Abstract
Cyanotoxins are potent toxic compounds produced by cyanobacteria during algal blooms, which threaten drinking water supplies. These compounds can poison and kill animals and humans. The host—guest interactions of α-, β-, and γ-cyclodextrins (CD) with problematic cyanotoxins, including microcystins (MCs) and nodularin (NOD), were investigated to demonstrate the potential application of CDs for the removal of these toxins from drinking water or applications related to their separation or purification. MCs and NOD have a hydrophobic Adda chain, which contains diene and benzene functional groups. The complexation of these cyanotoxins with CDs was monitored by nuclear magnetic resonance (NMR). The 1H NMR spectra for MCs are unchanged upon addition of α-CD (smallest host). However, addition of larger hosts, β-CD and γ-CD, leads to significant changes in chemical shifts of the benzene and diene resonances on the 3-amino-9-methoxy-2,6,8-trimethal-10-phenyldeca-4,6-dienoic acid (Adda) chain of MCs and NOD. Solution pH, natural organic matter, and salinity do not appreciably influence the host—guest complexation under our experimental conditions. The experimental binding constants for MCs and NOD with γ-CD are relatively strong, ranging from 1155 to 507 M−1. The observed changes in chemical shifts for specific protons and competitive binding experiments demonstrate a 1:1 inclusion complex between γ-CD and MCs or NOD, with the Adda chains threading through the CD ring, resulting in an inclusion complex. Our results suggest that CD-type substrates are useful hosts for the complexation of MCs and NOD. CDs can be readily attached to a number of polymeric or solid supports and their functionality tailored to strengthen specific host—guest interactions. With further development of such materials, CD host—guest chemistry may find direct application in the removal and/or separation science of these compounds.
INTRODUCTION
Cyanobacteria are a series of bacteria that obtain their energy through photosynthesis. Cyanobacteria produce potent toxic compounds during algal blooms, which can poison and even kill animals and humans.1–3 The cyclic peptide toxins of the microcystin and nodularin families are the most widespread and threatening compounds produced by cyanobacteria in blooms from fresh and brackish waters.4–6 They contain five (nodularin) and seven (microcystins) amino acids, with the two terminal amino acids of the peptide joined to form a cyclic compound (Figure 1). These toxins are hepatotoxins and tumor promoters.7 The lethal doses for 50% of the population (LD50) for approximately 70 different MCs range from 50 to 500 μg/kg.8 The hydrophobic Adda moiety, which is common to all microcystins and nodularin, is considered to be the key component for biological activity and their ability to bind and inhibit protein phosphatase.9
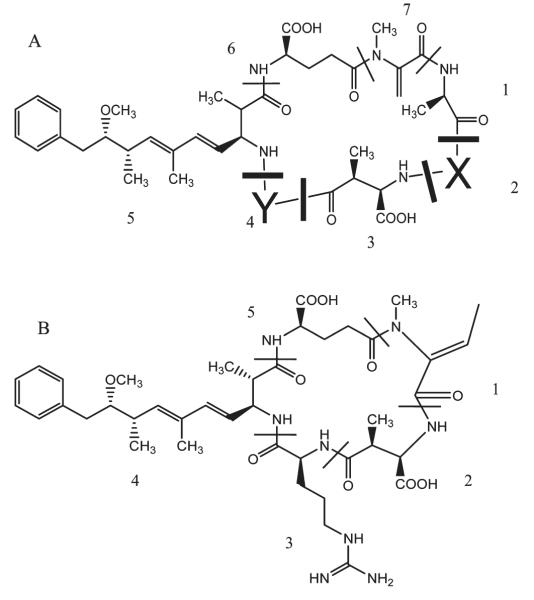
Figure 1.
(A) Microcystins [1, d-alanine; 2, X = variable d- and l-amino acid; 3, d-methylaspartic acid; 4,Y = variable d- and l-amino acid; 5, 3-amino-9-methoxy-2,6,8-trimethal-10-phenyldeca-4,6-dienoic acid (Adda); 6, d-glutamic acid; 7, N-methaldehydralanine] and (B) nodularin [1, 2-(methyl-amino)-2-dehydrobutyric acid; 2, methylaspartic acid; 3, arginine acid; 4, Adda; 5, d-glutamic acid].
Microcystins are extremely stable and resistant to chemical hydrolysis or oxidation. In natural waters and in the dark, microcystins can persist for months or years1 and are recalcitrant to conventional water treatment processes. The high toxicity of MCs and their impact on environmental, social, and economic aspects propelled the scientific interest for developing methods for the detection, characterization, and treatment of these toxins. Activated carbon adsorption10 and ozone and chlorine dioxide oxidation11,12 can effectively remove MCs from drinking water. However, the presence of natural organic materials causes a reduction of the operational lifetime and reduces the effectiveness. TiO2 photocatalysis and ultrasonic irradiation have been used to degrade MCs efficiently, and the associated toxicity is dramatically reduced.13–15 While TiO2 leads to degradation of MCs under bench-scale conditions, these methods are typically strongly influenced by water quality and are often not cost-effective.16 Moreover, the potential health risks associated with TiO2 nanoparticles in water have become a concern recently.17 Biodegradation by different types of bacteria has been studied, and the probable pathway was elucidated.18,19 Bioremediation of these toxins has been reported and has potential as a treatment method for these substrates, but it can also be highly sensitive to water quality and require extended periods of time (20—30 days).20,21 The combination of bioremediation with advanced oxidation technologies is another possible treatment strategy to consider for the treatment of cyanotoxins. Although CDs have been used as agents (cages) to trap and remove a variety of organic pollutants by host—guest complexation,22–24 the use of CDs for chemical remediation of cyanotoxins is unexplored.
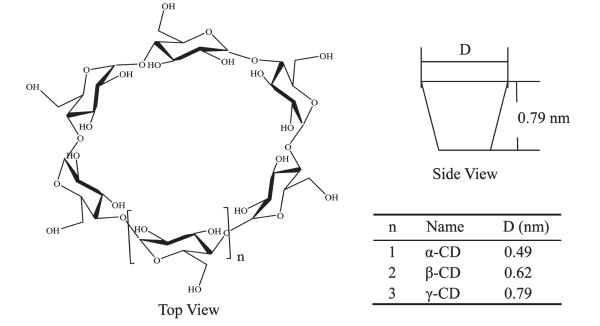
CDs are hollow open-ended cone-shaped molecules composed of repetitive d-glucopyranose (cyclic sugar) units joined through α (1—4) linkages, as illustrated in Figure 2. Secondary hydroxyl groups at the second and third positions of the cyclic sugar units define the wider opening of the CD substrates, while primary hydroxyl groups at the sixth position of the individual sugar units define the perimeter of the narrower opening. The interiors of the CD rings are lined with ether oxygens and methane functional groups.25 Thus cyclodextrins have hydrophilic openings and hydrophobic interiors. α-, β-, and γ-CDs have six, seven, and eight glucopyranose units, respectively. They are inexpensive, nontoxic, and readily available. It is well-known that cyclodextrins can act as hosts for hydrophobic guests and exhibit specific complexation with a number of organic compounds.26 Formation of cyclodextrin complexes is an equilibrium process where free guest molecules are in equilibrium with incapsulated molecules. In general, the complexation is controlled by the size, structural shape, and hydrophobic properties of the guests. The detection and characterization of these complexes have been explored through calorimetric,27,28 X-ray diffraction,29,30 NMR,31–33 computational studies,34 and ESI-MS.35 Among these analytical methods, NMR is one of the most useful methods due to its easy application and the detailed structural information on the nature of the complex that can be obtained. NMR is widely used in host—guest chemistry because it can readily distinguish encapsulation and surface or peripheral binding of the substrate to the host. Moreover, specific shifts of the proton signals are a direct indication of the specific nature/region of a substance that is encapsulated.31–33
Figure 2.
The structure and dimension of α-, β-, and γ-cyclodextrins.
The current study was conducted to demonstrate and characterize the complex of cyanotoxins with α, β, and γ-CDs as hosts (complexing agents) for the potential removal of MCs and/or NOD from drinking water sources. The strength and nature of the binding of these cyanotoxins with the different CDs and the influence of water quality on the complexation are reported here.
EXPERIMENTAL SECTION
Chemicals
Microcystin-LR (MCLR) was purified according to a method by Song.36 All reagents were HPLC grade or analytical grade, including microcystin-RR (MCRR); nodularin; α-, β-, and γ-cyclodextrins (Sigma-Aldrich); methanol (Fisher Scientific); and adamantanecarboxylic acid (Sigma-Aldrich). The sample solutions for 1H NMR measurement were prepared by dissolving MCs, NOD, and CDs in the D2O solution.
Solution Preparation
MCLR was mixed with three types of CDs in different molar ratios from 1:1 to 1:64, respectively. MC variants and NOD were individually mixed with γ-CD at a specific molar ratio 1:12 at a high concentration. The sample solutions were subsequently diluted to several specific concentrations, and complexation was monitored by 1H NMR to determine the equilibrium binding constant. The desired solution pH was obtained by addition of appropriate volumes of stock DCl or NaOD solutions. The effect of salinity and humic materials was determined by preparing solutions containing specific amounts of NaCl or humic acid. The salt and humic material were carefully weighed using an analytical balance and all solutions were prepared using volumetric glassware.
NMR Experiments
All NMR experiments were conducted in 99.9% D2O with 0.1% DSS [sodium 3-(trimethylsilyl)propionate-2,2,3,3-d4 (δ = 0.00 ppm)] as the internal standard. The NMR experiments were carried out on a Bruker DRX 400 spectrometer at 20 °C.
RESULTS AND DISCUSSION
Complex of MCLR with α-, β-, and γ-Cyclodextrins
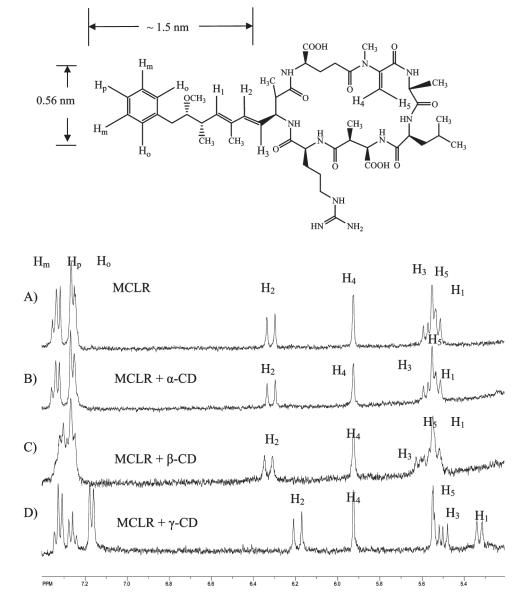
MCLR (C49H74O12N10) is a relatively large compound with a molecular weight of 994 g/mol. The 1H NMR spectrum for MCLR is complicated with extensive overlapping resonances in the aliphatic region (4.0—0.5 ppm). The host CD also possesses a multitude of overlapping peaks in the aliphatic region. Fortunately, upfield resonances of protons on the benzene ring and diene moieties of the Adda chain of the potential guest occur in a region of the 1H NMR spectra that is unique and unobstructed by other peaks (8.0—5.0 ppm). The 1H NMR peaks in this region correspond to the ortho, para, and meta protons on the benzene ring, Ho, Hm, Hp, and the diene protons, H1, H2, and H3 (Figure 3).
Figure 3.
1HNMR spectra of D2O solutions of MCLR (A) in the presence of (B) α-CD, (C) β-CD, and (D) γ-CD at a ratio of 1:2.
1H NMR spectra of pure MCLR and MCLR in the presence of three different CDs at a molar ratio of 1:2 are also shown in Figure 3. α-CD does not induce significant change in the chemical shift for protons on the Adda hydrophobic chain of MCLR, and the lack of a shift indicates that there is no measurable interaction between α-CD and the Adda chain. The 1H NMR spectra of MCLR in the presence of β-CD and γ-CD show distinct changes in the chemical shift for the aromatic ring and diene moieties relative to free MCLR. Modest chemical shift changes of proton peaks are observed for the complex of MCLR and β-CD, but the complexation with γ-CD yields the largest changes of chemical shift for the proton associated with the Adda chain.
The formation of a distinct stable complex between MCLR and CD would yield two sets of MCLR peaks, one set for complexed MCLR and the other set for free MCLR. We did not observe two sets of peaks, but rather observed a shift of the aromatic and olefinic peaks for MCLR upon addition of β and γ-CD, indicating that the exchange of MCLR between the free and host—guest complexed environments is fast relative to the 1H NMR time scale (0.5 ms). The resonances for protons on aromatic and diene moieties shift upfield (to lower chemical shift in ppm) upon complexation, indicating increased shielding of the protons from the magnetic field. This is consistent with the protons (Adda) being complexed within the CD ring, a hydrophobic environment.
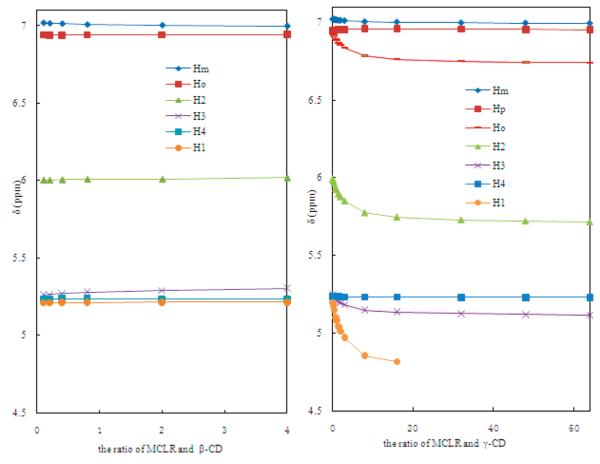
For the benzene proton resonances for Ho, Hm, and Hp were assigned on the basis of their multiplicity and integration.37 For free MCLR, Ho and Hp, the protons ortho and para relative to the alkyl chain, will be more shielded and thus have a lower chemical shift relative to the meta proton, Hm. The individual proton resonances of the benzene ring (Ho, Hm) shift only slightly upon complexation with β-CD (Figure 4). The Hm triplet on the benzene ring of MCLR shifts downfield, while olefinic proton peaks do not shift. We suggest this is the result of the smaller size of the β-CD cavity (i.d. 0.62 nm) that can only encapsulate the benzene ring (i.d. 0.56 nm) of the Adda chain. More pronounced changes in chemical shift are observed for the olefinic and benzene protons in the presence of γ-CD (i.d. 0.78 nm) compared to β-CD (Figure 4). The protons Ho (doublet), H1 (triplet), H2 (doublet), and H3 (quartet) shift downfield upon addition of γ-CD. Moreover, chemical shift for the protons on the OCH3 group of Adda chain also changes from 3.0 to 3.2 ppm. The significant changes in chemical shifts indicate MCLR may be encapsulated within γ-CD; thus, the benzene and olefinic protons are exposed to different regions of the γ-CD (top and bottom). These observations suggest γ-CD complexes the middle of the Adda chain, analogous to a bracelet fitting onto an arm or a bead threading a string. The olefinic protons (H4 and H5) within the cyclic structure of MCLR do not shift, implying that there is minimal or no measurable specific interaction between the cyclic region of MCLR and γ-CD.
Figure 4.
Chemical shift changes on benzene ring and diene group on MCLR in the presence of (A) β-CD and (B) γ-CD by increasing the ratio of CDs and MCLR. Each curve represents the middle point of multiplets for each hydrogen. Hp in B is not included because of overlap by other H peaks.
Characterization Nature of Complex
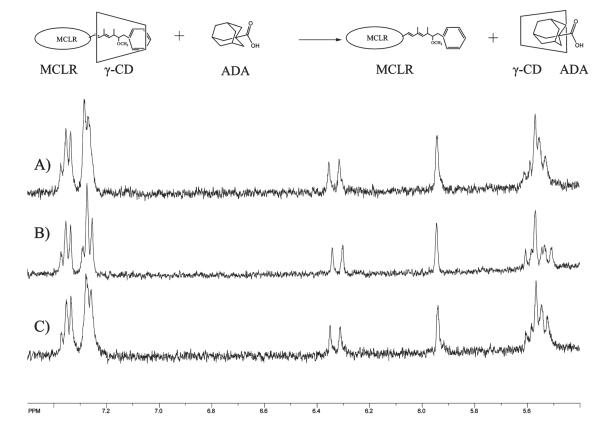
Different complexing modes, for example, internal host—guest complex or external association not involving the cavity of CD, are possible. The pronounced chemical shift change for the protons on the Adda moiety suggests that the host—guest complex involves Adda. In an attempt to confirm that the nature of the interaction of MCLR and γ-CD occurs via an inclusion complex, a powerful competitive guest, adamantanecarboxylic acid (ADA), with a binding constant of 34 000 M−1, known to form 1:1 inclusion complex,38 was added into MCLR and γ-CD solution. ADA undergoes the strongest binding with γ-CD at neutural pH.34 If the MCLR:γ-CD complex involves an inclusion complex, the resonances for several protons on benzene ring and diene group of MCLR shift upfield upon addition of γ-CD, as illustrated in Figure 5. When ADA is added into the mixture of MCLR and γ-CD, the NMR spectrum of the free MCLR is observed, indicating that ADA replaces MCLR in the cavity of γ-CD.
Figure 5.
1HNMR spectra resulting from addition of adamantanecarboxylic acid (ADA) to MCLR—γ-CD solution for (A) MCLR, (B) MCLR:γ-CD, 1:2, (C) MCLR:γ-CD:ADA, 1:2:10.
Effects of Solution pH, Salinity, and NOM
To probe the effects of water quality on the complexation of MCLR and γ-CD, the effects of solution pH and ionic strength were investigated. The affinity of the neutral/hydrophobic compounds for the hydrophobic cavity of CDs may be enhanced relative to ionized forms in solution of increased ionic strength.39 The solution pH has a direct effect on the charge and hydrophobicity of MCLR, which in turn could influence the host—guest interaction with CDs. We investigated the effect of solution pH on the MC:CD complex under the acidic, basic, and the neutral conditions normally associated with typical natural waters. A series of MCLR:γ-CD solutions at various solution pH values (1, 3, 5, 7, 8, 9, and 10) was characterized by 1H NMR. Since MCLR possesses acidic COOH groups, a stronger binding may be expected under acid conditions, where the nonionized MC is more hydrophobic. However, no appreciable change was observed in the complexation as a function of solution pH over the range from 1 to 7. The very small change for H3 observed at pH 5 is assigned to a chemical environment change due to protonation of carboxylic acid in the vicinity of H3 or changes in conformation of MCLR. The other resonances were not influenced by solution pH. Under basic conditions, the binding becomes slightly weaker, likely the result of ionization of functional groups on the cyclic peptide region.
Microcystins are most widely distributed in freshwaters, but they have also been identified in marine environments 1; thus, the effect of salinity on the complexation was investigated. It is reported that CD solubilization of organic compounds can be enhanced by increasing the ionic strength of the solution due to formation of guest and cyclodextrin complex aggregates.40 NaCl was added to the solution of MCLR and γ-CD to increase the ionic strength of the solution. Even at 5 mol/L NaCl no changes in the binding were observed on the basis of the NMR spectra. According to Loftsson et al., salts enhance the contribution of the noninclusion complexation to the overall guest solubility.40 The absence of a salt effect is consistent with the proposal that MCLR and γ-CD form an inclusion complex. The presence of natural organic matter (NOD) has a negative effect on the removal of cyanotoxins by several common methods, such as activated carbon adsorption,41 ozonation,42 and TiO2 photodegradation.43 In our study, up to 5 g/L humic acid was added into the solution of MCLR and γ-CD, yet no changes were observed in the binding or complexation of the toxin, a distinct advantage over existing treatment methods, which are negatively impacted by the presence of humic materials.
Binding Constant for the Complex of γ-CD with Different MCs and NOD
Binding constant for MCLR, MCRR, and NOD with γ-CD were obtained according to the method described by Ramstad et al.31 The binding isotherm for a 1:1 molecular stoichiometry can be obtained by measuring the chemical shift change as a function of host at a specific ratio of the host and γ-CD, 1:12. In accordance with the theory, Δmax, the maximum of chemical shift difference, cannot be experimentally obtained in the rare instance that complete binding is achieved. However, the binding constant, K, and Δmax may be calculated from eq 2 through application of nonlinear curve fitting to the empirical data.
| (1) |
| (2) |
where Δ is the chemical shift difference, K is the binding constant, [γ-CD] is the the concentration of γ-CD, and Δmax is the the maximum of chemical shift difference.
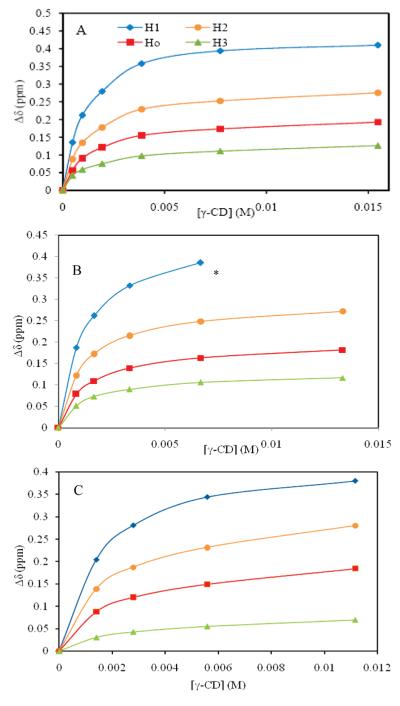
The data for calculating binding constants were obtained by monitoring chemical shift change as a function of [γ-CD]. The solutions employed for these experiments were prepared using serial dilution. The change in chemical shift was monitored relative to protons of the internal standard dextran sulfate sodium (DSS) at 0 ppm. The proton resonances for H1, H2, H3, and Ho shift upfield as the [CD] increases. Table 1 and Figure 6 show the measured data for chemical shift difference of H1, H2, H3, and Ho for MCLR. H1 exhibits the largest chemical shift change, which is consistent with a complexation made containing H1 (the diene) in the middle of the CD ring, the most hydrophobic and shielded environment of the CD interior, as discussed earlier. Relatively small shifts for H3 suggest that this proton may be at the edge of the CD ring or just outside, and thus the influence is less pronounced. To further probe the specific position of CD on the Adda chain, advanced NMR techniques (NOESY) were carried out in an attempt to determine the distances between protons on the host and guest molecules. We were unable to detect specific interactions between the host and guest molecules from our NOESY experiments. This could be due to experimental limitations, dynamic properties of the host—guest complexation, or movement of the host within the guest, i.e., rotating and sliding within the complex.
Table 1.
Chemical Shift Difference Data in the Function of [γ-CD] for Binding Curve at the Ratio of [MCLR]:[γ-CD], 1:12
| Δδ (ppm) |
||||
|---|---|---|---|---|
| γ-CD (mM) | Ho | H1 | H2 | H3 |
| 0.48 | 0.06 | 0.14 | 0.09 | 0.04 |
| 0.96 | 0.09 | 0.21 | 0.13 | 0.06 |
| 1.9 | 0.12 | 0.28 | 0.18 | 0.07 |
| 3.9 | 0.15 | 0.36 | 0.23 | 0.09 |
| 7.7 | 0.17 | 0.39 | 0.25 | 0.11 |
| 15.5 | 0.19 | 0.41 | 0.28 | 0.12 |
Figure 6.
Chemical shift change of hydrogens on MCLR as a function of [γ-CD] by complexation of MCLR (A), MCRR (B), NOD (C), and γ-CD at the molar ratio of 1:12 in D2O solution. *The peak after this point was overlapped by other peaks and incalculable.
The binding constant (K) and Δmax were obtained using curve-fitting analyses. Ideally each proton on a particular host would yield the same K. Although the value of binding constant is expected to be independent of the proton measured, K values for each measured proton are different, due to slightly different chemical environment.44 The average binding constant for the complex of MCLR and γ-CD is 1045 ± 144 M−1 and Δmax varies from 0.12 to 0.45 ppm (Table 2). Excellent modeling of the experimental data by eq 1 indicates a 1:1 complex and not a higher order complex.
Table 2.
Parameters for the Complexation of γ-CD and MCLR, MCRR, and NOD, respectively
| parameter | Ho | H1 | H2 | H3 | |
|---|---|---|---|---|---|
| MCLR | Δmax (ppm) | 0.20 | 0.45 | 0.29 | 0.12 |
| K (M−1) | 1014 | 1112 | 1155 | 902 | |
| MCRR | Δmax (ppm) | 0.19 | 0.45 | 0.29 | 0.13 |
| K (M−1) | 858 | 739 | 856 | 884 | |
| NOD | Δmax (ppm) | 0.21 | 0.44 | 0.31 | 0.08 |
| K (M−1) | 538 | 757 | 643 | 506 |
At least 70 different MC variants have been identified. All MC variants and NOD possess the Adda side chain and thus should be complexed by γ-CD.8,9 To test the broader applicability of CD complexation, experiments were conducted with MCRR and NOD substrates. The differences between the structure of MCRR and MCLR are the two variable l-amino acids at the 2 and 4 positions. Titration of the chemical shift change as a function of [γ-CD] leads to an average binding constant of 835 ± 77 M−1 for the complex of MCRR and γ-CD, slightly lower than the value for MCLR (1045 ± 144 M−1). The values of Δmax for MCRR are from 0.13 to 0.45 ppm (Table 2). Although MCRR and MCLR have different amino acid residues with the cyclic peptide ring structure, the similar K values further indicate the complexation occurs at the Adda chain common to both compounds. MC’s are composed of seven amino acid residues, while NOD consists of five residues (Figure 1B), but all possess the Adda chain. The average binding constant for the complex of NOD and γ-CD is 612 ± 145 M−1, which is lower than that of MCLR. The values for Δmax are from 0.08 to 0.44 ppm (Table 2). The different structures on the cyclic peptide of NOD and MCs affect their ability to complex with CDs.
The NMR studies demonstrate that α-CD does not exhibit a detectable host—guest interaction with MCLR. The slightly larger β-CD induces chemical shift changes in the benzene protons in the Adda side chain, but these shifts were too small to allow accurate determination of the K for β-CD with MCLR. Since the shifts are only observed for the benzene proton resonances, we propose the binding occurs at the end the Adda chain and the interaction between MCLR and β-CD is weak. However, γ-CD exhibits relatively strong binding with MC variants and NOD. 1H NMR was used to obtain the binding isotherms, curves of the observed chemical shifts versus free ligand (γ-CD) concentrations, which yield binding constants for MCLR, MCRR, and NOD of 1045, 835, and 612 M−1, respectively. To probe the nature of the MCLR:γ-CD complex, ADA, which forms a strong inclusion complex with γ-CD, was added to the MC:γ-CD complex. Upon addition of ADA, the MCLR:γ-CD complex disappears with the complementary formation of free MCLR. This observation indicates that the ADA replaces MCLR and the MCLR:γ-CD interactions occur via an inclusion complex. Our studies demonstrate that specific CDs form strong inclusion complexes with MCs and NOD, which are not influenced over a range of solution pH, by salinity, or in the presence of NOM.
Our results suggest that CDs are promising substrates for complexing MCs and NOD under a variety of different water qualities, thus forming the basis for potential treatment/removal methods. The applications of this process will require improvement in the binding properties of the host, but CDs can be readily tailored to enhance the binding properties through simple functional transformations of the peripheral hydroxyl groups.45 The practical application of this process for the removal or trapping of MCs and NOD will also require immobilized CD substrates, such as those employed for the removal of dyes, organic pollutants, and metal ions from wastewater.22–24,46 While testing modified CD materials for removal of MCs and NOD is beyond the scope of this paper, we are planning future studies in this direction.
ACKNOWLEDGMENT
The authors gratefully acknowledge support from the U.S. Environmental Protection Agency (RD-83322301).
REFERENCES
- (1).Kuiper TG, Falconer I, Fitzgerald J. Toxic cyanobacteria in water: A guide to their public health consequences. In: Chorus I, Bartram J, editors. Monitoring and Management. World Health Organization; Geneva: 1999. [Google Scholar]
- (2).Smith JL, Boyer GL, Zimba PV. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture. 2008;280:5–2008. [Google Scholar]
- (3).Burkholder JM. Harmful algal blooms. In: Likens GE, editor. Encyclopedia of Inland Waters. Elsevier; New York: 2008. [Google Scholar]
- (4).Camean A, Moreno IM, Ruiz MJ, Pico Y. Determination of microcystins in natural blooms and cyanobacterial strain cultures by matrix solid-phase dispersion and liquid chromatography—mass spectrometry. Anal. Bioanal. Chem. 2004;380:537–544. doi: 10.1007/s00216-004-2755-2. [DOI] [PubMed] [Google Scholar]
- (5).Moore RE. Cyclic peptides and depsipeptides from cyanobacteria: A review. J. Ind. Microbiol. Biotechnol. 1996;16:134–143. doi: 10.1007/BF01570074. [DOI] [PubMed] [Google Scholar]
- (6).Zhang DW, Xie P, Liu YQ, Qiu T. Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health. Sci. Total Environ. 2009;407:2191–2199. doi: 10.1016/j.scitotenv.2008.12.039. [DOI] [PubMed] [Google Scholar]
- (7).Hitzfeld BC, Hoger SJ, Dietrich DR. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000;108:113–122. doi: 10.1289/ehp.00108s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Poon KF, Lam MHW, Lam PKS, Wong BSF. Environmental chemistry-determination of microcystins in cyanobacterial blooms by solid-phase microextraction—high-performance liquid chromatography. Environ. Toxicol. Chem. 2001;20:1648–1655. [PubMed] [Google Scholar]
- (9).Matsushima RN, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, Carmichael WW, Fujiki H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992;118:420–424. doi: 10.1007/BF01629424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Huang WJ, Cheng BL, Cheng YL. Adsorption of microcystin-LR by three types of activated carbon. J. Hazard. Mater. 2007;141:115–122. doi: 10.1016/j.jhazmat.2006.06.122. [DOI] [PubMed] [Google Scholar]
- (11).Brooke S, Newcombe G, Nicholson B, Klass G. Decrease in toxicity ofmicrocystins LA and LR in drinking water by ozonation. Toxicon. 2006;48:1054–1059. doi: 10.1016/j.toxicon.2006.08.010. [DOI] [PubMed] [Google Scholar]
- (12).Rodrıguez E, Gretchen D, Onstad TPJ, Kullc JS, Metcalf JLA, Gunten UV. Oxidative elimination of cyanotoxins: comparison of ozone, chlorine, chlorine dioxide and permanganate. Water Res. 2007;41:3381–3393. doi: 10.1016/j.watres.2007.03.033. [DOI] [PubMed] [Google Scholar]
- (13).Song WH, Teshiba T, Rein K, O’Shea KE. Ulitrasonically induced degradation and detoxification of Microcystin-LR (cyanobacterial toxin) Environ. Sci. Technol. 2005;39:6300–6305. doi: 10.1021/es048350z. [DOI] [PubMed] [Google Scholar]
- (14).Liu I, Lawton LA, Cornish B, Robertson PKJ. Mechanistic and toxicity studies of the photocatalytic oxidation of microcystin-LR. J. Photochem. Photobiol. A Chem. 2002;148(1—3):349–354. [Google Scholar]
- (15).Liu I, Lawton LA, Robertson PKJ. Mechanistic studies of the photocatalytic oxidation of microcystin-LR: An investigation of byproducts of the decomposition process. Environ. Sci. Technol. 2003;37(14):3214–3219. doi: 10.1021/es0201855. [DOI] [PubMed] [Google Scholar]
- (16).Robertson PKJ, Lawton LA, Cornish BJPA. The involvement of phycocyanin pigment in the photodecomposition of the cyanobacterial toxin, microcystin-LR. J. Porphyrins Phthalocyanines. 1999;3(6—7):544–551. [Google Scholar]
- (17).Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implicationfor nanoparticle neurotoxicity. Environ. Sci. Technol. 2006;40(14):4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- (18).Valeria AM, Ricardo EJ, Stephan P, Alberto WD. Degradation of microcystin-RR by Sphingomonas sp. CBA4 isolated from San Roque reservoir (Cordoba—Argentina) Biodegradation. 2006;17:447–455. doi: 10.1007/s10532-005-9015-9. [DOI] [PubMed] [Google Scholar]
- (19).Wang H, Ho L, Lewis DM, Brookes JD, Newcombe G. Descriminating and assessing adsorption and biodegradation removal mechanisms during activated carbon filtration of microcystin toxins. Water Res. 2007;41:4262–4270. doi: 10.1016/j.watres.2007.05.057. [DOI] [PubMed] [Google Scholar]
- (20).Edwards C, Graham D, Fowler N, Lawton LA. Biodegradation of microcystins and nodularin in freshwater. Chemosphere. 2008;73:1315–1321. doi: 10.1016/j.chemosphere.2008.07.015. [DOI] [PubMed] [Google Scholar]
- (21).Lemesa GAF, Kersanach R, Pinto LS, Dellagostin OA, Yunes JS, Matthiensen A. Biodegradation of microcystins by aquatic Burkholderia sp. from a South Brazilian coastal lagoon. Ecotoxicol. Environ. Saf. 2008;69:358–365. doi: 10.1016/j.ecoenv.2007.03.013. [DOI] [PubMed] [Google Scholar]
- (22).Phan TNT, Bacquet M, Morcellet M. The removal of organic pollutants from water using new silica-supported β-cyclodextrin derivatives. React. Funct. Polym. 2002;52:117–125. [Google Scholar]
- (23).Gazpio C, Sanchez M, Isasi JR, Velaz I, Martin C, Oharriz CM, Zornoza A. Sorption of pindolol and related compounds by a β-cyclodextrin polymer: Isosteric heat of sorption. Carbohydr. Polym. 2008;71:140–146. [Google Scholar]
- (24).Crini G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigm. 2008;77:415–426. [Google Scholar]
- (25).Bender ML, Komiyama M. Cyclodextrin Chemistry. Spring-Verlag; New York: 1978. [Google Scholar]
- (26).Saenger W. Cyclodextrin inclusion complexes in research and industry. Angew. Chem. Int. Ed. Engl. 1980;19:344–362. [Google Scholar]
- (27).Bradshaw JS, Jones BA, Davidson RB, Christensen JJ, Lamb JD, Izatt RM, Morin FG, Grant DM. Chiral recognition by the S,S and R, R enantiomers of dimethyl dioxopyridino-18-crown-6 as measured by temperature-dependent proton NMR spectroscopy in CD2Cl2, titration calorimetry in methanol at 25°, and selective crystallization. Org. Chem. 1982;47:3362–3364. [Google Scholar]
- (28).Terekhova IV. Volumetric and calorimetric study on complex formation of cyclodextrins with amino benzoic acids. Mendeleev Commun. 2009;19:110–112. [Google Scholar]
- (29).Harata KI. Structural aspects of stereo differentiation in the solid state. Chem. Rev. 1998;98:1803–1827. doi: 10.1021/cr9700134. [DOI] [PubMed] [Google Scholar]
- (30).Lippold I, Vlay K, Gorls H, Plass W. Cyclodextrin inclusion compounds of vanadium complexes: Structural characterization and catalytic sulfoxidation. J. Inorg. Biochem. 2009;103:480–486. doi: 10.1016/j.jinorgbio.2008.12.014. [DOI] [PubMed] [Google Scholar]
- (31).Ramstad T, Hadden CE, Martin GE, Speaker SM, Teagarden DL, Thamann TJ. Determination by NMR of the binding constant for the molecular complex between alprostadil and α-cyclo-dextrin: Implications for a freeze-dried formulation. Int. J. Pharm. 2005;296:55–63. doi: 10.1016/j.ijpharm.2005.02.018. [DOI] [PubMed] [Google Scholar]
- (32).Cao YJ, Lu RH. 1HNMR titration and quantum calculation for the inclusion complexes of cis-cyclooctene, cis,cis-1, 3-cyclooctadiene and cis,cis-1, 5-cyclooctadiene with β-cyclodextrin. Spectrochim. Acta (A) 2009;73:713–718. doi: 10.1016/j.saa.2009.03.012. [DOI] [PubMed] [Google Scholar]
- (33).Yamamoto T, Kobayashi T, Matsui Y, Takahashi T, Aso YA. 1HNMR spectroscopic study on the tryptophan residues of lysozyme included by glucosyl-β-cyclodextrin. J. Mol. Struct. 2009;920:264–269. [Google Scholar]
- (34).Cromwell WC, Byströom K, Eftink MR. Cyclodextrin—adamantanecarboxylate inclusion complexes: Studies of the variation in cavity size. J. Phys. Chem. 1985;89:326–332. [Google Scholar]
- (35).Dotsikas Y, Loukas YL. Efficient determination and evaluation of model cyclodextrin complex binding constants by electrospray mass spectrometry. J. Am. Soc. Mass. Spectrom. 2003;14:1123–1129. doi: 10.1016/S1044-0305(03)00451-3. [DOI] [PubMed] [Google Scholar]
- (36).Song W, Bardowell S, O’Shea KE. Mechanistic study and the influence of oxygen on the photosensitized transformationsof microcystins (cyanotoxins) Environ. Sci. Technol. 2007;41(15):5336–5341. doi: 10.1021/es0630660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Gunther H. NMR spectroscopy: Basic principles, concepts, and applications in chemistry. John Wiley& Sons; New York: 1995. [Google Scholar]
- (38).Gelb RI, Schwartz LM, Laufer DA. Cycloamylose complexation of adamantane derivatives. Life Sci. 1983;33(1):83–85. doi: 10.1016/0024-3205(83)90714-2. [DOI] [PubMed] [Google Scholar]
- (39).Tommasini S, Calabrò ML, Raneri D, Ficarra P, Ficarra R. Combined effect of pH and polysorbates with cyclodextrins on solubilization of naringenin. J. Pharm. Biomed. Anal. 2004;36:327–333. doi: 10.1016/j.jpba.2004.06.013. [DOI] [PubMed] [Google Scholar]
- (40).Loftsson T, Matthiasson K, Masson M. The effects of organic salts on the cyclodextrin solubilization of drugs. Int. J. Pharm. 2003;262:101–107. doi: 10.1016/s0378-5173(03)00334-x. [DOI] [PubMed] [Google Scholar]
- (41).Donati C, Drikas M, Hayes R, Newcombe G. Microcystin-LR adsorption by powdered activated carbon. Water Res. 1994;28:1735–1742. [Google Scholar]
- (42).Shawwa AR, Smith DW. Kinetics of microcystin-LR oxidation by ozone. Ozone. Sci. Eng. 2001;23(2):161–170. [Google Scholar]
- (43).Feitz AJ, Waite TD. Kinetic modeling of TiO2-catalyzed photodegradation of trace levels of microcystin-LR. Environ. Sci. Technol. 2003;37(3):561–568. doi: 10.1021/es0256010. [DOI] [PubMed] [Google Scholar]
- (44).Wiese M, Cordes HP, Chi H, Seydel JK, Bachensfeld T, Muler BW. Interaction of prostaglandin E, with a-cyclodextrin in aqueous systems: Stability of the inclusion complex. J. Pharm. Sci. 1991;80:153–156. doi: 10.1002/jps.2600800213. [DOI] [PubMed] [Google Scholar]
- (45).Yilmaz A, Yilmaz E, Yilmaz M, Bartsch RA. Removal of azo dyes from aqueous solutions using calix[4]arene and β-cyclodextrin. Dyes Pigm. 2007;74(1):54–59. [Google Scholar]
- (46).Crini G. Studies of adsorption of dyes on beta-cyclodextrin polymer. Biores. Technol. 2003;90:193–198. doi: 10.1016/s0960-8524(03)00111-1. [DOI] [PubMed] [Google Scholar]