Abstract
Ascorbic acid (Vitamin C) has a critical role in bone formation and osteoblast differentiation, but very little is known about the molecular mechanisms of ascorbic acid entry into bone marrow stromal cells (BMSCs). To address this gap in knowledge, we investigated the identity of the transport system that is responsible for the uptake of ascorbic acid into bone marrow stromal cells (BMSCs). First, we examined the expression of the two known isoforms of the sodium-coupled ascorbic acid transporter, namely SVCT1 and SVCT2, in BMSCs (Lin-ve Sca1+ve) and bone at the mRNA level. Only SVCT2 mRNA was detected in BMSCs and bone. Uptake of ascorbic acid in BMSCs was Na+-dependent and saturable. In order to define the role of SVCT2 in BMSC differentiation into osteoblasts, BMSCs were stimulated with osteogenic media for different time intervals, and the activity of SVCT2 was monitored by ascorbic acid uptake. SVCT2 expression was up-regulated during the osteogenic differentiation of BMSCs; the expression was maximal at the earliest phase of differentiation. Subsequently, osteogenesis was inhibited in BMSCs upon knock-down of SVCT2 by lentivirus shRNA. We also found that the expression of the SVCT2 could be negatively or positively modulated by the presence of oxidant (Sin-1) or antioxidant (Ascorbic acid) compounds, respectively, in BMSCs. Furthermore, we found that this transporter is also regulated with age in mouse bone. These data show that SVCT2 plays a vital role in the osteogenic differentiation of BMSCs and that its expression is altered under conditions associated with redox reaction. Our findings could be relevant to bone tissue engineering and bone related diseases such as osteoporosis in which oxidative stress and aging plays important role.
Keywords: bone marrow stromal cells, SVCT2, Ascorbic acid, Osteogenesis, oxidative stress, Aging
Introduction
Ascorbic acid (AA; vitamin C) is a water-soluble vitamin and the primary antioxidant with ability to scavenge reactive oxygen and nitrogen species (Levine et al., 1999; Conner et al 1996). It decreases oxidative stress associated with the bone-resorptive process and might help in the prevention of osteoporosis (Basu et al., 2001; Ruiz-Ramos et al., 2010). Ascorbic acid is also an essential cofactor for prolyl hydroxylase (Togari et al., 1995) and in-vitro differentiation of osteoblasts and other mesenchymal-derived cells (Franceschi et al., 1992). The bone matrix contains over 90% of protein as collagen (Termine et al., 1990) and it is well known that ascorbic acid is an essential cofactor for collagen synthesis and maturation (Peterkofsky et al., 1991). Animal studies have demonstrated that deficiency of this vitamin leads to impaired bone mass, cartilage, and connective tissue (Poal-Manresa et al., 1970; Kipp et al., 1996; Miyajima et al 1995). In addition to its involvement in collagen synthesis, ascorbic acid has also been reported to be necessary for the proliferation and multilayering of osteoblastic cells (Bellows et al., 1986). Being highly water-soluble, ascorbic acid cannot permeate the hydrophobic plasma membrane of cells and hence requires a specific transport process to enter into mammalian cells. The entry of ascorbic acid into mammalian cells is mediated by a Na+-dependent transport system (Wilson et al., 1989; Franceschi et al., 1995). This is an active process and it enables cells to concentrate ascorbic acid against a concentration gradient. Most of the studies in literature related to ascorbic acid and bone metabolism have been carried out using the murine osteoblastic-like cell line MC3T3-E1. MC3T3-E1 cells are the pre-osteoblastic and are commonly employed for studies of osteoblast differentiation (Schindeler et al., 2010). In contrast, bone marrow stromal cells (BMSCs) are progenitor cells which differentiate into osteoblasts, osteocytes, adipocytes, and cartilage (Prockop et al., 1997; Pittenger et al., 1999). BMSCs can be harvested from bone marrow, expanded in culture, and induced to differentiate. Ascorbic acid and collagen synthesis are important aspects of BMSC differentiation and bone formation (Miyajima et al., 1995). However, little is known about the molecular aspects of the transport process that is responsible for active accumulation of ascorbic acid in these pluripotent stem cells.
The purpose of this investigation was to provide the first detailed analysis of the ascorbic acid uptake process in mouse primary BMSCs as well as its regulation during osteogenesis and oxidative stress. To this end, we isolated lineage-negative Sca1-positive BMSCs and used them for the characterization of the ascorbic acid transport process at the functional and molecular levels in undifferentiated state and during their differentiation into osteoblasts. In addition, we induced oxidative stress in cell culture system using Sin-1 and performed uptake assay to investigate SVCT2 regulation and protective effect of ascorbic acid on BMSCs.
Material and methods
Animals
This study was approved by the Institutional Animal Care and Use Committee of the Georgia Health Science University.
Isolation of BMSCs from Mice
Murine BMSCs were isolated from the long bones of 6 month-old C57BL/6 mice (n=6). The mice were euthanized and the femurs and humeri removed. The marrow was then flushed with phosphate-buffered saline (PBS) and the cellular material harvested. The cellular material was then centrifuged, the supernatant discarded and the pellet washed with PBS. The cells were then plated in 100-cm2 culture plates with DMEM, supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U/mL penicillin/streptomycin, and 2 mM L-glutamine. After 24 h, the supernatant were removed and the adherent stromal cells trypsinized for negative selection. A negative selection process was used to deplete hematopoietic cell lineages (T- and B-lymphocytic, myeloid and erythroid cells) using a commercially available kit (BD biosciences), thus retaining the progenitor (stem) cell population. The positive fractions were collected using the following parameters: negative for CD3e (CD3 ε chain), CD11b (integrin αM chain), CD45R/B220, Ly-6G and Ly-6C (Gr-1), and TER-119/Erythroid Cells (Ly-76). Next, positive selections were performed using the anti-Stem cell antigen-1 (Sca-1) column magnetic bead sorting kit (Miltenyi Biotec,).
Isolation of RNA, synthesis of cDNA, and real-time PCR
Total RNA was isolated from long bone (femur), calvaria, BMSCs and liver of mice. For aging studies, 12 month (n=5) and 24 month (n=5) mice were sacrificed and femora were excised. The epiphyses of the femur were removed and the marrow was flushed out with PBS. The diaphyses were then snap-frozen in liquid nitrogen. In the case of femur, calvaria, and liver, the tissue was ground in liquid N2 with a pestle and mortar, and the powdered tissue was dissolved in Trizol for RNA isolation. For BMSCs, cell pellet was directly dissolved in trizol. RNA was isolated using the Trizol method following manufacturer’s instructions, and the quality of the RNA preparations was monitored by absorbance at 260 and 280 nm (Helios-Gamma, Thermo Spectronic, Rochester, NY). The RNA was reverse-transcribed into complementary deoxyribonucleic acid (cDNA) using iScript reagents from Bio-Rad on a programmable thermal cycler (PCR-Sprint, Thermo Electron, Milford, MA). 50 ng of cDNA was amplified in each real-time PCR using a Bio-Rad iCycler, ABgene reagents (Fisher scientific) using appropriate primers (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for normalization.
Table 1.
Nucleotide sequences of mouse primers used for RT-PCR
| Gene | Primer | Product size in base pair | Annealing temperature (°C) | Reference/Accession Number |
|---|---|---|---|---|
| GAPDH | CAT GGC CTC CAA GGA GTA AGA GAG GGA GAT GCT CAG TGT TGG |
105 | 60 | M32599 |
| SVCT1 | GGC ATC ATT GAG TCC ATC GG ATA GCC CCG CGA TGA TGC AG |
117 | 60 | Kuo et al 2004 |
| SVCT2 | TAA TCC TGG CTA TCC TCG TG CAT CTG TGC GTG CAT AGT AGC |
105 | 60 | Kuo et al 2004 |
| Collagen type I | GCC CAT TAG CCG GTA TGT TAT TA TCC CTG GTA CCT ATG GAG ACT GT |
112 | 60 | U50767.1 |
| BMP-2 | TGT TTG GCC TGA AGC AGA GA TGA GTG CCT GCG GTA CAG AT |
83 | 60 | NM_007553.2 |
| RUNX-2 | GGA AAG GCA CTG ACT GAC CTA ACA AAT TCT AAG CTT GGG AGG A |
103 | 60 | NM_009820 |
| Osteocalcin | ATT TAG GAC CTG TGC TGC CCT A GGA GCT GCT GTG ACA TCC ATA C |
120 | 60 | U11542.1 |
Immunocytochemical analysis of SVCT2 expression in mice BMSCs
The mouse BMSCs (Lin-ve Sca-1+) cells were grown on multi-chamber slide for 24hrs and then fixed in ice-cold 4% paraformaldehyde for immunostaining. The slides were stained with goat anti-SVCT-2 polyclonal antibody (1:50 dilution) (Santa Cruz) followed by FITC-labeled anti-goat IgG as the secondary antibody (1:200) (Sigma-Aldrich). The slides were counterstained with Hoechst dye to label nuclei and then mounted in aqueous medium
Uptake Studies
BMSCs were seeded in 24-well plates at an initial density of 0.01 ×106 cells/well. Uptake of [14C] ascorbic acid was measured on 3rd day post-seeding. The medium was removed by aspiration and the cells washed with uptake buffer once. Uptake was initiated by adding 0.25 mL of uptake buffer containing [14C] ascorbic acid (20 nM). Initial experiments were carried out to determine the time course of uptake. Subsequent uptake measurements were made with 15 min incubation representing initial uptake rates. Uptake was terminated by aspiration of the uptake buffer from the cells. The cell monolayers were quickly washed twice with ice-cold uptake buffer without the radiolabeled substrate. Cells were then lysed in 0.5 ml of 1% SDS/0.2N NaOH and the radioactivity associated with the cells was quantified. The composition of uptake buffer in most experiments was: 25 mM HEPES, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5 mM glucose, and 140 mM NaCl. When sodium-free buffer was used, NaCl in the uptake buffer was replaced iso-osmotically with N-methyl-D-glucamine chloride (NMDG.Cl). When chloride-free buffer was used, NaCl in the uptake buffer was replaced iso-osmotically with sodium gluconate. In addition, the transport buffer was supplemented with 1 mM dithiothreitol (DTT) to maintain ascorbic acid in the reduced state. Samples were analyzed using liquid scintillation counter (Beckman Instruments Inc., Fullerton, CA, USA, model LS-6500) and rate of uptake was normalized to the protein content of each well. The amount of protein in the cell lysate was measured using the BCA Protein Estimation Kit.
The time course of [14C] ascorbic acid (20 nM ) uptake in Na+ -containing buffer (NaCl) and Na+-free (choline chloride) buffer was established by conducting uptake at 2.5, 5, 15, 30, 45 and 60 min at 37 °C. Studies of concentration dependency (range, 10 μM – 1 mM) were conducted at 37 °C using 15 min incubation representing initial uptake rates. For analysis of substrate specificity of the transport process, uptake of [14C] ascorbic acid was measured in the absence and presence of various unlabeled vitamins (ascorbic acid, thiamine, riboflavin, myoinositol, and nicotinic acid; concentration, 5 mM).
Differentiation of BMSCs under Osteogenic Conditions
BMSCs were trypsinized, harvested, washed and plated in 24-well plates at a density of 5,000 cells/cm2. The cells were allowed to adhere and grow to ~90% confluence before being changed to an osteogenic medium (OM). The OM contained DMEM supplemented with 5% FBS, 50 U/mL penicillin/streptomycin, 10nM dexamethasone, 0.25 mM L-ascorbic acid and 10 mM β-glycerophosphate. Uptake assays were done after culturing the cells in osteogenic medium for 24 h, 48 h, 4 days, 6 days, 9 days and 12 days. RNA isolation was done at 3, 6, and 9 day time points whereas alizarin red assay were done at 3 days, 6 days, 9 days, 12 days, and 16 day time points. BMSCs were also stimulated with each component of osteogenic media separately (1 μM dexamethasone, 0.25 mM L-ascorbic acid and 10 mM β-glycerophosphate) for 24 h, and 48 h then used for measurements of ascorbic acid uptake.
Alizarin Red Assay
To quantify matrix calcification by the cultured BMSCs, we used Alizarin Red S binding assay as described in the literature (Hessle et al., 2002) with some modifications. In brief, BMSCs (5,000 cells/cm2) were plated in 24-well plates with osteogenic media. At each time point, the medium was removed, and washed with PBS twice. The cells were fixed with 70% ethanol for 30 min at 4 °C. The cells were then stained with Alizarin red solution (40 mM; 300 μl/well) for 10 min and then washed with PBS until the supernatant was clear. The staining extent was recorded by photography and the retained dye was then extracted with 0.25 ml of 10% (wt/vol) cetylpyridinium chloride solution for 10 min. The solution was diluted at a ratio of 1:10 and read at 570 nm with a spectrophotometer.
Knockdown of SVCT2 activity using Lentivirus
All work with lentiviruses was performed under BSL2 conditions. The lentiviral particles shSVCT2 (sc-41008-V), shControl (sc-108080), Polybrene® (sc-134220) and puromycin (sc-108071) was purchased from Santa Cruz Biotechnology, Inc. USA. In short, BMSCs were plated at 30–50% confluence and transfected with appropriate dilutions of lentivirus particles and polybrene. Forty-eight hours after transfection, the cells were cultured in growth medium containing puromycin (2ug/ml) to obtain the stable, transfected BMSC cells. The efficiency of shRNA activity was analysis by real-time PCR and uptake assay as describes above. Osteogenic differentiation studies of transfected cell were done as described above.
Regulation of SVCT2 transporter by Redox reaction
In order to investigate the effect of redox reaction on ascorbic acid uptake in BMSCs, we preincubated the cells with oxidant (Sin-1 200 μM, 400 μM and 600 μM), antioxidant (Ascorbic acid 250μM) and a combination of oxidant and antioxidant for 18hrs and then used the cells for uptake experiments.
Statistical Analysis
Data are expressed as the mean ± SD. Differences in measured variables between experimental and control groups were assessed using Student’s t-test. A p-value < 0.05 was considered statistically significant in between control and treatment groups.
Results
Expression of Vitamin C transporters in BMSCs, long bone and calvaria
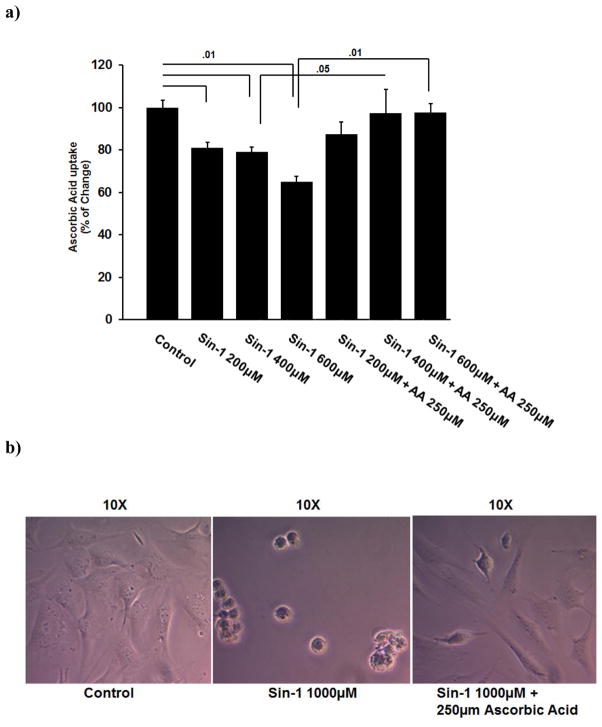
The SVCT family consists of SVCT1 and SVCT2, and both isoforms transport ascorbic acid into cells in a Na+-coupled manner. Therefore, it was not possible to establish the identity of the isoform that was responsible for ascorbic acid uptake in BMSCs by functional studies alone. Hence, prior to performing uptake studies, we analyzed the expression of SVCT1 mRNA and SVCT2 mRNA in mouse BMSCs, long bone, calvaria and liver (positive control) by RT–PCR. We used specific primers (Kuo et al., 2004) for each isoform of the transporter. We found no evidence of expression of SVCT1 in BMSCs, bone, and calvaria (Fig. 1a). In contrast, SVCT2 mRNA was detectable in BMSCs, bone, and calvaria. These results showed that BMSCs, bone, and calvaria in mice express only SVCT2. Liver RNA was used as a positive control for both isoforms of the transporter.
Figure 1.
RT-PCR showing the absence of SVCT1 and presence of SVCT2 in mouse BMSCs, Bone and cavaria. The primers used and other experimental details for RT-PCR are provided in Materials and Methods. (a) Lane 1: Mouse BMSCs, Lane 2: Mouse Bone, Lane 3: Mouse cavaria Lane 4: Positive control (Mouse liver). Upper panel shows SVCT1 and Lower panel shows SVCT2 (b) Immunohistochemical localization of SVCT2 protein in mice bone marrow. (c) Immunohistochemical localization of SVCT2 protein in mice BMSCs. The immunofluorescence analysis was performed using a polyclonal antibody specific for SVCT2. Secondary antibody was anti-rabbit IgG labeled with FITC. The nuclei were stained with Hoechst dye.
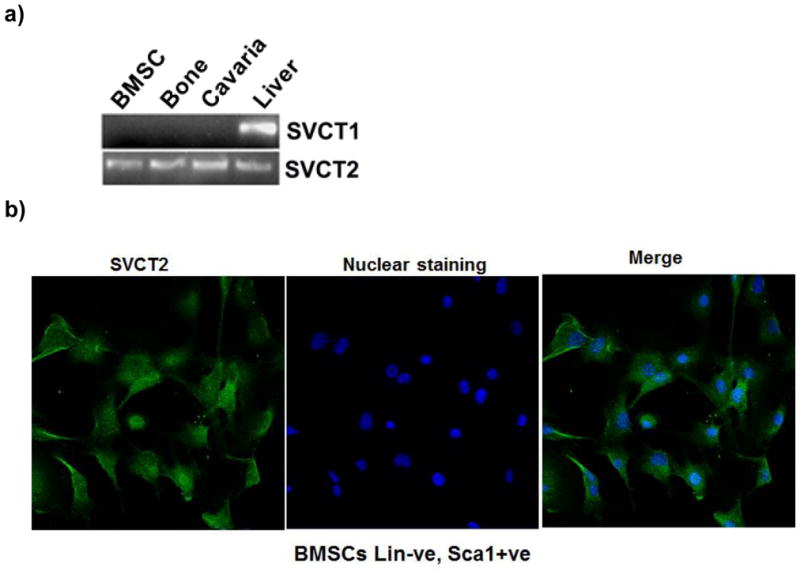
Confocal analysis of SVCT2 protein expression
To investigate the expression and localization of SVCT2 protein in purified BMSCs (lin-negative and Sca1-positive), slides were stained with a specific antibody against SVCT2. The fluorescence imaging of the immuno-positive signal indicated that SVCT2 protein was present abundantly in BMSCs (Fig. 1b). In BMSCs, the protein was located predominantly on the plasma membrane.
Functional activity of SVCT2 in BMSCs
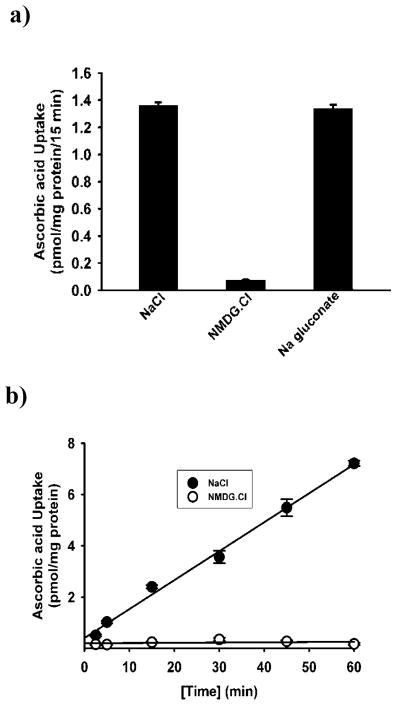
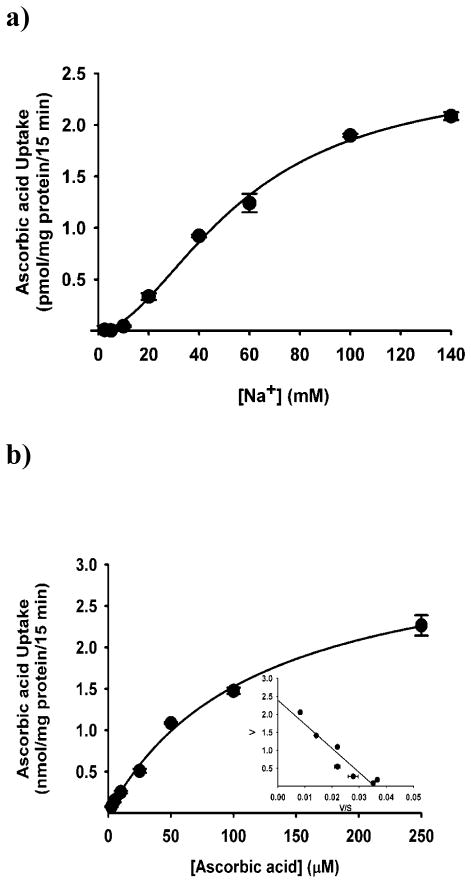
To confirm the functional involvement of SVCT2 in BMSCs, we carried out uptake experiments. BMSCs showed sodium-dependent uptake of ascorbic acid (Fig. 2a). Removal of Na+ in the uptake buffer abolished the uptake almost completely, indicating the obligatory requirement of Na+ for the uptake process. In contrast, there was no effect of chloride on the uptake process as the uptake rates were similar in the presence and absence of this anion. The substrate specificity of the transport system was investigated by assessing the ability of various vitamins to compete with [14C]-ascorbic acid for the transport process. Unlabeled ascorbate was able to compete with [14C]-ascorbate effectively at a concentration of 5 mM. None of the other vitamins such as thiamine, riboflavin, myoinositol and nicotinic acid were able to compete with [14C]-ascorbic acid, showing the specificity of this transport system (Fig. 2c). Time-dependency of the uptake was also done in presence and absence of Na+. The uptake of [14C]-ascorbic acid was linear in the Na+-containing buffer over a time period of 30 min (Fig. 2b). In the absence of Na+, the uptake was reduced markedly (<3% of uptake measured in the presence of Na+). An incubation period of 15 min was selected for subsequent uptake experiments to represent linear uptake rates. The uptake process was found to be concentration-dependent and saturable (Fig. 3a). The Michaelis constant for ascorbic acid was 56 ± 5 μM. The value for the maximal velocity was 1.84 ± 0.18 nmol/mg protein/15 min. Since the transport of [14C]-ascorbate was completely Na+-dependent, we performed Na+-activation kinetics. The uptake of [14C]-ascorbate was measured in BMSCs in the presence of varying concentrations of Na+ (0–140 mM). The relationship between the uptake rate and the Na+ concentration was sigmoidal, suggesting the involvement of more than one Na+ in activation process (Fig. 3B). We were able to calculate the Hill coefficient (i.e., the number of Na+ involved in the activation process) by fitting the data to the Hill equation. The value was 2.1 ± 0.2.
Figure 2.
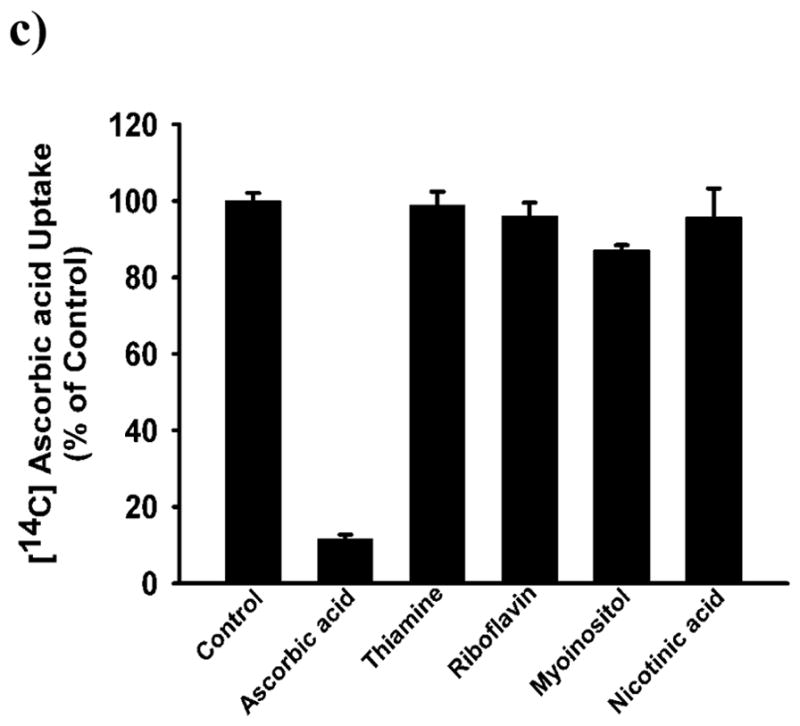
Ion dependency, time course and, substrate specificity of SVCT2 in mouse BMSCs cells. (a), Uptake of [14C]-ascorbate (20 nM) in the presence of NaCl (presence of Na+ and Cl−) or NMDG.Cl [(N-methyl-D-glucamine chloride) absence of Na+ but presence of Cl−] and Na gluconate (presence of Na+ and absence of Cl−) in mice BMSCs. (b), Uptake of [14C]-ascorbate (20 nM) was measured for different periods of time in the presence of NaCl (presence of Na+ and Cl−) or NMDG.Cl (c), Uptake of [14C]-ascorbate (20 nM) was measured for 15 min in the presence of NaCl with or without various vitamins (5 mM). Uptake measured in the absence of competing vitamins was taken as the control (100 %) and uptake measured in the presence of competing vitamins is given as a percentage of this control value (means ± SD, n = 4).
Figure 3.
Na+-activation and Saturation kinetics of SVCT2 in mouse BMSCs cells. (a), Uptake of [14C]-ascorbate (20 nM) was measured for 15 min in the presence of NaCl at increasing concentrations of Na+ (2.5 – 140 mM) but in the presence of a fixed concentration of Cl− (140 mM). The concentration of Na+ was varied by replacing NaCl with NMDG.Cl iso-osmotically. (b)Uptake of [14C]-ascorbate (20 nM) was measured for 15 min in the presence of NaCl at increasing concentrations of unlabeled ascorbate (2.5 – 250 μM). Radiolabeled ascorbate (20 nM) was present as the tracer. Eadie-Hofstee plot [ascorbate uptake (V) versus ascorbate uptake/ascorbate concentration (V/S)] (means ± SD, n = 4).
Expression of SVCT2 during BMSC differentiation into osteoblasts (osteogenesis)
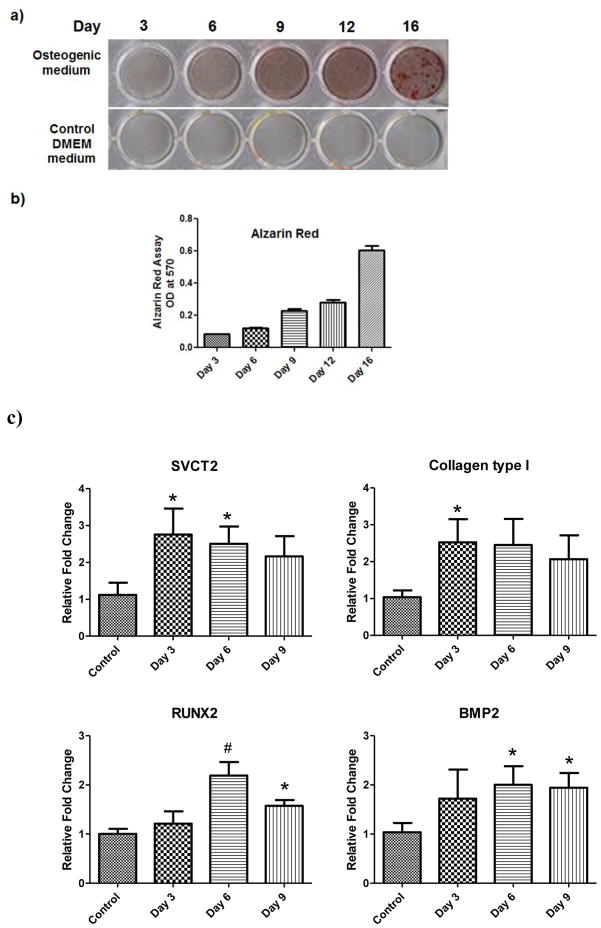
BMSCs expressed SVCT2 under undifferentiated conditions. Since these cells differentiate into osteoblasts when cultured in an osteogenic medium, we wanted to determine if the expression of the transporter is altered during differentiation. BMSCs were cultured in osteogenic medium for 3–16 days and then stained Alizarin Red for quantification of mineralization. The extent of mineralization increased in the cultures as the time of exposure to osteogenic medium increased (Fig. 4a&b). Real-time PCR analysis of osteogenic markers such as BMP-2, RUNX-2 and type I Collagen was performed on these cultures. The expression of all these three markers was increased significantly during differentiation of BMSCs into osteoblasts (Fig. 4c). SVCT2 expression was also upregulated significantly.
Figure 4.
a) Mouse BMSCs cultured in osteogenic medium and stained for mineralized nodule by Alizarin red S assay. (b) Quantitative analysis of the extent of mineralization in alizarin red S assay using elution of dye by 10% (wt/vol) cetylpyridinium chloride (means ± SD, n = 4) (c) Real time PCR analysis of steady-state levels of mRNA for SVCT2, type I collagen, RUNX-2 and BMP-2 expression in BMSCs after treatment of osteogenic media for 3 days, 6 days and 9days. Data for each sample were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Data (means ± SD, n = 4) are represented as the fold change in expression compared to control. *P < 0.05, # P < 0.01.
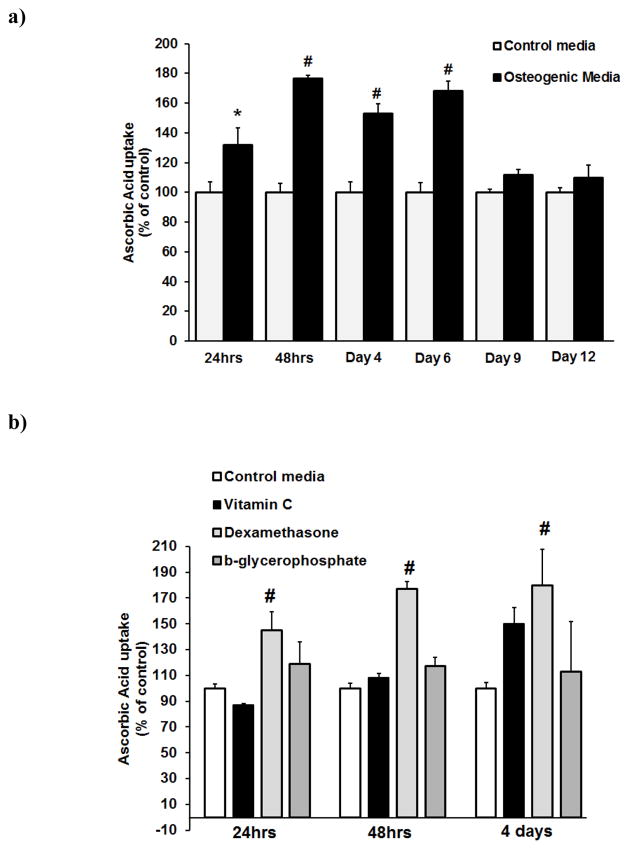
We then monitored the function of SVCT2 by measuring ascorbic acid uptake in BMSCs during differentiation. The cells were cultured in normal medium and in osteogenic medium for various time periods prior to uptake measurements. The culture of the cells in osteogenic medium increased the uptake of ascorbic acid (Fig. 5a). The differentiation-specific increase was maximal (50–80%) at early time points (1–6 days in culture); this effect decreased significantly as the culture was continued for longer time periods. The osteogenic medium consists of dexamethasone, ascorbic acid, and β-glyerophosphate. To determine the identity of the chemical in the osteogenic medium that was responsible for the induction of SVCT2 in BMSCs, we cultured the cells for 24hrs and 48hrs in the presence of dexamethasone, ascorbic acid or β-glyerophosphate at concentrations found in the osteogenic medium and then measured ascorbic acid uptake. The uptake increased significantly in a time-dependent manner in dexamethasone-treated BMSCs (Fig. 5b). Exposure to ascorbic acid and β-glyerophosphate did not show significant change.
Figure 5.
Effects of osteogenic media, vitamin C, dexamethasone and b-glycerophosphate on MSCs culture for different time point on ascorbic acid uptake. (a) BMSCs cells were incubated with osteogenic media for 24hrs, 48hrs, 4day, 6 day, 9 day, and 12 day. These cells were then used for uptake measurements. Uptake of [14C]-ascorbic acid (20 nM) was measured for 15 min in the presence of NaCl. (b) BMSCs cells were incubated with vitamin C (0.25mM), Dexamethasone (10nM) and b-glycerophosphate (10 mM) for different time points. These cells were then used for uptake measurements. Uptake of [14C]-ascorbic acid (20 nM) was measured for 15 min in the presence of NaCl. Values (means ± SD, n = 4)) are expressed relative to the control. *P < 0.05, # P < 0.01.
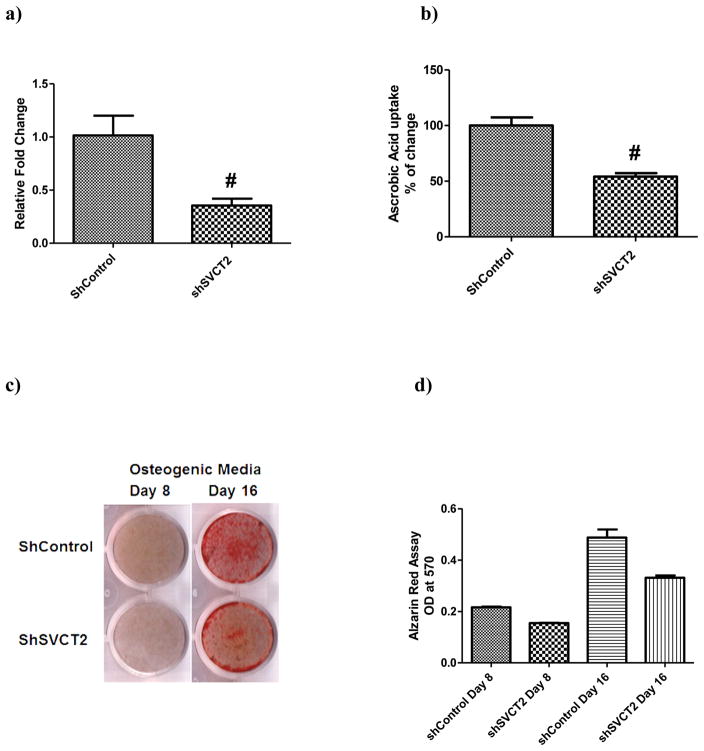
Knockdown of SVCT2 greatly impaired osteogenesis of mouse BMSCs
To further investigate the role of SVCT2 in osteogenesis, a lentivirus-based shRNA knockdown system was used. BMSCs were transduced with lentivirus containing either SVCT2-specific shRNA or non-target control shRNA (shNTC) and then subjected to osteogenic induction for 8 and 16 days. The knockdown efficiency of lentivirus shSVCT2 in BMSCs was 50–60% as assessed by real-time PCR and vitamin C uptake assay (Fig. 6 a & b). Compared to BMSCs infected with shNTC, shSVCT2-expressing BMSCs showed greatly impaired osteogenic activity, as revealed by alizarin red staining (Fig. 6 c & d). Real time PCR showed down-regulation of bone markers BMP2, collagen type I, RUNX2 and osteocalcin in shSVCT2-expressing BMSCs in both, 8 and 16 days of osteogenic induction (Fig. 6 e).
Figure 6.
Lentivirus-mediated SVCT2 knockdown a) Quantitative real-time PCR analysis of total SVCT2 transcripts from stable, SVCT2 shRNA transfected and non-targeting shRNA (shNTC) mouse BMSCs. Data for each sample were normalized with GAPDH mRNA. Data (means ± SD, n = 3) are represented as the fold change in expression compared to control. *P < 0.05, # P < 0.01. b) Uptake of [14C]-ascorbate (20 nM) was measured for 15 min in SVCT2 shRNA and non-targeting shRNA (shNTC) in mouse BMSCs. Uptake measured in control non-targeting shRNA (shNTC) was taken as the control (100 %) and uptake measured in the SVCT2 shRNA given as a percentage of this control value (means ± SD, n = 3). c) SVCT2 knockdown reduced BMSC osteogenesis. BMSCs were transduced with lentivirus containing either SVCT2 specific shRNA or non-targeting shRNA (shNTC) and then subjected to osteogenic media for 8 and 16 days and stained for mineralized nodule by Alizarin red S assay, (d) quantification of Alizarin red S assay and (e) quantitative real-time PCR analysis of steady-state levels of mRNA for BMP2, type I collagen, RUNX-2 and osteocalcin expression. Data for each sample were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Data (means ± SD, n = 4) are represented as the fold change in expression compared to control. *P < 0.05, # P < 0.01.
Regulation of ascorbic acid uptake by redox reaction
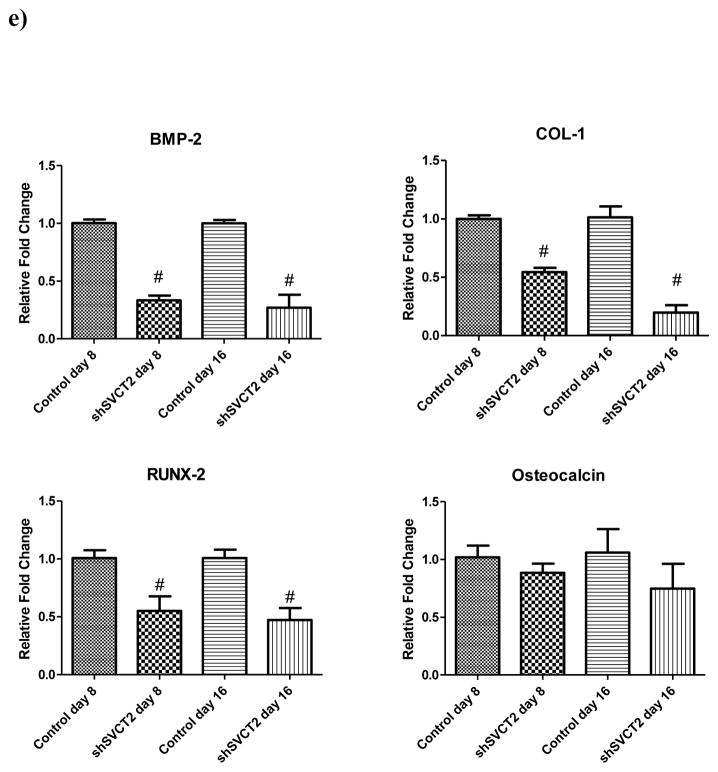
To determine the effect of redox reaction on ascorbic acid uptake in BMSCs, experiments were carried out in presence of oxidant and antioxidant for 18hrs. For our studies, we used an oxidant Sin-1 which slowly decomposes to release both superoxide and NO (More et al., 1998). The antioxidant ascorbic acid was used to counter act the effect of oxidant sin-1. We used various concentrations of Sin-1 (200μM, 400μM and 600μM) and found that ascorbic acid uptake decrease with increase in the concentration of oxidant. The 600uM of sin-1 caused the most 40% decrease in AA uptake where as 400uM and 200uM cause 30% and 20% decrease in uptake, respectively (Fig. 7a). The antioxidant ascorbic acid did not showed significant effect on AA uptake. When cells were cultured in presence of both, oxidant (Sin-1 200μM, 400μM and 600μM) and antioxidant (ascorbic acid 250uM), there was not significant different in AA uptake compare to control (Fig. 7a). The higher concentration of Sin-1 (1000μM) was toxic to cells and substantially reduced cell viability but AA protects cells from cytotoxicity of Sin-1(Fig. 7b). This indicates that antioxidants nullify the effect of sin-1. Altogether, these findings suggested that AA uptake activity may be redox regulated.
Figure 7.
Effects of Redox reaction on BMSCs ascorbic acid uptake. (a) BMSCs cells were incubated with Sin-1(200μM, 400μM and 600μM) and Sin-1+ ascorbic acid (250). These cells were then used for uptake measurements. Uptake of [14C]-ascorbic acid (20 nM) was measured for 15 min in the presence of NaCl. Values (means ± SD, n= 7)) are expressed relative to the control. (b) Ascorbic acid protect cells from Sin1 induce death. BMSCs cells were incubated with no treatment, Sin-1(1000μM) and Sin-1+ ascorbic acid (250μM) for 24hrs. Microscopic imaging (10X) was performed using pixelink camera.
Effect of aging on Bone SVCT2 expression
SVCT2 expression was analyzed in femora from mice 12- and 24-months of age, along with other known bone markers. We found that relative expression of the SVCT2 gene in bone was markedly decreased with age. Compared to 12 month mice, SVCT2 expression was down regulated two-fold in 24 month old mice (Fig. 8). The Collagen type 1, BMP-2 and RUNX-2 were also down regulated with age (data not shown).
Figure 8.
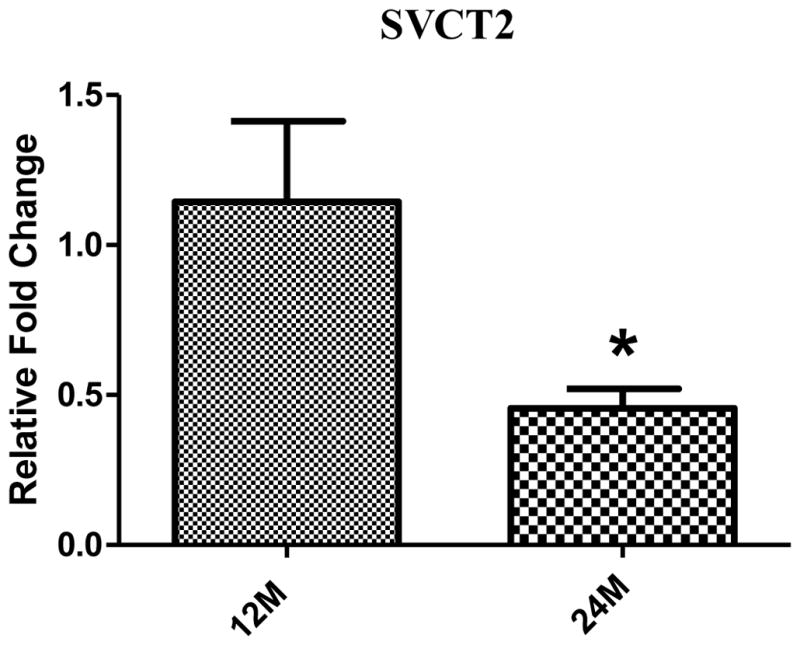
Age-related declines in expression of SVCT2 at mRNA levels in mouse bone. After reverse transcription of total RNA, cDNA was amplified by quantitative real-time PCR. Data for each sample were normalized with GAPDH mRNA. Data (means ± SD, n= 5) are represented as the fold change in expression compared to 12 month mouse. *P < 0.05.
Discussion
The expression of SVCT transporter has been studied in osteoblast (Tsukaguchi et al.,1999), MC3T3-E1 (Fujita et al., 2001), and articular cartilage (Clark et al., 2002; McNulty et al., 2005) but there are no data until now on ascorbic acid uptake or SVCT expression in BMSCs under undifferentiated conditions as well as during osteogenic differentiation. This report presents the first study of the characterization of ascorbic acid uptake in BMSCs. These studies show convincingly that BMSCs exhibit robust ascorbic acid uptake activity and that the uptake process is Na+-dependent. Our molecular studies reveal that BMSCs express only SVCT2 isoform of the Na+-coupled ascorbic acid transporter. SVCT1, another isoform of ascorbic acid transporter that is also capable of Na+-coupled transport, is not expressed in BMSCs. It has been reported that osteoblasts (Tsukaguchi et al., 1999) and MC3T3-E1 (mouse osteoblastic cell line) (Fujita et al., 2001) express SVCT2 but not SVCT1. The characteristics of ascorbic acid uptake in BMSCs include absolute requirement for Na+, saturability with high affinity for its substrate, specific for ascorbic acid, and a Na+:ascorbic acid stoichiometry of 2:1. The Michaelis constant for the uptake process was ~56 μM. This value is in the range of previously reported data for SVCT2 in different cell types in different species (Rajan et al., 1999, Liang et al., 2001; Manfredini et al., 2002, Savini et al., 2008).
We also investigated changes in the expression of SVCT2 in BMSCs during their osteogenic differentiation. Ascorbic acid uptake increases within 24 h after addition of the osteogenic medium to the culture. This effect persisted for 6 days but decreased at longer periods of time. Type I collagen expression was also upregulated in the initial phase of osteogenesis. Similar findings have been reported for MC3T3-El mouse calvaria-derived cell line, showing dramatically increased procollagen type I mRNA within 24 h during osteogenesis and the decreased effect at longer treatment times (Franceschi et al., 1992). As expected, bone-specific markers BMP-2 and Runx-2 were also upregulated during differentiation of BMSCs into osteoblasts and this was accompanied with increased mineralization. We et al (2004) showed that MC3T3-E1 osteoblasts SVCT2-overexpressing cells exhibited more ability to promote mineralization and increase calcium deposition under the stimulation of osteogenic media. Our results match up with the We et al (2004) finding that SVCT2 plays important role in osteogenesis. Our findings suggest that SVCT2 is upregulated during the initial phase of differentiation of BMSCs into osteoblasts, which helps to promote collagen synthesis. This is followed by increased mineralization. There are reports that SVCT2 expression and ascorbic acid uptake are regulated by zinc, calcium and phosphate in murine osteoblastic cell line MC3T3-E1 during osteogenesis (We et al., 2003a; 2003b). Analysis of the differential effects of the three important components of the osteogenic medium reveals that dexamethasone is primarily responsible for the observed increase in ascorbic acid uptake. Fujita et al (2001) also reported that dexamethasone induces SVCT2 mRNA expression and uptake of AA in a mouse osteoblastic cell line MC3T3-E1. To further clarify the role of SVCT2 in the osteogenic differentiation of BMSCs, we knockdown SVCT2 expression utilizing lentivirus-based shRNA system and osteogenic analysis was performed. The knockdown of SVCT2 transporter significantly inhibits osteogenesis which clarify the importance of this transporter in bone formation
We also investigated the relationship between SVCT2 and oxidative stress in undifferentiated BMSCs. It is generally accepted that osteoporosis and aging is associated with oxidative stress. Oxidative stress stimulates osteoclast differentiation through receptor activator of nuclear factor-kappaB ligand (RANKL) and induces bone loss (Lee et al., 2005; Wauquier et al., 2009). However, there are no data available concerning the impact of oxidative stress on SVCT2 regulation in BMSCs. In this study, we show that BMSCs cells modulated the AA uptake according to their redox balance. The AA uptake was down regulated in Sin-1-treated BMSCs, while Sin-1 along with antioxidant supplementation compensates AA uptake. Completely opposite findings were previously reported in other cell types, where oxidative stress induced SVCT-2 expression (Kannan et al., 2001; Savinin et al., 2007, May et al., 2010, Portugal et al.,2012) and antioxidant supplementation reversed this effect (Savini et al., 2007). Our study and previous studies show that antioxidant treatment ablates the effect that oxidative stress imposes upon SVCT2 expression. The inconsistent AA uptake regulation among these cell types may be attributable to differences in cellularity, but it seems that AA uptake is in fact an adaptive response to redox reaction. We also found that Sin-1 treatment (1000μM) substantially reduced cell viability and AA protected cells from cytotoxicity of Sin-1.
As this transporter is regulated by redox reaction, we investigated whether there was any change in SVCT2 expression with age. Our study shows that there is an age dependent decrease in SVCT2 transcript number in old mice femur along with other bone marker such as Collagen type 1, BMP-2, and RUNX2 (data not shown). Similar results were reported in rat liver showing down regulation of SVCT with age (Michels et al., 2003). Liver express both sub-types, SVCT1 and SVCT2, and only SVCT1 significantly down regulated (Michels et al., 2003). Aging is a complicated process and is associated with a number of systemic changes in the body. The changes in SVCT2 expression with age in bone could be a consequence of systemic changes such as an increasingly pro-oxidant environment, redox active metal accumulation, and change in growth hormone and growth factors expression in aged tissues. In aged bone, there is a decrease in osteoblast activity and an increase in osteoclast activity. Oxidative stress increased osteoblast apoptosis and compromises osteoblast differentiation (Wauquier et al., 2009; Almeida et al., 2010). The changes in SVCT2 expression that we observed with age suggest that it could play an important role in bone formation. Thus, future studies should explore the regulation of SVCT2 transporter by redox reaction and age-associated changes in various growth factors and sex steroids.
In conclusion, the present study demonstrates for the first time that bone and BMSCs express only the SVCT2 transporter, and this transporter plays an important role in the osteogenic differentiation of BMSCs. Furthermore, this transporter is regulated with age in bone, and by dexamethasone and redox reaction in BMSCs. There is a need to further investigate the role of SVCT2 in bone biology to better understand the relevance of this transporter to bone metabolism and osteoporosis.
Highlights.
Mouse bone marrow stromal cells and Bone express only SVCT2 and not SVCT1
SVCT2 plays an important role in the osteogenic differentiation of BMSCs.
Down-regulation of SVCT2 significantly reduces BMSCs osteogenesis.
The expression of the SVCT2 is modulated by redox reaction.
SVCT2 is down-regulated with age in mouse bone.
Acknowledgments
Funding for this research was provided in part by the AO foundation (S-11-12F) and National Institute on Aging (P01AG036675)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol. 2010 Oct;24(10):2030–7. doi: 10.1210/me.2010-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288:275–9. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Aubin JE, Heersche JNM, Antosz ME. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Clark AG, Rohrbaugh AL, Otterness I, Kraus VB. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol. 2002;21:175–84. doi: 10.1016/s0945-053x(01)00193-7. [DOI] [PubMed] [Google Scholar]
- Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–7. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- Franceschi RT. The role of ascorbic acid in mesenchymal differentiation. Nutr Rev. 1992 Mar;50(3):65–70. doi: 10.1111/j.1753-4887.1992.tb01271.x. Review. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Wilson JX, Dixon SJ. Requirement for Na-dependent ascorbic acid transport in osteoblast function. Am J Physiol. 1995;268:C1430–C1439. doi: 10.1152/ajpcell.1995.268.6.C1430. [DOI] [PubMed] [Google Scholar]
- Fujita I, Hirano J, Itoh N, Nakanishi T, Tanaka K. Dexamethasone induces sodium-dependant vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. Br J Nutr. 2001 Aug;86(2):145–9. doi: 10.1079/bjn2001406. [DOI] [PubMed] [Google Scholar]
- Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Stolz A, Ji Q, Prasad PD, Ganapathy V. Vitamin C transport in human lens epithelial cells: evidence for the presence of SVCT-2. Exp Eye Res. 2001;73:159–65. doi: 10.1006/exer.2001.1024. [DOI] [PubMed] [Google Scholar]
- Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18:281–8. doi: 10.1016/8756-3282(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Kuo SM, MacLean ME, McCormick K, Wilson JX. Gender and sodium-ascorbate transporter isoforms determine ascorbate concentrations in mice. J Nutr. 2004 Sep;134(9):2216–21. doi: 10.1093/jn/134.9.2216. [DOI] [PubMed] [Google Scholar]
- Lee NK, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- Levine M, Rumsey SC, Daruwala RC, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol. 2001 Jan-Mar;18(1):87–95. doi: 10.1080/09687680110033774. Review. [DOI] [PubMed] [Google Scholar]
- Manfredini S, Pavan B, Vertuani S, Scaglianti M, Compagnone D, Biondi C, Scatturin A, Tanganelli S, Ferraro L, Prasad P, Dalpiaz A. Design, synthesis and activity of ascorbic acid prodrugs of nipecotic, kynurenic and diclophenamic acids, liable to increase neurotropic activity. J Med Chem. 2002 Jan 31;45(3):559–62. doi: 10.1021/jm015556r. [DOI] [PubMed] [Google Scholar]
- May JM, Li L, Qu ZC. Oxidized LDL up-regulates the ascorbic acid transporter SVCT2 in endothelial cells. Mol Cell Biochem. 2010 Oct;343(1–2):217–22. doi: 10.1007/s11010-010-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty AL, Vail TP, Kraus VB. Chondrocyte transport and concentration of ascorbic acid is mediated by SVCT2. Biochim Biophys Acta. 2005 Jun 30;1712(2):212–21. doi: 10.1016/j.bbamem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Michels AJ, Joisher N, Hagen TM. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch Biochem Biophys. 2003 Feb 1;410(1):112–20. doi: 10.1016/s0003-9861(02)00678-1. [DOI] [PubMed] [Google Scholar]
- Miyajima K, Ito R, Matsuyama T, Togari A, Matsumoto S, Iizuka T. Morphological differences in the skull of ascorbic acid-deficient ODS rats. Arch Oral Biol. 1995 Apr;40(4):293–7. doi: 10.1016/0003-9969(94)00174-a. [DOI] [PubMed] [Google Scholar]
- Moro MA, Fernández-Tomé P, Leza JC, Lorenzo P, Lizasoain I. Neuronal death induced by SIN-1 in the presence of superoxide dismutase: protection by cyclic GMP. Neuropharmacology. 1998 Aug;37(8):1071–9. doi: 10.1016/s0028-3908(98)00104-x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:S1135–40. doi: 10.1093/ajcn/54.6.1135s. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poal-Manresa J, Little K, Trueta J. Some observations on the effects of vitamin C deficiency on bone. Br J Exp Pathol. 1970;51:372–8. [PMC free article] [PubMed] [Google Scholar]
- Portugal CC, da Encarnação TG, Socodato R, Moreira SR, Brudzewsky D, Ambrósio AF, Paes-de-Carvalho R. Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-κB (NF-κB) J Biol Chem. 2012 Feb 3;287(6):3860–72. doi: 10.1074/jbc.M111.260166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun. 1999 Sep 7;262(3):762–8. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ramos M, Vargas LA, Fortoul Van der Goes TI, Cervantes-Sandoval A, Mendoza-Nunez VM. Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J Nutr Health Aging. 2010 Jun;14(6):467–72. doi: 10.1007/s12603-010-0099-5. [DOI] [PubMed] [Google Scholar]
- Savini I, Rossi A, Catani MV, Ceci R, Avigliano L. Redox regulation of vitamin C transporter SVCT2 in C2C12 myotubes. Biochem Biophys Res Commun. 2007 Sep 21;361(2):385–90. doi: 10.1016/j.bbrc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Savini I, Rossi A, Pierro C, et al. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347–55. doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- Schindeler A, Morse A, Peacock L, Mikulec K, Yu NY, Liu R, Kijumnuayporn S, McDonald MM, Baldock PA, Ruys AJ, Little DG. Rapid cell culture and pre-clinical screening of a transforming growth factor-beta (TGF-beta) inhibitor for orthopaedics. BMC Musculoskelet Disord. 2010 May 28;11:105. doi: 10.1186/1471-2474-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine JD. Bone matrix proteins and the mineralization process. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 1. American Society for Bone and Mineral Research; Kelseyville, California: 1990. [Google Scholar]
- Togari A, Arai M, Nakagawa S, Banno A, Aoki M, Matsumoto S. Alteration of bone status with ascorbic acid deficiency in ODS (osteogenic disorder Shionogi) rats. Jpn J Pharmacol. 1995 Jul;68(3):255–61. doi: 10.1254/jjp.68.255. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999 May 6;399(6731):70–5. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009 Oct;15(10):468–77. doi: 10.1016/j.molmed.2009.08.004. Review. [DOI] [PubMed] [Google Scholar]
- Wilson JX, Dixon SJ. High-affinity sodium-dependent uptake of ascorbic acid by rat osteoblasts. J Membr Biol. 1989;111:83–91. doi: 10.1007/BF01869211. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T, Nakanishi T, Tanaka K. Requirement of calcium and phosphate ions in expression of sodium-dependent vitamin C transporter 2 and osteopontin in MC3T3-E1 osteoblastic cells. Biochim Biophys Acta. 2003 Jun 17;1641(1):65–70. doi: 10.1016/s0167-4889(03)00065-x. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T, Nakanishi T, Tatsu Y, Yumoto N, Tanaka K. Zinc-induced sodium-dependent vitamin C transporter 2 expression: potent roles in osteoblast differentiation. Arch Biochem Biophys. 2003 Dec 1;420(1):114–20. doi: 10.1016/j.abb.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T, Hirano J, Nakanishi T, Tanaka K. Stimulation of differentiation in sodium-dependent vitamin C transporter 2 overexpressing MC3T3-E1 osteoblasts. Biochem Biophys Res Commun. 2004 May 14;317(4):1159–64. doi: 10.1016/j.bbrc.2004.03.158. [DOI] [PubMed] [Google Scholar]