GliR (repressor) and favoring the processing of Gli into GliA, the activator that drives the expression of different target genes
Shh signaling plays an important role during morphogenesis, patterning, and growth of different tissues and organs, and thus defects in this cascade can readily explain several phenotypes characteristic of the ciliopathies, such as neural tube defects, polydactyly, and craniofacial defects (for some reviews see [6, 9, 74, 115]). In addition, different lines of evidence are implicating Shh signaling to the cystic phenotype. For example, ARL13b, a gene mutated in JS, a ciliopathy characterized by brain malformations but also the formation of cysts in the kidney, among other features, was originally identified as a cystogenic zebrafish mutant, scorpion (sco) and was shown to play a role in ciliogenesis [43, 116, 117]. A null mutation in Arl13b has been reported in the mouse hennin (hnn) mutant, which presents defects in cilia structure and perturbed Shh signaling [118]. It has also recently been shown that Mks1 is required for ciliogenesis and Shh signaling in the mouse [66], while the identification of NPHP7 further supports the role of Shh in the etiology of cystic kidney disease since the gene encodes for the transcription factor Gli-similar protein 2 (GLIS2) [41].
Despite these associative observations, the role of Shh during cystogenesis is not well understood. Shh signaling, together with Wnt, epidermal growth factor (EGF), fibroblast growth factor (FGF), and TGFβ, have been implicated in several morphogenetic processes, such as the epidermal to mesenchymal transition (EMT) ([119] and references therein). Notably, the kidney phenotype in, for example, NPHP and BBS is characterized by increased fibrosis, a feature that might be explained—at least in part—by deregulated EMT as a consequence of defective Shh signaling. Supporting this possibility, differential gene expression analysis of NPHP7/Glis2 mutants shows an upregulation of genes favoring EMT [41].
Cell proliferation and cystic kidney disease
The formation of cysts is characterized by the deregulation of the balance between cell proliferation and differentiation, and cilia appear to play a role in maintaining this balance through sensing the extracellular milieu, responding to mechanical cues, and modulating different signaling cascades. In addition to Wnt and Shh, platelet-derived growth factor receptor alpha (PDGFRα), a pathway intimately linked to cell cycle regulation, has also been shown to depend on the cilium (reviewed in [10]). Also, the cilium and different ciliary proteins appear to have an active role in cell cycle regulation. Cilia are postmitotic structures, and thus both ciliogenesis and ciliary disassembly are tightly regulated with cell division (reviewed in [120]). For example, mutations in Nek1 and Nek8/NPHP9, members of the family of NIMA-related protein kinases postulated to coordinate ciliary function with the cell cycle, result in cystic kidney disease [121–123].
Importantly, several ciliary proteins appear to control cell proliferation, likely through cilia-independent mechanisms. While polaris is required for the formation and maintenance of cilia, it has also been demonstrated that this protein can remain associated with the centrosome throughout the cell cycle, even in the absence of cilia, and regulate the G1–S transition. Over-expression of polaris in non-ciliated cells leads to cell cycle arrest through a mechanism that involves the inhibition of Che1, which normally counteracts the activity of the negative cell cycle regulator retinoblastoma (Rb) [124]. As mentioned earlier, the polycystins also play an important role during cilia-mediated mechanosensation and Ca2+ signaling and can modulate cell proliferation and differentiation. In addition, PC1 is subjected to proteolytic cleavage, releasing its cytoplasmic C-terminal tail, which is then translocated into the nucleus to regulate gene transcription mediated by the signal transducer and activator of transcription protein 6 (STAT6), a process that is inhibited by mechanical stimuli and appears to be misregulated due to ciliary dysfunction [125, 126]. Also, PC1 can regulate the expression of p21, a tumor suppressor that inhibits cyclin-dependent kinases, leading to cell cycle arrest, whereas depletion of PC1 results in the acceleration of the G1–S transition, leading to increased cell proliferation [127, 128]. Additionally, it has also been shown that PC1 can regulate the mTOR (mammalian target of rapamycin) pathway, providing yet another mechanism by which to regulate cell proliferation and differentiation [129, 130]. Therefore, the polycystins represent a clear example of the complexity underlying the biological role(s) of the cilium and the proteins that compose it and highlight the need for further work to fully understand their biological role(s) in the context of cystic kidney disease.
Although cilia are post-mitotic structures, it is not clear whether re-absorption of cilia precede cycling or whether it is a consequence of cells engaging in the cell cycle. To start understanding this issue, mutations that unlink the ciliary and mitotic spindle roles of a given ciliary protein will be extremely useful. One example of such mutations has recently been reported for BBS4, where a leucine to proline substitution at position 327 of BBS4 (L327P) leads to the mislocalization of BBS4 from the pericentriolar region in postmitotic cells while retaining the centriolar localization to the mitotic spindles of dividing cells. Interestingly, this mutant behaves as a dominant negative in an in vivo rescue assay, interfering with the correct transduction of the Wnt signaling pathway in a manner that is similar to the inhibition of wild-type BBS4, suggesting that its main effect is a consequence of its absence from the basal body of cilia in post-mitotic cells [131]. Further studies will be required to address the behavior of this type of mutant with regards to cell cycle regulation.
Concluding remarks
Remarkable progress has been made towards gaining an understanding of the genetic, cellular, and molecular basis of the ciliopathies/cystic diseases of the kidney. These data highlight the complexity of the cilium, an organelle that not only participates in multiple biological processes but also likely acts as a relaying center in which different signals and pathways are integrated to correctly process and interpret the different stimuli to which cells are subjected. In this context, it is not surprising that a single mechanism is likely not sufficient to explain the different types of disease presentation, progression, and outcome that characterize the different renal cystic disorders. However, and despite current limitations, this increased body of knowledge is already translating into different examples of successful therapeutic interventions in different animal models of cystic disease. For example, rapamycin, a drug that inhibits mTOR signaling, has been used to slow cyst progression and rescue renal function in different mouse, rat, and zebrafish models of cystic kidney disease [130, 132–134]. Furthermore, treatment with rapamycin for immune suppression after renal transplantation in ADPKD patients has shown promising results in reducing renal volume [130]. However, mTOR signaling is not only involved in cyst formation but is required for a number of biological processes and, therefore, its inhibition is likely to produce undesired secondary effects that need to be carefully evaluated (for an in-depth review on the topic, see [135]). In another example and due to the characteristic misregulation in cell proliferation during cystogenesis, Bukanov and colleagues decided to test the cyclin-dependent kinase inhibitor roscovitine in two animal models of PKD, the jck and the cpk mouse [136]. Importantly, administration of roscovitine does ameliorate the cystic phenotype in these animals as well as in zebrafish models [133, 136]. More recently, the Src inhibitor SKI-606 has also been use to treat renal cyst formation in mouse and rat models of ARPKD [137]. Despite these advances, however, the challenge ahead is to fully dissect the biological role(s) of cilia and ciliary proteins in the pathogenesis of the different phenotypes that characterize the ciliopathies, including but also extending beyond the kidney. Undoubtedly, this will be necessary to continue exploring novel therapeutic venues with an increasing level of efficacy and specificity.
Acknowledgments
First of all we apologize to all those scientists whose contribution on ciliary biology and the process of cystogenesis could not be properly cited in this manuscript due to space limitations. JLB is supported by the Genzyme Renal Innovations Program (GRIP), JLB and CG by PEDECIBA and ANII–Agencia Nacional de Investigación e Innovación, Programa de Apoyo Sectorial a la Estrategia Nacional de Innovación–INNOVA URUGUAY, DCI–ALA/2007/19.040, and NK by the National Institutes of Health grants HD04260, DK072301, and DK075972. NK is the George W. Brumley Professor.
Contributor Information
Cecilia Gascue, Institut Pasteur de Montevideo, Mataojo 2020, Montevideo, CP 11400, Uruguay.
Nicholas Katsanis, Email: katsanis@cellbio.duke.edu, Center for Human Disease Modeling, Duke University Medical Center, Box 3709, Durham, NC 27710, USA.
Jose L. Badano, Institut Pasteur de Montevideo, Mataojo 2020, Montevideo, CP 11400, Uruguay
References
- 1.Beales PL, Parfrey PS, Katsanis N. The Bardet-Biedl and Alstrom Syndromes. In: Maher E, Saggar-Malik A, editors. Genetics of renal disease. Oxford: Oxford University Press; 2004. pp. 361–398. [Google Scholar]
- 2.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 3.Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–356. doi: 10.1038/ng0803-355. [DOI] [PubMed] [Google Scholar]
- 4.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emergin class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 5.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:263–280. doi: 10.1002/ajmg.c.30227. [DOI] [PubMed] [Google Scholar]
- 10.Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- 11.Gerdes JM, Katsanis N. Ciliary function and Wnt signal modulation. Curr Top Dev Biol. 2008;85:175–195. doi: 10.1016/S0070-2153(08)00807-7. [DOI] [PubMed] [Google Scholar]
- 12.Haycraft CJ, Serra R. Cilia involvement in patterning and maintenance of the skeleton. Curr Top Dev Biol. 2008;85:303–332. doi: 10.1016/S0070-2153(08)00811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 14.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 16.Consortium TEPKD. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki T, Wu G, Hayashi T, Xenophontos S, Veldhuisen B, Saris J, Renolds D, Cai Y, Gabow P, Pierides A, Kimberling W, Breuning M, Deltas C, Peters D, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 18.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 21.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans . Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 22.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- 23.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- 26.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 27.Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George ALJ, Coffey RJ, Feng ZP, Wu G. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19:455–468. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH, LaRusso NF, Harris PC, Ward CJ. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int. 2007;72:328–336. doi: 10.1038/sj.ki.5002294. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 33.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in ORPK mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 34.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 35.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 37.O'Toole JF, Liu Y, Davis EE, Westlake CJ, Attanasio M, Otto EA, Seelow D, Nurnberg G, Becker C, Nuutinen M, Kärppä M, Ignatius J, Uusimaa J, Pakanen S, Jaakkola E, van den Heuvel LP, Fehrenbach H, Wiggins R, Goyal M, Zhou W, Wolf MT, Wise E, Helou J, Allen SJ, Murga-Zamalloa CA, Ashraf S, Chaki M, Heeringa S, Chernin G, Hoskins BE, Chaib H, Gleeson J, Kusakabe T, Suzuki T, Isaac RE, Quarmby LM, Tennant B, Fujioka H, Tuominen H, Hassinen I, Lohi H, van Houten JL, Rotig A, Sayer JA, Rolinski B, Freisinger P, Madhavan SM, Herzer M, Madignier F, Prokisch H, Nurnberg P, Jackson PK, Khanna H, Katsanis N, Hildebrandt F. Individuals with mutations in XPNPEP3, which encodes a mitochondrial protein, develop a nephronophthisis-like nephropathy. J Clin Invest. 2010;120:791–802. doi: 10.1172/JCI40076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto EA, Tory K, Attanasio M, Zhou W, Chaki M, Paruchuri Y, Wise EL, Utsch B, Wolf MT, Becker C, Nürnberg G, Nürnberg P, Nayir A, Saunier S, Antignac C, Hildebrandt F. Hypomorphic mutations in Meckelin (MKS3/TMEM67) cause nephronophthisis with liver dibrosis (NPHP11) J Med Genet. 2009;46:663–670. doi: 10.1136/jmg.2009.066613. [DOI] [PubMed] [Google Scholar]
- 40.Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attanasio M, Uhlenhaut NH, Sousa VH, O'Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nürnberg G, Becker C, Chudley AE, Nürnberg P, Hildebrandt F, Treier M. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet. 2007;39:1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 42.Edvardson S, Shaag A, Zenvirt S, Erlich Y, Hannon GJ, Shanske AL, Gomori JM, Ekstein J, Elpeleg O. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am J Hum Genet. 2010;86:93–97. doi: 10.1016/j.ajhg.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C:326–340. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, Adams M, Szymanska K, Mazzotta A, Lee JE, Tolentino JC, Swistun D, Salpietro CD, Fede C, Gabriel S, Russ C, Cibulskis K, Sougnez C, Hildebrandt F, Otto EA, Held S, Diplas BH, Davis EE, Mikula M, Strom CM, Ben-Zeev B, Lev D, Sagie TL, Michelson M, Yaron Y, Krause A, Boltshauser E, Elkhartoufi N, Roume J, Shalev S, Munnich A, Saunier S, Inglehearn C, Saad A, Alkindy A, Thomas S, Vekemans M, Dallapiccola B, Katsanis N, Johnson CA, Attié-Bitach T, Gleeson JG. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, Wallingford JB. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, Dollfus H, Beales PL, Badano JL, Katsanis N. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 48.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJF, Sang L, Giles RH, Liu Q, Coene KLM, Estrada-Cuzcano A, Collin RWJ, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, MacDonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford L, Neumann HPH, Obermüller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Bettencourt Dias M, Zhang X, Cavalcoli JD, Nürnberg G, Nürnberg P, Pierce EA, Jackson P, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42:840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, Hamel C, de Ravel TJ, Lewis RA, Friederich E, Thibault C, Danse JM, Verloes A, Bonneau D, Katsanis N, Poch O, Mandel JL, Dollfus H. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 51.Badano JL, Leitch CC, Ansley SJ, May-Simera H, Lawson S, Lewis RA, Beales PL, Dietz HC, Fisher S, Katsanis N. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature. 2006;439:326–330. doi: 10.1038/nature04370. [DOI] [PubMed] [Google Scholar]
- 52.Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, Green JS, Lewis RA, van Haelst MM, Parfrey PS, Baillie DL, Beales PL, Katsanis N, Davidson WS, Leroux MR. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat Genet. 2004;36:989–993. doi: 10.1038/ng1414. [DOI] [PubMed] [Google Scholar]
- 53.Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36:462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 54.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomic identification of conserved flagellar and basal body proteins that includes a novel gene for Bardet-Biedl syndrome. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 55.Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15:854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 57.Alexiev BA, Lin X, Sun CC, Brenner DS. Meckel-Gruber syndrome: pathologic manifestations, minimal diagnostic criteria, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1236–1238. doi: 10.5858/2006-130-1236-MS. [DOI] [PubMed] [Google Scholar]
- 58.Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, Esculpavit C, Toutain A, Moraine C, Parent P, Marcorelles P, Dauge MC, Roume J, Le Merrer M, Meiner V, Meir K, Menez F, Beaufrère AM, Francannet C, Tantau J, Sinico M, Dumez Y, MacDonald F, Munnich A, Lyonnet S, Gubler MC, Génin E, Johnson CA, Vekemans M, Encha-Razavi F, Attié-Bitach T. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Berthélémé JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Rüther U, Schneider-Maunoury S, Attié-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 60.Kyttälä M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestilä M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 61.Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, VHn G, Harris PC, Johnson CA. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38:191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 62.Tallila J, Jakkula E, Peltonen L, Salonen R, Kestilä M. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet. 2008;82:1361–1367. doi: 10.1016/j.ajhg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, Nürnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nürnberg P, Gretz N, Lo C, Lienkamp S, Schäfer T, Walz G, Benzing T, Zerres K, Omran H. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T, Afford SC, Copp AJ, Kelly DA, Gull K, Johnson CA. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet. 2007;16:173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- 65.Sayer JA, Otto EA, O'Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 66.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 2009;18:4565–4575. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet-Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12:1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- 68.Beales PL, Badano JL, Ross AJ, Ansley SJ, Hoskins BE, Kirsten B, Mein CA, Froguel P, Scambler PJ, Lewis RA, Lupski JR, Katsanis N. Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am J Hum Genet. 2003;72:1187–1199. doi: 10.1086/375178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR. Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science. 2001;293:2256–2259. doi: 10.1126/science.1063525. [DOI] [PubMed] [Google Scholar]
- 70.Hoefele J, Wolf MT, O'Toole JF, Otto EA, Schultheiss U, Dêschenes G, Attanasio M, Utsch B, Antignac C, Hildebrandt F. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- 71.Karmous-Benailly H, Martinovic J, Gubler MC, Sirot Y, Clech L, Ozilou C, Auge J, Brahimi N, Etchevers H, Detrait E, Esculpavit C, Audollent S, Goudefroye G, Gonzales M, Tantau J, Loget P, Joubert M, Gaillard D, Jeanne-Pasquier C, Delezoide AL, Peter MO, Plessis G, Simon-Bouy B, Dollfus H, Le Merrer M, Munnich A, Encha-Razavi F, Vekemans M, Attie-Bitach T. Antenatal presentation of Bardet-Biedl syndrome may mimic Meckel syndrome. Am J Hum Genet. 2005;76:493–504. doi: 10.1086/428679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frank V, den Hollander A, Brüchle NO, Zonneveld MN, Nürnberg G, Becker C, Du Bois G, Kendziorra H, Roosing S, Senderek J, Nürnberg P, Cremers FP, Zerres K, Bergmann C. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum Mutat. 2008;29:45–52. doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- 73.Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, Fazzi E, Signorini S, Louie CM, Bellacchio E, Bertini E, Dallapiccola B, Gleeson JG Group IJSRDS. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 74.Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- 75.Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Gröne HJ, Lopez I, Gudiseva HV, O'Toole JF, Vallespin E, Ayyagari R, Ayuso C, Cremers FP, den Hollander AI, Koenekoop RK, Dallapiccola B, Ghiggeri GM, Hildebrandt F, Valente EM, Williams DS, Gleeson JG. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet. 2010;42:175–180. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander A, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, Macdonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attié-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 78.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 79.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 80.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signalling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang P, Luo Y, Chasan B, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, Cantero Mdel R, Ramos AJ, Goldmann WH, Zhou J, Cantiello HF. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet. 2009;18:1238–1251. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Praetorius HA, Frøkiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol. 2005;288:F133–F141. doi: 10.1152/ajprenal.00238.2004. [DOI] [PubMed] [Google Scholar]
- 85.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geng L, Segal Y, Pavlova A, Barros EJ, Löhning C, Lu W, Nigam SK, Frischauf AM, Reeders ST, Zhou J. Distribution and developmentally regulated expression of murine polycystin. Am J Physiol. 1997;272:F451–F459. doi: 10.1152/ajprenal.1997.272.4.F451. [DOI] [PubMed] [Google Scholar]
- 88.Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet. 2009;75:107–117. doi: 10.1111/j.1399-0004.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 89.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 90.McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol. 2009;20:2104–2111. doi: 10.1681/ASN.2008111173. [DOI] [PubMed] [Google Scholar]
- 91.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 92.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 94.Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- 95.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 96.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 97.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 98.Kim I, Ding T, Fu Y, Li C, Cui L, Li A, Lian P, Liang D, Wang DW, Guo C, Ma J, Zhao P, Coffey RJ, Zhan Q, Wu G. Conditional mutation of Pkd2 causes cystogenesis and upregulates beta-catenin. J Am Soc Nephrol. 2009;20:2556–2569. doi: 10.1681/ASN.2009030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 100.Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 104.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 105.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–R228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 107.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21:295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aanstad P, Santos N, Corbit KC, Scherz PJ, Trinh LA, Salvenmoser W, Huisken J, Reiter JF, Stainier DY. The extracellular domain of smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr Biol. 2009;19:1034–1039. doi: 10.1016/j.cub.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 110.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 112.Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev Cell. 2010;18:237–247. doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 114.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 115.Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: primary cilia in development and disease. Curr Top Dev Biol. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
- 116.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG Group IJSRDS. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 118.Caspary T, Larkins CE, Anderson KV. The graded response to sonic hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 119.Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA (review) Int J Mol Med. 2008;22:271–275. [PubMed] [Google Scholar]
- 120.Santos N, Reiter JF. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn. 2008;237:1972–1981. doi: 10.1002/dvdy.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond IA, Beier DR. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 122.Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA. 2000;97:217–221. doi: 10.1073/pnas.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robert A, Margall-Ducos G, Guidotti JE, Brégerie O, Celati C, Bréchot C, Desdouets C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci. 2007;120:628–637. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- 125.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 127.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 128.Kim H, Bae Y, Jeong W, Ahn C, Kang S. Depletion of PKD1 by an antisense oligodeoxynucleotide induces premature G1/S-phase transition. Eur J Hum Genet. 2004;12:433–440. doi: 10.1038/sj.ejhg.5201136. [DOI] [PubMed] [Google Scholar]
- 129.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 130.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zaghloul NA, Liu Y, Gerdes JM, Gascue C, Oh EC, Leitch CC, Bromberg Y, Binkley J, Leibel RL, Sidow A, Badano JL, Katsanis N. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc Natl Acad Sci USA. 2010;107:10602–10607. doi: 10.1073/pnas.1000219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 133.Tobin JL, Beales PL. Restoration of renal function in zebrafish models of ciliopathies. Pediatr Nephrol. 2008;23:2095–2099. doi: 10.1007/s00467-008-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 135.Torres VE, Boletta A, Chapman A, Gattone V, Pei Y, Qian Q, Wallace DP, Weimbs T, Wüthrich RP. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol. 2010;5:1312–1329. doi: 10.2215/CJN.01360210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 137.Sweeney WEJ, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol. 2008;19:1331–1341. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]