Abstract
Objective
To determine the contribution of hyperinsulinemia to atherosclerosis development.
Methods and Results
Apolipoprotein E (Apoe) null mice which had knockout of one allele of the insulin receptor (Insr) gene were compared with littermate Apoe null mice with intact insulin receptors. Plasma insulin levels in Insr haploinsufficient/Apoe null mice were 50% higher in the fasting state and up to 69% higher during a glucose tolerance test, but glucose tolerance was not different in the two groups. C-peptide levels, insulin sensitivity, and post-receptor insulin signaling in muscle, liver, fat, and aorta were not different between groups, whereas disappearance in plasma of an injected insulin analogue was delayed in Insr haploinsufficient/Apoe null mice, indicating that impaired insulin clearance was the primary cause of hyperinsulinemia. No differences were observed in plasma lipids or blood pressure. Despite the hyperinsulinemia, atherosclerotic lesion size was not different between the two groups at time points up to 52 weeks of age when measured as en face lesion area in the aorta, cross-sectional plaque area in the aortic sinus, and cholesterol abundance in the brachiocephalic artery.
Conclusions
Hyperinsulinemia, without substantial vascular or whole-body insulin resistance and without changes in plasma lipids or blood pressure, does not change susceptibility to atherosclerosis.
Introduction
Numerous epidemiological studies have shown that hyperinsulinemia is a major independent risk factor for cardiovascular disease [1-4]. An increase in circulating insulin levels is usually considered a surrogate measure of insulin resistance, which is often thought to have a closer causal relationship with cardiovascular disease than hyperinsulinemia [5, 6]. However, insulin can activate several mechanisms considered proatherosclerotic through direct effects on vascular or inflammatory cells [7-14]. Whether hyperinsulinemia changes susceptibility to atherosclerosis independently of associated metabolic changes such as insulin resistance, dyslipidemia, or hypertension is not known. A barrier to answering this question has been the unavailability of an atherosclerosis-susceptible animal model with endogenous hyperinsulinemia, but without a substantial impairment of insulin action and with no changes in circulating lipids or blood pressure.
We recently reported that knockout of the insulin receptor targeted to endothelial cells increases atherosclerotic lesion area in the aorta by up to 2.9-fold in the absence of changes in glucose or lipid metabolism [15]. Downregulation of endothelial cell vascular cell adhesion molecule-1 (VCAM-1) by insulin appeared to contribute to this effect [15]. Since mice with endothelial cell insulin receptor knockout did not have changes in circulating insulin levels, the results imply that normal levels of insulin protects against atherosclerosis development, . However, in that study we could not address the influence, if any, of hyperinsulinemia itself on atherogenesis. Insulin can activate several mechanisms considered proatherosclerotic through direct effects on vascular cells, including increased endothelial cell expression of endothelin-1 [8, 9] and plasminogen activator inhibitor-1 (PAI-1) [10, 11] and increased smooth muscle cell proliferation [12]. Insulin may also augment activation of macrophages infiltrating the vascular wall, and conditional deletion of the insulin receptor (Insr) gene in macrophages has been shown to decrease the rate of atherosclerosis development in some [13, 14], but not all [16] studies.
During characterization of mice bred to create tissue-specific insulin receptor knockout [15], we also created mice with whole-body knockout of one allele of the insulin receptor combined with total knockout of the apolipoprotein E (Apoe) gene. These animals had hyperinsulinemia, but no signs of insulin resistance, suggesting they would be an attractive model for studying the effect of hyperinsulinemia on atherosclerosis development. Although insulin receptor knockout was created by cre recombinase activity in the germline of mice with a “floxed” mutation in the insulin receptor (Insr) gene, animals used for experiments were offspring of cre-negative mice and therefore had an inherited deletion of one allele of the Insr gene. Metabolic studies showed that compared with littermate controls, these mice had hyperinsulinemia primarily due to decreased insulin clearance, with no detectable impairment of insulin signaling and no change in glucose tolerance or whole-body insulin sensitivity measured by insulin tolerance tests. Plasma lipid levels and blood pressure was not different in the two groups. This model has allowed us to determine whether hyperinsulinemia can alter atherosclerosis development.
Research Design and Methods
Additional details of the experimental procedures are included in the online Supplemental Material at http://atvb.ahajournals.org.
Animals
Cross-breeding of mice with exon 4 of the insulin receptor (Insr) gene flanked by loxP sequences (“floxed”), Tie2-cre transgenic mice, and Apoe null mice have been described previously [15]. When breeding Apoe null mice homozygous for a floxed Insr gene, a whole-body null recombination of one allele of the Insr gene occurred if the female breeder harbored the Tie2-cre transgene, presumably because of cre-mediated recombination in the female germline (Figure I in the online-only Data Supplement) as described previously for this transgene [17]. For the current study, we used breeders negative for the cre transgene which ensured that a null mutation in one Insr allele was always inherited. Females breeders had a null recombination of one allele of the Insr gene (Insr flox/Δ Apoe−/−). Male breeders had floxed, but intact Insr genes ( Insr flox/ flox Apoe−/−). The resultant offspring which were used for experiments had the genotypes Insr flox/Δ Apoe−/− (“haploinsufficient”) or Insr flox/ flox Apoe−/− (“controls”) (Figure I).
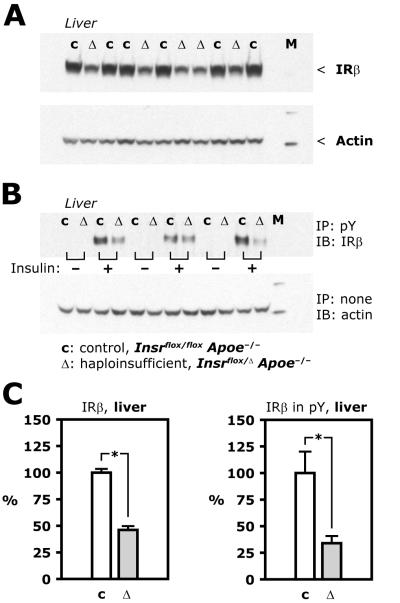
Figure 1. Insulin receptor expression and tyrosine phosphorylation in liver.
A, Representative Western blots based on lysate of liver from control (c) and haploinsufficient (Δ) mice (Insr flox/ flox Apoe−/− and Insr flox/Δ Apoe−/−, respectively). M, molecular weight marker. B, Insulin (50 mU/g) or insulin diluent was injected in the vena cava during pentobarbital anesthesia. After 5 minutes, organs were isolated and flash frozen. Representative Western blots based on phospho-tyrosine immunoprecipitates from liver lysate using tissue from control (c) and haploinsufficient (Δ) mice. C, Average values from 6 control and 5 haploinsufficient animals (left) and from 6 control and 6 haploinsufficient animals (right).
The mice had been partly backcrossed to the C57 background, with previous genetic characterization of animals from the same colony showing that animals had 87.6±0.8% of the C57BL/6 background, using an array genotyping 377 single nucleotide polymorphisms [15]. Littermate controls were used in all analysis. Female mice were used for experiments and fed a regular chow diet with 9.0% (w/w) fat and 0.221 ppm cholesterol (Mouse Diet 9F, LabDiet). All protocols for animal use and euthanasia were reviewed and approved by the Animal Care Committee of the Joslin Diabetes Center and were in accordance with NIH guidelines.
Insulin injection and tissue isolation
Mice were fasted 4 hours before sampling of blood for analysis of plasma lipids and overnight (16 hours) before isolation of tissues after insulin stimulation. Anaesthesia was induced with pentobarbital. In some animals, insulin or insulin diluent was injected into the vena cava after laparotomy. Blood was drawn by cardiac puncture in a syringe containing EDTA immediately before opening the thorax.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed as described [15].
Insulin clearance
Human insulin with a proline B28 to aspartate mutation, also called insulin aspart (NovoLog, Novo Nordisk) was administered at a dose of 0.5 mU/g as an intravenous bolus in the jugular vein through a catheter placed 3 days previously. Capillary blood from a tail cut was sampled in heparinized capillary tubes immediately before insulin aspart injection and 15, 30, 45, and 60 minutes after injection. Plasma was stored at −70 °C and subsequently analyzed with an ELISA which does not cross-react with native insulin [18]. Accordingly, the assay showed no detectable value in plasma samples obtained before insulin aspart injection.
Quantitation of atherosclerotic lesion size in the aorta
Quantitation of en face atherosclerotic lesion size in the aorta, cross-sectional plaque area in the aortic sinus, and brachicephalic artery cholesterol abundance was performed as described previously [15].
Statistical analysis
Responses in glucose and insulin tolerance tests were analyzed by calculating the area under the concentration versus time curve using the linear trapezoidal rule. Analysis of differences between atherosclerotic lesion size was done with Wilcoxon rank-sum test. All other analysis were made using paired or unpaired t-test. Statistical significance was defined as p<0.05. In text and graphs, data are presented as the mean ± standard error of the mean.
Results
In Apoe null mice with haploinsufficiency of the Insr gene (“haploinsufficient mice”), insulin receptor protein expression was reduced in all tissues tested when compared to littermate Apoe null controls with a “floxed” but intact Insr gene (“control mice”). In liver of haploinsufficient mice protein expression of the β subunit of the insulin receptor was 46% of levels in control mice (Figure 1A and C, p<0.01) and in skeletal muscle it was 25% of control levels (Figure 2A, p<0.01). Similar to what was previously reported for for Insr+/− mice [19], body mass was 13% lower in haploinsufficient mice than in controls at 24 weeks of age (Table, p=0.02). Dual-energy X-ray absorptiometry (DEXA) scanning showed that fat mass was 12.7% lower and lean mass 4.5% lower in haploinsufficient mice compared with controls (Table), although these differences were not statistically significant. Food intake was 3.3±0.6 and 3.4±0.6 g per day in control and haploinsufficient mice, respectively (p=0.9). Therefore, the lower body mass in the haploinsufficient mice was likely caused primarily by a reduction in fat mass, although more detailed characterization of these changes would require further study.
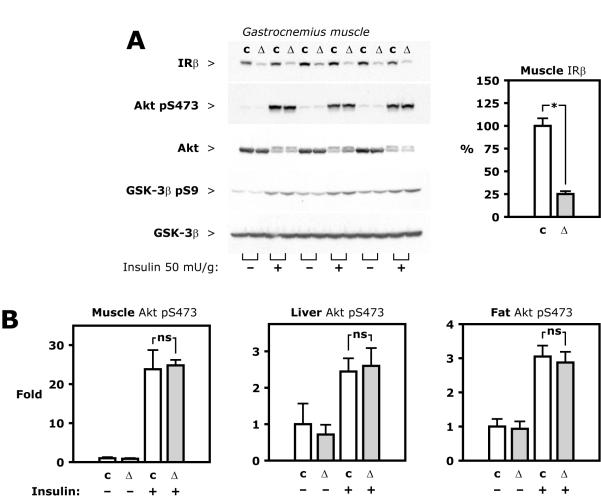
Figure 2. Insulin signaling in muscle, liver and fat.
Insulin at a dose of 50 mU/g or insulin diluent was injected into the vena cava during pentobarbital anesthesia. Organs were isolated and flash frozen after 5 minutes. A, Representative Western blots based on lysate of gastrocnemius muscle from control (c) or haploinsufficient (Δ) mice (Insr flox/ flox Apoe−/− and Insr flox/Δ Apoe−/−, respectively). B, Average values calculated from densitometry of Western blots based on tissue lysate from 6 control and 6 haploinsufficient animals.
Table.
Characteristics of haploinsufficient animals (Δ) and controls.
| controls | Δ | n | P | ||
|---|---|---|---|---|---|
| Unit | Mean ± SEM | Mean ± SEM | controls;Δ | ||
| Body mass | g | 30.1 ± 1.1 | 26.1 ± 1.1 | 10;10 | 0.02 |
| Lean mass | g | 6.4 ± 1.9 | 5.6 ± 1.5 | 12;7 | 0.7 |
| Fat mass | g | 21.9 ± 1.0 | 20.9 ± 0.9 | 12;7 | 0.4 |
| Food intake | g/day | 3.3 ± 0.6 | 3.4 ± 0.6 | 7;8 | 0.9 |
| C-peptide | pM | 322 ± 81 | 315 ± 65 | 9;10 | 0.9 |
| Fasting plasma cholesterol | mg/dl | 295 ± 31 | 313 ± 23 | 10;10 | 0.7 |
| Fasting plasma triglyceride | mg/dl | 131 ± 30 | 106 ± 7 | 8;9 | 0.4 |
| Systolic blood pressure | mmHg | 113 ± 3 | 114 ± 3 | 11;11 | 0.6 |
| Diastolic blood pressure | mmHg | 92 ± 3 | 95 ± 3 | 11;11 | 0.6 |
| Pulse | 1/min | 522 ± 32 | 549 ± 24 | 11;11 | 0.5 |
Intravenous injection of an insulin bolus (50 mU/g body weight) increased insulin receptor expression in phospho-tyrosine immunoprecipitate from liver lysate of control mice, but less so in haploinsufficient mice, in which it was 34±7% of the control value (Figure 1B and C, p<0.01). Despite reduction in insulin receptor expression and levels of tyrosine-phosphorylated receptors, downstream insulin signaling was not affected, similar to what was previously found in Insr+/− mice [20]. In skeletal muscle in the current study, intravenous injection of insulin stimulated phosphorylation at Ser473 of Akt in control and haploinsufficient mice 23.8±4.9 and 24.8±1.4-fold, respectively, from the levels of control mice injected with vehicle (Figure 2A and B).
Similarly, insulin-stimulated phospho-Akt in liver was 245±36% and 260±49%, respectively, of the levels of control mice injected with vehicle and in perigonadal fat 305±32% versus 288±31% (Figure 2B, all statistically non-significant). Insulin-stimulated phosphorylation of glycogen synthase kinase-3β (GSK-3β) at Ser9 also was not significantly different in muscle, liver, and fat in the two groups (Figure 2A and data not shown). These experiments were repeated with a lower intravenous dose of insulin, 0.5 mU/g, which resulted in plasma insulin concentrations of 214 μU/ml or less in blood sampled just before tissue isolation. Again, insulin-stimulated phospho-Akt and phospho-GSK-3β was similar in the two groups in liver, muscle, and fat (Figure II in the online-only Data Supplement).
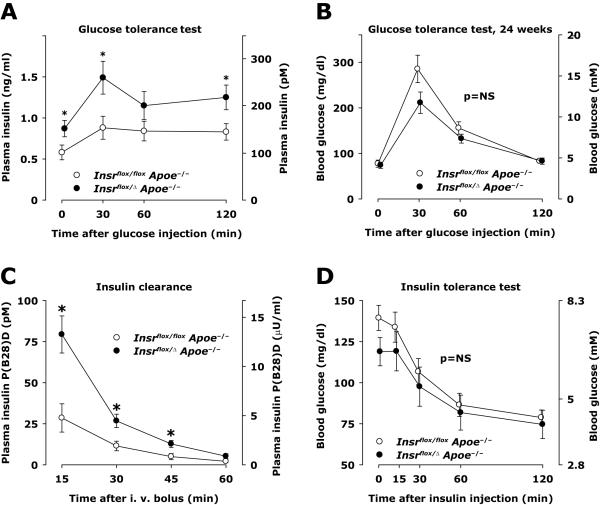
Hyperinsulinemia without glucose intolerance was previously reported for Insr+/− mice [19]. To examine whether Insr haploinsufficiency in our mouse model had a similar phenotype, glucose tolerance tests were performed by intraperitoneal injection of glucose in fasting mice. Plasma insulin levels were elevated in haploinsufficient mice at every time point studied (Figure 3A); in the basal condition before glucose injection, it was 50% higher (p<0.05), and 30 minutes after glucose injection it was 69% higher in haploinsufficient mice compared to controls (1.49±0.20 versus 0.88±0.14 ng/ml at 30 minutes, respectively, Figure 3A, p=0.02). Haploinsufficient mice had somewhat better glucose tolerance, although this was not significantly different between the two groups (Figure 3B). Furthermore, plasma C-peptide in the random fed state was similar in the two groups in the current study, 322±81 pM in controls and 315±65 pM in haploinsufficient animals (Table, p=0.9), suggesting that there was no difference in insulin production.
Figure 3. Glucose and insulin tolerance, insulin production and clearance.
A and B, Glucose tolerance tests were performed in 10 control and 10 haploinsufficient mice (Insr flox/ flox Apoe−/− and Insr flox/Δ Apoe−/−, respectively) in the morning after an overnight fast. D-glucose 2 mg per gram body weight was injected intraperitoneally. Capillary blood was sampled from a tail cut and analyzed immediately for glucose concentration. Heparinized plasma was analyzed for insulin concentrations by ELISA. C, Insulin aspart (containing a P(B28)D mutation) at a dose of 0.5 mU/g was injected intravenously in the random fed state. Capillary blood was sampled from a tail cut and heparinized plasma was analyzed by an ELISA without cross-reactivity to endogenous insulin. D, Insulin tolerance test performed in the random fed state using intraperitoneal injection of insulin (0.6 mU/g).
Because hyperinsulinemia was not readily explained by insulin resistance or increased insulin production, we measured insulin clearance. Exogenous insulin was given as an intravenous injection of human insulin with a proline B28 to aspartate mutation (NovoLog, Novo Nordisk), an insulin analogue with receptor affinity and potency similar to that of regular human insulin [21], but allowing specific immunodetection of exogenous insulin. Disappearance of B28-Asp insulin in plasma was substantially slower in Insr haploinsufficient mice compared with controls, with plasma concentrations of B28-Asp insulin up to 2.7-fold higher in haploinsufficient animals (Figure 3C, p<0.01 for area under the curve).
During an insulin tolerance test in the random fed state, mice with Insr haploinsufficiency showed a decrease in blood glucose no different than in control mice during a course of 2 hours, indicative of similar insulin sensitivity (Figure 3D, p>0.3). Overall, this characterization is compatible with hyperinsulinemia caused primarily by decreased insulin clearance.
Apoe null mice spontaneously develop hypercholesterolemia on a regular chow diet. In haploinsufficient animals, serum concentrations of free fatty acids (Figure III panel A in the online-only Data Supplement) and plasma concentrations of total cholesterol and total triglyceride (Figure III panel B in the online-only Data Supplement) were not different in haploinsufficient and control mice. Distribution of cholesterol and triglycerides in different lipoprotein moieties was measured as lipid concentration in fractions separated by fast protein liquid chromatography (FPLC), and was not different in the two groups (Figure III panel C in the online-only Data Supplement). Therefore, Insr haploinsufficiency in an Apoe null background does not change major lipid parameters involved in atherosclerosis development.
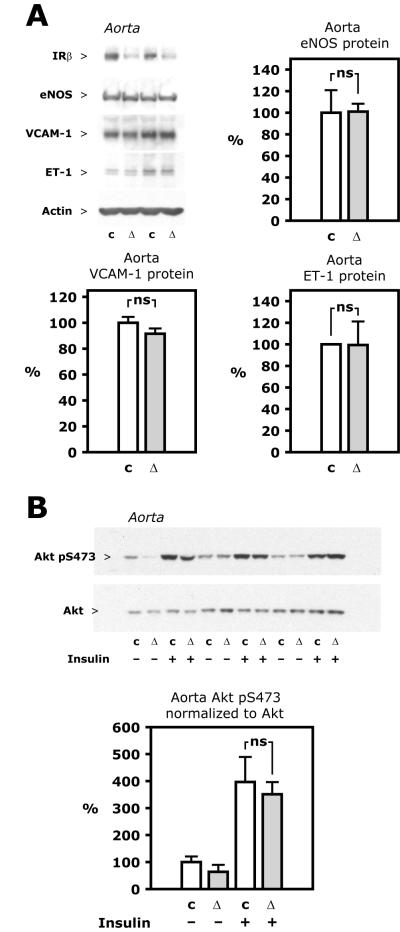
Analysis of protein expression in the aorta showed that the β subunit of the insulin receptor was reduced by 50.0±8.4% (Figure 4A and data not shown, p<0.001). Expression of eNOS protein in the aorta was not different in the two groups (Figure 4A, p=0.96). Vascular cell adhesion molecule-1 (VCAM-1), which is upregulated in endothelial cells in mice with insulin receptor knockout targeted to endothelial cells [15], or ET-1 were also not different between haploinsufficient and control animals (Figure 4A). Vascular wall insulin sensitivity was assessed by insulin-stimulated Akt phosphorylation in vivo. Akt Ser473 phosphorylation in the aorta after an intravenous insulin bolus, as in muscle, liver and fat, was similar between groups (Figure 4B). The insulin-stimulated level relative to control animals injected with vehicle was 397±93% in controls and 351±45% in haploinsufficient animals, respectively (Figure 4B, p=0.6). Decreased insulin receptor expression could potentially change IGF-1 signaling by decreasing the number of hybrid insulin/IGF-1 receptors or by other mechanisms. However, intravenous injection of IGF-1 increased phosphorylation of Akt in the aorta to the same degree in control and haploinsufficient mice (to 222±42 and 216±48% of control levels, Figure IV in the online-only Data Supplement, p=0.9). There was no difference between the two groups of animals in terms of systolic or diastolic blood pressure or pulse rate (Table).
Figure 4. Aorta protein expression and insulin signaling.
A, Representative Western blots (top left) based on lysate of aorta. Graphs show average protein expression calculated from densitometry of Western blots based on tissue from 6 or 8 animals in each group, except for ET-1, where quantitation was based on 5 animals in each group. B, Insulin (50 mU/g) or insulin diluent was injected into the vena cava during pentobarbital anesthesia. After 5 minutes, the aorta was isolated and flash frozen. Representative Western blots (top) based on lysate of aorta. Average expression (bottom) calculated from densitometry of Western blots based on tissue from 3 animals in each group (total of 6 control (c) and 6 haploinsufficient (Δ) mice, Insr flox/ flox Apoe−/− and Insr flox/Δ Apoe−/−, respectively).
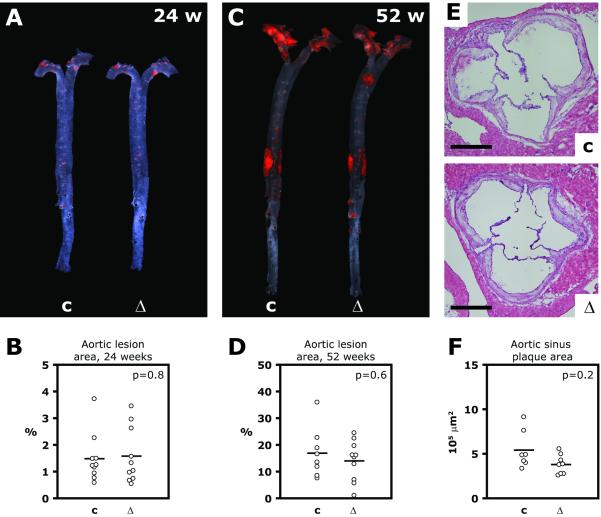
Atherosclerosis in the aorta was measured as the fraction of the surface area in en face flat preparations stained by Sudan IV (Figure 5A through D). At 24 weeks of age, the lesion area, on average, was almost identical in the two groups; it was 1.5±0.3% in haploinsufficient animals and 1.6±0.3% in controls (Figure 5B, p=0.8). At 52 weeks, lesion area remained without significant differences (16.9±3.5 and 14.0±2.5% in control and haploinsufficient mice, respectively, Figure 5D, p=0.6). At this age, the cross-sectional plaque area in the aortic sinus and the macrophage content in the aortic sinus was also not statistically different in the two groups (plaque area 541,489±86,632 and 379,405±41,244 μm2, respectively, Figure 5E and F, p=0.2; F4/80-positive area relative to plaque area 14.1±2.9 and 12.4±2.4%, respectively, Figure V panel A and B in the online-only Data Supplement, p=0.6).
Figure 5. Atherosclerosis.
A through D, Aortas were dissected free of perivascular tissue, stained with Sudan IV and mounted in a flat preparation (en face preparation). A and C, Representative photographs of aortas from an haploinsufficient mouse and its littermate control at 24 (A) and 52 (C) weeks of age. B and D, Average atherosclerotic lesion area in 10 control (c) and 10 haploinsufficient (Δ) mice (Insr flox/ flox Apoe−/− and Insr flox/Δ Apoe−/−, respectively) at 24 weeks of age (B) and in 8 control and 10 haploinsufficient animals at 52 weeks of age (D). E and F, Cryosections of aortic sinus were made from animals at 52 weeks of age and stained with hematoxylin and eosin. E, Representative microphotographs; scale bar represents 500 μm. F, Mean plaque area in 7 control and 8 haploinsufficient animals.
As a separate analysis of atherosclerotic plaque development, cholesterol content was analyzed in extracts of the brachiocephalic artery at 36 weeks of age. This measure has been shown to correlate well with lesion area in histological cross-sections [22]. Free cholesterol abundance was 8.4±2.4 and 8.6±1.6 nmole (Figure V panel C in the online-only Data Supplement) and cholesterol ester was 8.6±3.6 and 8.4±1.9 nmole (Figure V panel D) in haploinsufficient animals and littermate controls, respectively (p>0.9). This indicated that no significant difference existed in atherosclerotic lesion size in haploinsufficient mice and their controls when measured at different time points, in various locations, and by different approaches.
Discussion
High circulating insulin concentrations present in conditions like obesity and type 2 diabetes have for decades been suspected to promote atherosclerosis through direct effects on the vascular wall [23-25]. However, animal models to test this hypothesis have been lacking. Hyperinsulinemia in models of obesity and diabetes is associated with changes in plasma lipids which confound the association between plasma insulin and atherosclerosis development. Furthermore, hyperinsulinemia is usually associated with significant impairment of insulin signaling in the vascular wall, which in itself can promote atherosclerosis [15]. In the current study, we describe a mouse model with levels of hyperinsulinemia which are relevant for human disease and primarily due to decreased insulin clearance, with little impairment of insulin action in the vasculature or in other tissues. Atherosclerosis development in these mice and their controls is caused by hypercholesterolemia due to deletion of the Apoe gene. However, we found no difference in atherosclerotic lesion size in hyperinsulinemic mice compared with controls. These data indicate that abnormally high insulin concentrations alone, without substantial impairment of vascular insulin signaling, and in the absence of significant changes in glucose or lipid metabolism, are not sufficient to accelerate atherosclerosis.
Plasma insulin in the Insr haploinsufficient mice was 50% higher than in controls in the fasting state and up to 69% higher during a glucose tolerance test. This level of hyperinsulinemia is appropriate as a model of hyperinsulinemia in humans at risk for cardiovascular disease. For example, in the Quebec Heart Study, the mean plasma insulin in the case group of patients with cardiovascular disease was 18% higher than in controls (92.1 vs. 78.2 pM) with an odds ratio of 1.7 for each standard deviation of increase in plasma insulin [4]. In human conditions and in animal models of obesity and insulin resistance, hyperinsulinemia is often an index of peripheral insulin resistance. Therefore, it may seem counterintuitive that hyperinsulinemia in the haploinsufficient mice in the current study is present together with preserved insulin sensitivity and glucose tolerance. However, it is possible that clearance of insulin, which involves binding of the hormone to its receptor on hepatocytes, may be impacted more by a reduction in insulin receptor numbers than post-receptor insulin signaling or insulin-stimulated glucose uptake.
In the current study, neither mice used for experiments nor their parents carried a cre transgene. Therefore, haploinsufficiency of the Insr gene in Insr flox/Δ Apoe−/− mice was due to an inherited mutation and cre-mediated deletion in any tissue would not have occurred. The metabolic phenotype of Insr flox/Δ Apoe−/− mice may be modified by the Apoe null mutation, but is generally compatible with data from Insr+/− mice, in which the Insr deletion was created by targeted mutation in stem cell culture [19]. In the publication first describing the phenotype of Insr+/− mice, it was noted that animals had hyperinsulinemia in the fasting state and during glucose tolerance test, but no glucose intolerance [19]. Subsequent study of these mice showed a modest [26, 27] or no [20] reduction in insulin signaling events in liver, muscle, or fat, such as p85 co-immunoprecipitation with phospho-tyrosine or IRS proteins or PI3K activity in phospho-tyrosine immunoprecipitates.
In the current study, we observed no decrease in insulin signaling between haploinsuffient and control mice over a 100-fold range in insulin dose, from 0.5 to 50 mU/g. In contrast, mice fed a high-fat diet develop insulin resistance with considerable inhibition of insulin-stimulated phospho-Akt [28] at insulin doses similar to the high dose (50 mU/g) used in the current study. Haploinsufficient mice had a modest reduction of body mass relative to control mice, although this had no apparent effect on insulin sensitivity, other metabolic parameters or circulating lipids. Insulin sensitivity in both haploinsufficient and control mice in our study was likely affected by Apoe deletion, as Apoe null mice have lower fat mass in adipose tissue, are more insulin sensitive, and are protected against insulin resistance induced by diet-induced obesity [29-31], primarily because of decreased triglyceride uptake and turnover in adipose tissue [31-33]. It is possible that haploinsufficient mice could have decreased insulin signaling compared to control mice at an even lower insulin dose, or that insulin sensitivity, which was not different between the two groups in insulin tolerance tests, would be impaired during a euglycemic hyperinsulinemic clamp. Regardless, the primary cause of hyperinsulinemia in haploinsufficient mice appears to be impaired insulin clearance. Measurement of insulin clearance in Insr+/− mice has not been published, but mice with deletion of the Insr gene specifically in hepatocytes have plasma insulin concentrations that are more than 20-fold higher than their controls [34] confirming that the majority of insulin clearance occurs in the liver after binding of the hormone to its receptor [8, 35].
Plasma cholesterol and triglyceride in plasma and in lipoprotein fractions were not different in haploinsufficient animals and controls. This made it possible to compare the effect of hyperinsulinemia on atherosclerosis when accompanied with no or only mild insulin resistance, without any confounding effect of changes in plasma lipids or blood pressure. Insulin receptor knockout mice with a transthyretin transgene, which rescues these mice from neonatal death by expression of less than 10% of control levels of insulin receptor protein in the liver, have decreased atherosclerosis [36]. However, these animals have changes in several determinants of atherosclerosis development, including abolished insulin signaling in the aorta and reduced plasma VLDL and LDL cholesterol as well as reduced hepatic VLDL secretion as [36].
In mice with normal circulating insulin levels and loss of insulin signaling in endothelial cells, atherosclerotic lesion area was increased in the aorta by up to 2.9-fold [15]. Together with data from the current study, these results suggest that in the metabolic syndrome and type 2 diabetes, activation of potentially proatherosclerotic mechanisms in vascular cells by hyperinsulinemia has little effect on atherogenesis, whereas substantial insulin resistance in endothelial cells by itself can promote atherosclerosis development. This implies that in terms of the contribution of abnormal vascular insulin signaling to atherosclerosis, our efforts may be better focused on improving endothelial cell insulin resistance than preventing effects of hyperinsulinemia on the vascular wall.
Supplementary Material
Acknowledgements
Measurement of plasma insulin and serum free fatty acids was performed by the Joslin Diabetes Center Specialized Assay Core, blood pressure and DEXA scanning by Joslin’s Animal Physiology Core, and cryosections made by Joslin’s Advanced Microscopy Core, all supported by NIH grant P30DK036836. Plasma lipid profiles were measured at the Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotype Centers with support from NIH grant U24DK059637. We are grateful to Dr. Birgitte Andersen at the Hagendorn Institute, Denmark, who arranged for analysis using the insulin aspart ELISA, which was generously provided by Novo Nordisk, Denmark. We thank Dr. C. Ronald Kahn at the Joslin Diabetes Center for providing mice used in this study.
Sources of Funding The study was supported by NIH grants K08EY018677 to Dr. Rask-Madsen and R01DK053105 to Dr. King. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Disclosure Dr. Rekhter is an employee of Eli Lilly & Co. The authors declare no other conflict of interest in reporting this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pyorala K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care. 1979;2:131–141. doi: 10.2337/diacare.2.2.131. [DOI] [PubMed] [Google Scholar]
- 2.Ducimetiere P, Eschwege E, Papoz L, Richard JL, Claude JR, Rosselin G. Relationship of plasma insulin levels to the incidence of myocardial infarction and coronary heart disease mortality in a middle-aged population. Diabetologia. 1980;19:205–210. doi: 10.1007/BF00275270. [DOI] [PubMed] [Google Scholar]
- 3.Orchard TJ, Eichner J, Kuller LH, Becker DJ, McCallum LM, Grandits GA. Insulin as a predictor of coronary heart disease: interaction with apolipoprotein E phenotype. A report from the Multiple Risk Factor Intervention Trial. Ann Epidemiol. 1994;4:40–45. doi: 10.1016/1047-2797(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 5.Howard G, O’Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 6.Rewers M, Zaccaro D, D’Agostino R, Haffner S, Saad MF, Selby JV, Bergman R, Savage P. Insulin sensitivity, insulinemia, and coronary artery disease: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:781–787. doi: 10.2337/diacare.27.3.781. [DOI] [PubMed] [Google Scholar]
- 7.Stout RW. Insulin as a mitogenic factor: role in the pathogenesis of cardiovascular disease. Am J Med. 1991;90:62S–65S. doi: 10.1016/0002-9343(91)90041-u. [DOI] [PubMed] [Google Scholar]
- 8.Oliver FJ, de la Rubia G, Feener EP, Lee ME, Loeken MR, Shiba T, Quertermous T, King GL. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem. 1991;266:23251–23256. [PubMed] [Google Scholar]
- 9.Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- 10.Nordt TK, Sawa H, Fujii S, Bode C, Sobel BE. Augmentation of arterial endothelial cell expression of the plasminogen activator inhibitor type-1 (PAI-1) gene by proinsulin and insulin in vivo. J Mol Cell Cardiol. 1998;30:1535–1543. doi: 10.1006/jmcc.1998.0719. [DOI] [PubMed] [Google Scholar]
- 11.Grenett HE, Benza RL, Fless GM, Li XN, Davis GC, Booyse FM. Genotype-specific transcriptional regulation of PAI-1 gene by insulin, hypertriglyceridemic VLDL, and Lp(a) in transfected, cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1803–1809. doi: 10.1161/01.atv.18.11.1803. [DOI] [PubMed] [Google Scholar]
- 12.Banskota NK, Taub R, Zellner K, King GL. Insulin, insulin-like growth factor I and platelet-derived growth factor interact additively in the induction of the protooncogene c-myc and cellular proliferation in cultured bovine aortic smooth muscle cells. Mol Endocrinol. 1989;3:1183–1190. doi: 10.1210/mend-3-8-1183. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Navarro H, Vinue A, Vila-Caballer M, Fortuno A, Beloqui O, Zalba G, Burks D, Diez J, Andres V. Molecular mechanisms of atherosclerosis in metabolic syndrome: role of reduced IRS2-dependent signaling. Arterioscler Thromb Vasc Biol. 2008;28:2187–2194. doi: 10.1161/ATVBAHA.108.175299. [DOI] [PubMed] [Google Scholar]
- 15.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 17.de Lange WJ, Halabi CM, Beyer AM, Sigmund CD. Germ line activation of the Tie2 and SMMHC promoters causes noncell-specific deletion of floxed alleles. Physiol Genomics. 2008;35:1–4. doi: 10.1152/physiolgenomics.90284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen L, Jorgensen PN, Jensen LB, Walsh D. A new insulin immunoassay specific for the rapid-acting insulin analog, insulin aspart, suitable for bioavailability, bioequivalence, and pharmacokinetic studies. Clin Biochem. 2000;33:627–633. doi: 10.1016/s0009-9120(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 19.Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 20.Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest. 2005;115:3494–3505. doi: 10.1172/JCI26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brange J, Owens DR, Kang S, Volund A. Monomeric insulins and their experimental and clinical implications. Diabetes Care. 1990;13:923–954. doi: 10.2337/diacare.13.9.923. [DOI] [PubMed] [Google Scholar]
- 22.Kuo MS, Kalbfleisch JM, Rutherford P, Gifford-Moore D, Huang XD, Christie R, Hui K, Gould K, Rekhter M. Chemical analysis of atherosclerotic plaque cholesterol combined with histology of the same tissue. J Lipid Res. 2008;49:1353–1363. doi: 10.1194/jlr.D700037-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Stout RW. Insulin and atheroma. 20-yr perspective. Diabetes Care. 1990;13:631–654. doi: 10.2337/diacare.13.6.631. [DOI] [PubMed] [Google Scholar]
- 24.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–582. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 25.Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol. 2004;286:H76–82. doi: 10.1152/ajpheart.00539.2003. [DOI] [PubMed] [Google Scholar]
- 26.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 27.Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J, Katagiri H, Ishigaki Y, Yamada T, Ogihara T, Imai J, Uno K, Hasegawa Y, Kanzaki M, Yamamoto TT, Ishibashi S, Oka Y. Involvement of apolipoprotein E in excess fat accumulation and insulin resistance. Diabetes. 2007;56:24–33. doi: 10.2337/db06-0144. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima Y, Chen J, Sun H, Lann D, Hajjar RJ, Yakar S, Leroith D. Apolipoprotein E deficiency abrogates insulin resistance in a mouse model of type 2 diabetes mellitus. Diabetologia. 2009;52:1434–1441. doi: 10.1007/s00125-009-1378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann SM, Perez-Tilve D, Greer TM, Coburn BA, Grant E, Basford JE, Tschop MH, Hui DY. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 2008;57:5–12. doi: 10.2337/db07-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 33.Huang ZH, Reardon CA, Mazzone T. Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- 34.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 35.Mora ME Valera, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003;22:487–493. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- 36.Han S, Liang CP, Westerterp M, Senokuchi T, Welch CL, Wang Q, Matsumoto M, Accili D, Tall AR. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest. 2009;119:1029–1041. doi: 10.1172/JCI36523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.