Abstract
Background
Reduced right ventricular ejection fraction (RVEF) is associated with poor outcomes in patients with chronic systolic heart failure (HF). Although most HF patients are older adults, little is known about the relationship between low RVEF and outcomes in older adults with systolic HF.
Methods
Of the 2008 Beta-Blocker Evaluation of Survival Trial (BEST) participants with systolic HF (left ventricular ejection fraction ≤35%) 822 were ≥65 years and had data on baseline RVEF estimated by gated-equilibrium radionuclide ventriculography. Using RVEF ≥40% (n=308) as reference, we examined association of RVEF 30–39% (n=214), 20–29% (n=206) and <20% (n=94) with outcomes using Cox regression models.
Results
All-cause mortality occurred in 36%, 40%, 39% and 56% of patients with RVEF ≥40%, 30–39%, 20–29% and <20% respectively. Compared with RVEF ≥40%, unadjusted hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality associated with RVEF 30–39%, 20–29% and <20% were 1.19 (0.90–1.57; P=0.220), 1.13 (0.84–1.51; P=0.423) and 1.97 (1.43–2.73; P<0.001) respectively. Respective multivariable-adjusted HR’s (95% CI’s) for all-cause mortality were 1.19 (0.88–1.60; P=0.261), 1.00 (0.73–1.39; P=0.982) and 1.70 (1.14–2.53; P=0.009). Adjusted HR’s (95% CI’s) associated with RVEF <20% (versus ≥40%) for cardiovascular mortality and HF mortality were 1.79 (1.17–2.76; P=0.008) and 1.97 (1.02–3.83; P=0.045) respectively. RVEF had no independent association with sudden cardiac death, all-cause or HF hospitalization.
Conclusions
Abnormally low RVEF is a significant independent predictor of mortality, but not of HF hospitalization, in older adults with systolic HF.
Keywords: Heart Failure, Older Adults, Right Ventricle, Mortality, Morbidity
1. Introduction
We have recently demonstrated that in a relatively young (mean age, 60 years) cohort of systolic heart failure (HF) patients, low right ventricular ejection fraction (RVEF) was an independent predictor of increased all-cause mortality and HF hospitalization [1]. In that study, we have also observed that systolic HF patients with reduced RVEF were significantly younger than those with preserved RVEF. Although most HF patients are 65 years or older [2], most prior studies of the association between RVEF and outcomes in HF were conducted in younger HF patients [3–10] and little is known about the specific relationship between reduced RVEF and outcomes in older adults with systolic HF. Therefore, in the current study we examined the relationship between RVEF and outcomes in older adults with advanced chronic systolic HF.
2. Material and methods
2.1. Study design
The Beta-Blocker Evaluation of Survival Trial (BEST) was a randomized clinical trial of the beta-blocker bucindolol in HF conducted at 30 Veterans Administration Hospital (VA) sites and 60 non-VA sites in the United States and Canada between May 1995 and December 1998. The study was funded by the National Heart, Lung, and Blood Institute (NHLBI) and the Department of Veterans Affairs Cooperative Studies Program. The BEST protocol and results have been previously detailed elsewhere [11, 12]. Briefly, 2708 patients with moderate-to-severe chronic systolic HF were randomized to receive bucindolol or placebo and were followed up for a mean of 2 years. All patients gave written informed consent and the protocol was approved by the institutional review board of each site. For the purpose of the current analysis we used a public-use copy of the BEST data obtained from the NHLBI. The public-use version of the data is similar to the original data except for de-identification and that one patient did not consent to be included in these de-identified datasets.
2.2 Patients
Of the 2707 patients in the public-use copy of the data, 2008 had data on baseline RVEF, who were the subjects of our previous study [1]. The current analysis is restricted to the 822 (41%) patients who were 65 years or older at baseline. All patients had a LVEF ≤35%, and were in New York Heart Association (NYHA) functional class III (92%) or IV (8%). The majority was receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (>90%), diuretics (>90%) and digoxin (>90%).
2.3. Estimation of LVEF and RVEF
All patients underwent a baseline gated-equilibrium radionuclide ventriculographic assessment of LVEF and RVEF during randomization or within 60 days prior to randomization [1]. The lower limit of normal RVEF by gated-equilibrium radionuclide ventriculography is 40% [13, 14]. Patients were categorized into four RVEF groups: ≥40% (n=308 or 37%), 30–39% (n=214 or 26%), 20–29% (n=206 or 25%) and <20% (n=94 or 11%).
2.4. Study outcomes
The primary end point for the current analysis was all-cause mortality which was also the primary end point in BEST and was centrally adjudicated. Secondary outcomes included cardiovascular and HF mortality, and all-cause and HF hospitalization.
2.5. Statistical analysis
We used chi-square tests and analysis of variance tests, as appropriate, for descriptive analyses to compare baseline characteristics between the four RVEF groups. Kaplan–Meier plots were constructed to determine associations of RVEF groups with all-cause mortality. Associations of various RVEF categories with outcomes were determined using Kaplan–Meier survival analysis and Cox proportional hazard models. RVEF category ≥40% was used as the reference category and dummy variables were used for RVEF categories 30%–39%, 20%–29% and <20%. Variables were entered into the model in multiple steps in the following order: step 1 (unadjusted: dummy variables for RVEF 30–39%, 20–29% and <20%), and step 2 (step 1 plus LVEF), step 3 (step 2 plus demographics), step 4 (step 3 plus medical history), step 5 (step 4 plus medications), step 6 (step 5 plus clinical findings), and step 7 (step 6 plus laboratory findings). The same model was used for all the outcomes. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. All statistical tests were evaluated using two-tailed 95% confidence levels and tests with p-value <0.05 were considered significant. Data analyses were performed using SPSS for Windows, Rel. 15. 2006. Chicago: SPSS Inc.
3. Results
3.1. Baseline characteristics
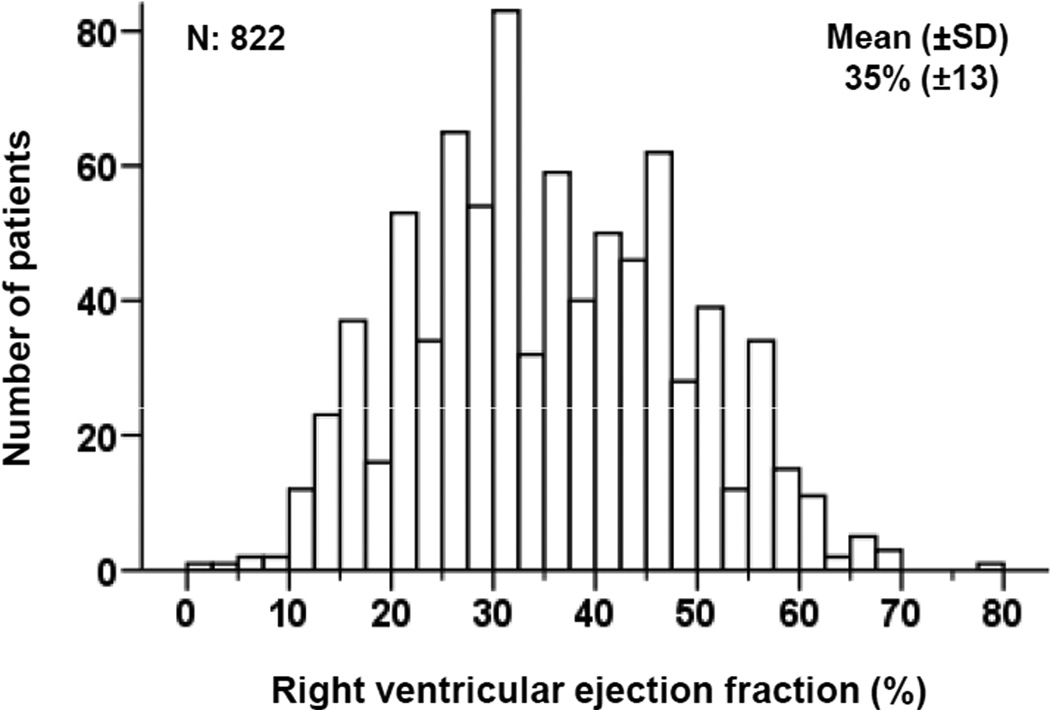
Patients had a mean age of 72 (±5) years, 16% were women and 14% were African Americans. Patients in the lower RVEF categories were more likely to be African Americans with characteristics suggesting more advanced HF, including higher NYHA functional class, higher heart rate, lower systolic blood pressure, lower LVEF, and more signs of peripheral or pulmonary congestion (Tables 1 and 2). Mean RVEF was 35% (±13) and its distribution among the participants is displayed in Figure 1.
Table 1.
Baseline patient characteristics by right ventricular ejection fraction (RVEF) categories
| n (%) or mean (±SD) | RVEF ≥40% (n=308) |
RVEF 30 to 39% (n=214) |
RVEF 20 to 29% (n=206) |
RVEF <20% (n=94) |
P-value |
|---|---|---|---|---|---|
| Age, years | 73 (±5) | 71 (±5) | 72 (±5) | 72 (±5) | 0.014 |
| Female | 62 (20) | 31 (15) | 27 (13) | 12 (13) | 0.099 |
| African American | 30 (10) | 29 (14) | 37 (18) | 22 (23) | 0.003 |
| Veteran | 127 (41) | 92 (43) | 91 (44) | 34 (36) | 0.601 |
| Current smoker | 40 (13) | 20 (9) | 22 (11) | 6 (6) | 0.269 |
| New York Heart Association class III | 281 (91) | 196 (92) | 176 (85) | 74 (79) | 0.002 |
| Body mass index, kg/m2 | 34 (±7) | 35 (±7) | 33 (±6) | 33 (±7) | 0.025 |
| Heart rate, beats per minute | 77 (±12) | 77 (±11) | 79 (±12) | 83 (±11) | <0.001 |
| Systolic blood pressure, mm Hg | 123 (±19) | 118 (±17) | 115 (±18) | 114 (±19) | <0.001 |
| Diastolic blood pressure, mm Hg | 69 (±10) | 69 (±10) | 68 (±10) | 69 (±11) | 0.514 |
| Left ventricular ejection fraction, % | 26 (±6.3) | 24 (±6.4) | 21 (±7.1) | 18 (±5.8) | <0.001 |
| Past medical history | |||||
| Duration of heart failure, months | 52 (±48) | 57 (±52) | 64 (±57) | 58 (±61) | 0.099 |
| Idiopathic dilated cardiomyopathy | 66 (21) | 43 (20) | 35 (17) | 15 (16) | 0.318 |
| Coronary artery disease | 223 (72) | 153 (72) | 153 (74) | 70 (75) | 0.904 |
| Coronary artery stenosis >70% | 171 (56) | 116 (54) | 123 (60) | 51 (54) | 0.666 |
| Angina pectoris | 180 (58) | 121 (57) | 115 (56) | 59 (63) | 0.689 |
| ST segment elevation myocardial infarction | 122 (40) | 85 (40) | 87 (42) | 44 (47) | 0.610 |
| Anterior ST segment elevation myocardial infarction | 69 (22) | 32 (15) | 35 (17) | 19 (20) | 0.155 |
| Lateral ST segment elevation myocardial infarction | 28 (9) | 16 (8) | 21 (10) | 9 (10) | 0.800 |
| Inferior-posterior ST segment elevation myocardial infarction | 45 (15) | 38 (18) | 26 (13) | 16 (17) | 0.480 |
| Coronary artery bypass surgery | 111 (36) | 90 (42) | 87 (42) | 36 (38) | 0.419 |
| Percutaneous coronary interventions | 54 (18) | 35 (16) | 26 (13) | 10 (11) | 0.251 |
| Hypertension | 188 (61) | 139 (65) | 128 (62) | 66 (70) | 0.394 |
| Diabetes mellitus | 107 (35) | 82 (38) | 65 (32) | 38 (40) | 0.360 |
| Hyperlipidemia | 145 (47) | 102 (48) | 76 (37) | 37 (39) | 0.061 |
| Atrial fibrillation | 90 (29) | 77 (36) | 78 (38) | 23 (25) | 0.043 |
| Peripheral arterial disease | 69 (22) | 52 (24) | 38 (18) | 15 (16) | 0.263 |
| Chronic kidney disease* | 186 (60) | 107 (50) | 120 (58) | 55 (59) | 0.115 |
| Medications | |||||
| Bucindolol | 149 (48) | 105 (49) | 94 (46) | 55 (59) | 0.223 |
| Angiotensin-converting enzyme inhibitors / angiotensin II receptor blockers | 291 (95) | 202 (94) | 195 (95) | 88 (94) | 0.987 |
| Digitalis | 274 (89) | 197 (92) | 190 (92) | 91 (97) | 0.103 |
| Diuretics | 288 (94) | 197 (92) | 196 (95) | 94 (100) | 0.040 |
| Vasodilators | 157 (51) | 111 (52) | 95 (46) | 48 (51) | 0.637 |
| Anticoagulants | 166 (54) | 142 (66) | 119 (58) | 48 (51) | 0.018 |
Estimated glomerular filtration rate <60 mL/min per 1.73 m2 of body surface area
Table 2.
Baseline clinical and laboratory characteristics by right ventricular ejection fraction (RVEF) categories
| n (%) or mean (±SD) | RVEF ≥40% (n=308) |
RVEF 30 to 39% (n=214) |
RVEF 20 to 29% (n=206) |
RVEF <20% (n=94) |
P-value |
|---|---|---|---|---|---|
| Clinical findings | |||||
| Elevated jugular venous pressure at 30 degrees | 141 (46) | 98 (46) | 123 (60) | 49 (52) | 0.009 |
| S3 gallop | 122 (40) | 85 (40) | 90 (44) | 45 (48) | 0.439 |
| S4 gallop | 53 (17) | 45 (21) | 27 (13) | 14 (15) | 0.172 |
| Pulmonary rales | 57 (19) | 37 (17) | 51 (25) | 28 (30) | 0.029 |
| Hepatomegaly | 32 (10) | 29 (14) | 30 (15) | 19 (20) | 0.093 |
| Lower extremity edema | 80 (26) | 64 (30) | 73 (35) | 40 (43) | 0.010 |
| Chest x-ray findings | |||||
| Pulmonary edema | 21 (7) | 16 (8) | 32 (16) | 28 (30) | <0.001 |
| Cardiothoracic ratio | 54.5 (±7.1) | 54.7 (±7.0) | 57.3 (±7.3) | 59.5 (±7.0) | <0.001 |
| Electrocardiographic findings | |||||
| Left ventricular hypertrophy | 54 (18) | 37 (17) | 39 (19) | 20 (21) | 0.830 |
| Right ventricular hypertrophy | 1 (0.3) | 2 (1) | 2 (1) | 3 (3) | 0.105 |
| Atrial fibrillation | 38 (12) | 41 (19) | 40 (19) | 8 (9) | 0.015 |
| Left bundle branch block | 94 (31) | 64 (30) | 56 (27) | 27 (29) | 0.869 |
| Right bundle branch block | 19 (6) | 23 (11) | 22 (11) | 9 (10) | 0.203 |
| Q-T interval (corrected) | 443 (±45) | 452 (±45) | 455 (±45) | 444 (±45) | 0.014 |
| Laboratory values | |||||
| Creatinine, mg/dL | 1.38 (±0.43) | 1.31 (±0.41) | 1.38 (±0.37) | 1.40 (±0.39) | 0.192 |
| Potassium, mEq/L | 4.4 (±0.2) | 4.4 (±0.3) | 4.4 (±0.2) | 4.4 (±0.3) | 0.818 |
| Sodium, mEq/L | 139 (±3.4) | 139 (±3.5) | 139 (±3.5) | 139 (±3.2) | 0.250 |
| Magnesium, mg/dL | 1.8 (±0.2) | 1.8 (±0.3) | 1.8 (±0.2) | 1.8 (±0.3) | 0.279 |
| Blood urea nitrogen, mg/dL | 27 (±15.0) | 29 (±17.0) | 30 (±15.6) | 32 (±20.8) | 0.046 |
| Glucose, mg/dL | 137 (±77) | 139 (±76) | 124 (±67) | 126 (±69) | 0.099 |
| Uric acid, mg/dL | 7.84 (±2.25) | 8.05 (±2.22) | 8.55 (±2.37) | 8.95 (±2.34) | <0.001 |
| Total cholesterol, mg/dL | 193 (±42) | 191 (±57) | 180 (±41) | 179 (±42) | <0.002 |
| Triglycerides, mg/dL | 222 (±170) | 226 (±208) | 157 (±143) | 145 (±104) | <0.001 |
| Albumin, g/dL | 4.1 (±0.38) | 4.0 (±0.37) | 4.0 (±0.38) | 3.9 (±0.44) | 0.004 |
| Norepinephrine, pg/mL (n=1580) | 510 (±232) | 526 (±235) | 620 (±335) | 671 (±449) | <0.001 |
| Hemoglobin, g/dL | 13.6 (±1.6) | 13.7 (±1.6) | 14.0 (±1.7) | 14.0 (±1.8) | 0.048 |
| White blood cell count, 103/µL | 7.5 (±1.98) | 7.5 (±2.98) | 7.2 (±1.91) | 7.2 (±1.92) | 0.240 |
| Platelet count, 103/µL | 209 (±58) | 211 (±62) | 295 (±59) | 203 (±44) | 0.017 |
Figure 1.
Histogram displaying frequency distribution of right ventricular ejection fraction (%)
3.2. Association between RVEF and mortality
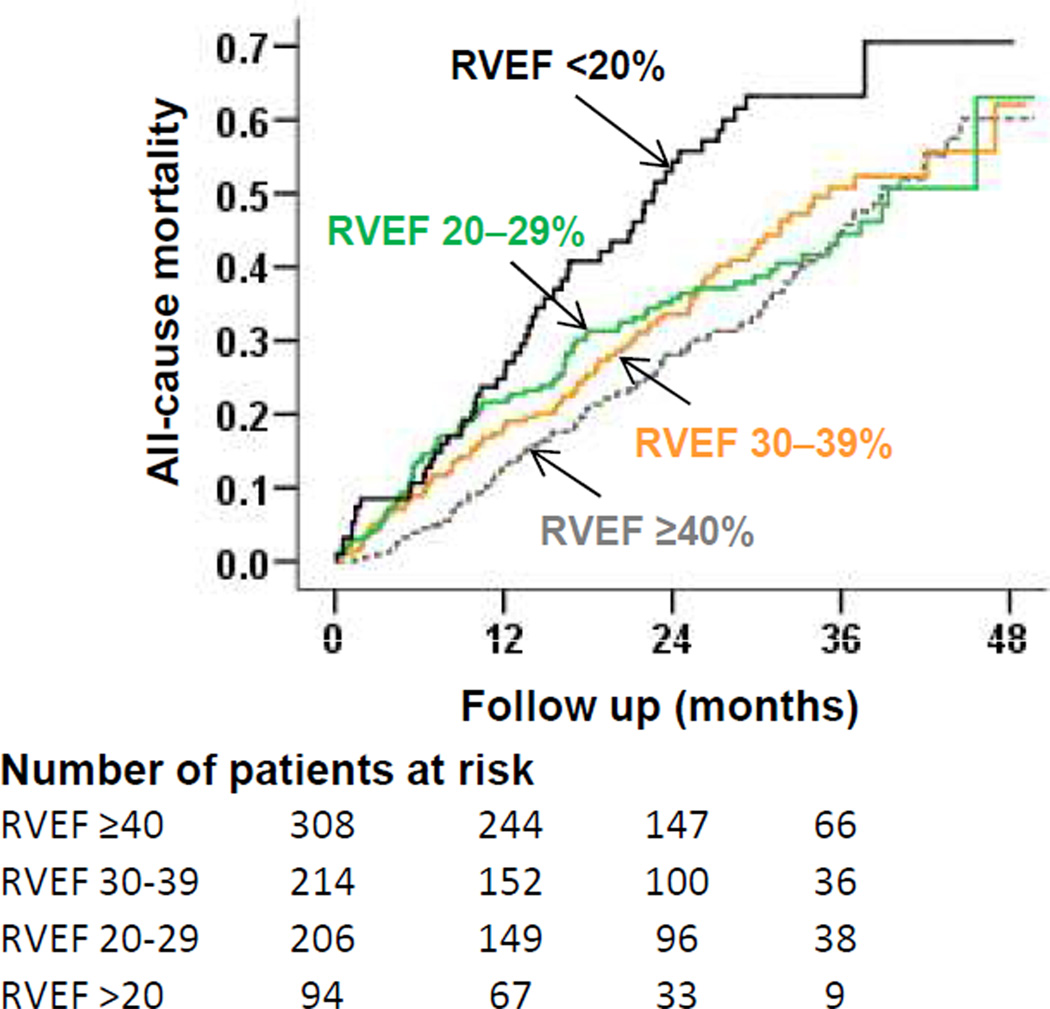
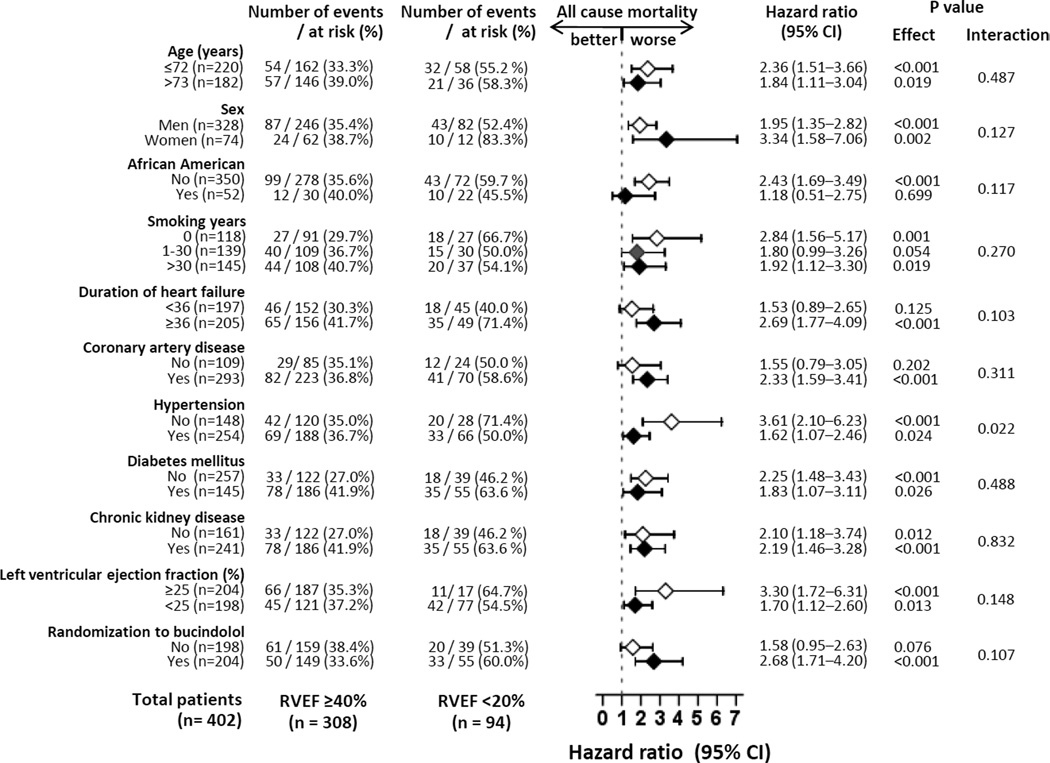
Unadjusted rates for all-cause mortality in patients with RVEF ≥40%, 30–39%, 20–29% and <20% were 36%, 40%, 39% and 56%, respectively (Table 3 and Figure 2). When compared to patients with RVEF ≥40%, unadjusted hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality for those with RVEF 30–39%, 20–29% and <20% were 1.19 (0.90–1.57; P=0.220), 1.13 (0.84–1.51; P=0.423) and 1.97 (1.43–2.73; P<0.001) respectively. Respective multivariable-adjusted HR’s (95% CI’s) for all-cause mortality associated with RVEF 30–39%, 20–29% and <20% were 1.19 (0.88–1.60; P=0.261), 1.00 (0.73–1.39; P=0.982) and 1.70 (1.14–2.53; P=0.009) respectively. The associations between RVEF <20% (versus ≥40%) and all-cause mortality were homogenous across various subgroups (Figure 3). Unadjusted and adjusted HR’s (95% CI’s) for cause-specific mortalities are displayed in Table 4.
Table 3.
Associations of right ventricular ejection fraction (RVEF) and all-cause mortality
| Hazard ratio (95% confidence interval); P-value | ||||
|---|---|---|---|---|
| RVEF ≥40% (n=308) |
RVEF 30 to 39% (n=214) |
RVEF 20 to 29% (n=206) |
RVEF <20% (n=94) |
|
| Unadjusted mortality, n (%) | 111 (36%) | 86 (40%) | 81 (39%) | 53 (56%) |
| Step 1: Unadjusted | 1.00 (Reference) | 1.19 (0.90–1.57); P=0.220 | 1.13 (0.84–1.51) P=0.423 | 1.97 (1.43–2.73) P<0.001 |
| Step 2: Step 1 + LVEF* | 1.00 (Reference) | 1.15 (0.87–1.52); P=0.323 | 1.06 (0.79–1.43) P=0.700 | 1.77 (1.26–2.51) P=0.001 |
| Step 3: Step 2 + demographics** | 1.00 (Reference) | 1.18 (0.89–1.56); P=0.246 | 1.10 (0.81–1.48) P=0.555 | 1.80 (1.27–2.57) P=0.001 |
| Step 4: Step 3 + medical history*** | 1.00 (Reference) | 1.15 (0.86–1.52); P=0.348 | 1.08 (0.79–1.47) P=0.631 | 1.83 (1.27–2.65) P=0.001 |
| Step 5: Step 4 + medications**** | 1.00 (Reference) | 1.15 (0.87–1.53); P=0.336 | 1.08 (0.79–1.48) P=0.621 | 1.80 (1.12–2.62) P=0.002 |
| Step 6: Step 5 + clinical findings***** | 1.00 (Reference) | 1.17 (0.88–1.57); P=0.284 | 0.93 (0.67–1.27) P=0.636 | 1.67 (1.14–2.46) P=0.008 |
| Step 7: Step 6 + laboratory findings****** | 1.00 (Reference) | 1.19 (0.88–1.60); P=0.261 | 1.00 (0.73–1.39) P=0.982 | 1.70 (1.14–2.53) P=0.009 |
LVEF=left ventricular ejection fraction
Demographics: age, sex, and race.
Medical history: duration of smoking, duration of heart failure, New York Heart Association class, coronary artery disease, angina pectoris, diabetes mellitus, hypertension, hyperlipidemia, peripheral vascular disease, atrial fibrillation, >70% coronary artery stenosis, positive stress perfusion test
Medications: bucindolol, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, digitalis, diuretics, and anticoagulants
Clinical findings: body mass index, heart rate, systolic and diastolic blood pressure, S3 gallop, pulmonary râles, and x-ray findings of cardiothoracic ratio and pulmonary edema
Laboratory findings: creatinine, potassium, sodium, magnesium, blood urea nitrogen, glucose, uric acid, total cholesterol, albumin, hemoglobin, white blood cells, and platelets
Figure 2.
Kaplan–Meier plots for all-cause mortality by right ventricular ejection fraction (RVEF)
Figure 3.
Association of right ventricular ejection fraction (RVEF) <20% (versus RVEF ≥40%) with all-cause mortality in various patient subgroups (CI = confidence interval)
Table 4.
Associations of right ventricular ejection fraction (RVEF) and cause-specific outcomes
| Events, n (%) | Unadjusted hazard ratio (95% confidence interval); P-value |
Adjusted hazard ratio* (95% confidence interval); P-value |
|
|---|---|---|---|
| Cardiovascular mortality | |||
| RVEF ≥40% | 90 (29%) | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30 to 39% | 61 (29%) | 1.05 (0.76–1.44); P=0.789 | 1.00 (0.71–1.40); P=0.987 |
| RVEF 20 to 29% | 64 (31%) | 1.11 (0.80–1.53); P=0.544 | 0.97 (0.68–1.39); P=0.867 |
| RVEF <20% | 46 (49%) | 2.09 (1.47–2.97); P<0.0001 | 1.79 (1.17–2.76); P=0.008 |
| Heart failure mortality | |||
| RVEF ≥40% | 37 (12%) | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30 to 39% | 25 (12%) | 1.01 (0.61–1.66); P=0.971 | 1.06 (0.61–1.85); P=0.836 |
| RVEF 20 to 29% | 29 (14%) | 1.12 (0.68–1.85); P=0.651 | 0.93 (0.53–1.61); P=0.783 |
| RVEF <20% | 21 (22%) | 2.27 (1.34–3.85); P=0.002 | 1.97 (1.02–3.83); P=0.045 |
| Sudden cardiac death | |||
| RVEF ≥40% | 41 (13%) | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30 to 39% | 33 (15%) | 1.26 (0.80–1.98); P=0.327 | 1.15 (0.70–1.87); P=0.586 |
| RVEF 20 to 29% | 28 (14%) | 1.10 (0.67–1.79); P=0.711 | 0.95 (0.55–1.65); P=0.860 |
| RVEF <20% | 23 (25%) | 2.26 (1.36–3.75); P=0.002 | 1.61 (0.86–3.00); P=0.135 |
| All-cause hospitalization | |||
| RVEF ≥40% | 205 (67%) | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30 to 39% | 148 (69%) | 1.09 (0.88–1.34); P=0.443 | 1.07 (0.86–1.34); P=0.543 |
| RVEF 20 to 29% | 135 (66%) | 1.02 (0.82–1.28); P=0.838 | 0.92 (0.72–1.17); P=0.496 |
| RVEF <20% | 68 (72%) | 1.44 (1.09–1.89); P=0.009 | 1.19 (0.86–1.63); P=0.292 |
| Heart failure hospitalization | |||
| RVEF ≥40% | 122 (40%) | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30 to 39% | 86 (40%) | 1.11 (0.85–1.46); P=0.451 | 1.06 (0.79–1.42); P=0.706 |
| RVEF 20 to 29% | 87 (42%) | 1.20 (0.91–1.58); P=0.209 | 0.92 (0.67–1.26); P=0.590 |
| RVEF <20% | 48 (51%) | 1.82 (1.30–2.54); P<0.001 | 1.26 (0.84–1.87); P=0.260 |
Multivariable model based on model 7 from Table 3.
3.3. Association between RVEF and hospitalization
Unadjusted rates for HF hospitalization in patients with RVEF ≥40%, 30–39%, 20–29% and <20% were 40%, 40%, 42% and 51%, respectively (Table 4). Compared to patients with RVEF ≥40%, unadjusted HR for HF hospitalization for those with RVEF <20% was 1.82 (95% CI, 1.30–2.54; P<0.001) but lost significance after multivariable-adjustment (1.26, 95% CI =0.84–1.87; P=0.260). Unadjusted and adjusted HR’s (95% CI’s) for all-cause hospitalization are displayed in Table 4.
4. Discussion
4.1. Summary and relevance of the key findings
Findings from our study demonstrate that in older adults with advanced systolic HF, compared to normal RVEF (≥40%), those with severely reduced RVEF (<20%) had increased risk of all-cause, cardiovascular and HF mortalities and sudden cardiac death, and all-cause and HF hospitalizations. However, only the association with all-cause, cardiovascular and HF mortalities were independent of other confounders including LVEF. Milder impairment of RVEF (20 to 39%), on the other hand, had no association with mortality or HF hospitalization. These findings suggest that in older adults with systolic HF, a severely reduced RVEF may be used as a marker of poor prognosis and evaluation of RVEF may be considered a part of a comprehensive assessment of these patients. These findings are important because the majority of HF patients are 65 years and older and most of HF-related mortality occurs in these patients [15, 16].
4.2. Potential explanation and mechanism of the key findings
Low RVEF in HF patients with reduced LVEF may occur early as a result of a disease process involving both ventricles but more commonly, it may be the consequence of LVEF impairment through complex hemodynamic, mechanical and neurohormonal ventricular interactions [1, 17–21]. RV failure, in turn, may compromise adequate LV preload and further reduce LV output, which creates a positive loop of feedback enhancing neurohormonal activation and precipitating end-organ hypoperfusion [1, 19]. The association of reduced RVEF with mortality in elderly patients is therefore mechanistically coherent since low RVEF is primarily a long-term consequence of low LVEF and may also lead to further LVEF impairment and disease progression.
Interestingly, in contrast to the patients with systolic HF in general [1], RVEF was not associated with HF hospitalization in this older cohort with systolic HF. Potential explanations for increased mortality without associated increase in hospitalization include sudden death or death not associated with acute exacerbation of symptoms. However, RVEF <20% in our study was not association with sudden cardiac death. This is also unlikely to be explained by small sample size or event size, as the number of events for HF hospitalization (51%) in those with RVEF <20% was similar to that for total mortality (56%) and CV mortality (49%), both of which were significantly increased. Finally, an alternative explanation might be that this association occurred by chance.
4.3. Comparison with findings from relevant published literature
Several studies have reported the prognostic value of RVEF in HF using different techniques of assessment of RVEF [3–10]. However, patients included in these studies had a mean age of 50 to 60 years, and many were based exclusively on candidates for heart transplant [4, 5, 9]. In contrast, our previous report of the relationship between RVEF and outcomes was the largest, was based on ambulatory systolic HF patients, and nearly half of the patients were older adults. To the best of our knowledge, this is the first report of the effect of RVEF on the natural history of systolic HF in ambulatory older adults. The findings from the current study suggest that RVEF may provide useful prognostic information in ambulatory older adults with systolic HF and whenever possible RVEF should be estimated as a part of the comprehensive evaluation of HF in these patients. Radionuclide ventriculography has been extensively validated for the estimation of RVEF. However, echocardiographic assessment of the right ventricle using the apical 4-chamber view can also provide overall qualitative assessments of right ventricular size and function [22]. Finally, three-dimensional echocardiography appears very promising in RVEF measurement [23].
4.4. Clinical and public health importance
The management of RV failure in patients with chronic systolic HF is poorly understood and remains largely empirical [17]. The presence of reduced RVEF may be used in a near future not only to assess prognosis but also to refine the therapeutic management of these patients. Preliminary data from patients with nonischemic hear disease suggest that those with low RVEF are less likely to experience an increase in LVEF from beta-blocker therapy [24]. Patients with low RVEF are also less likely to respond to cardiac resynchronization therapy [25] but more likely to respond to therapy with sildenafil [26]. Cardiac resynchronization therapy has been shown to be associated with a slight improvement in RVEF (by about 2%; P=0.016) after a mean follow-up of 9 months [25]. Data from patients with systolic HF and pulmonary hypertension also suggest that therapy with sildenafil may also improve RVEF [26].
4.5. Potential limitations and future direction
Several limitations of our study must be acknowledged. RVEF may have changed during follow-up resulting in regression dilution and potential underestimation of the observed associations between RVEF and outcomes [27]. Radionuclide ventriculography has now been replaced by cardiac magnetic resonance imaging (MRI) as the gold standard for measuring RVEF [28]. However, routine use of MRI in the assessment of HF patients is still limited by lack of availability, costs and the wide use of devices that are not MRI-compatible yet. Also, RVEF is an imperfect measure of RV systolic function as it is dependent on loading conditions [17, 28], and thus may be affected by volume status, pulmonary pressure, and tricuspid regurgitation, none of which was specifically evaluated in our study. However, the same limitations also apply to many other measurements of RV systolic function. Finally, BEST participants were not receiving beta-blockers approved for HF, which may limit generalizability of these findings to contemporary patients with systolic HF.
4.6. Conclusions
In conclusion, in older adults with advanced chronic systolic HF, severely reduced RVEF (<20%) is an independent predictor of increased mortality but had no association with hospitalization. Measurement of RVEF should be considered in these patients, and when available, should be used to stratify patients for prognostic and therapeutic purposes. Future studies need to develop and test new therapies to improve outcomes in older adults with systolic HF and low RVEF.
Acknowledgment
The Beta-Blocker Evaluation of Survival Trial (BEST) is conducted and supported by the NHLBI in collaboration with the BEST Study Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the BEST or the NHLBI.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [29].
Funding Sources: Dr. Ahmed is supported by grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masoudi FA, Havranek EP, Krumholz HM. The burden of chronic congestive heart failure in older persons: magnitude and implications for policy and research. Heart Fail Rev. 2002;7:9–16. doi: 10.1023/a:1013793621248. [DOI] [PubMed] [Google Scholar]
- 3.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–224. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 4.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 5.Gavazzi A, Berzuini C, Campana C, et al. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997;16:774–785. [PubMed] [Google Scholar]
- 6.Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 7.de Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 8.La Vecchia L, Paccanaro M, Bonanno C, Varotto L, Ometto R, Vincenzi M. Left ventricular versus biventricular dysfunction in idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:120–122. A9. doi: 10.1016/s0002-9149(98)00795-4. [DOI] [PubMed] [Google Scholar]
- 9.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 10.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 11.The BEST Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 12.The BEST Steering Committee. Design of the Beta-Blocker Evaluation Survival Trial (BEST) Am J Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- 13.Manno BV, Iskandrian AS, Hakki AH. Right ventricular function: methodologic and clinical considerations in noninvasive scintigraphic assessment. J Am Coll Cardiol. 1984;3:1072–1081. doi: 10.1016/s0735-1097(84)80368-x. [DOI] [PubMed] [Google Scholar]
- 14.Hesse B, Lindhardt TB, Acampa W, et al. EANM/ESC guidelines for radionuclide imaging of cardiac function. Eur J Nucl Med Mol Imaging. 2008;35:851–885. doi: 10.1007/s00259-007-0694-9. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 16.Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2009;49:165–171. doi: 10.1016/j.archger.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right Ventricular Function in Cardiovascular Disease, Part II: Pathophysiology, Clinical Importance, and Management of Right Ventricular Failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 18.Setaro JF, Cleman MW, Remetz MS. The right ventricle in disorders causing pulmonary venous hypertension. Cardiol Clin. 1992;10:165–183. [PubMed] [Google Scholar]
- 19.Voelkel NF, Quaife RA, Leinwand LA, et al. Right Ventricular Function and Failure: Report of a National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 20.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 21.Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 22.Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr. 2010;11:81–96. doi: 10.1093/ejechocard/jep234. [DOI] [PubMed] [Google Scholar]
- 23.Leibundgut G, Rohner A, Grize L, et al. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr. 2010;23:116–126. doi: 10.1016/j.echo.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Ramahi TM, Longo MD, Cadariu AR, et al. Left ventricular inotropic reserve and right ventricular function predict increase of left ventricular ejection fraction after beta-blocker therapy in nonischemic cardiomyopathy. J Am Coll Cardiol. 2001;37:818–824. doi: 10.1016/s0735-1097(00)01162-1. [DOI] [PubMed] [Google Scholar]
- 25.Burri H, Domenichini G, Sunthorn H, et al. Right ventricular systolic function and cardiac resynchronization therapy. Europace. 2010;12:389–394. doi: 10.1093/europace/eup401. [DOI] [PubMed] [Google Scholar]
- 26.Lewis GD, Shah R, Shahzad K, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 27.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. American journal of epidemiology. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 28.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right Ventricular Function in Cardiovascular Disease, Part I: Anatomy, Physiology, Aging, and Functional Assessment of the Right Ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 29.Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. International journal of cardiology. 2010;144:1–2. [Google Scholar]