Abstract
Background
There are robust sex differences for alcohol phenotypes, with men reporting more drinking and alcohol use disorder (AUD) symptoms than women. However, the sources of these effects are not completely understood. Sex hormones, a substantial biological sex difference, exert neurobehavioral influences and are candidates for influencing sex differences in alcohol phenotypes. The current study investigated the effects of prenatal androgens based on the hypothesis of prenatal hormone transfer, which posits that hormones from one twin influence the development of a co-twin.
Methods
The current study compared female twins from opposite-sex (OSF) and same-sex (SSF) pairs to investigate associations between prenatal androgens and alcohol phenotypes. Additional analyses distinguished prenatal and postnatal effects by comparing OSFs and SSFs with a close-in-age older (CAO) brother.
Results
OSFs endorsed more lifetime AUD symptoms than SSFs (d = 0.14). Females with a CAO brother reported greater intoxication frequency (d = 0.35), hangover frequency (d = 0.24), typical drinking quantity (d = 0.33), and max drinks (i.e., the most drinks ever consumed in a 24-hour period; d = 0.29). Controlling for postnatal effects, OSFs still endorsed more lifetime AUD symptoms than SSFs with a CAO brother (d = 0.16).
Conclusions
Prenatal exposure to a male co-twin was associated with increases in AUD symptoms, above the effect of postnatal exposure to a male sibling. Prenatal exposure to a male co-twin was not associated with increases in other alcohol-related phenotypes, but postnatal exposure to older male siblings produced medium effect sizes for indicators of alcohol consumption. Sex differences in AUDs, but not alcohol use, may be partially due to the neurodevelopmental effects of prenatal androgens. However, sibling effects may be larger than any effect of prenatal androgen exposure.
Keywords: prenatal, androgen, alcohol, alcohol use disorder, twins, sibling, familial
Robust sex differences have been shown for alcohol use and alcohol use disorders (AUDs), with men reporting more drinking and alcohol-related consequences than women (Kessler et al., 1994). The sources of this effect, however, are not well understood. Factors potentially contributing to sex differences in alcohol-related outcomes include genetics, perceived social norms, coping styles, and personality traits (for review, see Nolen-Hoeksema, 2004). Sex hormones are one of the most substantial biological sex differences and are known to affect behavior, making them an additional candidate for influencing alcohol-related sex differences (for review, see Collaer and Hines, 1995).
Sex hormones are typically classified as estrogens (i.e., feminizing) or androgens (i.e., masculinizing), and may also be classified based on the timing of exposure (i.e., prenatally or postnatally; Goy and McEwen, 1980). For example, prenatal androgen secretion has been attributed to various masculinizing behaviors (e.g., aggression Cohen-Bendahan et al., 2005a). Although present in the body throughout the lifespan, the strongest surges of sex hormones typically occur prenatally (when driving the development of primary sex characteristics and neuroanatomical organization) and during puberty (when driving the development of secondary sex characteristics and interacting with neural systems already in place).
Postnatal androgen levels have been consistently associated with alcohol use and AUDs. In adolescent males, relationships between postnatal androgens and AUD symptoms and diagnoses have been observed, even after controlling for physical maturity (Eriksson et al., 2005). In a college population, after puberty has likely ended and testosterone levels have stabilized, non-pathological alcohol use remains positively correlated with testosterone levels in male and female college students (La Grange et al., 1995). In a study of 4,462 male military veterans (mean age = 37), Dabbs and Morris (1990) found that males highest in testosterone (10th percentile) endorsed more AUD symptoms (16.4%) than the rest of the sample (11.5%; risk-ratio = 1.8).
Animal studies have demonstrated similar associations between testosterone and alcohol-related outcomes. Apter and Eriksson (2003) found alcohol-preferring rodent strains to have higher testosterone levels than non-preferring strains, both at baseline and after alcohol administration. In an experimental study, castrated male rats given daily testosterone injections for four months developed alcohol preference more quickly and to a greater degree (consuming 45-times more ethanol than water) than those that received placebo injections (consuming 8-times more ethanol than water; Lakoza and Barkov, 1980). Findings have therefore led some to consider postnatal androgens a biomarker for AUDs (Stålenheim et al., 1998). The involvement of prenatal androgens, however, is less clear.
Prenatal hormones and teratogens profoundly affect prenatal neurodevelopment and, in turn, may influence postnatal behavior (Collaer and Hines, 1995). Furthermore, prenatal hormone exposure may be an epigenetic trigger (Nagel and vom Saal, 2004). Some studies on prenatal hormones have used direct measures of hormone levels present in the umbilical artery or vein (Auyeung et al., 2009), but more frequently exposure is inferred (Miller, 1994). For example, in rats, hormones in the amniotic fluid diffuse across the membrane of the amniotic sac, into the uterine lumen, and across the membrane of adjacent amniotic sacs sharing the womb (Even et al., 1992). Ex vivo hormone exposure for a fetus can, therefore, be inferred from the gender of adjacent fetuses. This is referred to as prenatal hormone transfer (Miller, 1994). Animal studies utilizing this method have found several postnatal behaviors to be masculinized in female fetuses adjacent to male fetuses, such as masculinized sexual behavior (guinea pigs; Phoenix et al., 1959), scent marking (gerbils; Clark et al., 1990), and increased aggressive behavior (mice; vom Saal, 1989).
In human research, the hypothesis of prenatal hormone transfer has been applied to opposite-sex twin pairs, with elevated exposure to sex hormones of the opposite sex being inferred (e.g., increased androgen exposure in females; Loehlin and Martin, 2000; Miller and Martin, 1995; Resnick et al., 1993). Such studies have shown that females from opposite-sex twin pairs are masculinized on sensation seeking (Resnick et al., 1993; Slutske et al., 2011), aggression (Cohen-Bendahan et al., 2005a), and the 2D:4D digit ratio – a key indicator of prenatal androgen exposure (Voracek and Dressler, 2007). Some studies, however, have failed to find such effects for the the 2D:4D digit ratio (Medland et al., 2008) and stereotypic masculine-feminine interests (Rose et al., 2002), and additional replication attempts are needed (for review, see Tapp et al., 2011). Additional evidence for prenatal androgens influencing behavioral and physical development has come from research on females with congenital adrenal hyperplasia (CAH; a genetic condition leading to elevated androgen production). Females with CAH tend to exhibit masculinized patterns of play behavior, aggression, visuospatial abilities, and learning disabilities, as well as more bisexual or homosexual tendencies (for review, see Collaer and Hines, 1995).
The current study utilized females from opposite-sex (OSFs) and same-sex (SSFs) twin pairs to investigate the relationship between prenatal androgen exposure on alcohol use and AUDs. We hypothesized that OSFs would have elevated prenatal androgen exposure via amniotic diffusion and, consequently, report elevated levels of alcohol use and AUD symptoms. In addition to this prenatal environmental difference, it is likely that OSFs and SSFs have different postnatal environments related to alcohol use, such as exposure to sibling modeling (e.g., masculinized drinking). To account for this potential confound and more rigorously test for an effect of prenatal environment, the current study included additional OSF-SSF comparisons matched on exposure to male siblings (i.e., SSFs with a close-in-age older brother). If effects found for OSF-SSF comparisons fail to persist under these comparisons, it would suggest that factors related to the postnatal environment, rather than prenatal environment, are more likely the source of any effects found in OSF-SSF comparisons.
Methods
Participants
Participants were members of the national community-based Australian Twin Registry (ATR) Cohort II (Slutske et al., 2009) who took part in a study that primarily focused on gambling. In 2004 – 2007, 4,764 participants (2,727 females, 2,037 males) completed a structured psychiatric telephone interview that also included measures of alcohol use and AUD symptoms. Female participants consisted of 2,106 SSFs, of which there were 1,191 monozygotic (MZ) and 915 dizygotic (DZ) twins, and 621 OSF twins. There were 1,517 male participants from SS pairs (847 MZ, 670 DZ).
Trained lay interviewers conducted the telephone interviews under the supervision of a clinical psychologist. All interviews were recorded and some were randomly sampled for quality-assurance review. Participants were 32 – 43 years of age when interviewed (M = 37.67, SD = 2.31). The participation rate for this interview survey was 80% (see Slutske et al., 2009 for more details about sample characteristics and zygosity determination). All data collection was approved by the Institutional Review Boards at the University of Missouri and the Queensland Institute of Medical Research (QIMR).
Measures
Alcohol use
Participants were asked about their drinking over the previous year. If there was a 12-month period in which they drank more than in the past 12 months, alcohol use during that period was also queried. For the purpose of this paper, we focused on alcohol use during the participants’ heaviest drinking period (regardless of whether it occurred during or prior to the previous 12 months). Note that if the participant judged their alcohol use to be stable, their current age was used as the age at which their “heaviest drinking period” occurred. For participants whose heaviest drinking period did not include the past year (n = 1,930), the average age at which the heaviest drinking period began was 22.7 (SD = 5.7). (Unfortunately, the age at which the heaviest drinking period began was not ascertained if it included the past year).
The measures of alcohol use during the heaviest drinking period included: (1) drinking frequency (days per year consuming at least one drink), (2) intoxication frequency (days per year had slurred speech, unsteady gait when drinking), (3) hangover frequency (days per year did not feel well the day after drinking), and (4) typical drinking quantity (number of drinks per drinking occasion). In addition, the most drinks ever consumed in a 24-hour period (“max drinks”) was assessed. Of the 2,727 female and 1,517 male participants, there were complete alcohol data for 2,721 and 1,508 participants, respectively. For lifetime alcohol abstainers, frequency measures were coded as zero and quantity measures (including max drinks) were coded as missing.
A small sub-sample of the participants (N = 167) were re-interviewed after several months (mean interval = 3.4 months, SD = 1.4 months, range = 1.2–9.5 months) to establish the test-retest reliability of the measures. These data provide evidence for adequate reliability in respondent recall for: (1) drinking frequency: r = .74; (2) drinking quantity: r = .73; (3) intoxication frequency: r = .82; (4) hangover frequency: r = .64; and (5) max drinks: r = .80. All correlations were significant at the p < .0001 level.
Alcohol use disorder
The alcohol use disorder section from the World Health Organization Composite International Diagnostic Interview (CIDI) was used to assess AUD symptoms (Robins et al., 1988). The CIDI is a structured interview based on mental health disorder symptoms from the DSM-IV and International Classification of Diseases Tenth Version, and has been used in other national community-based studies, such as the National Comorbidity Survey (Kessler et al., 1998).
Alcohol dependence and abuse symptoms were combined into a single 11-item symptom count. This is consistent with research demonstrating that symptoms for both diagnoses comprise a single unidimensional factor (Kahler and Strong, 2006) and the proposed DSM-5 criteria for combining the current alcohol dependence and abuse symptoms into a single disorder. In the ATR sample, the 11 AUD symptoms demonstrated adequate internal consistency for self-reports of lifetime symptom counts (α = .80).
Family composition
Information about siblings, including number, gender (male or female), and type (i.e., full, half, step, adoptive) of siblings, was obtained from a previous telephone interview (see Slutske et al., 2009 for more details). Birthdates for siblings were obtained from a twin-family database maintained at the QIMR Genetic Epidemiology Unit. For the purpose of this study, only data pertaining to brothers of participants were used. Of the 2,102 SSF participants with alcohol data, 2,019 (96%) had information on male siblings from the previous interview and 1,341 (66%) had at least one full brother. Of those with at least one full brother, 999 (75%) had birthdate information in the QIMR database. Thus, data about brothers were available for 47% of SSFs (549 MZ, 450 DZ) in the ATR Cohort II. Although data on non-full siblings (i.e., half, step, adopted) were available, these were not included in analyses because it was unclear if and when participants began to live with these siblings. Inferences based on non-full siblings are, therefore, ambiguous.
Of the 999 female participants with a full brother and sibling birthdate information, 747 (395 MZ, 352 DZ) had at least one older brother. Older siblings consistently predict alcohol use in younger siblings, but the inverse has not been shown (Trim et al., 2006). We therefore focused solely on the effects of having an older brother. In addition, only close-in-age older (CAO) siblings (no more than three years older) were considered because sibling effects on drinking attenuate as siblings become more disparate in age (McGue et al., 1996). Of respondents with an older brother, 317 (185 MZ, 132 DZ) had a CAO brother.
Data Analysis
Analytic Plan
Because alcohol use and AUD symptom measures are highly skewed and kurtotic, analyses were performed on log- and rank-transformed as well as raw data. However, there were minimal differences between the results of the analyses of log- and rank-transformed data, and only log-transformed data will be discussed to simplify presentation. To ease interpretation, all tables include means and standard deviations that were based on the variables prior to data transformation, although the results reported are from analyses conducted with log-transformed data.
The current study included tests for four effects. First, sex differences were assessed to determine the magnitude of sex effects for alcohol use and AUD measures, as behaviors with the largest between-sex effect sizes are those most likely to be influenced by sex hormones (Cohen-Bendahan et al., 2005b). Tests of sex differences were conducted using only data from SS twin pairs. Second, OSF-SSF comparisons were conducted to determine whether any alcohol-related outcomes are potentially influenced by prenatal androgens. Third, sibling effects by CAO brothers included two groups of SSFs: those without any brother (younger or older; full, adopted, or half) and those with at least one CAO brother. Finally, prenatal environment (while accounting for postnatal effects of having a CAO brother) was assessed by comparing OSFs to SSFs with a CAO brother. That is, the ultimate test was a comparison of females who differed in prenatal, but not postnatal, exposure to a male sibling. To eliminate any potential confounds (e.g., placentation effects) and ensure that groups differed only in prenatal exposure to an adjacent male fetus, all OSF-SSF comparisons included only DZ twin pairs.
All analyses used a saturated, univariate twin model as a baseline model against which nested models were compared. The twin pair covariance was included in all analyses to account for the non-independence of having data for two participants from the same family. For tests of mean differences, a nested model with means constrained to be equal across groups (e.g., SSFs and OSFs) was compared to the baseline model to produce a chi-square difference test. That is, chi-square difference tests were used to infer whether group means for alcohol use and AUD measures differed significantly. Similar analyses were conducted for tests of variance differences. All analyses were run using OpenMx (Boker et al., 2011).
Results
Sample Description
Of the female participants in the current study, 34 (2%) reported never drinking alcohol. Data were therefore available for 1,584 female participants (MZ SSF = 532, DZ SSF = 444, OSF = 608).
Sex Differences
The alcohol-related outcomes for same-sex males (SSMs) and SSFs, and their respective significance tests, are displayed in Table 1. Mean sex differences were found across all measures, with males reporting more drinking and AUD symptoms than females. Effect sizes (Cohen’s d; Cohen, 1988) for sex differences ranged from medium (drinking frequency = 0.36, hangover frequency = 0.37) to large (max drinks = 0.98). Excluding quantity measures (which are confounded by body composition differences), the largest sex differences were found for drinking frequency (d = 0.49) and lifetime AUD symptoms (d = 0.44).
Table 1.
Differences in drinking phenotypes between women and men.
| Means (SD) | Mean Tests | Variance Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Same-Sex Males a |
Same-Sex Females b |
Χ2 | p-value | Χ2 | p-value | ||
| Drinking Frequency c | 161.85 | (112.13) | 120.20 | (106.20) | 46.15 | p<.01 | 11.99 | 0.11 |
| Intoxication Frequency c | 44.37 | (69.89) | 24.45 | (51.80) | 66.92 | p<.01 | 1.71 | 0.19 |
| Hangover Frequency c | 29.09 | (51.01) | 17.20 | (37.81) | 48.71 | p<.01 | 4.25 | 0.04 |
| Drink Quantity | 5.19 | (4.35) | 3.49 | (2.73) | 82.77 | p<.01 | 29.67 | p<.01 |
| Max Drinks | 21.98 | (14.45) | 9.89 | (7.90) | 261.71 | p<.01 | 12.99 | p<.01 |
| Lifetime AUD Symptoms | 1.73 | (2.11) | 1.00 | (1.62) | 65.41 | p<.01 | 14.04 | p<.01 |
761 twin pairs
1,057 twin pairs
All frequency phenotypes are measured in days per year.
NOTE: Means and standard deviations presented are based on raw data, statistical tests were based on log-transformed data. The twin pair covariance was included in all analyses to account for the non-independence of having data for two participants from the same family.
Variance differences, again with males having larger values than females, were found across nearly all measures. Drinking frequency (p = .11) and intoxication frequency (p = .19) were the only measures without variance differences. These mean and variance between-sex differences provided a baseline against which to evaluate subsequent within-sex comparisons.
OSF-SSF Comparisons
Results for OSF-SSF comparisons are displayed in Table 2. OSFs reported more lifetime AUD symptoms (M = 1.24) than DZ SSFs (M = 0.98), although the effect size associated with this mean difference was small (d = .14, p = .01). Follow-up analyses of each of the individual AUD symptoms revealed that the symptom prevalences were larger among the OSFs than the DZ SSFs for all 11 symptoms, and significantly so for three (see online supplementary material, Table S1). There was also a trend (d = .09, p = .11) for OSFs (M = 11.02) to report more max drinks than DZ SSFs (M = 10.23). No other differences were found for any alcohol use or AUD measures.
Table 2.
Tests of overall effect: Differences in drinking phenotypes between opposite-sex and same-sex dizygotic female twins.
| Means (SD) | Mean Tests | Variance Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Opposite-Sex Females a |
Same-Sex Females b |
Χ2 | p-value | Χ2 | p-value | ||
| Drinking Frequency c | 130.87 | (107.57) | 122.74 | (107.15) | 2.09 | 0.15 | 0.14 | 0.70 |
| Intoxication Frequency c | 32.47 | (61.96) | 27.72 | (53.27) | 0.76 | 0.38 | 0.80 | 0.37 |
| Hangover Frequency c | 22.23 | (46.52) | 20.96 | (47.02) | 0.86 | 0.35 | 0.18 | 0.67 |
| Drink Quantity | 3.83 | (3.18) | 3.67 | (2.90) | 0.41 | 0.52 | 1.73 | 0.19 |
| Max Drinks | 11.02 | (8.18) | 10.23 | (7.79) | 2.61 | 0.11 | 0.45 | 0.50 |
| Lifetime AUD Symptoms | 1.24 | (1.88) | 0.98 | (1.57) | 6.82 | 0.01 | 5.87 | 0.02 |
621 twin pairs
459 twin pairs
All frequency phenotypes are measured in days per year.
NOTE: Means and standard deviations presented are based on raw data, statistical tests were based on log-transformed data. The twin pair covariance was included in all analyses to account for the non-independence of having data for two participants from the same family.
Only variances for lifetime AUD symptoms were significantly different. Among OSFs the variance for AUD symptoms was greater than among DZ SSFs (p = .02), but the variance for all other measures was only marginally greater in OSFs.
Sibling Effects
Results for sibling effects tests (comparing SSFs exposed to a CAO full brother and SSFs without such exposure) are displayed in Table 3. SSFs with CAO brothers reported greater intoxication frequency (d = 0.35, p < .01), hangover frequency (d = 0.24, p = .03), drinking quantity (d = 0.33, p < .01), and max drinks (d = 0.29, p = .01). In addition, there was a trend in the same direction for drinking frequency (d = 0.18, p = .11) and lifetime AUD symptoms (d = 0.17, p = .13).
Table 3.
Tests of sibling effect: Differences in drinking phenotypes between same-sex female twins with a close-in-age older brother and without a brother.
| Means (SD) | Mean Tests | Variance Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Same-Sex Females with a Close-in-Age Older Brother a |
Same-Sex Females with no Brother b |
Χ2 | p-value | Χ2 | p-value | ||
| Drinking Frequency c | 136.66 | (114.75) | 113.50 | (103.59) | 2.62 | 0.11 | 0.24 | 0.62 |
| Intoxication Frequency c | 37.97 | (70.74) | 22.45 | (51.94) | 9.51 | < 0.01 | 1.30 | 0.25 |
| Hangover Frequency c | 21.75 | (43.67) | 15.94 | (35.61) | 4.81 | 0.03 | 0.45 | 0.50 |
| Drink Quantity | 4.02 | (3.29) | 3.10 | (2.39) | 8.73 | < 0.01 | 4.88 | 0.03 |
| Max Drinks | 12.10 | (10.65) | 9.18 | (7.08) | 6.36 | 0.01 | 1.13 | 0.29 |
| Lifetime AUD Symptoms | 1.22 | (1.79) | 0.95 | (1.57) | 2.30 | 0.13 | 1.66 | 0.20 |
167 twin pairs
306 twin pairs
All frequency phenotypes are measured in days per year.
NOTE: Means and standard deviations presented are based on raw data, statistical tests were based on log-transformed data. The twin pair covariance was included in all analyses to account for the non-independence of having data for two participants from the same family.
Significant variance differences were obtained only for drinking quantity. Among SSFs exposed to a CAO brothers, the variance for drinking quantity was greater than among SSFs without a brother (p = .03).
Prenatal Environment
Results from tests of prenatal environment (comparing OSFs and DZ SSFs with a CAO brother) are displayed in Table 4. Lifetime AUD symptom counts (d = 0.16, p = .14) were marginally higher in OSFs (M = 1.24) compared to the SSF comparison group (M = 1.01). Unexpectedly, all frequency measures produced trends in the opposite direction from these effects and from the initial OSF-SSF comparisons, as SSFs with a CAO brother reported marginally greater frequencies on all measures, suggesting that the magnitude of the effect of having a CAO brother is greater than the magnitude of the effect associated with having a same-age twin brother.
Table 4.
Tests of prenatal environment: Differences in drinking phenotypes between opposite-sex female twins and dizygotic same-sex female twins with a close-in-age older brother.
| Means (SD) | Mean Tests | Variance Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| Phenotype | Opposite-Sex Females a |
Same-Sex Females with a Close-in-Age Older Brother b |
Χ2 | p-value | Χ2 | p-value | ||
| Drinking Frequency c | 130.87 | (107.57) | 133.89 | (111.00) | 0.38 | 0.54 | 2.03 | 0.15 |
| Intoxication Frequency c | 32.47 | (61.96) | 34.78 | (65.19) | 0.38 | 0.54 | 0.00 | 0.96 |
| Hangover Frequency c | 22.23 | (46.52) | 27.08 | (55.97) | 0.10 | 0.75 | 0.69 | 0.41 |
| Drink Quantity | 3.83 | (3.18) | 3.75 | (3.00) | 0.03 | 0.87 | 0.05 | 0.83 |
| Max Drinks | 11.02 | (8.18) | 10.09 | (6.43) | 0.36 | 0.55 | 1.88 | 0.17 |
| Lifetime AUD Symptoms | 1.24 | (1.88) | 1.01 | (1.74) | 2.17 | 0.14 | 0.31 | 0.58 |
621 twin pairs
65 twin pairs
All frequency phenotypes are measured in days per year.
NOTE: Means and standard deviations presented are based on raw data, statistical tests were based on log-transformed data. The twin pair covariance was included in all analyses to account for the non-independence of having data for two participants from the same family.
No variance differences were observed for any of the tests of the prenatal environment.
Effect Sizes
Effect sizes (Cohen’s d) for log-transformed data across all analyses (except tests for sex differences) are displayed with standard errors (Nakagawa and Cuthill, 2007) in Figure 1, with positive effect sizes indicating masculinizing effects (i.e., more alcohol use or AUD symptom in the group with prenatal and/or postnatal exposure to male siblings). All alcohol use measures and AUD symptom counts were in the hypothesized direction of masculinized drinking for OSF-SSF and sibling effect comparisons. However, only drinking quantity items and AUD symptom counts were in the direction consistent with masculinization for prenatal environment (i.e., having a male co-twin vs. a male brother). All other tests of prenatal environment produced small effects in the opposite direction.
Figure 1.
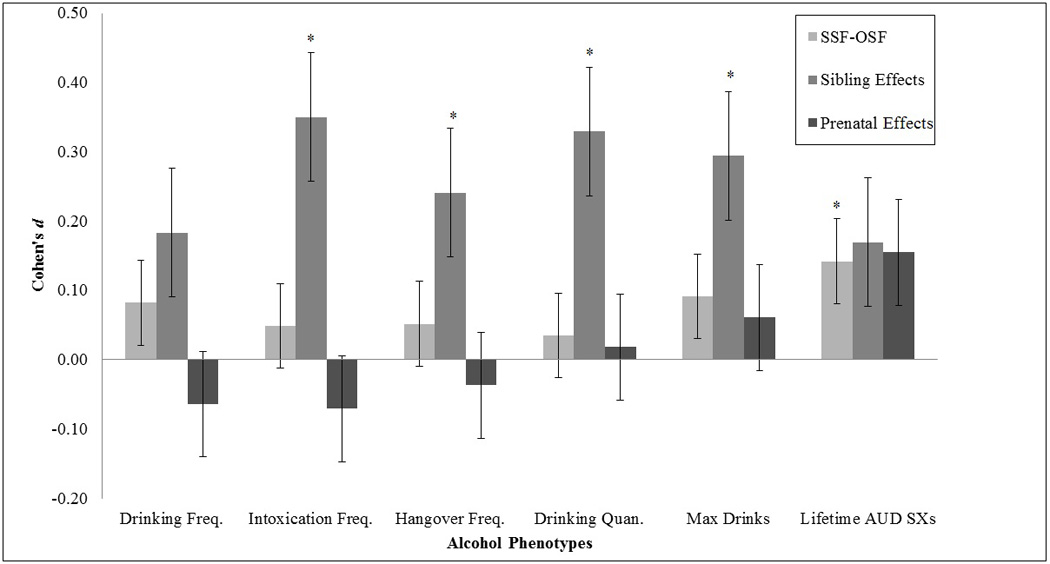
Effect sizes of mean comparisons of: (a) same-sex versus opposite-sex females (SSF-OSF), (b) same-sex females with a close-in-age older brother versus no brother at all (sibling effects), and (c) opposite-sex versus dizygotic same-sex females with a close-in-age older brother (prenatal environment). (Note: Freq. = frequency; Quan. = quantity; SXs = symptoms; * = significant mean difference at .05 significance level; effect size [Cohen’s d] is calculated as (M1 - M2) / σpooled (using log-transformed data); error bars represent one standard error above and below the mean calculated using formulae from Nakagawa and Cuthill, 2007).
Most tests involving OSFs (i.e., OSF-SSF, prenatal effects) did not yield effect sizes greater than .10. In fact, this occurred only for lifetime AUD symptoms (OSF vs. SSF = 0.14, prenatal effects = 0.16). Notably, prenatal effects on AUD symptom counts did not reach significance, but the effect size exceeded that from the initial OSF-SSF comparisons, which was statistically significant. This indicates a loss of power but not necessarily an absence of an effect.
Unexpectedly, the largest effects were found for sibling effects by CAO brothers, for which effect sizes exceeded .20 (hangover frequency, max drinks) and .30 (drinking quantity, intoxication frequency).
Discussion
This study is the first investigation of the influence of prenatal androgen exposure on female alcohol use and AUDs, and is one of the first to account for postnatal sibling effects when testing the prenatal hormone-transfer hypothesis. Under the assumptions of the prenatal hormone-transfer hypothesis (Miller, 1994), analyses revealed small effects suggesting prenatal androgens were associated with AUDs. Having a male cotwin (rather than a male sibling) produced negligible to small effect sizes, the largest being for lifetime AUD symptoms (d = 0.16). All effect sizes for frequency measures (days drinking, intoxicated, and hung over), however, were actually in the opposite direction. These findings suggest that sibling effects by a CAO brother are associated with female alcohol use (d = 0.18–0.35) and AUD symptoms (d = 0.17), but that prenatal androgens are associated only with AUD symptoms (d = 0.16).
To put results from within-sex comparisons in perspective, effect sizes for between-sex comparisons can be used as an upper limit for expected effect sizes. That is, within-sex differences are unlikely to exceed between-sex differences on these alcohol-related outcomes. Thus, a ratio of within-sex to between-sex effect sizes (when testing a factor that likely influences behavior typical of the opposite sex) can gauge the degree to which that factor may contribute to between-sex differences.
This ratio shows that the effect size for prenatal environment was 35% of that for sex differences in AUD symptoms (lifetime AUDs d = 0.16 vs. 0.44), but only 6% of that for max drinks (d = 0.06 vs. 0.98). These findings suggest that prenatal androgen exposure influences problematic, but not necessarily normative, alcohol use. This is consistent with other studies of the prenatal hormone-transfer hypothesis, which suggest that sensation seeking (a strong correlate of AUDs) is also associated with prenatal androgen exposure (Resnick et al., 1993; Slutske et al., 2011).
In the current study, OSFs (relative to SSFs with a CAO brother) endorsed more AUD symptoms, but slightly lower levels of alcohol use. Follow-up analyses comparing OSFs and SSFs, both with a CAO brother, yielded similar findings (see online supplementary material, Table S2). Previous research suggests at least one pathway by which prenatal androgens might influence AUDs. The orbitofrontal cortex (OFC), is associated with addictive behavior (Volkow and Fowler, 2000), is densely populated with sex hormone receptors and is much larger in females than in males (Goldstein et al., 2001). This region is associated with personality traits (e.g., disinhibition; Udry and Talbert, 1988) that are associated with both AUDs and androgen levels. In addition, scores on measures of behavioral undercontrol, a masculinized trait sharing a large degree of general and genetic variance with AUDs (Slutske et al., 2002), may be higher among OSFs than SSFs (Resnick et al., 1993).
The largest effects obtained in this study were the effects of having an older male sibling on both normative (e.g., quantity and frequency of use) and problematic alcohol use (i.e., AUD symptoms) among younger sisters. Follow-up analyses, comparing OSFs with a CAO brother with OSFs without a brother, yielded similar findings (see online supplementary material, Table S3). The magnitude of these effects is especially noteworthy because they were based on having a brother, which are consistent with a growing literature on sibling influences in alcohol-related outcomes. Younger siblings’ alcohol use norms mirror those of their older siblings (Brody et al., 1998), and older siblings’ frequency and quantity of use predicts younger siblings’ use both concurrently and over time (Trim et al., 2006; Van Der Vorst et al., 2007). Older siblings have the strongest influence on younger siblings’ alcohol use when they are close in age (McGue et al., 1996; Trim et al., 2006). These sibling effects might be explained by a number of processes, including modeling or increased access to alcohol. In addition, while younger siblings often misperceive their older siblings’ drinking behaviors, these perceptions guide their own drinking regardless of their accuracy (D'Amico and Fromme, 1997).
Older siblings’ attitudes and behaviors related to alcohol use are most impactful during adolescence and emerging adulthood (Van Der Vorst et al., 2007) when individuals often have their most formative experiences with alcohol. Notably, alcohol use in the current study was reported for the heaviest drinking period, which typically occurs in emerging adulthood (Sher and Gotham, 1999). It is therefore likely that participants' heaviest drinking was close in temporal proximity to their exposure to siblings' alcohol use.
Shared environmental factors have a greater impact on early life drinking experiences than the development and maintenance of problems (Rhee et al., 2003). The magnitude of such environmental effects as sibling attitudes and behaviors, therefore, likely tapers off over time. Indeed, additional analyses yielded findings consistent with this hypothesis: previous year drinking was not elevated in SSFs with a CAO brother (results not shown).
Limitations / Future Directions
There are at least four limitations to attributing behavioral differences to prenatal hormone exposure that must be addressed. First, the effects produced by prenatal hormone exposure may be threshold-dependent (Loehlin and Martin, 2000), with the amount of prenatal androgen exposure occurring in OSFs being insufficient to cause significant behavioral effects on normative alcohol use. Consistent with this possibility, ancillary analyses of the current dataset yielded minimal differences between SSMs and opposite-sex males (OSMs) on alcohol use and AUD symptoms (see online supplementary material, Tables S4 and S5). That is, these null findings suggest that SSMs are not exposed to sufficient prenatal androgens to hypermasculinized their normative or problematic alcohol use.
Second, hormone exposure occurs in two surges—prenatally and during puberty—and remains present throughout the lifespan. It is possible that hormone exposure at one stage of life (e.g., prenatally) influences sensitivity later in life (e.g., priming neural receptors for that hormone; Cohen-Bendahan et al., 2005a). That is, androgen receptors may proliferate to a greater degree while in utero in OSFs (relative to SSFs), causing OSFs to be more sensitive to androgens during pubertal hormone surges. Thus, the current findings leave open the possibility that prenatal androgen exposure indirectly leads to observed effects via postnatal androgen activity.
Third, the current study relies on retrospective reports, which are less readily available for recall and, consequently, may be less accurate. Most drinking trajectories peak in young adulthood and eventually decline (Littlefield et al., 2009), and about 60% of participants in the current study were not currently in the midst of their heaviest drinking period and had to recall their drinking patterns several years prior. However, given the high test-retest reliability, it seems unlikely that recall bias is a significant concern.
Finally, the test of prenatal environment that compared OSFs to SSFs with a CAO brother assumed that the effect of a same-age and CAO brother were similar. It is possible, however, that same-age and CAO brothers have different effects on the alcohol use of their sisters. To investigate this possibility, follow-up analyses compared OSFs and SSFs, both with a CAO brother (see online supplementary material, Tables S2). Consistent with the presence of prenatal effects, OSFs with a CAO brother reported more lifetime AUD symptoms (d = 0.27) and, unexpectedly, more max drinks (d = 0.37), but only marginally greater alcohol use (d = 0.08–0.16). Further research into the effects of sibship composition on alcohol involvement may expand on the sibling effects in the current study and help to develop more appropriate comparison groups for tests of putative prenatal androgenization in OSF twins. Furthermore, comparing twins and singletons may clarify whether the mere presence of a same-age sibling (i.e., co-twin) is associated with alcohol use and AUD symptoms.
Conclusions
This study is the first to investigate the influences of prenatal androgen exposure on alcohol-related outcomes in females. The current findings suggest that prenatal androgen exposure influenced problematic alcohol use, but not alcohol use per se, beyond exposure to postnatal factors associated with having a close-in-age male sibling. Sex differences in alcohol use disorder, but not alcohol use, may be partially due to the neurodevelopmental effects of prenatal androgens.
This study is also one of the first to account for postnatal sibling effects when testing the prenatal hormone-transfer hypothesis among twins. The results of these follow-up analyses suggested that the effect of having a CAO brother was substantially larger than the effect of having a same-age twin brother among female twins. Not only does this imply that the effect of having an older brother is stronger than the effect of having a same-age twin brother, but also that the sibling effect may be larger than any effect of prenatal androgen exposure. The robust sibling effects obtained in the present study reinforces the notion that future alcohol research might benefit from shifting the focus in the family from the parents (e.g., Slutske et al., 2008) to the siblings.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants MH66206 and T32AA13526.
Footnotes
Author Note
Jarrod M. Ellingson, Wendy S. Slutske, and Leah S. Richmond-Rakerd, Department of Psychological Sciences, University of Missouri; Nicholas G. Martin, Genetic Epidemiology Laboratory, Queensland Institute of Medical Research. This work was supported by National Institutes of Health Grants MH66206 and T32AA13526. Thanks to Dixie Statham, Bronwyn Morris, and Megan Fergusson for coordinating the data collection for the twins, and to David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. We thank the Australian Twin Registry twins for their continued participation.
References Cited
- Apter SJ, Eriksson CJ. The effect of alcohol on testosterone concentrations in alcohol-referring and non-referring rat lines. Alcohol Clin Exp Res. 2003;27:1190–1193. doi: 10.1097/01.ALC.0000075832.83254.81. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci. 2009;20:144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Flor DL, Hollett-Wright N, McCoy JK. Children's development of alcohol use norms: Contributions of parent and sibling norms, children's temperaments, and parent–child discussions. J Fam Psychol. 1998;12:209–219. [Google Scholar]
- Clark MM, Malenfant SA, Winter DA, Galef BG. Fetal uterine position affects copulation and scent marking by adult male gerbils. Physiol Behav. 1990;47:301–305. doi: 10.1016/0031-9384(90)90146-u. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, Buitelaar JK, van Goozen SH, Orlebeke JF, Cohen-Kettenis PT. Is there an effect of prenatal testosterone on aggression and other behavioral traits? A study comparing same-sex and opposite-sex twin girls. Horm Behav. 2005a;47:230–237. doi: 10.1016/j.yhbeh.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neurosci Biobehav Rev. 2005b;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychol Bull. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- D'Amico EJ, Fromme K. Health risk behaviors of adolescent and young adult siblings. Health Psychol. 1997;16:426–432. doi: 10.1037//0278-6133.16.5.426. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Morris R. Testosterone, social class, and antisocial behavior in a sample of 4,462 men. Psychol Sci. 1990;1:209–211. [Google Scholar]
- Eriksson CJ, Kaprio J, Pulkkinen L, Rose RJ. Testosterone and alcohol use among adolescent male twins: Testing between-family associations in within-family comparisons. Behav Genet. 2005;35:359–368. doi: 10.1007/s10519-005-3228-x. [DOI] [PubMed] [Google Scholar]
- Even MD, Dhar MG, vom Saal FS. Transport of steroids between fetuses via amniotic fluid in relation to the intrauterine position phenomenon in rats. J Reprod Fertil. 1992;96:709–716. doi: 10.1530/jrf.0.0960709. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual Differentiation of the Brain. Cambridge, MA: MIT Press; 1980. [Google Scholar]
- Kahler CW, Strong DR. A Rasch model analysis of DSM-IV alcohol abuse and dependence items in the National Epidemiological Survey on Alcohol and Related Conditions. Alcohol Clin Exp Res. 2006;30:1165–1175. doi: 10.1111/j.1530-0277.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short-Form (CIDI-SF) Int J Methods Psychiatr Res. 1998;7:171–185. [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- La Grange L, Jones TD, Erb L, Reyes E. Alcohol consumption: Biochemical and personality correlates in a college student population. Addict Behav. 1995;20:93–103. doi: 10.1016/0306-4603(94)00049-5. [DOI] [PubMed] [Google Scholar]
- Lakoza GN, Barkov NK. The role of testosterone in the development of experimental alcoholism. Bull Narc. 1980;32:41–48. [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, Wood PK. Is “maturing out” of problematic alcohol involvement related to personality change? J Abnorm Psychol. 2009;118:360–374. doi: 10.1037/a0015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehlin JC, Martin NG. Dimensions of psychological masculinity-femininity in adult twins from opposite-sex and same-sex pairs. Behav Genet. 2000;30:19–28. doi: 10.1023/a:1002082325784. [DOI] [PubMed] [Google Scholar]
- McGue M, Sharma A, Benson P. Parent and sibling influences on adolescent alcohol use and misuse: Evidence from a U.S. adoption cohort. J Stud Alcohol. 1996;57:8–18. doi: 10.15288/jsa.1996.57.8. [DOI] [PubMed] [Google Scholar]
- Medland SE, Loehlin JC, Martin NG. No effects of prenatal hormone transfer on digit ratio in a large sample of same-and opposite-sex dizygotic twins. Pers Individ Diff. 2008;44:1225–1234. [Google Scholar]
- Miller EM. Prenatal sex hormone transfer: A reason to study opposite-sex twins. Pers Individ Diff. 1994;17:511–529. [Google Scholar]
- Miller EM, Martin NG. Analysis of the effects of hormones on opposite-sex twin attitudes. Acta Geneticae Medicae et Gemellologiae. 1995;44:41–52. doi: 10.1017/s0001566000001884. [DOI] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS. Endocrine control of sexual differentiation: Effects of the maternal-fetal environment and endocrine disrupting chemicals. Advances in Molecular and Cell Biology. 2004;34:15–37. [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Gottesman II, McGue M. Sensation seeking in opposite-sex twins: An effect of prenatal hormones? Behav Genet. 1993;23:323–329. doi: 10.1007/BF01067432. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, Sartorius N, Towle LH. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Kaprio J, Winter T, Dick DM, Viken RJ, Pulkkinen L, Koskenvuo M. Femininity and Fertility in sisters with twin brothers: Prenatal androgenization? Cross-sex socialization? Psychol Sci. 2002;13:263–267. doi: 10.1111/1467-9280.00448. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ. Pathological alcohol involvement: A developmental disorder of young adulthood. Dev Psychopathol. 1999;11:933–956. doi: 10.1017/s0954579499002394. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Bascom EN, Meier MH, Medland SE, Martin NG. Sensation seeking in females from opposite- versus same-sex twin pairs: Hormone transfer or sibling imitation? Behav Genet. 2011;41:533–542. doi: 10.1007/s10519-010-9416-3. [DOI] [PubMed] [Google Scholar]
- Slutske WS, D'Onofrio BM, Turkheimer E, Emery RE, Harden KP, Heath AC, Martin NG. Searching for an environmental effect of parental alcoholism on offspring alcohol use disorder: A genetically informed study of children of alcoholics. J Abnorm Psychol. 2008;117:534–551. doi: 10.1037/a0012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PAF, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002;111:124–124. [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian Twin Study of Gambling (OZ-GAM): Rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Res Hum Genet. 2009;12:63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Stålenheim EG, Eriksson E, von Knorring L, Wide L. Testosterone as a biological marker in psychopathy and alcoholism. Psychiatry Res. 1998;77:79–88. doi: 10.1016/s0165-1781(97)00143-1. [DOI] [PubMed] [Google Scholar]
- Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: A review of the empirical evidence. Horm Behav. 2011;60:713–722. doi: 10.1016/j.yhbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Trim RS, Leuthe E, Chassin L. Sibling influence on alcohol use in a young adult, high-risk sample. J Stud Alcohol Drugs. 2006;67:391–398. doi: 10.15288/jsa.2006.67.391. [DOI] [PubMed] [Google Scholar]
- Udry JR, Talbert LM. Sex hormone effects on personality at puberty. J Pers Soc Psychol. 1988;54:291–295. doi: 10.1037//0022-3514.54.2.291. [DOI] [PubMed] [Google Scholar]
- Van Der Vorst H, Engels RCME, Meeus W, Dekovic M, Van Leeuwe J. Similarities and bi-directional influences regarding alcohol consumption in adolescent sibling pairs. Addict Behav. 2007;32:1814–1825. doi: 10.1016/j.addbeh.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- vom Saal FS. Sexual differentiation in litter-bearing mammals: Influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- Voracek M, Dressler SG. Digit ratio (2D:4D) in twins: Heritability estimates and evidence for a masculinized trait expression in women from opposite-sex pairs. Psychol Rep. 2007;100:115–126. doi: 10.2466/pr0.100.1.115-126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


