Abstract
The nodulus and uvula (lobules X and IX of the vermis) receive mossy fibers from both vestibular afferents and vestibular nuclei neurons and are thought to play a role in spatial orientation. Their properties relate to a sensory ambiguity of the vestibular periphery: otolith afferents respond identically to translational (inertial) accelerations and changes in orientation relative to gravity. Based on theoretical and behavioral evidence, this sensory ambiguity is resolved using rotational cues from the semicircular canals. Recordings from the cerebellar cortex have identified a neural correlate of the brain's ability to resolve this ambiguity in the simple spike activities of nodulus/uvula Purkinje cells. This computation, which likely involves the cerebellar circuitry and its reciprocal connections with the vestibular nuclei, results from a remarkable convergence of spatially- and temporally-aligned otolith-driven and semicircular canal-driven signals. Such convergence requires a spatio-temporal transformation of head-centered canal-driven signals into an estimate of head reorientation relative to gravity. This signal must then be subtracted from the otolith-driven estimate of net acceleration to compute inertial motion. At present, Purkinje cells in the nodulus/uvula appear to encode the output of this computation. However, how the required spatio-temporal matching takes place within the cerebellar circuitry and what role complex spikes play in spatial orientation and disorientation remains unknown. In addition, the role of visual cues in driving and/or modifying simple and complex spike activity, a process potentially critical for long-term adaptation, constitutes another important direction for future studies.
Keywords: Cerebellum, Vestibular, Purkinje cell, Vermis, Nodulus
Introduction
The vestibulo-cerebellum is the oldest part of the cerebellum, and thus, its role in vestibular processing has long been thought to provide significant insights into the functions and computations this important brain structure performs. Historically, much attention has been focused on the flocculus and ventral paraflocculus and their role in motor learning of the vestibulo-ocular reflex [1–4]. However, another important component of the vestibulo-cerebellum that is phylogenetically well-preserved among different species is the nodulus and uvula (lobules X and IX of the vermis, collectively referred to here as “NU”). These areas of the cerebellar cortex are unique in that they receive projections from more than 70% of vestibular primary afferents (including afferents from both the otolith organs and the semicircular canals), which terminate ipsilaterally as mossy fibers [5–10]. The major source of secondary afferent mossy fibers originates from the vestibular nuclei [11–17]. Vestibular information also reaches the NU through climbing fibers, which arise from the contralateral inferior olive, primarily through projections from the β-nucleus and dorsomedial cell column [16, 18–22].
Lesions of the NU result in balance problems, spatial disorientation, and deficits in the spatio-temporal properties of the vestibulo-ocular reflex [23–26]. Thus, its function has been globally linked to spatial orientation/disorientation, although its exact role has remained unknown. Until very recently, characterization of NU Purkinje cell responses was limited to rotations and static tilt, tested exclusively in anesthetized animals [27–33]. Using such limited vestibular testing, it was reported that simple and complex spike responses reflect activation of the vertical canals and otolith organs [30–33]. In contrast, despite a clear horizontal canal input [10, 34], NU Purkinje cells do not appear to modulate during yaw rotation [32, 33, 35].
Here, we review recent work [36, 37], emphasizing a potentially very important role of the NU in resolving a sensory ambiguity in the peripheral vestibular system. As the basic features of cerebellar cortical circuitry are conserved throughout the entire cerebellum [38, 39], it is likely that computations similar to those performed by the NU are performed by other cerebellar areas. We start with a brief outline of the problem.
A Vestibular Sensory Ambiguity
The peripheral otolith system faces a sensory encoding ambiguity: primary otolith afferents, the sensors that detect linear accelerations, respond identically to an inertial motion stimulus (e.g., translational motion during lateral or forward motion) and a change in head orientation relative to gravity (e.g., roll or pitch tilt) [40–43]. The ambiguity is illustrated with an example of responses from an otolith afferent during translation, tilt, and combinations of these stimuli in Fig. 1a. This example also illustrates the experimental protocol used to address the problem and its solution at the level of single neurons.
Fig. 1.
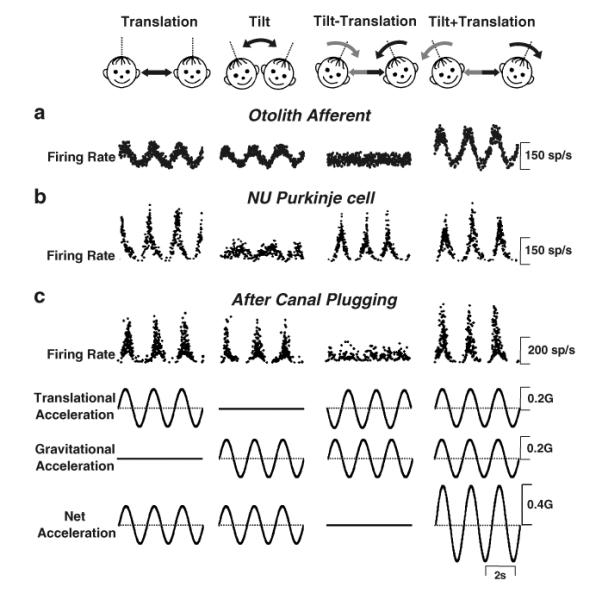
Experimental protocol (top cartoons) and firing rates from (a) an otolith afferent, (b) simple spike responses of an NU Purkinje cell in labyrinthine-intact animals, and (c) simple spike responses of a NU Purkinje cell in canal-plugged animals. From left to right, stimuli were translation, tilt, tilt–translation, and tilt+translation (0.5 Hz), all delivered in complete darkness. Note that the translational and tilt stimuli were matched in both amplitude and direction to elicit an identical linear acceleration in the horizontal plane (bottom traces). Straight black and curved gray arrows denote translation and tilt axes of stimulation, respectively. Modified and replotted with permission from Angelaki [40] and Yakusheva [37]
Starting from the traces on the left, the first stimulus was a pure left/right translation (1st column, “translation”) and the second stimulus was a pure roll tilt (2nd column “tilt”), both at a frequency of 0.5 Hz. All stimuli were delivered in darkness to exclude contributions from visual cues. The peak amplitude of the sinusoidal tilt was adjusted to produce a horizontal linear acceleration component (due to changes in head orientation relative to gravity) that was the same as that during translation [40, 44]. Note that, even though the two movements were quite different, the responses from the otolith afferent were similar for tilt and translation. To facilitate interpretation of cell responses, the bottom traces illustrate the “translation” stimulus, the “tilt” stimulus, as well as the “net acceleration” stimulus. Clearly, the otolith afferent activity was best correlated with the net acceleration stimulus [40, 45, 46].
This observation is further illustrated in the last two columns of Fig. 1, where the tilt and translation stimuli were combined either out of phase (so that the gravitational and inertial accelerations canceled each other) such that the net horizontal linear acceleration was zero (3rd column, “tilt−translation”) or in-phase, such that net acceleration doubled (4th column, “tilt+translation”). Clearly, if the brain relied only on the net acceleration information provided by otolith afferents, the actual motion would not be correctly perceived, resulting in problems of spatial disorientation. However, typically, this does not happen during daily activities. For example, as we swing in the playground, a motion that combines both tilt and translation, we correctly perceive our motion even when our eyes are closed (thus, excluding visual cues).
Indeed, there is now substantial evidence that the brain finds a solution to this ambiguity by combining otolith signals with extra-otolith rotational signals that arise from either the semicircular canals [44, 47, 48] or the visual system [49, 50]. Several computational studies have also tackled this problem to show that theoretically angular velocity signals arising from the semicircular canals can be transformed into estimates of head reorientation relative to gravity. The latter can then be subtracted from net gravitoinertial acceleration, the signal coded by primary otolith afferents, to extract translational motion information [44, 47, 51–61].
A Neural Solution in the Cerebellar Cortex
A neural correlate of the solution to the sensory ambiguity inherent in the peripheral otolith system has been found in the simple spike activities of NU Purkinje cells [36, 37]. As illustrated in Fig. 1b, the example Purkinje cell selectively responded during inertial motion (translation), while ignoring head reorientations relative to gravity [37]. Particularly notable is the fact that Purkinje cells modulate during the tilt−translation stimulus, although otolith afferents do not respond to this stimulus (because the net horizontal plane linear acceleration is zero; compare Fig. 1b with 1a; see also Angelaki [40]). Direct evidence that canal signals are used to compute an internal estimate of tilt relative to gravity that combines with otolith signals to estimate translation is illustrated in Fig. 1c, which plots a typical NU Purkinje cell response after all six semicircular canals were inactivated [37, 62]. After canal plugging, instead of encoding translation, the Purkinje cell activity resembles the otolith afferent response (i.e., it follows net acceleration). This shows that the tilt−translation responses observed in the intact animal (Fig. 1b, 3rd column) indeed arise from the semicircular canals, as illustrated by the lack of such modulation after canal plugging (Fig. 1c, 3rd column).
Population data in labyrinthine-intact and canal-plugged animals are shown in Fig. 2. Responses were quantified using a partial correlation analysis in which the responses of each cell to all four stimuli (translation, tilt, tilt−translation, and tilt+translation) were simultaneously fitted with “net acceleration-like” and “translation-coding” models; these models assume that neural firing rate modulation is either due to the net acceleration or due to the translational acceleration component (see the stimuli of Fig. 1; see also Angelaki [40]; Green [55] for details). Computed partial correlation coefficients were converted to z-score equivalents, to facilitate comparison between the two model fits (e.g., see Angelaki [40] for details). Data points falling in the upper/left quadrant (defined by the dashed lines corresponding to a 0.01-level of significance) illustrate cells which were significantly better fit with the translation-coding model. In contrast, data points falling in the lower/right quadrant correspond to cells that were significantly better fit with the net acceleration-like model. Data from a few otolith afferents fell, as expected, in the lower/right quadrant (Fig. 2, open triangles; data from Angelaki [40]). In contrast, data from NU Purkinje cells fell in the upper/left quadrant consistent with their encoding of translation (Fig. 2, black circles; data from Yakusheva [37]). Notably, however, after canal plugging, NU Purkinje cell data fell in the lower/right quadrant, illustrating that, in the absence of canal signals, the modulation of simple spikes became significantly better fit by the net acceleration-like model (Fig. 2, gray circles; data from Yakusheva [37]). These data clearly demonstrate that, when canal signals are no longer available to estimate the component of linear acceleration due to changes in head orientation relative to gravity, NU Purkinje cells respond similarly to tilt and translation.
Fig. 2.
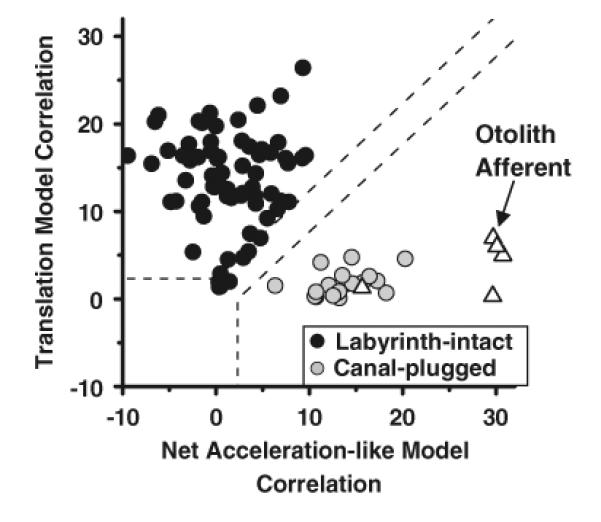
Scatter plots of z-transformed partial correlation coefficients for the fits of each cell's responses with the “translation-coding” and “net acceleration-like” models. Black circles represent NU simple spike responses from labyrinthine-intact animals, whereas gray circles represent data from canal-plugged animals. Open triangles illustrate responses from otolith afferents. The superimposed dashed lines divide the plots into three regions: an upper/left area corresponding to cell responses that were significantly better fit (p<0.01) by the translation-coding model; a lower/right area that includes neurons which were significantly better fit by the net acceleration model; and an in-between area that would include cells that were not significantly better fit by either model. Modified and replotted with permission from Angelaki [40] and Yakusheva [37]
In summary, by combining signals from both the otolith organs and the semicircular canals, NU Purkinje cells have solved the linear acceleration problem and selectively encode translation. In the next section, we dig deeper into the properties of the two vestibular signals that converge onto Purkinje cells. Specifically, the otolith contribution can be characterized by studying cell responses during pure translation whereas the canal drive can be isolated by studying cell responses during the tilt–translation stimulus (when the dynamic acceleration stimulus to the otoliths is zero and Purkinje cell modulation arises exclusively from activation of the semicircular canals).
Probing the Neural Computation: Spatio-temporally Matched Otolith/Canal Convergence
We start by describing the preferred directions of the otolith-driven (translation, Fig. 3a) and canal-driven (tilt–translation, Fig. 3b) components of the simple spike response. The preferred directions for both response components in the horizontal plane clustered around the oblique axes (Fig. 3a, b, top). Here, each dot corresponds to a cell with the distance to the origin denoting response gain, and its angular location denoting its preferred direction (see cartoon drawing). This clustering about oblique axes is particularly notable as it reflects the oblique orientation of the vertical semicircular canals in the head. The clustering of preferred directions close to the canal activation axes is further emphasized by histogram plots (Fig. 3a, b, bottom) illustrating the population distribution of preferred response directions over a range of 0° to 180° (i.e., data have been folded about the 0° to 180° axis). Importantly, even when the preferred directions for Purkinje cells were tested fully in 3D, they remained clustered along the preferred axes of the three semicircular canals [36].
Fig. 3.
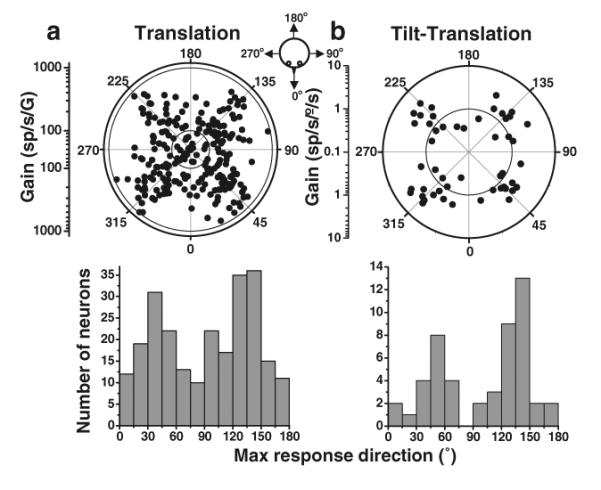
Preferred directions for simple spike responses to (a) translation and (b) tilt–translation (0.5 Hz). Top: Polar plot, where the radius corresponds to response gain and the polar angle illustrates the preferred (i.e., maximum response) direction in the horizontal plane. The inner and outer circles mark the tick marks of the gain scale. Each data point corresponds to one NU Purkinje cell. Bottom: Same data, now plotted as the distribution of preferred directions in the range (0°, 180°). Replotted with permission from Yakusheva [36]
It was particularly striking that the preferred directions during translation and tilt–translation were precisely matched on a cell-by-cell basis. This is illustrated in Fig. 4a, which plots the distribution of the absolute difference in preferred direction between the otolith-driven and canal-driven components of each Purkinje cell simple spike response. This precise matching also held true for response amplitude (Fig. 4b) and response phase (Fig. 4c). The latter is particularly remarkable since the neuronal response phase during translation varies broadly from cell to cell (e.g., Angelaki and Dickman [63]; Dickman and Angelaki [64]; Shaikh [65]), yet the canal-driven response for each Purkinje cell was processed dynamically relative to the velocity-encoding canal afferent signal such that it varied from cell to cell in a nearly identical way.
Fig. 4.

Spatio-temporal matching of canal-driven (tilt−translation) and otolith-driven (translation) signals. (a) Distribution of the difference in preferred directions between the 0.5 Hz tilt−translation and translation stimulus conditions. (b, c) Response amplitude and phase during the 0.5 Hz tilt−translation stimulus (canal-driven component) is plotted as a function of the respective amplitude and phase during translation (otolith-driven component). Replotted with permission from Yakusheva [37]
Evidence of Spatio-temporal Transformation of Canal Signals Within the Cerebellar Cortex
The evidence summarized so far illustrates that canal signals contribute to estimating translation by canceling the gravitational acceleration component of the net acceleration signal encoded by otolith afferents during tilt. But exactly how does this happen? Specifically, since semicircular canal afferents encode angular velocity, how is this information used to create a central estimate of gravitational acceleration? Indeed, to compute this estimate, canal signals must be altered in two important ways [37, 53, 55]:
First, they should make a contribution only when the head reorients relative to gravity. Note that some rotations change head orientation relative to gravity (e.g., pitch/roll from upright, as in Fig. 1), whereas others do not (e.g., yaw while upright). A rotation is described as “earth-horizontal (ωEH)” if its rotational axis is perpendicular to gravity, while a rotation is described as “earth-vertical (ωEV)” if its rotational axis aligns with gravity. Rotations around earth-horizontal axes (e.g., pitch/roll from upright, as in Fig. 1) change the head orientation with respect to gravity while rotations around earth-horizontal axes (e.g., yaw while upright) do not. Consequently, to accurately signal reorientation relative to gravity, it is important that Purkinje cells selectively encode only the earth-horizontal component of rotation, ωEH, but neither ωEV nor ω = ωEH + ωEV (the signal encoded by canal afferents; see Fig. 5). Second, this canal-driven, spatially-transformed signal must be temporally integrated (ʃωEH in Fig. 5), so that, rather than encoding velocity, they encode tilt position (i.e., a signal approximately proportional to gravitational acceleration).
Fig. 5.
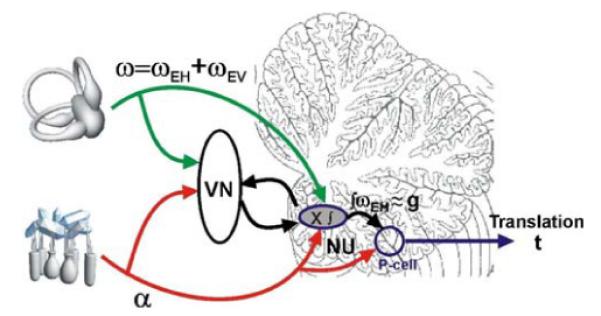
Schematic illustrating our working hypothesis regarding the relationship between the processing of semicircular canal and otolith signals within the NU. Semicircular canal afferents carry head-referenced angular velocity (ω), but the NU encodes only the earth-horizontal component (ωEH). This signal is temporally integrated (ʃωEH) and used to cancel the gravitational component (g) of net linear acceleration (α), the signal carried by otolith afferents. The resulting output is the inertial component (t)
Both of these predictions were shown to be true when examining the properties of the canal-driven response component of NU Purkinje cells [37]. Specifically, it was shown that, for head orientations close to upright, NU Purkinje cells only encoded the earth-horizontal component of rotation, ωEH (rather than ωEV or ω). This observation is of particular note because it provides a simple explanation for the curious observation that, despite a sizeable horizontal canal input, NU Purkinje cells do not modulate during upright yaw rotation [32, 33, 36, 37, 66]. The absence of yaw responsiveness makes sense because, as long as the animal remains upright, the horizontal canal signal during yaw rotation indicates rotation about an earth-vertical axis (ωEV), when there is no change in head orientation relative to gravity. However, as summarized in the previous section, NU Purkinje cells appear to use canal signals to extract changes in head reorientation relative to gravity and do not respond to ωEV. Note, however, that if yaw rotation were instead delivered with the animal oriented supine or ear-down such that the head did reorient relative to gravity (i.e., the yaw rotation now represents an ωEH stimulus; e.g., see Green [55]; Green and Angelaki [53, 54]), it is expected that the horizontal canal inputs to the nodulus/uvula would now be effective in driving Purkinje cell responses. This prediction, however, remains to be tested.
In summary, the two transformations outlined above ensure that: (1) a canal-driven gravitational estimate is only created during rotations that change head orientation relative to gravity; (2) this estimate is a signal proportional to gravitational acceleration (i.e., a tilt position rather than velocity-like signal) which is temporally appropriate to cancel the gravitational acceleration component of otolith afferent activities. Note also that this represents a simplification that is mathematically accurate only during small tilts relative to gravity when the horizontal gravitational acceleration stimulus activating otolith afferents is proportional to tilt angle (see Green [55]; Green and Angelaki [53, 54] for a more accurate description of the mathematical solution in 3D). Such a canal-driven estimate of head orientation could then be subtracted from the net linear acceleration signal provided by the otoliths and used to estimate inertial motion during navigation. Importantly, for this subtraction to work, the canal-driven signal unmasked by the tilt–translation stimulus must be both temporally and spatially matched on a cell-by-cell basis with the cell's response during translation, as already shown in Fig. 4. This “matched” convergence is a necessary condition for an effective solution to the tilt/translation ambiguity problem.
Recently, new evidence that this spatio-temporal transformation of canal signals occurs within the cerebellar cortex has been provided by pharmacological manipulations. Local injection of the GABA-a antagonist, Gabazine (using multi-barrel pipettes), destroys the ability of NU Purkinje cells to encode inertial motion; simple spikes instead respond to net linear acceleration (T.A. Yakusheva, D.E. Angelaki, and P.M. Blazquez, unpublished observations). Thus, removal of inhibition locally in the Purkinje cell layer preserves the otolith-driven response but eliminates the canal-driven response component. These preliminary results suggest that the spatio-temporal transformation needed to convert canal afferent information into an estimate of tilt position relative to gravity might utilize the intricate circuitry of the NU cortex.
Efferent Targets of the Nodulus/Uvula
The computations performed in NU could be used for both perception and motor functions. NU Purkinje cells project primarily to the vestibular nuclei but also to the rostral medial cerebellar (fastigial) nuclei [22, 67, 68]. Unlike the selective coding of translation by all NU Purkinje cells, a mixture of cells encoding inertial motion (translation) or net acceleration was found in these nuclei [40, 55, 62]. It is possible that there is a correlation between the signals vestibular and fastigial nuclei cells encode (i.e., translation versus net linear acceleration) and their connectivity with the nodulus/uvula. Our working hypothesis is that net linear acceleration-coding cells might project to the NU, whereas translation-coding cells are NU-target neurons. This hypothesis is currently being investigated by recording from vestibular nuclei neurons that have been identified as NU-target or NU-projecting neurons based on electrical stimulation of the nodulus/uvula.
A mixture of translation- and net linear acceleration-coding cells was also found in the ventral posterior nuclei of the thalamus to which the vestibular and fastigial/interposed nuclei project [69]. Other than the NU, the only brain area tested so far in which all neurons reliably encoded translation (as opposed to net acceleration) is cortical area MSTd (dorsal medical superior temporal area) in the dorsal visual stream [70]. This area is thought to be functionally linked to visual/vestibular multisensory integration for heading perception [71–73].
The remarkable similarities between the properties of MSTd neurons and those of NU Purkinje cells [70] might represent a physiological signature of a yet-unidentified multi-synaptic interconnectivity. We have hypothesized that there is a functional link between MSTd (and perhaps other extrastriate cortical areas with a role in heading perception) and the nodulus/uvula, which could be mediated by closed-loop anatomical circuits, an emerging architecture of cerebro–cerebellar interactions [74].
The Vestibular System Fails to Resolve the Ambiguity at Low Frequencies
The observations summarized above were made at 0.5 Hz, a frequency commonly experienced in everyday head movements. It is important to clarify that the vestibular system cannot resolve the tilt−translation ambiguity at low frequencies. Below ~0.1 Hz, in the absence of other, extra-vestibular cues (e.g., from vision), linear accelerations are incorrectly interpreted as tilt even when generated by translational motion [51, 57, 58, 75, 76]. In fact, it is typically at these low frequencies that perceptual illusions occur (“somatogravic/oculogravic illusions”; see Graybiel [77]; Clark and Graybiel [78, 79]). This is because, at low frequencies, the semicircular canals no longer provide a veridical estimate of rotation [80], and extra-vestibular information, most notably from visual cues, becomes critical in order to avoid spatial disorientation [49, 50, 81, 82].
Remarkably, simple spike NU activity follows these behavioral observations [36]. That is, at low frequencies, canal signals are no longer temporally matched to the otolith response, and thus they are no longer appropriate to cancel the otolith gravitational acceleration component and extract translation. As a result, as frequency decreases, NU Purkinje cells progressively increase their modulation in response to tilt [31, 32, 36].
Conclusions and Future Directions
The exquisitely elegant circuitry of the cerebellar cortex has often been described as ideal for precise spatial and temporal computations [38, 83–85]. Here, we have summarized recent evidence that part of this circuitry is likely instrumental in solving a computationally challenging problem related to vestibular function. The mathematical solution to this physical ambiguity involves solving a vector differential equation where estimation of inertial motion along a given direction requires knowledge of the gravitational acceleration along the other directions [44, 53–55]. We have summarized here evidence that, for the range of motions considered thus far (i.e., small tilts and translation from an upright head/body orientation), the simple spike responses of NU Purkinje cells combine precisely matched otolith- and canal-driven signals to appropriately recover inertial motion information, fundamental for heading perception and spatial navigation.
Although this work has provided a small glimpse into the potential contributions of the cerebellar NU in spatial orientation, it is important to acknowledge that this is just the beginning. We have gained some understanding of the properties of simple spike modulation during translations and tilts at different frequencies, but we still know little about their properties under conditions of sensory conflict and spatial disorientation. Furthermore, the role that complex spikes and other cerebellar cells play in performing the required computations remains unknown. Equally important is the question of how visual cues drive and/or modify simple and complex spike activity and the role of these signals in long-term adaptation. A particularly important focus of future studies will be to investigate the functional connectivity of the NU with cortical areas that have been implicated in heading perception, navigation, and spatial orientation using modern neuroanatomical techniques, including transynaptic tracers. This will provide new insights into the flow of information processing under situations of spatial disorientation, as well as the short- and long-term changes observed in altered gravity environments. Investigations of the midline vestibulo-cerebellum thus promise to provide new insights into the role of the cerebellar cortex in complex spatio-temporal computations and multisensory integration and will continue to help unravel general functional and computational principles of organization of this fundamental brain structure.
Acknowledgements
The work was supported by NIH R01 EY12814.
References
- 1.Blazquez PM, Hirata Y, Highstein SM. The vestibulo-ocular reflex as a model system for motor learning: what is the role of the cerebellum? Cerebellum. 2004;3(3):188–192. doi: 10.1080/14734220410018120. [DOI] [PubMed] [Google Scholar]
- 2.Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- 3.du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci. 1995;18:409–441. doi: 10.1146/annurev.ne.18.030195.002205. [DOI] [PubMed] [Google Scholar]
- 4.Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 5.Barmack NH, Baughman RW, Errico P, Shojaku H. Vestibular primary afferent projection to the cerebellum of the rabbit. J Comp Neurol. 1993;327:521–534. doi: 10.1002/cne.903270405. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter MB, Stein BM, Peter P. Primary vestibulocerebellar fibers in the monkey: distribution of fibers arising from distinctive cell groups of the vestibular ganglia. Am J Anat. 1972;135:221–249. doi: 10.1002/aja.1001350209. [DOI] [PubMed] [Google Scholar]
- 7.Kevetter GA, Perachio AA. Distribution of vestibular afferents that innervate the sacculus and posterior canal in the gerbil. J Comp Neurol. 1986;254(3):410–424. doi: 10.1002/cne.902540312. [DOI] [PubMed] [Google Scholar]
- 8.Korte GE. The cerebellar projection of the vestibular nerve in the cat. J Comp Neurol. 1979;184(2):265–277. doi: 10.1002/cne.901840204. [DOI] [PubMed] [Google Scholar]
- 9.Gerrits NM, Epema AH, van Linge A, Dalm E. The primary vestibulocerebellar projection in the rabbit: absence of primary afferents in the flocculus. Neurosci Lett. 1989;105(1–2):27–33. doi: 10.1016/0304-3940(89)90006-2. [DOI] [PubMed] [Google Scholar]
- 10.Kevetter GA, Leonard RB, Newlands SD, Perachio AA. Central distribution of vestibular afferents that innervate the anterior or lateral semicircular canal in the Mongolian gerbil. J Vestib Res. 2004;14(1):1–15. [PubMed] [Google Scholar]
- 11.Ono S, Kushiro K, Zakir M, Meng H, Sato H, Uchino Y. Properties of utricular and saccular nerve-activated vestibulocerebellar neurons in cats. Exp Brain Res. 2000;134(1):1–8. doi: 10.1007/s002210000424. [DOI] [PubMed] [Google Scholar]
- 12.Brodal A, Brodal P. Observations on the secondary vestibulocerebellar projections in the macaque monkey. Exp Brain Res. 1985;58(1):62–74. doi: 10.1007/BF00238954. [DOI] [PubMed] [Google Scholar]
- 13.Epema AH, Gerrits NM, Voogd J. Secondary vestibulocerebellar projections to the flocculus and unulo-nodular lobule of the rabbit: a study using HRP and double fluorescent tracer techniques. Exp Brain Res. 1990;80(1):72–82. doi: 10.1007/BF00228849. [DOI] [PubMed] [Google Scholar]
- 14.Akaogi K-I, Ikarashi K, Kawasaki T. Mossy fiber projections from the brain stem to the nodulus in the cat: an experimental study comparing the nodulus, the uvula and the flocculus. Brain Res. 1994;638(1–2):12–20. doi: 10.1016/0006-8993(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Kanda K-I, Ikarashi K, Kawasaki T. Differential mossy fiber projections to the dorsal and ventral uvula in the cat. J Comp Neurol. 1989;279(1):149–164. doi: 10.1002/cne.902790113. [DOI] [PubMed] [Google Scholar]
- 16.Ruigrok TJ. Collateralization of climbing and mossy fibers projecting to the nodulus and flocculus of the rat cerebellum. J Comp Neurol. 2003;466(2):278–298. doi: 10.1002/cne.10889. [DOI] [PubMed] [Google Scholar]
- 17.Bigare F, Voogd J. Cerebello-vestibular projections in the cat. Acta Morphol Neerl Scand. 1977;15(4):323–325. [PubMed] [Google Scholar]
- 18.Bernard J-F. Topographical organization of olivocerebellar and corticonuclear connections in the rat-An WGA-HRP study: I. Lobules IX, X and the flocculus. J Comp Neurol. 1987;263(2):241–258. doi: 10.1002/cne.902630207. [DOI] [PubMed] [Google Scholar]
- 19.Groenewegen HJ, Voogd J. Parasagittal zonation within the olivocerebellar projection. I. Climbing fiber distribution in the vermis of cat cerebellum. J Comp Neurol. 1977;174(3):417–488. doi: 10.1002/cne.901740304. [DOI] [PubMed] [Google Scholar]
- 20.Brodal P, Brodal A. Further observation on the olivocerebellar projection in the monkey. Exp Brain Res. 1982;45:71–83. doi: 10.1007/BF00235764. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman GD, Mustari MJ, Miselis RR, Perachio AA. Transneuronal pathways to the vestibulocerebellum. J Comp Neurol. 1996;370:501–523. doi: 10.1002/(SICI)1096-9861(19960708)370:4<501::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Voogd J, Gerrits NM, Ruigrok TJH. Organization of the vestibulocerebellum. Ann N Y Acad Sci. 1996;781:553–579. doi: 10.1111/j.1749-6632.1996.tb15728.x. [DOI] [PubMed] [Google Scholar]
- 23.Angelaki DE, Hess BJ. Lesion of the nodulus and ventral uvula abolish steady-state off-vertical axis otolith response. J Neurophysiol. 1995;73(4):1716–1720. doi: 10.1152/jn.1995.73.4.1716. [DOI] [PubMed] [Google Scholar]
- 24.Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. 1995;73(5):1729–1751. doi: 10.1152/jn.1995.73.5.1729. [DOI] [PubMed] [Google Scholar]
- 25.Wearne S, Raphan T, Cohen B. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol. 1998;79:2690–2715. doi: 10.1152/jn.1998.79.5.2690. [DOI] [PubMed] [Google Scholar]
- 26.Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. Lesions of the cerebellar nodulus and uvula in monkeys: effect on otolith-ocular reflexes. Prog Brain Res. 2008;171:167–172. doi: 10.1016/S0079-6123(08)00622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marini G, Provini L, Rosina A. Macular input to the cerebellar nodulus. Brain Res. 1975;99(2):367–371. doi: 10.1016/0006-8993(75)90038-4. [DOI] [PubMed] [Google Scholar]
- 28.Marini G, Provini L, Rosina A. Gravity responses of Purkinje cells in the nodulus. Exp Brain Res. 1976;24:311–323. doi: 10.1007/BF00235018. [DOI] [PubMed] [Google Scholar]
- 29.Precht W, Simpson JI, Llin SR. Responses of Purkinje cells in rabbit nodulus and uvula to natural vestibular and visual stimuli. Pflugers Arch. 1976;367(1):1–6. doi: 10.1007/BF00583649. [DOI] [PubMed] [Google Scholar]
- 30.Fushiki H, Barmack NH. Topography and reciprocal activity of cerebellar Purkinje cells in the uvula-nodulus modulated by vestibular stimulation. J Neurophysiol. 1997;78(6):3083–3094. doi: 10.1152/jn.1997.78.6.3083. [DOI] [PubMed] [Google Scholar]
- 31.Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in Purkinje cells. J Neurosci. 2003;23(21):7904–7916. doi: 10.1523/JNEUROSCI.23-21-07904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yakhnitsa V, Barmack NH. Antiphasic Purkinje cell responses in mouse uvula-nodulus are sensitive to static roll–tilt and topographically organized. Neuroscience. 2006;143(2):615–626. doi: 10.1016/j.neuroscience.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Barmack NH, Shojaku H. Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74(6):2573–2589. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- 34.Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Dev Brain Res. 2003;140(2):223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- 35.Barmack NH, Shojaku H. Vestibularly induced slow oscillations in climbing fiber responses of Purkinje cells in the cerebellar nodulus of the rabbit. Neuroscience. 1992;50(1):1–5. doi: 10.1016/0306-4522(92)90376-d. [DOI] [PubMed] [Google Scholar]
- 36.Yakusheva T, Blazquez PM, Angelaki DE. Frequency-selective coding of translation and tilt in macaque cerebellar nodulus and uvula. J Neurosci. 2008;28(40):9997–10009. doi: 10.1523/JNEUROSCI.2232-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54(6):973–985. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3–5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 40.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430(6999):560–564. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- 41.Dickman JD, Angelaki DE, Correia MJ. Response properties of gerbil otolith afferents to small angle pitch and roll tilts. Brain Res. 1991;556(2):303–310. doi: 10.1016/0006-8993(91)90320-u. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol. 1972;35(6):978–987. doi: 10.1152/jn.1972.35.6.978. [DOI] [PubMed] [Google Scholar]
- 43.Si X, Angelaki DE, Dickman JD. Response properties of pigeon otolith afferents to linear acceleration. Exp Brain Res. 1997;117(2):242–250. doi: 10.1007/s002210050219. [DOI] [PubMed] [Google Scholar]
- 44.Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJ. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci. 1999;19(1):316–327. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol. 1976;39(5):985–995. doi: 10.1152/jn.1976.39.5.985. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39(5):970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- 47.Green AM, Angelaki DE. Resolution of sensory ambiguities for gaze stabilization requires a second neural integrator. J Neurosci. 2003;23(28):9265–9275. doi: 10.1523/JNEUROSCI.23-28-09265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- 49.MacNeilage PR, Banks MS, Berger DR, Bulthoff HH. A Bayesian model of the disambiguation of gravitoinertial force by visual cues. Exp Brain Res. 2007;179(2):263–290. doi: 10.1007/s00221-006-0792-0. [DOI] [PubMed] [Google Scholar]
- 50.Zupan LH, Merfeld DM. Neural processing of gravito-inertial cues in humans. IV. Influence of visual rotational cues during roll optokinetic stimuli. J Neurophysiol. 2003;89(1):390–400. doi: 10.1152/jn.00513.2001. [DOI] [PubMed] [Google Scholar]
- 51.Glasauer S. Linear acceleration perception: frequency dependence of the hilltop illusion. Acta Otolaryngol. 1995;S520:37–40. doi: 10.3109/00016489509125184. [DOI] [PubMed] [Google Scholar]
- 52.Glasauer S, Merfeld DM. Modeling three-dimensional responses during complex motion stimulation. In: Fetter MTH, Misslisch H, Tweed D, editors. Three-dimensional kinematics of eye, head, and limb movements. Howard Academic Press; Amsterdam: 1997. pp. 387–398. [Google Scholar]
- 53.Green AM, Angelaki DE. An integrative neural network for detecting inertial motion and head orientation. J Neurophysiol. 2004;92(2):905–925. doi: 10.1152/jn.01234.2003. [DOI] [PubMed] [Google Scholar]
- 54.Green AM, Angelaki DE. Coordinate transformations and sensory integration in the detection of spatial orientation and self-motion: from models to experiments. Prog Brain Res. 2007;165:155–180. doi: 10.1016/S0079-6123(06)65010-3. [DOI] [PubMed] [Google Scholar]
- 55.Green AM, Shaikh AG, Angelaki DE. Sensory vestibular contributions to constructing internal models of self-motion. J Neural Eng. 2005;2(3):S164–S179. doi: 10.1088/1741-2560/2/3/S02. [DOI] [PubMed] [Google Scholar]
- 56.Merfeld DM. Modeling the vestibulo-ocular reflex of the squirrel monkey during eccentric rotation and roll tilt. Exp Brain Res. 1995;106(1):123–134. doi: 10.1007/BF00241362. [DOI] [PubMed] [Google Scholar]
- 57.Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined tilt & translation. J Neurophysiol. 2005;94(1):199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- 58.Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol. 2005;94(1):186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- 59.Merfeld DM, Zupan LH. Neural processing of gravitoinertial cues in humans. III. Modeling tilt and translation responses. J Neurophysiol. 2002;8792:819–833. doi: 10.1152/jn.00485.2001. [DOI] [PubMed] [Google Scholar]
- 60.Mergner T, Glasauer S. A simple model of vestibular canal-otolith signal fusion. Ann N Y Acad Sci. 1999;871:430–434. doi: 10.1111/j.1749-6632.1999.tb09211.x. [DOI] [PubMed] [Google Scholar]
- 61.Zupan LH, Merfeld DM, Darlot C. Using sensory weighting to model the influence of canal, otolith and visual cues on spatial orientation and eye movements. Biol Cybern. 2002;86(3):209–230. doi: 10.1007/s00422-001-0290-1. [DOI] [PubMed] [Google Scholar]
- 62.Shaikh AG, Green AM, Ghasia FF, Newlands SD, Dickman JD, Angelaki DE. Sensory convergence solves a motion ambiguity problem. Curr Biol. 2005;15(18):1657–1662. doi: 10.1016/j.cub.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84(4):2113–2132. doi: 10.1152/jn.2000.84.4.2113. [DOI] [PubMed] [Google Scholar]
- 64.Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol. 2002;88(6):3518–3533. doi: 10.1152/jn.00518.2002. [DOI] [PubMed] [Google Scholar]
- 65.Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005;93(2):853–863. doi: 10.1152/jn.00879.2004. [DOI] [PubMed] [Google Scholar]
- 66.Barmack NH, Shojaku H. Representation of a postural coordinate system in the nodulus of the rabbit cerebellum by vestibular climbing fiber signals. In: Shimazu H, Shinoda Y, editors. Vestibular and brain stem control of eye, head and body movements. Basel; S. Karger: 1992. pp. 331–338. [Google Scholar]
- 67.Shojaku H, Sato Y, Ikarashi K, Kawasaki T. Topographical distribution of Purkinje cells in the uvula and the nodulus projecting to the vestibular nuclei in cats. Brain Res. 1987;416(1):100–112. doi: 10.1016/0006-8993(87)91501-0. [DOI] [PubMed] [Google Scholar]
- 68.Wylie DR, De Zeeuw CI, Digiorgi PL, Simpson JI. Projections of individual Purkinje cells of identified zones in the ventral nodulus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol. 1994;349(3):448–463. doi: 10.1002/cne.903490309. [DOI] [PubMed] [Google Scholar]
- 69.Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci. 2007;27(50):13590–13602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu S, Angelaki DE. Vestibular signals in macaque extrastriate visual cortex are functionally appropriate for heading perception. J Neurosci. 2009;29(28):8936–8945. doi: 10.1523/JNEUROSCI.1607-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Britten KH, van Wezel RJ. Electrical microstimulation of cortical area MST biases heading perception in monkeys. Nat Neurosci. 1998;1(1):59–63. doi: 10.1038/259. [DOI] [PubMed] [Google Scholar]
- 72.Gu Y, Angelaki DE, Deangelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci. 2008;11(10):1201–1210. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10(8):1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 75.Seidman SH, Telford L, Paige GD. Tilt perception during dynamic linear acceleration. Exp Brain Res. 1998;119(3):307–314. doi: 10.1007/s002210050346. [DOI] [PubMed] [Google Scholar]
- 76.Kaptein RG, Van Gisbergen JA. Canal and otolith contributions to visual orientation constancy during sinusoidal roll rotation. J Neurophysiol. 2006;95(3):1936–1948. doi: 10.1152/jn.00856.2005. [DOI] [PubMed] [Google Scholar]
- 77.Graybiel A. Oculogravic illusion. AMA Arch Ophthalmol. 1952;48(5):605–615. doi: 10.1001/archopht.1952.00920010616007. [DOI] [PubMed] [Google Scholar]
- 78.Clark B, Graybiel A. Contributing factors in the perception of the oculogravic illusion. Am J Psychol. 1963;76:18–27. [PubMed] [Google Scholar]
- 79.Clark B, Graybiel A. Factors contributing to the delay in the perception of the oculogravic illusion. Am J Psychol. 1966;79(3):377–388. [PubMed] [Google Scholar]
- 80.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- 81.Dichgans J, Held R, Young LR, Brandt T. Moving visual scenes influence the apparent direction of gravity. Science. 1972;176:1217–1219. doi: 10.1126/science.178.4066.1217. [DOI] [PubMed] [Google Scholar]
- 82.Howard IP, Hu G. Visually induced reorientation illusions. Perception. 2001;30(5):583–600. doi: 10.1068/p3106. [DOI] [PubMed] [Google Scholar]
- 83.Jacobson GA, Rokni D, Yarom Y. A model of the olivocerebellar system as a temporal pattern generator. Trends Neurosci. 2008;31(12):617–625. doi: 10.1016/j.tins.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26(4):222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 85.Yarom Y, Cohen D. The olivocerebellar system as a generator of temporal patterns. Ann N Y Acad Sci. 2002;978:122–134. doi: 10.1111/j.1749-6632.2002.tb07561.x. [DOI] [PubMed] [Google Scholar]


