Abstract
This study examines the effect of TNFα on whole bovine intervertebral discs in organ culture and its association with changes characteristic of intervertebral disc degeneration (IDD) in order to inform future treatments to mitigate the chronic inflammatory state commonly found with painful IDD. Pro-inflammatory cytokines such as TNFα contribute to disc pathology and are implicated in the catabolic phenotype associated with painful IDD. Whole bovine discs were cultured to examine cellular (anabolic/catabolic gene expression, cell viability and senescence using β-galactosidase) and structural (histology and aggrecan degradation) changes in response to TNFα treatment. Control or TNFα cultures were assessed at 7 and 21 days; the 21 day group also included a Recovery group with 7 days TNFα followed by 14 days in basal media. TNFα induced catabolic and anti-anabolic shifts in the nucleus pulposus (NP) and annulus fibrosus (AF) at 7 days and this persisted until 21 days however cell viability was not affected. Data indicates that TNFα increased aggrecan degradation products and suggests increased β-galactosidase staining at 21 days without any recovery. TNFα treatment of whole bovine discs for 7 days induced changes similar to the degeneration processes that occur in human IDD: aggrecan degradation, increased catabolism, pro-inflammatory cytokines and nerve growth factor expression. TNFα significantly reduced anabolism in cultured IVDs and a possible mechanism may be associated with cell senescence. Results therefore suggest that successful treatments must promote anabolism and cell proliferation in addition to limiting inflammation.
Keywords: TNFα, Catabolism, Aggrecan degradation, Intervertebral disc degeneration (IDD)
INTRODUCTION
Pro-inflammatory cytokine expression increases with age and severity of intervertebral disc (IVD) degeneration (IDD) [1]. Pro-inflammatory cytokines can cause structural deterioration via increased catabolism in IVD cells and influence pain-related factors with up-regulation of substance P, NGF and VEGF [2,3,4], highlighting a role for inflammation in the pathogenesis of painful human IDD. While pro-inflammatory mediators appear to be involved in this pathology it remains unclear if a single inflammatory insult, for example resulting from a single injury, is sufficient to initiate an inflammatory cascade in a healthy IVD. It is also unclear whether pro-inflammatory mediators influence other biologic characteristics associated with IDD such as cellular senescence.
The pro-inflammatory cytokines principally associated with the progression of IDD are tumor necrosis factor-α (TNFα) and interleukin 1-β (IL-lβ) although others have also been implicated [2,4,5,6,7,8]. TNFα is expressed by IVD cells and has been suggested to be involved in the early onset of IDD through initiating an inflammatory cascade [9] although it remains unclear if a healthy IVD can recover following exposure to TNFα.
Along with the increases in TNFα expression that correlate with degenerative grade, a parallel increase in cellular senescence has been observed with the IDD [10,11,12]. In cartilage a relationship between pro-inflammatory cytokines and senescence has also been demonstrated where IL-1β was shown to induce premature senescence in osteoarthritic chondrocytes [13]. This suggests that increases in TNFα may also be related to the cellular senescence observed in IVD degeneration.
The aim of this study is to investigate a potential role of TNFα in the processes associated with degeneration of the IVD. We hypothesized that healthy bovine IVDs can recover from TNFα exposure and that TNFα exposure would cause a significant catabolic shift and increase the amount of senescent cells observed in the IVD. Bovine IVD organ culture models are widely utilized large animal models with similar size and composition as human IVDs, and these models allow multiple measurements of matrix accumulation and breakdown while maintaining in-situ cell matrix connectivity [14,15,16]. IVD organ culture also enables development of cytokine-matrix interactions that are known to strongly influence cellular responses in bone and cartilage tissues [17,18]. Organ culture models have substantial control over boundary conditions enabling investigation of the isolated effects of an individual cytokine in the IVD independent of systemic immune responses. Bovine caudal IVD explant cultures were used to examine responses at the cellular and structural levels at various time points with emphasis on cell viability, cellular senescence, gene expression for matrix structural proteins, enzymes, cytokines and symptom-modifying factors, along with assessment of matrix degradation through histology and western blot.
METHODS AND MATERIALS
Caudal IVDs were harvested from skeletally mature bovine tails obtained from a local abattoir (Green Village Packing Co., NJ) and endplates were removed to promote maximal transport and cell viability. After isolation, IVD height, diameter, and weight were recorded. Isolated IVDs were assigned to Control (cultured in control media consisting of high glucose DMEM, 10% FBS, 50ug/mL ascorbic acid, 1% penicillin/streptomycin and 0.5% fungizone) or TNFα (control media + 200ng/mL human recombinant TNFα; Invitrogen Cat #PHC3016) groups and cultured for 7 days (N=7/group). Additional cultures for 21 days (N=9/group) consisted of 3 groups; a Control, TNFα and a Recovery group which involved 7 days with TNFα exposure followed by 14 days of recovery in control media. All IVDs were cultured at 37°C & 5% CO2, and loaded under 0.2 MPa static compression. Media was continuously circulated and changed and collected every 3-4 days. Following culture, IVD height, diameter and wet weight were recorded. IVD tissue was divided into 4 sagittal sections (~4mm wide) using a custom dissection tool, and each section was used to assess tissue viability, qRT-PCR, histology, and Western blot analysis of aggrecan degradation respectively.
Tissue viability was assessed at 7 and 21 days via a double staining technique using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO) to stain viable cells, and 4′,6-diamidino-2-phenylindole (DAPI, Roche Diagnostics, Germany) to stain cell nuclei, with processing and quantification as previously described [16].
Real-time qRT-PCR was performed on 7 days and 21 days specimens. Tissue from the AF and NP regions were separated from the inner AF which was discarded and RNA extracted using the Qiagen RNEasy Kit (Qiagen, USA). Gene expression of housekeeping (18S), anabolic (Aggrecan, Collagen I, and Collagen II), catabolic (MMP-3, MMP-13, and ADAMTS-5) and pro-inflammatory genes (TNFα, IL-1β, IL-6) were assessed using SYBR green bovine specific primers [16]. Gene expression for angiogenic/pain-related genes (VEGF (Bt03213283_m1), NGF (Bt03817604_s1), Substance-P (Bt03259156_m1)) and 18S (Hs03928985_g1) were also assessed via TAQMAN assays on demand (Applied Biosystems, Bedford MA). Gene expression levels were analyzed using the ΔΔCT method and normalized to 18S and time-matched controls, as described [19].
IVD structure and composition were evaluated with Western blot, water content and histology. Western blot used an antibody specific to the G1 region of aggrecan to assess aggrecan degradation in AF and NP regions [20]. GAG loss to the culture media was analyzed using the dimethylmethylene blue (DMB) dye binding assay, and normalized to initial IVD wet weight [21]. AF and NP water content were calculated from wet and dry weights. Sagittal histology sections 10μm thick were stained with picosirius red and alcian blue [16] as a further measure of IVD structural integrity and composition. Histological sections were scored by 3 blinded reviewers using a semi-quantitative histological grading scheme modified from that described by Sive et al. [22] (Supplemental Figure A). Briefly the grading system assessed three histological characteristics; loss of demarcation between NP and AF (0-3), loss of proteoglycans from the NP (0-3) and the presence & extent of fissures (0-3), with a cumulative score range from 0 (healthy) to 9 (degenerated).
Histology samples from the 21 day timepoint were also processed for senescence associated β-galactosidase (SA-β-gal) immunohistochemistry (N=3/group) which is a common technique for evaluating senescence in the IVD [10]. Sections (10μm) were deparaffinized, a primary anti-rabbit β-galactosidase antibody (ab4761, Abcam, Cambridge, MA) and a goat anti-rabbit HRP polymer secondary antibody (ab94710, Abcam Cambridge, MA) were used with omission of primary antibody as a negative control. Samples were then counter stained with Toluidine Blue. Twenty images representative of the entire IVD were captured from the each disc at 40x magnification. The percent of positive β-galactosidase stained cells were calculated in each IVD.
Student’s t-test compared the ΔΔCT and water content values for the 7 day results (Control vs. TNFα). One-way ANOVA with a Tukey post hoc test compared the ΔΔCT’s, water content, viability, β-galactosidase quantification and GAG present in the media (DMB) for the 21 day results (Control, TNFα, and Recovery). One-way ANOVA with a Tukey post hoc test compared the semi-quantitative histological grading between 4 Groups (Control, 7 day TNFα, 21 day TNFα, Recovery). To assess whether exposure to TNFα increased β-galactosidase staining a one-tailed t-test compared Control to pooled TNFα groups (21 day TNFα + Recovery) was used. Statistical analyses were performed using GraphPad Prism 3 (La Jolla, Ca) with p<0.05 significant. Variance is given as standard deviations (SD) throughout.
RESULTS
All cultures remained viable throughout culture period with no differences between groups in all regions (AF, IAF, NP) and time points (Supplemental Figure B). Viability in AF was 80.3±11.4%, 78.2±11.3%, 80.1±8.9% for control, 21 day TNFα, and Recovery groups, respectively. The viability in IAF was 75.6±19.1%, 80.2±10.4%, 77.4±13.2% for Control, 21 day TNFα, and Recovery groups, respectively and in the NP was 61.9±20.4%, 81.8±10.6%, 82.0±12.4% for Control, 21 day TNFα, and Recovery groups, respectively.
In the AF, 7 days of TNFα exposure significantly down-regulated gene expression of aggrecan (−5.7 fold), and significantly up-regulated MMP-3 (3.9 fold), MMP-13 (3.1 fold), IL-6 (2.9 fold), and NGF (3.2 fold) (Figure 1). After 21 days of TNFα exposure AF gene expression was significantly up-regulated for MMP-3 (10.8 fold) only, and this was not up-regulated for the recovery group. In the NP, 7 days of TNFα exposure significantly down-regulated aggrecan (−18.3 fold), collagen I (−23.1 fold), collagen II (−15.7 fold), and MMP-3 (−14.7 fold) and significantly up-regulated IL-1β (45.0 fold), and IL-6 (56.4 fold). After 21 days, NP gene expression was significantly down-regulated in TNFα and Recovery groups for collagen I (−15.8 fold, −69.4 fold), collagen II (−17.3 fold, −23.5 fold), and ADAMTS-5 (−14.2 fold, −67.3 fold), respectively, while MMP-3 (−35.5 fold) was significantly down-regulated only in the Recovery group. TNFα mRNA was not consistently expressed in any samples.
Figure 1.
Gene expression results from the annulus fibrosus (AF) and nucleus pulposus (NP) normalized to 18S and time-matched controls, after (A) 7 days and (B) 21 days in culture. In general, there was a large catabolic shift with down-regulation of anabolic gene expression and up-regulation of some catabolic genes and pro-inflammatory cytokines for TNFα and Recovery groups in both AF and NP regions and for both time points. There were no differences between TNFα and Recovery groups. Significant differences from the value 1 are noted with * and bars represent differences between groups, p<0.05.
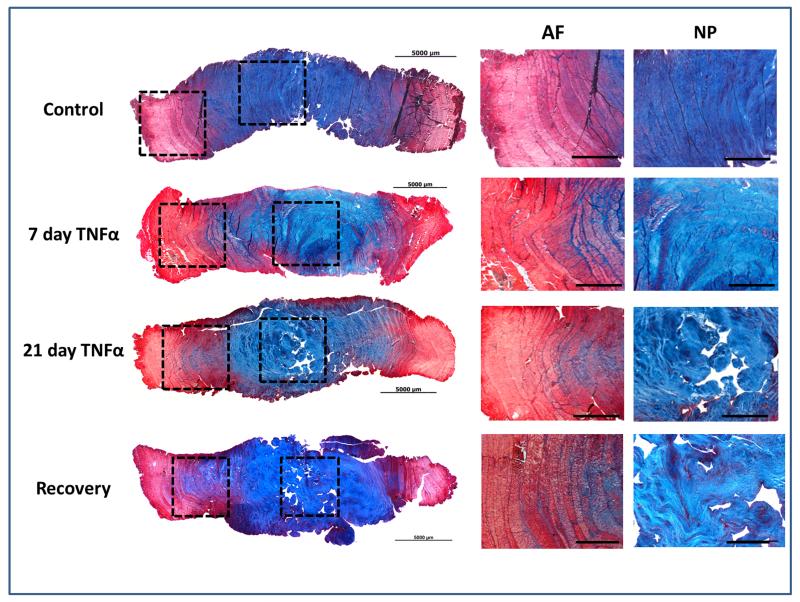
TNFα induced substantial matrix breakdown. Histological staining demonstrated a reduction in the intensity of alcian blue staining in the NP of TNFα treated samples at 7 and 21 days, suggesting a loss/degradation of proteoglycans relative to Control (Figure 2). Semi-quantitative analysis demonstrated a significant increase in degenerative grade in all groups treated with TNFα compared to control (Supplemental Figure C). The histological score was significantly worsened for all TNFα groups with values of 1.4±0.5, 5.8±0.8 (p<0.01), 4.1±2 (p<0.05), and 5.2±.7 (p<0.01) for Control, 7 day TNFα, 21 days TNFα, and Recovery groups, respectively. Western blot analysis confirmed an increase in degraded aggrecan products in the NP of TNFα treated IVDs at both 7 and 21 days (Figure 3A) and a trend of increased GAG in the culture medium was observed at 21 days (ANOVA, p=0.073) with values of 33.2±5.7, 43.4±17.8, and 52.2±22.0 μg GAG/(mg wet weight) for Control, 21 days TNFα, and Recovery groups, respectively (Figure 3B). Interestingly, at 21 days no differences were observed in the aggrecan degradation products between the 21 day TNFα and Recovery groups. There were no significant changes in water content or disc height between any samples at any time point.
Figure 2.
Structural histology for day 7 and day 21 experimental groups; no differences were observed between 7 and 21 day Control groups. Tissue stained with Picosiruis red\Alcian blue. All images were taken at 2.5x and scale bars represents 1000um. Notable morphological changes in TNFa and Recovery groups included loss of proteoglycan staining in the nucleus pulposus, loss of demarcation between AF & NP, and AF encroachment into the NP region, particularly for the 21 day groups.
Figure 3.
A Western blot for the aggrecan G1 domain in the NP and AF at 7 days and 21 days. There was substantially more degraded aggrecan in the NP of TNFα and Recovery groups at day 7 and 21 with minimal differences in degraded aggrecan observed in the AF region. B Total GAG loss to cell culture media at 21 days with μg GAG normalized to mg tissue wet weight.
There was an increased trend (p=0.108) of positively SA-β-gal stained cells in pooled TNFα treated IVDs (44.1±29.4%) compared to Control (19.0±15.3%) (Figure 4A & Figure 4B). A similar observation was made between Control (19.0±15.3%), 21 day TNFα (33.8±30.0%) and Recovery groups (54.5±30.6%) with p=0.32.
Figure 4.
A Immunohistochemistry for senescence associated β-galactosidase (SA-β-gal), demonstrating an increase in staining in the 3 week TNFα and Recovery groups relative to Control. Pictures were taken at 40x and scale bar represents 50μm. Annulus fibrosus (AF) and nucleus pulposus (NP). B The percentage of SA-β-gal stained cells in 21 Day TNFα and Recovery groups compared to Control.
DISCUSSION
This study assessed the role of TNFα in contributing to pathologic processes associated with IDD using a large animal IVD organ culture system. TNFα treatment for 7 days induced many of the structural and compositional degenerative changes known to be important in human IDD including large catabolic and anti-anabolic shifts on the gene level, up-regulation of genes predictive of painful conditions, rapid accumulation of degraded aggrecan fragments and structural alterations including AF encroachment into the NP. These degradation patterns were also observed following culture with TNFα for 21 days. However when TNFα was present for the first 7 days of culture and then removed for the last 14 days, there was no apparent recovery suggesting that IVDs cannot recover from TNFα exposure. Interestingly, TNFα was not a source of lost IVD cellularity but suggested an increase in the amount senescent cells which may provide a rationale for the lack of recovery and highlights a potential role for TNFα in the senescence associated with IDD. TNFα exposure also stimulated mRNA production of IL-1β, and IL-6 supporting and strengthening its role as an initiator and regulator of multiple pro-inflammatory cytokines in intact IVDs.
TNFα exposure induced a dramatic catabolic shift with proteoglycan degradation at 7 and 21 days that was not reversed following TNFα removal. Western blot indicated aggrecan degradation was primarily due to ADAMTS activity (2 bands between 60-80 kDa MW) and not MMP activity (~50 kDa MW) as suggested by the size of the degraded fragments. TNFα up-regulates ADAMTS-5 expression in NP cells through modulation of syndecan-4 via the NF-κB pathway [8]. This pathway is likely involved our model and is supported by our finding of degradation resulting primarily from ADAMTS and not MMP activity. Another potential mechanism that could be involved in the catabolic shift observed in this model is through TNFα inducing PHD3 expression that promotes NF-kB mediated catabolism [23].
Comparing the protein changes with gene expression in the NP suggests the majority of the aggrecan degradation may have occurred early in the culture period since there was already substantial aggrecan degradation and a decrease in ADAMTS-5 gene expression in the NP by day 7. The different responses in catabolic gene expression between the AF and NP observed at both 7 and 21 days were notable and may be explained by differences in the kinetic response between NP and AF cells based on their heterogeneity, distinct cell lineages and diverse tissue functions. However, the large amounts of aggrecan degradation in the NP suggest these regional differences are not related to insufficient dosage. While it is possible that ADAMTS-4 or other aggrecanases were expressed and activated, we focused on ADAMTS-5 in this study because it has more potent degradative potential [8,24].
TNFα exposure led to an increased trend in SA-β-gal staining which may have contributed to the continued IVD degradation and inability of the IVD to recover following removal of TNFα. Increases in cellular senescence have been observed in degenerated IVDs and micro-environmental stress-induced cellular senescence may be a potential mechanism [11,25,26]. The literature suggests possible upstream signaling pathways that could lead to pro-inflammatory cytokine induced senescence. TNFα treatment of NP cells increases Wnt/β-catenin signaling [24] and activity of this signaling pathway has also been shown to enhance cellular senescence in IVD cells [27]. Also, treatment of osteoarthritic chondrocytes with IL-1β induced pre-mature senescence mediated by caveolin-1, and IDD is known to be associated with increased caveolin-1 expression, pro-inflammatory cytokine expression and cellular senescence [10,11,26]. Our findings together with the literature suggest a possible mechanism by which excessive pro-inflammatory cytokine may increase cellular senescence in the IVD.
The dramatic catabolic shift in matrix synthesis at both the gene and protein levels induced by TNFα is likely also to be influenced by increases in other pro-inflammatory cytokines, and we measured significant increases in IL-1β and IL-6 gene expression, particularly at 7 days. These results support the hypothesis that TNFα can initiate matrix breakdown and an inflammatory cascade [9]. Cell culture studies have shown TNFα exposure can increase IL-1β mRNA [4,6,9] and in-situ zymography demonstrated IL-1β has a greater ability to increase catabolic enzyme activity than TNFα [28]. Results also reinforce the concept that pro-inflammatory cytokines likely play a role in painful IDD since we observed significantly increased NGF mRNA in the AF at 7 days. The literature further demonstrates that IL-1β and IL-6 can up-regulate nerve promoting factors such as NGF and BDNF and are correlated with painful disc degeneration [3,5,29].
The dose of TNFα used in this study was chosen to induce rapid catabolic changes in the IVD, and to mimic those changes observed following decades of pro-inflammatory cytokine exposure that occurs during human degeneration. Cytokines such as a TNFα are known to interact and bind proteoglycans in the matrix [30] thereby decreasing the ‘effective concentration’ that cells experience. Concentrations of TNFα of 100ng/mL have also been used in IVD and cartilage explant culture studies [8,31,32,33,34]. Because of the large size and relatively low cellularity of bovine IVDs, a pilot study of 100, 200 and 400ng was conducted and we chose the dose of 200ng since it consistently induced catabolism whilst maintaining cell viability. A recent study by Ponnappan et al. also explored the role of pro-inflammatory cytokines in disc degeneration using a cocktail of 100ng/mL TNFα & 10ng/mL IL-1β in a rat IVD organ culture model over 10 days [34]. They observed a similar suppression of collagen II and aggrecan mRNA providing confirmation that similar effects occur in small and large animal organ culture systems.
In conclusion, TNFα exposure in a bovine organ culture model induced several changes characteristic of the human IDD including a dramatic increase in degraded aggrecan, an anti-anabolic phenotype, up-regulation of catabolic and pain related genes and changes suggestive of cellular senescence. We demonstrated for the first time that TNFα treatment causes an unrecoverable shift in catabolic and anti-anabolic gene expression and matrix protein degradation. Our data suggested that this lack of recovery may be correlated with increased cell senescence induced by TNFα exposure. This lack of recovery following removal of TNFα therefore supports the concept that future therapeutic interventions are required to stimulate anabolic metabolism and cell proliferation in the IVD following TNFα exposure since long-term catabolic effects can persist long after the cytokine is cleared. This organ culture model system which maintains cell and matrix connectivity can be used in future studies to explore how anti-inflammatory and pro-anabolic interventions can be optimized for large animal IVDs in chronic pro-inflammatory states.
Supplementary Material
HIGHLIGHTS.
TNFα induced catabolic changes similar to human intervertebral disc degeneration
The metabolic shift induced by TNFα was sustained following removal
TNFα induced changes suggestive of cell senescence without affecting cell viability
Interventions are required to stimulate anabolism & increase cell proliferation
ACKNOWLEDGEMENTS
The authors gratefully acknowledge technical assistance of Ilana Stock. This study was funded by NIAMS/NIH grants R01AR051146 and R01AR057397.
ABBREVIATIONS
- IDD
Intervertebral disc degeneration
- TNFα
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the non-degenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- [5].Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- [6].Seguin CA, Bojarski M, Pilliar RM, Roughley PJ, Kandel RA. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25:409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [7].Gabr MA, Jing L, Helbling AR, Sinclair SM, Allen KD, Shamji MF, Richardson WJ, Fitch RD, Setton LA, Chen J. Interleukin-17 synergizes with IFNgamma or TNFalpha to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J Orthop Res. 2011;29:1–7. doi: 10.1002/jor.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Millward-Sadler SJ, Costello PW, Freemont AJ, Hoyland JA. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11:R65. doi: 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gruber HE, Ingram JA, Norton HJ, Hanley EN., Jr. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- [11].Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J. 2006;15(Suppl 3):S312–316. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K, Yudoh K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- [14].Korecki CL, MacLean JJ, Iatridis JC. Characterization of an in vitro intervertebral disc organ culture system. Eur Spine J. 2007;16:1029–1037. doi: 10.1007/s00586-007-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Walter BA, Korecki CL, Purmessur D, Roughley PJ, Michalek AJ, Iatridis JC. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage. 2011;19:1011–1018. doi: 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baker SM, Sugars RV, Wendel M, Smith AJ, Waddington RJ, Cooper PR, Sloan AJ. TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif Tissue Int. 2009;85:66–74. doi: 10.1007/s00223-009-9248-4. [DOI] [PubMed] [Google Scholar]
- [18].Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15:1334–1348. doi: 10.2174/138161209787846739. [DOI] [PubMed] [Google Scholar]
- [19].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [20].Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326(Pt 1):235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- [22].Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fujita N, Gogate SS, Chiba K, Toyama Y, Shapiro IM, Risbud MV. Prolyl Hydroxylase 3 (PHD3) Modulates Catabolic Effects of Tumor Necrosis Factor-alpha (TNF-alpha) on Cells of the Nucleus Pulposus through Co-activation of Nuclear Factor kappaB (NF-kappaB)/p65 Signaling. J Biol Chem. 2012;287:39942–39953. doi: 10.1074/jbc.M112.375964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Junger S, Gantenbein-Ritter B, Lezuo P, Alini M, Ferguson SJ, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009;34:1264–1271. doi: 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]
- [25].Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [26].Heathfield SK, Le Maitre CL, Hoyland JA. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res Ther. 2008;10:R87. doi: 10.1186/ar2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- [29].Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- [30].Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs) J Cell Commun Signal. 2009;3:323–335. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stevens AL, Wheeler CA, Tannenbaum SR, Grodzinsky AJ. Nitric oxide enhances aggrecan degradation by aggrecanase in response to TNF-alpha but not IL-1beta treatment at a post-transcriptional level in bovine cartilage explants. Osteoarthritis Cartilage. 2008;16:489–497. doi: 10.1016/j.joca.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vuolteenaho K, Moilanen T, Hamalainen M, Moilanen E. Effects of TNFalpha-antagonists on nitric oxide production in human cartilage. Osteoarthritis Cartilage. 2002;10:327–332. doi: 10.1053/joca.2002.0521. [DOI] [PubMed] [Google Scholar]
- [33].Stevens AL, Wishnok JS, White FM, Grodzinsky AJ, Tannenbaum SR. Mechanical injury and cytokines cause loss of cartilage integrity and upregulate proteins associated with catabolism, immunity, inflammation, and repair. Mol Cell Proteomics. 2009;8:1475–1489. doi: 10.1074/mcp.M800181-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ponnappan RK, Markova DZ, Antonio PJ, Murray HB, Vaccaro AR, Shapiro IM, Anderson DG, Albert TJ, Risbud MV. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Res Ther. 2011;13:R171. doi: 10.1186/ar3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.