Abstract
Multidrug resistance-associated protein 2 (MRP2; ABCC2) mediates the biliary excretion of glutathione, glucuronide and sulfate conjugates of endo- and xenobiotics. Single nucleotide polymorphisms (SNPs) of MRP2 contribute to interindividual variability in drug disposition and ultimately in drug response. The transport function of human MRP2 (WT) and four SNP variants, S789F, A1450T, V417I and T1477M, was characterized following their expression in Sf9 cells using recombinant baculovirus infection. The kinetic parameters [Km, (µM); Vmax, (pmol·mg−1·min−1); Hill coefficient (HC)] of ATP-dependent transport of leukotriene C4 (LTC4), estradiol-3-glucuronide (E23G), estradiol-17β-glucuronide (E217G) and tauroursodeoxycholic acid (TUDC) were determined in Sf9-derived plasma membrane vesicles. Transport activity, normalized for expression level, was decreased for all substrates for S789F and A1450T, except for unchanged E217G transport by A1450T. V417I showed decreased apparent affinity for LTC4, E23G and E217G, while transport was similar between WT and T1477M, except for a modest increase in TUDC transport. Examination of substrate-stimulated MRP2-dependent ATPase activity of S789F and A1450T, SNPs located in MRP2 nucleotide binding domains (NBDs), demonstrated significantly decreased ATPase activity and only modestly decreased affinity for ATP compared to WT. In conclusion, SNPs in the NBDs (S789F in the D loop of NBD1, or A1450T near the ABC signature motif of NBD2) variably decreased the transport of all substrates. V417I in membrane spanning domain 1 selectively decreased the apparent affinity for the glutathione and glucuronide conjugated substrates, while the T1477M SNP in the carboxyl terminus altered only TUDC transport.
Keywords: MRP2/ABCC2, ABC transporter, biliary excretion, renal excretion, oral absorption
INTRODUCTION
MRP2 (ABCC2) is a 190-kDa multidrug resistance protein that belongs to subfamily C of the ABC transporter superfamily [1, 2]. It is composed of 17 transmembrane helices forming three membrane-spanning domains (MSD0, MSD1 and MSD2) and two highly conserved nucleotide binding domains (NBD1 and NBD2). MSD0 and the adjacent linker region are essential for apical targeting of the protein [3]. MSD1 and MSD2 are important in substrate recognition and specificity, while the NBDs catalyze ATP hydrolysis, the energy from which drives the efflux of substrates. MRP2 is expressed in the canalicular membrane of the hepatocyte, the apical luminal membrane of epithelial cells of the small intestine and kidney, in endothelial cells of the blood-brain barrier and in the apical syncytiotrophoblast membrane of the placenta. MRP2 is a major efflux transporter of various endo- and exogenous organic anionic substrates such as glucuronide, glutathione and sulfate conjugates. It also efficiently transports unconjugated drugs, such as statins, angiotensin II receptor antagonists and methotrexate. MRP2 substrates also include carcinogens, anticancer drugs, immunosuppression drugs, antibiotics and toxins, resulting in their efflux and contributing to their detoxification [4–6].
Inter-individual variability of drug response is a widely recognized determinant of drug toxicities, especially for those drugs with narrow therapeutic windows. Genetic polymorphisms are a major cause of such variability, often resulting in altered pharmacokinetics and subsequent pharmacological and toxicological effects of drugs [7]. We now know that in addition to well-studied metabolic pathways, such as those of the cytochrome P450 enzymes, single nucleotide polymorphisms (SNPs) in membrane transporters can play a key role in variable drug responsiveness [8–11]. Owing to its expression in major clearance organs, i.e., liver and kidney, and its broad substrate specificity that includes drugs and toxicants, MRP2 polymorphisms can critically affect the pharmacological response and toxicity of its substrate drugs. Early studies identified SNPs in the MSDs and NBDs of MRP2 that caused a rare autosomal recessive disorder, termed the Dubin-Johnson syndrome in humans, characterized by mild conjugated hyperbilirubinemia and deposition of dark pigment in liver. These variants resulted in rapid degradation of mRNA, impaired protein maturation or inappropriate protein trafficking, leading to non-functional MRP2 [12–14]. A number of less damaging SNPs have been described more recently. The MRP2 SNP 3972C>T is reported to be associated with a higher risk of developing intrahepatic cholestasis of pregnancy, which can cause increased fetal risks [15]. Several studies have reported a strong association between MRP2 SNPs and altered disposition of substrate drugs. The −24C>T SNP in the 5-untranslated region of MRP2 has been associated with altered pharmacokinetics for various drugs, including methotrexate [16], irinotecan and its active metabolite SN-38 [17, 18] and mycophenolic acid [19]. The variant 1446C>G is associated with decreased exposure to pravastatin, possibly due to increased MRP2 expression that could be in linkage with other polymorphisms [20]. The 1271A>G SNP, located in MSD1 of MRP2, altered methotrexate transport and thus impaired its elimination, resulting in renal toxicity [21]. These studies document the importance and need for more detailed analysis of MRP2 SNPs. However, to date, there is no comprehensive study of the functional relevance of SNPs located in various regions of MRP2 protein.
In this study, we systematically characterized the effects of four nonsynonymous polymorphisms on MRP2 transport function. The SNPs were selected based on their location in different regions of human MRP2, i.e., MSD1, NBD1, NBD2 and the carboxy terminal region, as shown in Table 1. Because the effect of SNPs on transport activity could depend on the substrate tested, we compared the transport kinetics for each of four substrates between WT MRP2 and each of the four SNPs using vesicular uptake studies in Sf9 plasma membranes. We further examined the effect of SNPs in the NBDs on substrate-stimulated ATPase activity and the Km for ATP in Sf9 plasma membranes. An understanding of the influence of SNPs on intrinsic transport function of MRP2 would significantly add to our knowledge of structural features of MRP2 that are critical for differential substrate binding and transport. Our results indicated that the SNPs altered MRP2 protein expression and significantly affected its function in a substrate-specific manner.
Table 1.
Nucleotide sequence of variants in MRP2 leading to amino acid changes in MRP2 protein and their allelic frequency1
| SNP Position |
Amino acid change |
Exon | Location | Allelic frequency | Functional Consequences2 |
|---|---|---|---|---|---|
| C2366T | S789F | 18 | NBD1 (D loop) | 0.01 (Japanese) | 52 |
| G4348A | A1450T | 31 | NBD2 (immediately after the ABC signature motif) | 0.01 (Japanese) | 52 |
| G1249A | V417I | 10 | MSD1 (between transmembrane helices 7 and 8) | 0.125/0.312/0.184 (Japanese/Iranian/ Moroccan) | 27,46,47,52,58,59 |
| C4430T | T1477M | 31 | Carboxy terminal | 0.006 (Japanese) |
Allelic frequency taken from [5].
References in text.
Methods
Materials
[3H]E217G (40–45 Ci/mmol), [3H] E23G (53–57 Ci/mmol), and [3H] LTC4 (190 Ci/mmol) were obtained from PerkinElmer Life and Analytical Sciences (Boston, MA), and [3H] TUDC (9 Ci/mmol) was obtained from Dr. Alan F. Hofmann (University of California, San Diego). All radiolabels were used at radiochemical purity ≥97%; purity of 3H-TUDC (99%) was verified by high performance liquid chromatography [22]. Unlabeled estrogen conjugates, TUDC, LTC4, ATP, AMP, creatine phosphate, and creatine phosphokinase were obtained from Sigma-Aldrich (St. Louis, MO) or Calbiochem (La Jolla, CA). All restriction enzymes were obtained from Invitrogen (Carlsbad, CA). Sf9 insect cells were obtained from Invitrogen (Cat. No. 11496-015) and were adapted to serum-free suspension culture in Sf-900 II SFM (Invitrogen, Cat. No.10902-104). All other chemicals used were commercially available and of analytical grade.
Construction of recombinant baculovirus containing MRP2
The plasmid (pEF6/V5-His-TOPO; Invitrogen) containing the wild type MRP2 (WT) (NM_000392) or its SNP variants S789F, A1450T, V417I, and T1477M, was used to create the MRP2 baculovirus expression vector. The pENTR™4 vector (Invitrogen) was mutated to generate a Hind III site using Quik Change II site-directed Mutagenesis Kit (Stratagene; La Jolla, CA) using primers Hind IIIF and Hind IIIR (Hind IIIF: AGGCTCCACCATGGGAAGCTTCAGTCGACTGGATC; Hind IIIR: GATCCAGTCGACTGAAGCTTCCCATGGTGGAGCCT). The mutated pENTR™4 was named pENTR™4M. The plasmid pEF6/V5-His-TOP containing MRP2 and MRP2 SNP variants was digested with HindIII and NotI to release the 4.7 kb fragment of MRP2 or its SNP variants. The 4.7 Kb fragment was gel purified with Gel Purification kit according to the manufacturer’s instructions (Qiagen; Valencia, CA). The purified fragment was inserted into the corresponding sites of the pENTR™4M vector. The pENTR4M containing the WT, S789F, A1450T, V417I, and T1477M SNPs were sequenced (MWG Biotech, Inc., Huntsville, AL). Recombinant baculovirus expressing the variant forms of MRP2 were generated with the Baculodirect expression system (Invitrogen) according to the manufacturer’s instructions. The recombinant baculovirus preparations were amplified and transfected using Cell-Fectin (Invitrogen) into Sf9 insect cells. The supernatant containing the recombinant baculovirus was harvested, amplified, and titered by a viral plaque assay. The titered virus was stored at 4°C for short durations (< 3 mos) and at −80°C for long term storage (> 6 mos) and titered again before use.
Baculovirus infection of Sf9 insect cells and preparation of the plasma membrane vesicles
Recombinant baculovirus infection conditions in Sf9 cells were optimized with respect to MRP2 protein expression and transport activity. Sf9 cells (1 × 106 cells/ml) were infected with the recombinant baculovirus in the presence of 5% fetal bovine serum using a multiplicity of infection (MOI) of six and cultured in Sf-900 II SFM (Invitrogen Cat No.10902-104). Two days after the infection, cells were harvested by centrifugation. Plasma membrane vesicles were isolated by layering on 38% sucrose and collecting the layer at the buffer-sucrose interface [23]. Membranes were vesiculated, snap frozen in liquid nitrogen, and stored at −80°C. Protein concentrations were determined by a modification of the method described using bovine serum albumin as a standard [24].
SDS-PAGE, immunoblotting and quantification of MRP2 in plasma membranes
Expression of human MRP2 and its variants was determined by immunoblot as described [25] using 2 µg of sucrose-fractionated membrane protein. Proteins were pretreated with mercaptoethanol before loading onto an 8–12% Tris/glycine polyacrylamide Novex precast gel (Invitrogen), separated by standard electrophoresis, and transferred onto Polynitran nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Membranes were blocked using 5% nonfat milk at room temperature for 1 h. Immunoblotting was performed by using 1: 4000 dilution of primary antibody [mouse anti-human MRP2 (M2III-6 or M2 I-4; Alexis Biochemicals, San Diego, CA) and 1:5000 dilution of secondary antibody (sheep anti-mouse, horseradish peroxidase-conjugated; Amersham Biosciences Inc., Piscataway, NJ) in 5% nonfat milk at room temperature for 1 h. Chemiluminescence detection was done using ECL-Plus (Amersham Biosciences Inc.) and exposure to Biomax MR film (Kodak, Rochester, NY) [27]. Protein expression of each variant was determined relative to WT MRP2 and transport activity was normalized to that of WT MRP2 expression.
Vesicle transport studies
ATP-dependent transport into inside–out membrane vesicles was measured as described [25]. All saturation kinetics studies were performed under conditions of linearity with respect to time and protein concentration (data not shown). LTC4 saturation kinetics transport assays were performed using eight LTC4 concentrations (0.1 to 20 µM) for 2 min at 23°C and 5 µg of plasma membrane vesicle protein, while transport of [3H]E217G and [3H]TUDC was measured at 37°C using 10 µg of plasma membrane vesicle protein over a substrate concentration range of 1 to 300 µM. [3H]E23G saturation kinetic studies were performed at 37oC using substrate concentrations from 10 to 1000 µM. Km and Vmax values for ATP-dependent transport were calculated by subtracting uptake in the presence of AMP from that in the presence of ATP. The final transport activity was also corrected for that detected in Sf9 membranes expressing only the empty vector (EV). Experiments to study the effect of SNPs in the NBDs on the ATP-dependence of transport were measured in the presence of ATP concentrations ranging from 50 µM to 7 mM at a fixed concentration of 300 µM [3H]E217G for 2 min at 37°C.
Kinetic analysis
Vesicular transport data were fitted by nonlinear regression analysis with the computer program GraphPad Prism version 4 (GraphPad Software, San Diego, CA). The results are expressed as mean and the 95% Confidence Limits (shown in brackets) obtained from two independent assays, each determined in triplicate. Non-overlapping 95% Confidence Limits were considered indicative of a significant difference at p<0.05. To determine allostery, data from concentration-dependent transport assays were analyzed according to the Hill equation
where v = rate, Vmax = maximum velocity, [S] = initial substrate concentration, Km is the substrate concentration at half-maximum velocity, and n = Hill coefficient. The data were then compared with a fit to a one-binding site version of Equation 1, the Michaelis-Menten equation. To decide which of the two models best fit the data, the extra sum-of-squares F-test was used.
Membrane ATPase measurements
ATPase activity of WT, S789F and A1450T was measured as the level of sodium vanadate-sensitive release of inorganic phosphate from ATP [26]. Membrane proteins (20 µg) were first incubated at 37°C in buffer containing 50 mM Tris-Mes (pH 6.8), 2 mM EGTA, 2 mM dithiothreitol, 50 mM KCl, and 5 mM sodium azide, the ATPase reaction was started by the addition of 5 mM MgATP, and the mixtures incubated for 60 min at 37°C. Vanadate-sensitive ATPase activity was calculated as the difference between activities obtained in the absence and presence of 300 µM vanadate. Substrate-induced ATPase activity was measured in the absence and presence of 300 µM of E217G, which was added in dimethyl sulfoxide (1% final concentration). Control experiments indicated that dimethyl sulfoxide at this concentration had no appreciable effect on ATPase activity. The reactions were stopped by the addition of 0.1 ml 5% SDS and the amount of inorganic phosphate determined immediately as described [26]. The results were obtained from the means of triplicate determinations.
RESULTS
The non-synonymous SNPs characterized in this study are shown in Table 1, and are distributed in MSD1, NBDs 1 and 2 and in the carboxy-terminal region of MRP2. Although the SNPs S789F and A1405T are reported to have low allelic frequencies, they were of interest for inclusion in this study due to their predicted functional relevance when analyzed using the Polyphen Database [5], likely due to their location in the catalytic NBD domains of MRP2. V417I located in MSD1 is reported to have a much higher allelic frequency in several different populations and is frequently associated with adverse drug reactions in human subjects [27–28]. There are no reports on the effects of SNP T1477M in the carboxy-terminal on MRP2 function or expression; despite its low allelic frequency, the location of this SNP was of interest because the function of this portion of MRP2 is rarely studied [29].
Protein expression of WT MRP2 and SNP variants in Sf9 cells
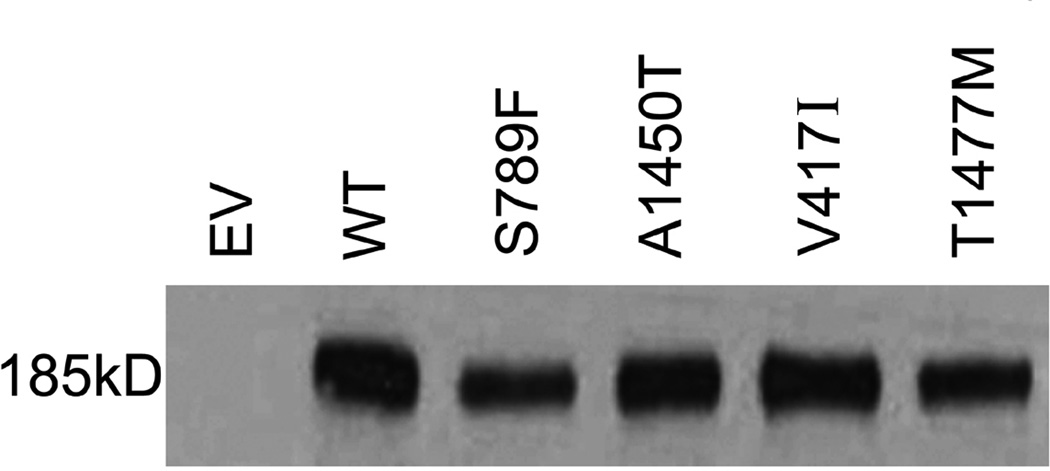
WT MRP2 and the SNP variants S789F, A1450T, V417I and T1477M were expressed in Sf9 cells using recombinant baculovirus. Quantitative immunoblotting of WT and the four MRP2 SNPs in the Sf9 plasma membranes was performed; a representative example of an immunoblot of WT and four MRP2 SNPs is shown in Fig. 1A. Human MRP2 antibody detected the bands corresponding to the underglycosylated human MRP2 protein (<190 kDa) as reported previously [30]. MRP2 was undetectable in EV membrane vesicles. Statistical analysis of triplicate experiments showed that MRP2 expression was significantly reduced about 40% for S789F and T1477M variants, located in NBD1 and carboxy terminal region, respectively. However there was no significant difference in the expression of V417I in MSD1 or of A1450T in NBD2 compared to WT MRP2 (Fig. 1B).
Figure 1.
Expression of WT MRP2 and its variants in Sf9 plasma membranes. A. Immunoblot analysis of wild-type and MRP2 SNP variants in plasma membranes. Membranes (1 µg protein) were isolated from Sf9 cells and MRP2 detected using M2 III-6 monoclonal antibody. EV, Empty Vector; WT, wild-type MRP2; S, serine; F, phenyalanine; A, alanine; T, threonine; V, valine; I, isoleucine; M, methionine. B. Densitometric analysis of western blots of WT and MRP2 SNP variants. Data represent mean ± SE from 3 independent experiments. Comparisons were made by Dunnett’s test following one-way analysis of variance. * p < 0.05, versus WT
Transport activity of WT MRP2 and SNP variants in Sf9 membrane vesicles
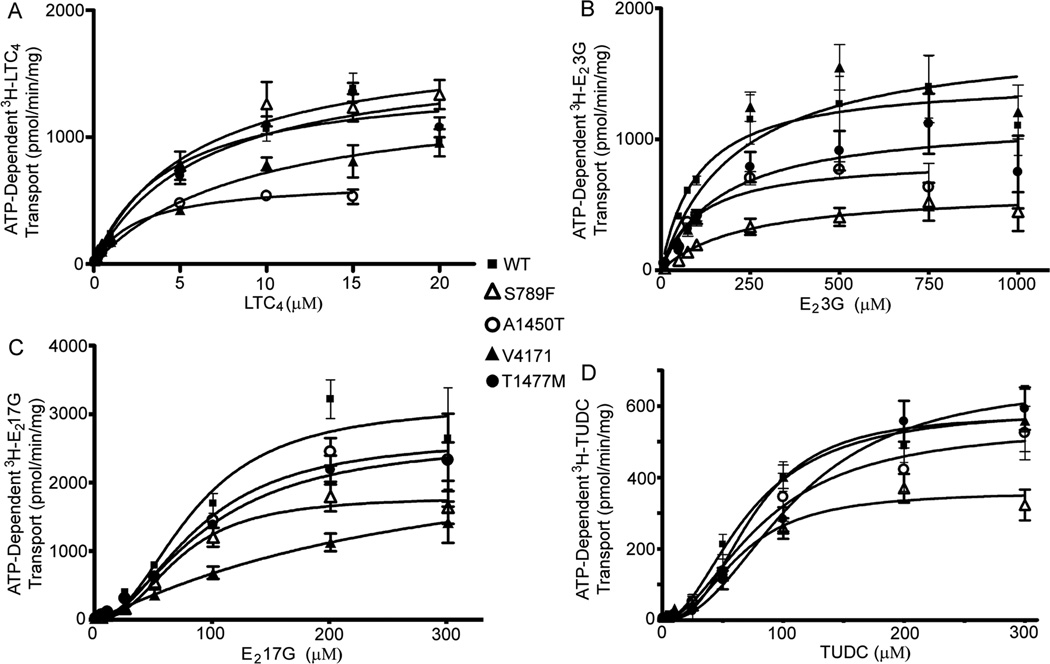
Vesicular transport studies were performed in Sf9 plasma membrane vesicles expressing WT and MRP2 SNP variants in order to characterize transport of typical endogenous substrates of MRP2, such as LTC4, E23G, E217G and TUDC. To ensure that the kinetic parameters obtained were attributable to MRP2, transport was corrected for that in membranes from Sf9 cells transfected with EV. Initial studies showed that Sf9 cells possess endogenous transporters for LTC4, E23G and E217G, hence it was necessary to correct for this endogenous transport activity to obtain accurate kinetic parameters. All transport studies were carried out in freshly prepared membrane vesicles that were less than 2 months old, since aged membranes showed decreased transport that was substrate specific (data not shown). WT MRP2 clearly transported all substrates tested in an ATP-dependent and saturable manner. LTC4 and E23G were transported with classic Michaelis-Menten kinetics by WT MRP2; however E217G and TUDC demonstrated positive cooperativity, as indicated by Hill Coefficients of 2.2 and 2.0, respectively. This is consistent with our previous findings [25, 31] and considered to be evidence of allosteric activation of substrate transport [32, 33]. In order to identify the intrinsic transport activity of MRP2 SNP variants, we normalized the expression of each SNP variant to that of WT MRP2 and the corrected protein levels were used for transport studies. The S789F variant located in the D loop of NBD1 showed markedly reduced transport capacity of all substrates tested compared to WT; Vmax values were decreased by 40 – 60% relative to WT MRP2 (Fig 2A–D, Table 2). The apparent affinity for substrates LTC4, E217G and TUDC were unchanged, however the Km for E23G was increased two-fold compared to WT MRP2 for the S789F SNP (Fig 2B, Table 2). The A1450T variant, which is located immediately following the ABC signature motif of NBD2, showed a 30–55% decrease in the Vmax for transport of LTC4, E23G and TUDC, but not for E217G, while the apparent affinity for all of the substrates tested was not different from that of WT MRP2 (Fig 2A–D, Table 2). The V417I SNP, located in MSD1 between the seventh and eighth transmembrane helices, showed a 2–3-fold increased Km for LTC4 and E217G. While the Km for E23G was increased, this was not statistically significant, as indicated by the overlapping 95% Confidence Limits; the apparent affinity for the bile acid TUDC was also unchanged (Fig 2, Table 2). Interestingly, the V417I SNP also abolished the positive cooperativity in E217G transport, decreasing the Hill Coefficient from 2.2 to 1.1. The T1477M SNP located in the carboxyl terminal region induced modest but significant changes, and showed both increased Km (50%) and Vmax (13%) for TUDC, but a decrease in the Vmax for E23G (20%), while it did not influence the transport of other substrates (Fig 2A–D, Table 2).
Figure 2.
ATP-dependent transport of wild-type and MRP2 SNP variants in Sf9 plasma membrane vesicles. Saturation kinetics are shown for (A) [3H]LTC4; (B) [3H]E23G; (C) [3H]TUDC; and [(D) 3H]E217G. Nonlinear regression was performed on saturation data (mean ± SEM from two independent experiments each determined in triplicate) by fitting the data to the Michaelis-Menten or Hill equation and choosing the best fit using Prism version 4.
Table 2.
Kinetic parameters for transport of substrates by wild-type MRP2 and four SNP variants
| Substrates | Kinetic parameters |
WT | S789F | A1450T | V417I | T1477M |
|---|---|---|---|---|---|---|
| LTC4 | Km | 4 (1.8 – 6.5) |
3 (1.8 – 4.4) |
3 (2 – 3.6) |
9 (7.8 – 12.7)* |
6 (3.5 – 9) |
| Vmax | 1464 (1314–1713) |
732 (648 – 816)* |
658 (618–699)* |
1366 (1200–1531) |
1659 (1373–1945) |
|
| E23G | Km | 103 (73 – 133) |
235 (173 – 296)* |
106 (64 – 148) |
212 (95 – 330) |
172 (88 – 230) |
| Vmax | 1458 (1339–1578) |
615 (558–671)* |
855 (746–964)* |
1794 (1454–2134) |
1157 (975–1338)* |
|
| E217βG | Km | 84 (64–104) |
72 (67–76) |
88 (75–100) |
263 (241–285)* |
94 (89–100) |
| Vmax | 3154 (2691–3617) |
1799 (1732–1866)* |
2665 (2422–2907) |
2647 (2520–2773) |
2646 (2555–2737) |
|
| HC | 2.2 (1.4 – 3) |
2.4 (2.1 – 2.6) |
2 (1.6 – 2.4) |
1.1 (1.1 – 1.2)* |
1.8 (1.7–1.9) |
|
| TUDC | Km | 71 (65–78) |
64 (71–96) |
71 (57–86) |
74 (70–79) |
109 (103–116)* |
| Vmax | 595 (563–627) |
359 (343–376)* |
424 (376–472)* |
577 (553–600) |
671 (645–699)* |
|
| HC | 2 (1.7–2.3) |
2.3 (1.9–2.6) |
1.8 (1.3–2.3) |
2.6 (2.3–2.8) |
2.2 (2–2.4) |
The kinetic parameters [Km (µM); Vmax (pmol·mg−1·min−1); Hill coefficient (HC)] of ATP-dependent transport of leukotriene C4 (LTC4), estradiol-3 glucuronide (E23G), estradiol-17-glucuronide (E217G) and tauroursodeoxycholic acid (TUDC) were determined in Sf9 plasma membrane vesicles. Values in brackets are the 95% Confidence Limits. The values were calculated from the data shown in Figure 2.
Significantly different from WT, as determined by non- overlapping 95% Confidence Limits.
Substrate-stimulated ATPase activity of MRP2 and SNP variants
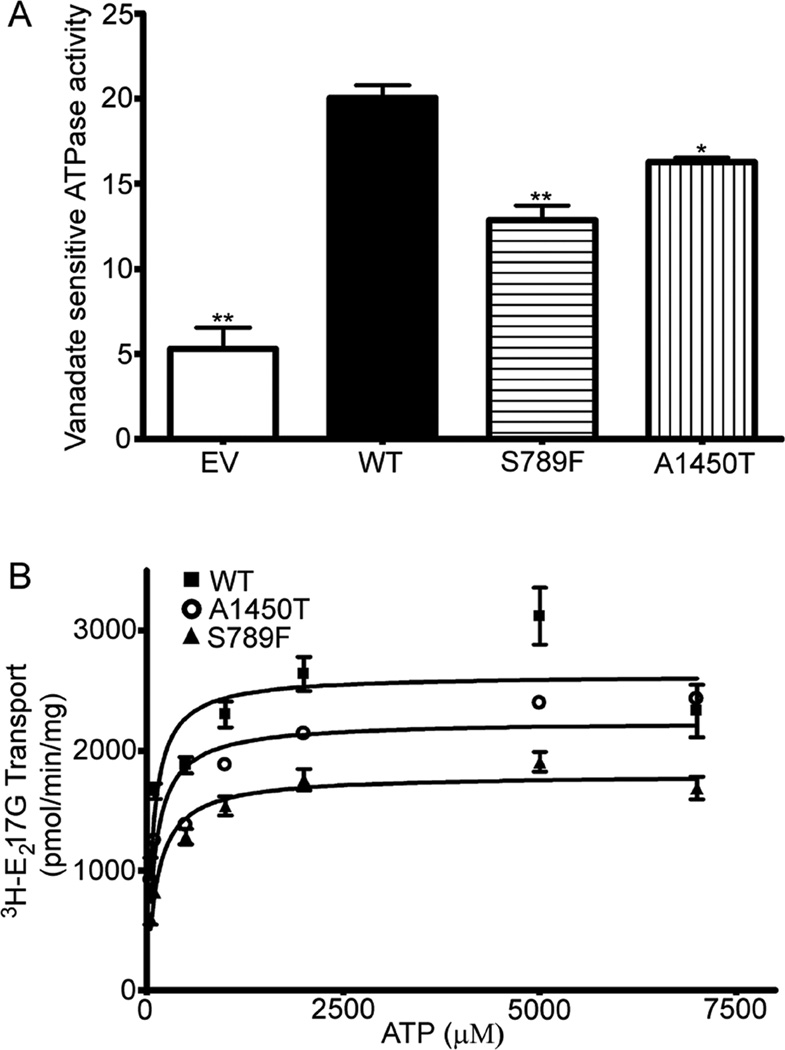
To identify the mechanism of reduced transport activity of the SNP variants S789F and A1450T, located in the ATP-binding domains, we determined their vanadate-sensitive ATPase activity in the presence of E217G (300 µM). Isolated plasma membrane preparations of Sf9 cells expressing WT MRP2 showed a high-capacity, drug-stimulated ATPase activity (Fig 3A), as reported previously [26]. EV membranes exhibited a vanadate-sensitive basal activity of <7 nmol of Pi /min/mg protein and demonstrated no E217G-stimulated ATPase activity (data not shown). S789F and A1450T exhibited vanadate-sensitive ATPase activity that was stimulated by E217G, however, the ATPase activity of S789F and A1450T was significantly decreased by 37% and 20%, respectively, compared to WT MRP2 (Fig 3A).
Figure 3.
A. ATPase activity of Sf9 membranes expressing wild-type MRP2 and the SNP variants S789F and A1450T. The membrane fractions were incubated with 5 mM ATP in the presence of 300 µM E217G and inorganic phosphate liberation determined [26]. Data represent mean ± SE from 3 independent experiments. Comparisons were made by Dunnett’s test following one-way analysis of variance. *, ** indicate p < 0.05, 0.01, respectively, versus WT. B. Determination of the effect of the MRP2 SNP variants on ATP dependence of E217G transport. Transport in the Sf9 membrane vesicles expressing WT, or variants S789F and A1450T was measured in the presence of various concentrations of ATP ranging from 50 µM to 7 mM at a fixed concentration of 3H-E217G (300 µM) for 2 min at 37°C. Each data point represents mean ± S.D. of triplicate determinations from representative experiments. Nonlinear regression was performed on saturation data (mean ± S.D.) by fitting the data to the Michaelis-Menten or Hill equation and choosing the best fit using Prism version 4.
To determine whether the maximal rates of ATP hydrolysis or affinity for the nucleotide were altered by the mutation seen in SNP variants S789F and A1450T, we compared their Km for ATP with that of WT MRP2 (Fig 3B; Table 3). Vesicular transport studies over a wide range of concentrations of ATP and a fixed E217G concentration (300 µM) showed that the apparent Km values for ATP were increased modestly but not significantly in both S789F (125 µM) and A1450T (101 µM), compared to WT MRP2 (79 µM) (Fig 3B; Table 3). The Vmax values were significantly decreased in to 68% S789F and to 85% in A1450T (Table 3), consistent with the decreased E217G Vmax values seen in the saturation kinetics study (Table 2).
Table 3.
Kinetic parameters for ATP in wild-type MRP2 and variants S789F and A1450T
| Kinetic parameters | WT | S789F | A1450T |
|---|---|---|---|
| Km | 79 (43–114) |
125 (96–155) |
101 (57–146) |
| Vmax | 2630 (2433–2927) |
1796 (1721–1870)* |
2242 (2071–2413)* |
Nonlinear regression results [Km, (µM); Vmax (pmol·mg−1·min−1)] were obtained from data in Figure 3B. Values in brackets are the 95% Confidence Limits.
Significantly different from WT, as determined by non- overlapping 95% Confidence Limits.
DISCUSSION
The major objective of the present study was to characterize the effects of selective MRP2 SNPs on the kinetics of transport of four distinct MRP2 substrates, two regioisomeric glucuronide conjugates of estradiol, E23G and E217G, a glutathione conjugate, LTC4, and a bile acid, TUDC, all MRP2 substrates that are well suited for vesicular transport studies. Four SNPs were selected for characterization, one in each of the two NBDs (S789F in NBD1 and A1450T in NBD2), one in MSD1 (V417I), and one in the carboxy terminal (T1477M), in order to probe how changes in these portions of MRP2 protein might impact its transport properties. Transport activity was normalized for the level of expression, so that the intrinsic transport properties of WT MRP2 and each SNP could be compared directly. The MRP2 variant S789F, located in the D-loop of NBD1, consistently decreased the Vmax for transport of all four substrates, while A1450T, located immediately following the ABC signature motif in NBD2, decreased the Vmax for all substrates except E217G. The NBDs are highly conserved among all of the ABC transporters, and contain the characteristic motifs essential for ATP binding and hydrolysis, the energy from which is used to efflux substrates. These motifs include the Walker A and B sequences, the ABC signature motif (LSGGQ), and the D-, H-, A-, and Q-loops. The two NBDs are arranged in a “head-to-tail” conformation that forms two nucleotide binding sites (NBS1 and NBS2) [34, 35]. NBS1 binds one molecule of ATP between the Walker A and B sequences, the A-, Q-, and H-loops of NBD1 and the ABC signature motif and D-loop of NBD2, while NBS2 binds the second molecule of ATP to the Walker A and B sequences, the A-, Q-, and H-loops of NBD2 and the ABC signature motif and D-loop of NBD1. In MDR1 (ABCB1), the two NBSs are functionally equivalent, while in MRP1 (ABCC1), NBS1 preferentially binds ATP, while NBS2 preferentially hydrolyzes ATP [36, 37]. While MRP1 and MRP2 are highly similar, the relative functions of NBS1 and NBS2 are not known for MRP2. We therefore questioned whether S789F, most likely located in NBS2, and A1450T, likely located in NBS1, might have differential effects on ATP binding and hydrolysis. ATPase activity was significantly inhibited in both S789F and A1450T (Figure 3A); while inhibition of ATPase activity was greater for S789F (37%) than A1450T (20%), the data did not permit unequivocal determination of the differential roles of NBS1 and NBS2 in ATP hydrolysis. The Km values for ATP were also equally but only modestly increased in both SNPs (Fig 3B; Table 3). These data imply that irrespective of their locations in NBS1 or NBS2, neither variant S789F nor A1450T is directly involved in ATP binding, but rather both variants impact transport by decreasing ATP hydrolysis.
The V417I SNP, located in MSD1, was found to have decreased apparent affinity for LTC4, E23G and E217G, but not for TUDC. These data imply that valine 417 plays a critical and selective role in binding of glutathione and glucuronide conjugates, but not the bile acid, TUDC. Numerous studies have shown that the binding sites for any two substrates are not identical in the MRP transporters, and substitutions of single amino acids can eliminate or reduce the transport of one substrate while leaving another intact [38–41]. Further, several studies have shown that charged amino acids, especially basic amino acids, in the MSDs play an important role in the substrate specificity of MRP proteins [42–44]. The present studies show for the first time that a nonpolar amino acid in MSD1 can also play an important role in substrate specificity and recognition. V417I also significantly reduced the Hill coefficient for E217G from 2.2 in WT MRP2 to 1.1 (Fig 2C and Table 2). MRP2 has been postulated to contain two binding sites for E217G, one of which is involved in the stimulation of E217G transport while the other is involved in its actual transport [25, 33, 45]. Replacing valine with isoleucine eliminated positive cooperativity in E217G transport, implying that this valine contributes to the modulatory site. Our data showing increased apparent Km values for three glucuronide or glutathione conjugate substrates by V417I could be of clinical relevance since this is a common SNP in the population, and has been detected at frequencies of more than 20% in Caucasians and somewhat less in Japanese subjects (Table 1) [16]. In fact, this SNP has been reported to be associated with various drug-induced pathological conditions, such as proximal tubulopathy induced by tenofovir [27], neurological adverse drug reactions caused by carbamazepine [28], and a suggested increased susceptibility to colorectal adenocarcinoma [46]. The V417I SNP was also suggested to play a role in altered pharmacokinetics of talinolol, resulting in its lowered oral bioavailability and increased clearance following intravenous administration [47]. However, in population studies of MRP2 haplotypes, V417I was not found to associate with cholestatic or toxic hepatitis [48], and there was no effect on MRP2 protein expression in liver ([49] or on serum conjugated bilirubin levels in individuals with this SNP [16].
Interestingly, the T1477M SNP located in the carboxy terminal region caused a selective decrease in the apparent affinity for TUDC but had no effect on the transport properties of other substrates. These data suggest that the amino acid residue at 1477 is involved in discriminating between glucuronide/glutathione conjugates and bile acid substrates, and implies that even this portion of the protein also participates in recognition and transport of MRP2 substrates.
Quantitative immunoblot analysis demonstrated a decreased expression of about 40% for two of the SNPs, S789F and T1477M, relative to WT MRP2. To ensure that the reduced expression was not antibody-dependent, we performed immunoblots using two antibodies, M2 III-6 and M2 I-4, which target epitopes located at different regions of the MRP2 protein, amino acids 1339–1541 and 215–310, respectively. The expression levels of the variants yielded similar results with both antibodies (data not shown), indicating that the reduced expression was a characteristic of the SNPs. Expression of S789F (located in NBD1) was decreased to 59% of that of WT MRP2; a nearby but different mutation in NBD1 (R768W) of MRP2 was shown to be localized in the cytoplasm with an endoplasmic reticulum-like distribution [50]. These data suggest that mutations in the NBD1 can induce a conformational change, resulting in a misfolded protein and/or defective sorting to the plasma membrane. Similarly, expression of T1477M, where the SNP is located 68 amino acid residues from the C-terminal of MRP2, was decreased to 62% of WT MRP2. A 15 C-terminal amino acid truncation of a GFP-MRP2 fusion protein impaired its apical localization in polarized HepG2 cells, either because part of a motif required for apical sorting is lost or because of a conformational change in the protein, impairing its maturation and leading to its retention in endoplasmic reticulum [29]. A significant contribution of a single leucine amino acid has been demonstrated for the apical expression of type IIb NaPi cotransporters [51]. While the amino acid change in T1477M could contribute to the localization signal, it is located a good distance from the C-terminal, suggesting an alternative mechanism for its decreased expression. From our studies, the data suggest that subjects with S789F and T1477M polymorphisms may have a lower expression level of MRP2 protein in the apical membrane of cells, and consequently, a reduced ability to export substrates.
Hirouchi et al [52] also characterized the expression and transport activity of several MRP2 variants (V417I, S789F, A1450T) using a Tet-off recombinant adenovirus system in LLC-PK1 cells. However, these authors did not detect any change in transport of E217G, LTC4 or 2,4-dinitrophenyl-S-glutathione by SNP V417I following normalization for expression levels, and further found that transport of these substrates was increased by S789F. These discrepancies are likely due to their characterization of transport at a single substrate concentration across various time points. Further analysis of the kinetic parameters showed that the Vmax for transport of 2,4-dinitrophenyl-S-glutathione by S789F was 1.3-fold greater than that of WT MRP2. Finally, these authors also showed a marked reduction in the expression of both S789F and A1450T to 24% and 18% of WT MRP2, respectively, such that transport of E217G by A1450T was too low to be normalized by its expression level [52]. The use of the Tet-off adenoviral system in mammalian cells by these authors [52] versus our use of baculovirus infection of Sf9 cells likely account for some of the observed differences in expression levels. These discrepancies, particularly in A1450T expression, indicate that the results of in vitro transporter expression should be extrapolated to in vivo situations with caution. Recent studies have shown that it is possible to quantify the absolute protein amount of transporter expression in tissue samples from these polymorphic subjects by LC/MS [53] and thus would better link the SNPs’ influence to transporter expression.
In conclusion, we compared the expression level and transport function of MRP2 SNP variants. V417I selectively inhibited the transport of glutathione and glucuronide conjugated substrates, while T1477M only altered the transport of TUDC, a hydrophilic bile acid. Even though the SNPs S789F and A1450T exist with low allelic frequency, the decreased expression and transport function of S789F and decreased transport activity of A1450T shown here could impact drug disposition in polymorphic subjects. Due most likely to the relative rarity of these SNPs, there are no reports of an association between S789F or A1450T and altered pharmacokinetics of MRP2 substrates. However, the clinical relevance of MRP2 polymorphisms in the disposition of drugs such as pravastatin [20] and anticancer drugs such as irinotecan [54], and recently, the response to tamoxifen that is widely used as adjuvant therapy for breast cancer, is well established [55]. These SNPs may also prove to be of toxicological and clinical relevance since MRP2 is an important component of the detoxification of environmental carcinogens, e.g., 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) [56] and arsenite glutathione complexes [57]. These data indicate that closer evaluation of the role of these MRP2 SNPs in haplotype analyses or genome wide association studies of adverse drug reactions and/or disease is warranted, as is careful monitoring of patients with the relatively frequent MRP2 SNP, V417I.
Acknowledgments
Supported by: National Institutes of Health grant HD58299
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Keppler D, Konig J. Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis. 2000;20(3):265–272. doi: 10.1055/s-2000-9391. [DOI] [PubMed] [Google Scholar]
- 2.Paulusma CC, Oude Elferink RP. The canalicular multispecific organic anion transporter and conjugated hyperbilirubinemia in rat and man. J Mol Med. 1997;75(6):420–428. doi: 10.1007/s001090050127. [DOI] [PubMed] [Google Scholar]
- 3.Bandler P, Westlake C, Grant C, Cole S, Deeley R. Identification of regions required for apical membrane localization of human multidrug resistance protein 2. Molecular pharmacology. 2008;74(1):9. doi: 10.1124/mol.108.045674. [DOI] [PubMed] [Google Scholar]
- 4.Fardel O, Jigorel E, Le Vee M, Payen L. Physiological, pharmacological and clinical features of the multidrug resistance protein 2. Biomed Pharmacother. 2005;59(3):104–114. doi: 10.1016/j.biopha.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453(5):643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 6.Chandra P, Brouwer KL. The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res. 2004;21(5):719–735. doi: 10.1023/b:pham.0000026420.79421.8f. [DOI] [PubMed] [Google Scholar]
- 7.Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112(2):457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Ayrton A, Morgan P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica. 2001;31(8–9):469–497. doi: 10.1080/00498250110060969. [DOI] [PubMed] [Google Scholar]
- 9.Kusuhara H, Sugiyama Y. Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J Control Release. 2002;78(1–3):43–54. doi: 10.1016/s0168-3659(01)00480-1. [DOI] [PubMed] [Google Scholar]
- 10.Sai K, Saito Y, Maekawa K, Kim SR, Kaniwa N, Nishimaki-Mogami T, Sawada J, Shirao K, Hamaguchi T, Yamamoto N, et al. Additive effects of drug transporter genetic polymorphisms on irinotecan pharmacokinetics/pharmacodynamics in Japanese cancer patients. Cancer Chemother Pharmacol. 2010;66(1):95–105. doi: 10.1007/s00280-009-1138-y. [DOI] [PubMed] [Google Scholar]
- 11.Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44(2):152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kartenbeck J, Leuschner U, Mayer R, Keppler D. Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology. 1996;23(5):1061–1066. doi: 10.1053/jhep.1996.v23.pm0008621134. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Sugiyama Y. Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Adv Drug Deliv Rev. 2002;54(10):1311–1331. doi: 10.1016/s0169-409x(02)00075-3. [DOI] [PubMed] [Google Scholar]
- 14.Tsujii H, Konig J, Rost D, Stockel B, Leuschner U, Keppler D. Exon-intron organization of the human multidrug-resistance protein 2 (MRP2) gene mutated in Dubin-Johnson syndrome. Gastroenterology. 1999;117(3):653–660. doi: 10.1016/s0016-5085(99)70459-2. [DOI] [PubMed] [Google Scholar]
- 15.Sookoian S, Castano G, Burgueno A, Gianotti TF, Pirola CJ. Association of the multidrug-resistance-associated protein gene (ABCC2) variants with intrahepatic cholestasis of pregnancy. J Hepatol. 2008;48(1):125–132. doi: 10.1016/j.jhep.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Gradhand U, Kim RB. Pharmacogenomics of MRP transporters (ABCC1–5) and BCRP (ABCG2) Drug Metab Rev. 2008;40(2):317–354. doi: 10.1080/03602530801952617. [DOI] [PubMed] [Google Scholar]
- 17.de Jong FA, Scott-Horton TJ, Kroetz DL, McLeod HL, Friberg LE, Mathijssen RH, Verweij J, Marsh S, Sparreboom A. Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther. 2007;81(1):42–49. doi: 10.1038/sj.clpt.6100019. [DOI] [PubMed] [Google Scholar]
- 18.Innocenti F, Undevia SD, Chen PX, Das S, Ramirez J, Dolan ME, et al. Pharmacogenetic analysis of interindividual irinotecan (CPT-11) pharmacokinetic (PK) Variability: Evidence for a functional variant of ABCC2. Proceedings of ASCO, 22 abstract. 2004 [Google Scholar]
- 19.Naesens M, Kuypers DR, Verbeke K, Vanrenterghem Y. Multidrug resistance protein 2 genetic polymorphisms influence mycophenolic acid exposure in renal allograft recipients. Transplantation. 2006;82(8):1074–1084. doi: 10.1097/01.tp.0000235533.29300.e7. [DOI] [PubMed] [Google Scholar]
- 20.Niemi M, Arnold KA, Backman JT, Pasanen MK, Godtel-Armbrust U, Wojnowski L, Zanger UM, Neuvonen PJ, Eichelbaum M, Kivisto KT, et al. Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet Genomics. 2006;16(11):801–808. doi: 10.1097/01.fpc.0000230422.50962.91. [DOI] [PubMed] [Google Scholar]
- 21.Hulot J, Villard E, Maguy A, Morel V, Mir L, Tostivint I, William-Faltaos D, Fernandez C, Hatem S, Deray G. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005;15(5):277. doi: 10.1097/01213011-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Roda A, Cerre C, Simoni P, Polimeni C, Vaccari C, Pistillo A. Determination of free and amidated bile acids by high-performance liquid chromatography with evaporative light-scattering mass detection. J Lipid Res. 1992;33:1393–1402. [PubMed] [Google Scholar]
- 23.Ito K, Suzuki H, Sugiyama Y. Single amino acid substitution of rat MRP2 results in acquired transport activity for taurocholate. Am J Physiol Gastrointest Liver Physiol. 2001;281(4):G1034–G1043. doi: 10.1152/ajpgi.2001.281.4.G1034. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 25.Gerk PM, Li W, Megaraj V, Vore M. Human multidrug resistance protein 2 transports the therapeutic bile salt tauroursodeoxycholate. J Pharmacol Exp Ther. 2007;320(2):893–899. doi: 10.1124/jpet.106.106922. [DOI] [PubMed] [Google Scholar]
- 26.Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267(7):4854–4858. [PubMed] [Google Scholar]
- 27.Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, Valantin MA, Lechat P, Deray AG. Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis. 2006;194(11):1481–1491. doi: 10.1086/508546. [DOI] [PubMed] [Google Scholar]
- 28.Kim WJ, Lee JH, Yi J, Cho YJ, Heo K, Lee SH, Kim SW, Kim MK, Kim KH, In Lee B, et al. A nonsynonymous variation in MRP2/ABCC2 is associated with neurological adverse drug reactions of carbamazepine in patients with epilepsy. Pharmacogenet Genomics. 2010;20(4):249–256. doi: 10.1097/FPC.0b013e328338073a. [DOI] [PubMed] [Google Scholar]
- 29.Nies AT, Konig J, Cui Y, Brom M, Spring H, Keppler D. Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2) Eur J Biochem. 2002;269:1866–1876. doi: 10.1046/j.1432-1033.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 30.Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, et al. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101(7):1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerk PM, Li W, Vore M. Estradiol 3-glucuronide is transported by the multidrug resistance-associated protein 2 but does not activate the allosteric site bound by estradiol 17-glucuronide. Drug Metab Dispos. 2004;32(10):1139–1145. doi: 10.1124/dmd.104.000372. [DOI] [PubMed] [Google Scholar]
- 32.Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B. Differential modulation of the human liver conjugate transporters MRP2 and MRP3 by bile acids and organic anions. J Biol Chem. 2003;278(26):23529–23537. doi: 10.1074/jbc.M303515200. [DOI] [PubMed] [Google Scholar]
- 33.Zelcer N, Huisman MT, Reid G, Wielinga P, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel AH, Borst P. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2) J Biol Chem. 2003;278(26):23538–23544. doi: 10.1074/jbc.M303504200. [DOI] [PubMed] [Google Scholar]
- 34.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17(4):412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Zaitseva J, Oswald C, Jumpertz T, Jenewein S, Wiedenmann A, Holland IB, Schmitt L. A structural analysis of asymmetry required for catalytic activity of an ABC-ATPase domain dimer. EMBO J. 2006;25(14):3432–3443. doi: 10.1038/sj.emboj.7601208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao M, Cui HR, Loe DW, Grant CE, Almquist KC, Cole SP, Deeley RG. Comparison of the functional characteristics of the nucleotide binding domains of multidrug resistance protein 1. J Biol Chem. 2000;275(17):13098–13108. doi: 10.1074/jbc.275.17.13098. [DOI] [PubMed] [Google Scholar]
- 37.Nagata K, Nishitani M, Matsuo M, Kioka N, Amachi T, Ueda K. Nonequivalent nucleotide trapping in the two nucleotide binding folds of the human multidrug resistance protein MRP1. J Biol Chem. 2000;275(23):17626–17630. doi: 10.1074/jbc.M000792200. [DOI] [PubMed] [Google Scholar]
- 38.Grant CE, Gao M, DeGorter MK, Cole SP, Deeley RG. Structural determinants of substrate specificity differences between human multidrug resistance protein (MRP) 1 (ABCC1) and MRP3 (ABCC3) Drug Metab Dispos. 2008;36(12):2571–2581. doi: 10.1124/dmd.108.022491. [DOI] [PubMed] [Google Scholar]
- 39.Ito K, Oleschuk CJ, Westlake C, Vasa MZ, Deeley RG, Cole SP. Mutation of Trp1254 in the multispecific organic anion transporter, multidrug resistance protein 2 (MRP2) (ABCC2), alters substrate specificity and results in loss of methotrexate transport activity. J Biol Chem. 2001;276(41):38108–38114. doi: 10.1074/jbc.M105160200. [DOI] [PubMed] [Google Scholar]
- 40.Ito K, Olsen SL, Qiu W, Deeley RG, Cole SP. Mutation of a single conserved tryptophan in multidrug resistance protein 1 (MRP1/ABCC1) results in loss of drug resistance and selective loss of organic anion transport. J Biol Chem. 2001;276(19):15616–15624. doi: 10.1074/jbc.M011246200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang DW, Cole SP, Deeley RG. Identification of an amino acid residue in multidrug resistance protein 1 critical for conferring resistance to anthracyclines. J Biol Chem. 2001;276(16):13231–13239. doi: 10.1074/jbc.M010008200. [DOI] [PubMed] [Google Scholar]
- 42.Haimeur A, Conseil G, Deeley RG, Cole SP. Mutations of charged amino acids in or near the transmembrane helices of the second membrane spanning domain differentially affect the substrate specificity and transport activity of the multidrug resistance protein MRP1 (ABCC1) Mol Pharmacol. 2004;65(6):1375–1385. doi: 10.1124/mol.65.6.1375. [DOI] [PubMed] [Google Scholar]
- 43.Haimeur A, Deeley RG, Cole SP. Charged amino acids in the sixth transmembrane helix of multidrug resistance protein 1 (MRP1/ABCC1) are critical determinants of transport activity. J Biol Chem. 2002;277(44):41326–41333. doi: 10.1074/jbc.M206228200. [DOI] [PubMed] [Google Scholar]
- 44.Ito K, Suzuki H, Sugiyama Y. Charged amino acids in the transmembrane domains are involved in the determination of the substrate specificity of rat Mrp2. Mol Pharmacol. 2001;59(5):1077–1085. doi: 10.1124/mol.59.5.1077. [DOI] [PubMed] [Google Scholar]
- 45.Borst P, Zelcer N, van de Wetering K. MRP2 and 3 in health and disease. Cancer Lett. 2006;234:51–61. doi: 10.1016/j.canlet.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 46.Nishioka C, Sakaeda T, Nakamura T, Moriya Y, Okamura N, Tamura T, Nakahara T, Aoyama N, Kamigaki T, Ohno M, Kuroda Y, Kasuga M, Okumura K. MDR1, MRP1 and MRP2 genotypes and in vitro chemosensitivity in Japanese patients with colorectal adenocarcinomas. Kobe J Med Sci. 2004;50(5–6):181–188. [PubMed] [Google Scholar]
- 47.Haenisch S, May K, Wegner D, Caliebe A, Cascorbi I, Siegmund W. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenet Genomics. 2008;18(4):357–365. doi: 10.1097/FPC.0b013e3282f974b7. [DOI] [PubMed] [Google Scholar]
- 48.Choi JH, Ahn BM, Yi J, Lee JH, Lee JH, Nam SW, Chon CY, Han KH, Ahn SH, Jang IJ, et al. MRP2 haplotypes confer differential susceptibility to toxic liver injury. Pharmacogenet Genomics. 2007;17:403–415. doi: 10.1097/01.fpc.0000236337.41799.b3. [DOI] [PubMed] [Google Scholar]
- 49.Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44(1):62–74. doi: 10.1002/hep.21214. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto K, Uchiumi T, Konno T, Ebihara T, Nakamura T, Wada M, Sakisaka S, Maniwa F, Amachi T, Ueda K, et al. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology. 2002;36(5):1236–1245. doi: 10.1053/jhep.2002.36368. [DOI] [PubMed] [Google Scholar]
- 51.Karim-Jimenez Z, Hernando N, Biber J, Murer H. Requirement of a leucine residue for (apical) membrane expression of type IIb NaPi cotransporters. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2916. doi: 10.1073/pnas.97.6.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirouchi M, Suzuki H, Itoda M, Ozawa S, Sawada J, Ieiri I, Ohtsubo K, Sugiyama Y. Characterization of the cellular localization, expression level, and function of SNP variants of MRP2/ABCC2. Pharm Res. 2004;21(5):742–748. doi: 10.1023/b:pham.0000026422.06207.33. [DOI] [PubMed] [Google Scholar]
- 53.Li N, Zhang Y, Hua F, Lai Y. Absolute difference of hepatobiliary transporter multidrug resistance-associated protein (MRP2/Mrp2) in liver tissues and isolated hepatocytes from rat, dog, monkey, and human. Drug Metab Dispos. 2009;37(1):66. doi: 10.1124/dmd.108.023234. [DOI] [PubMed] [Google Scholar]
- 54.Rosner G, Panetta J, Innocenti F, Ratain M. Pharmacogenetic pathway analysis of irinotecan. Clinical Pharmacology & Therapeutics. 2008;84(3):393–402. doi: 10.1038/clpt.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiyotani K, Mushiroda T, Imamura C, Hosono N, Tsunoda T, Kubo M, Tanigawara Y, Flockhart D, Desta Z, Skaar T. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. Journal of Clinical Oncology. 2010;28(8):1287. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dietrich CG, de Waart DR, Ottenhoff R, Schoots IG, Elferink RP. Increased bioavailability of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol Pharmacol. 2001;59(5):974–980. doi: 10.1124/mol.59.5.974. [DOI] [PubMed] [Google Scholar]
- 57.Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275(43):33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- 58.Vogelgesang S, Kunert-Keil C, Cascorbi I, Mosyagin I, Schroder E, Runge U, Jedlitschky G, Kroemer HK, Oertel J, Gaab MR, et al. Expression of multidrug transporters in dysembryoplastic neuroepithelial tumors causing intractable epilepsy. Clin Neuropathol. 2004;23(5):223–231. [PubMed] [Google Scholar]
- 59.Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, Haenisch S, Linnemann K, Fusch C, Cascorbi I, Kroemer HK. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos. 2005;33(7):896–904. doi: 10.1124/dmd.104.003335. [DOI] [PubMed] [Google Scholar]