Summary
Potassium perchlorate has been used at various times during the last 50 years to treat hyperthyroidism. Since World War II ammonium perchlorate has been used as a propellant for rockets. In 1997, the assay sensitivity for perchlorate in water was improved from 0.4 mg/L (ppm) to 4 µg/L (ppb). As a result, public water supplies in Southern California were found to contain perchlorate ions in the range of 5 to 8 ppb, and those in Southern Nevada were found to contain 5 to 24 ppb. Research programs have been developed to assess the safety or risk from these exposures and to assist state and regulatory agencies in setting a reasonable safe level for perchlorate in drinking water. This report reviews the evidence on the human health effects of perchlorate exposure. Perchlorate is a competitive inhibitor of iodine uptake. All of its pharmacologic effects at current therapeutic levels or lower are associated with inhibition of the sodium-iodide symporter (NIS) on the thyroid follicular cell membrane. A review of the medical and occupational studies has been undertaken to identify perchlorate exposure levels at which thyroid hormone levels may be reduced or thyrotropin levels increased. This exposure level may begin in the 35 to 100 mg/d range. Volunteer studies have been designed to determine the exposure levels at which perchlorate begins to affect iodine uptake in humans. Such effects may begin at levels of approximately 1 mg/d. Environmental studies have assessed the thyroidal health of newborns and adults at current environmental exposures to perchlorate and have concluded that the present levels appear to be safe. Whereas additional studies are underway both in laboratory animals and in the field, it appears that a safe level can be established for perchlorate in water and that regulatory agencies and others are now trying to determine that level.
Keywords: Perchlorate, Iodine uptake, Drinking water, Thyroid, Environment, Health effects
Perchlorate (ClO4−) is the dissociated anion of perchlorate salts such as potassium perchlorate, sodium perchlorate, and ammonium perchlorate. Potassium perchlorate was primarily used as a pharmaceutical agent for the treatment of hyperthyroidism. It is now mainly used in flares and automobile airbags, though it is still used to treat hyperthyroidism and for diagnostic purposes. Sodium perchlorate is used in the manufacture of slurry explosives. Ammonium perchlorate is used as the primary oxidant in solid propellant mixtures for rockets, missiles, fireworks, and certain munitions. As a powerful oxidizer, ammonium perchlorate is used in the propulsion systems of intercontinental ballistic missiles such as the peacekeeper missile, and as an accelerant in such well-known aerospace systems as the space shuttle and the Atlas and Minuteman missiles. Perchlorate is manufactured in the United States, France, Brazil, China, Egypt, Taiwan, and Japan. Perchlorate is manufactured today almost exclusively for the aerospace industry and secondarily for the production of fireworks.
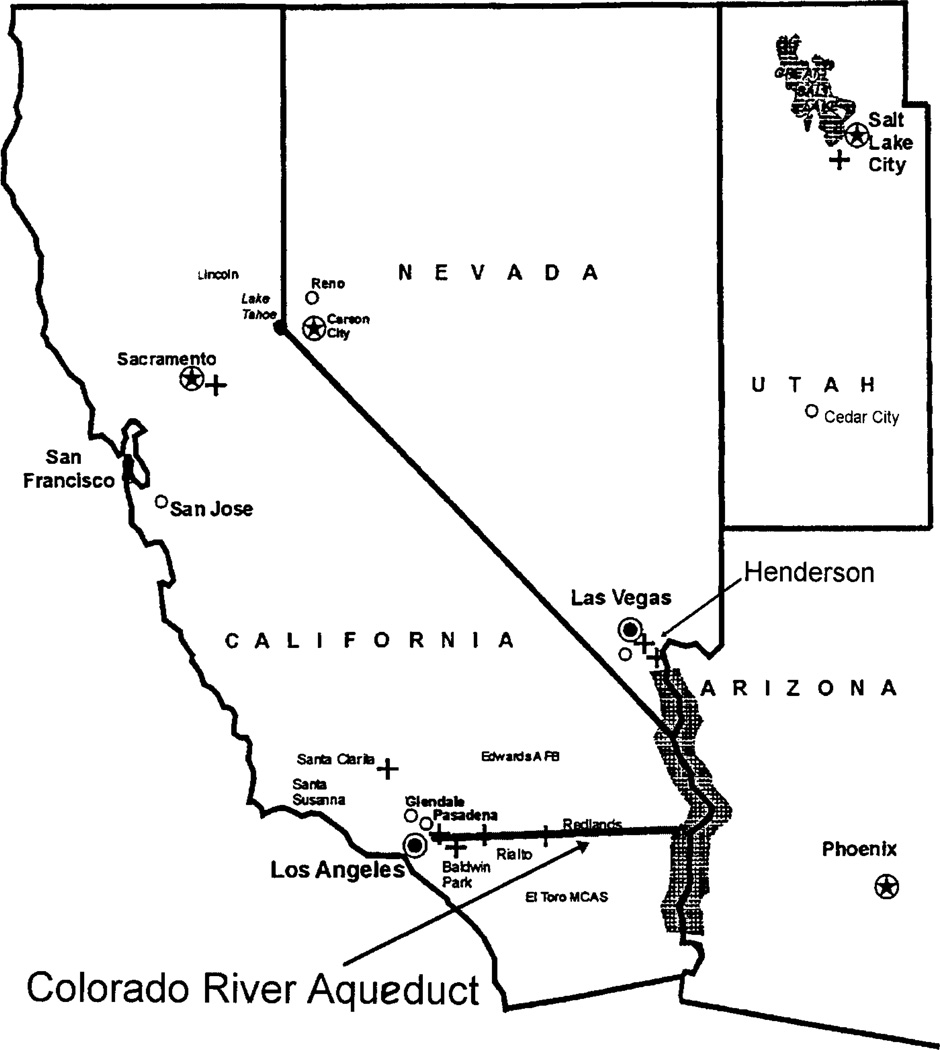
Perchlorate has been found in soil and in surface and ground water at sites where perchlorate salts were manufactured, stored, or used. Perchlorate salts are highly water soluble and fully ionize in water, releasing the perchlorate ion. The perchlorate ion is identical whether it is derived from potassium, sodium, or ammonium salts. Before 1997, the limit of detection of perchlorate in water was 0.4 mg/L (mg/L ≡ milligrams/liter ≡ parts per million ≡ ppm). At that time Sacramento, California was the only site in the United States where perchlorate-containing groundwater was suspected. In March 1997, researchers in the California Department of Health Services (Sanitation and Radiation Laboratory) developed an ion chromatographic analytic method with a detection limit of 4 µg/L µg/L ≡ micrograms/liter ≡ parts per billion ≡ ppb) (1). Using this newer analytic method, which allowed detection of perchlorate in water at one hundredth of its previous detection level, a survey of California water detected perchlorate in drinking water throughout Southern California in the 5 to 8 ppb range whereas higher levels were detected at several other sites. Although specific local industrial sites (fireworks factories, missile maintenance stations, etc.) were the sources of local environmental perchlorate, the major source of perchlorate in Southern California was perchlorate containing water brought in by an aqueduct from the Colorado River. The perchlorate found in the Colorado River water originated from a natural underground channel that carried perchlorate from under a former perchlorate manufacturing site in Henderson, Nevada to the Las Vegas Wash, which in turn carried the Las Vegas water outflow into Lake Mead and out into the Colorado River (Fig. 1). A recovery plant is now in place to remove the perchlorate from this paleochannel before it enters the Las Vegas Wash.
FIG. 1.
Map of southern California water distribution.
Perchlorate is now recognized as a persistent and pervasive contaminant of drinking-water supplies in Southern California (excluding Imperial County), Nevada (in Clark County), and Arizona (2). Concentrations down-stream from Lake Mead have generally been in the 5 to 8 ppb range. Concentrations in Lake Mead water supplied to Nevada have ranged from undetectable to 24 ppb (3). In Las Vegas Bay, where the Las Vegas Wash emerges, higher concentrations have occurred. The discovery in 1997 that perchlorate in the low 10-ppb range is present in the water supply of a large area of the United States has highlighted the need to identify the human health risks associated with perchlorate ingestion at various levels.
Our knowledge of the human health effects of perchlorate has come primarily from clinical studies and case reports on the use of potassium perchlorate in the treatment of hyperthyroidism. In 1952, potassium perchlorate was shown to be effective in blocking the uptake of iodide by the hyperactive thyroid and thus reducing thyroid hormone production (4). The site of action (sodium-iodide symporter [NIS]) and the exact mechanism of action (competitive inhibition of NIS) have subsequently been described (5–8). Clinical studies, clinical experience, and laboratory animal studies have now shown that the only significant biologic effect of perchlorate at less-than-toxic exposure levels is on the thyroid gland and through the effect on the NIS (9). This paper will review the scientific literature, concentrating on the human health effects of the perchlorate ion. Studies of environmental and daily low-level exposures and the basic physiology and pathophysiology of perchlorate at the tissue and cellular levels are discussed.
OCCURRENCE, MANUFACTURE, AND STABILITY OF PERCHLORATE SALTS
Large-scale production of perchlorate salts began in the United States in the mid-1940s to meet the increased demand for ammonium perchlorate for use in solid propellant mixtures for rockets and missiles. Perchlorate salts have also been used as components in nuclear reactors and electronic tubes, as additives in lubricating oils, in electroplating and aluminum refining, in tanning and finishing leather, and in the production of paints and enamels (10).
Perchlorate salts are found naturally at some sites. For example, in the Chilean plateau where nitrate is mined, deposits containing salts of iodate, chromate, and perchlorate also occur. As a result, perchlorate is present in Chilean nitrate fertilizer in concentrations ranging from 0.5 mg/kg to greater than 60 mg/kg (11–13). Nitrates have been mined in the Atacama Desert of northern Chile and exported to North America and Europe for use in gunpowder and fertilizer production for more than 200 years (14). Chilean potassium nitrate (saltpeter) was commonly used as a fertilizer for rye and tobacco crops in the United States several decades ago. Occasionally, damage to the crops was attributed to the potassium perchlorate present as a contaminant in the saltpeter. This saltpeter contained as much perchlorate as 1.5% by weight (14–16).
Extremely pure ammonium perchlorate can be produced by the reaction of ammonium and pure perchloric acid solution (17). The commercial manufacture of per-chlorate starts with the electrolysis of brine (sodium chloride in water) to first form sodium chlorate (NaClO3) and then sodium perchlorate (NaClO4). Ammonium chloride (NH4Cl) is formed from ammonia (NH3) and hydrochloric acid (HCl). The ammonium perchlorate is manufactured by a double-exchange reaction when the sodium perchlorate is reacted with the ammonium chloride to form ammonium perchlorate (NH4ClO4) and salt (NaCl). The solution is cooled, and the ammonium perchlorate crystals are dried and blended to specifications. Manufacturing, processing, and storing ammonium perchlorate requires low humidity and large amounts of electricity. Therefore in the United States, most of the ammonium perchlorate production has been concentrated in desert areas such as Southern Nevada and now in only a single plant in Cedar City, Utah. Under arid conditions, perchlorate can precipitate with cations, resulting in a long-term source for redissolution. With time, propellant manufactured from ammonium perchlorate decomposes, leading to a limited shelf life for these fuels and a need to replace them periodically (18). Perchlorate has been detected in surface water and groundwater at some perchlorate disposal sites and refueling sites, and at rocket testing sites and ordnance storage areas (19).
The toxicity of perchlorate salts is essentially that of the perchlorate ion. Ammonium perchlorate is hygroscopic and has high aqueous solubility (approximately the same as sodium chloride at body temperature). In water, it dissociates into its component ions; these are highly stable and can persist indefinitely under typical ground and surface water conditions.
IDENTIFICATION OF ENVIRONMENTAL PERCHLORATE EXPOSURES AND RISKS
The recent method to quantify perchlorate in water was developed by Okamoto et al. (1), refined by Jackson (20,21), and then established by the EPA as method 314.0 for detecting trace levels of perchlorate (Dionex IonPac AS11 and AS16 columns) (Sunnyvale, CA) (22). Monitoring for perchlorate in drinking water has been required since 1999 by the EPA in the United States under the Unregulated Contaminants Monitoring Rule (UCMR) (23). The method has been modified for measuring perchlorate levels in urine and for measuring perchlorate levels in serum. The limits of detection of perchlorate were 0.5 ppm in urine and 0.05 ppm in serum (24,25).
Detection of perchlorate by electrospray ionization–tandem mass spectrometry (ESI/MS/MS) method addresses the considerable signal suppression caused by anions that are typically present in groundwater, such as bicarbonate and sulfate (26). The detection limit for perchlorate in ground water was 0.5 ppb. The ESI/MS/MS method provides more accuracy and precision than does ion chromatography (IC). Results for the performance evaluation samples for ESI/MS/MS differed from the certified values by 4% to 13%, and precision ranged from 3% to 10% (relative standard deviation). However, the IC and ESI/MS/MS results were statistically indistinguishable (P > 0.05) for perchlorate concentrations above the detection limits of both methods (26).
Perchlorate is a monovalent anion that is freely soluble in water. The presence of perchlorate in soil, drinking water, and ground water raises important questions concerning both the potential exposure and the potential risk to exposed human populations. The level of perchlorate in drinking water would influence the steady state perchlorate levels in populations continuously consuming water with perchlorate (19).
In March 1997, at the International Toxicity Estimates for Risk Peer Review Meeting, an attempt was made to identify and quantify the risks associated with low levels of perchlorate in water. However, it was concluded that more research was needed to fully characterize the risk. Subsequently, the United States Environmental Protection Agency (EPA), the United States Department of Defense (through the Air Force), and the Perchlorate Study Group (a consortium of industrial manufacturers and users of perchlorate) have been sponsoring or conducting research to meet this need.
PERCHLORATE ECOLOGY: REMEDIATION OF WATER AND SOILS CONTAINING PERCHLORATE
Activated charcoal and reverse ion exchange have been used with varying results to treat perchlorate-containing water. Plants and vegetables grown in perchlorate-containing soil or water may incorporate perchlorate, posing a potential exposure if they are consumed (13,27,28). Perchlorate removal by bioaccumulation into plants and bioremediation of soils by bacterial transformation of perchlorate are currently being studied as potential methods to remove the perchlorate ion. Other methods of cleanup such as phyto-transformation have been developed (18). Perchlorate removal by phytoremediation using salt cedar (Tamarix ramosissima) planted along affected waterways has already been used (29). These shrubby trees mine the salts, including perchlorate salts, from the surrounding water. Stalks of the plant submerged in the Las Vegas Wash picked up 300 µg perchlorate per gram of tissue submerged. Even dry twigs growing well above the water accumulated 5 µg/g (29).
PERCHLORATE EFFECTS IN HUMANS
There are many studies on the effects of perchlorate in humans. Most of the human data available until recently were drawn from the clinical use of perchlorate in the therapy of hyperthyroidism, including Graves’ disease, in the 1950s and 1960s (4,30–33). The target sites of perchlorate action and the levels of exposure at which these actions might occur are quite consistent with the fundamental biochemical physiology of the thyroid. Most studies have focused on the mode of action and not on the determination of quantitative levels of action. Until recently, very few studies have evaluated the effects of subtherapeutic doses of perchlorate in healthy human subjects.
Pharmacology and Mechanism of Action
The two major thyroid hormones are the iodothyronines, thyroxine (T4) and tri-iodothyronine (T3), which are both synthesized and released from the thyroid. Iodine is essential for the synthesis of these hormones. Iodide is actively transported (trapped) from the blood into the thyroid by a protein located on the basolateral membrane of the thyroid follicular cell, the sodium-iodide symporter (NIS), cloned in 1996 by Carrasco and colleagues (34). Once trapped, the iodide is then oxidized to an active form by thyroid peroxidase, which is also essential for iodinating the amino acid tyrosine to form monoiodotyrosine (MIT) and diiodotyrosine (DIT), and for coupling MIT and DIT to generate T3 and T4. These synthetic processes occur at the colloid–follicular interphase within the large glycoprotein thyroglobulin. T3 and T4 are then hydrolyzed from thyroglobulin and secreted from the thyroid into the blood by an intricate series of steps involving proteolysis. Recent reviews summarize in detail the myriad of steps involving the intrathyroidal metabolism of iodine and T3 and T4 synthesis and secretion (35–38) .
Once secreted into the blood, both T3 and T4 are tightly bound by plasma proteins, primarily the inter-alpha globulin thyronine binding globulin (TBG), such that only approximately 0.03% T4 and 0.3% T3 are unbound or free and available to the cells to carry out a vast series of stimulatory metabolic effects, including protein synthesis and enhanced oxygen consumption. It is generally believed that T3 is the major metabolically active hormone and that T4 may serve, therefore, as a prohormone. To this end, almost all tissues have an enzyme termed the outer ring (5’) deiodinase which removes an iodine from the outer or phenolic ring of T4 to generate the active hormone, T3. Under normal circumstances approximately 80% of circulating T3 derives from this deiodination. Therefore only 20% results from thyroid secretion.
Thyroid gland function is tightly regulated by the classic negative feedback system. The thyrotrophs in the anterior pituitary synthesize and secrete the glycoprotein (α- and β- subunits) thyroid-stimulating hormone (thyrotropin, TSH). Synthesis of TSH is stimulated by the hypothalamic tripeptide hormone thyrotropin-releasing hormone (TRH), which reaches the anterior pituitary by the hypothalamic–pituitary portal circulation. Release of TSH into the peripheral circulation is controlled by the concentrations of free thyroid hormones in the peripheral circulation such that TSH decreases when free hormones rise and increase when free hormones fall. Thus, serum TSH concentrations are suppressed in hyperthyroidism and elevated in primary hypothyroidism.
Perchlorate is rapidly absorbed from the gastrointestinal (GI) tract after ingestion. It is excreted intact in the urine and has a half-life in humans of approximately 6 to 8 hours (30). Approximately 95% is recovered in the urine within 72 hours (30). Because of its similarity in ionic size to iodide, the perchlorate ion is a competitive inhibitor of NIS (8). Recent evidence indicates that perchlorate is not translocated into the thyroid follicular cell by the NIS (38–41). Perchlorate belongs to the Hofmeister ions series (Table 1) and inhibits the uptake of iodide at the cellular level. Nitrate (NO3−) is also in the Hofmeister series and is well known to suppress thyroid function. Perchlorate is a more potent inhibitor than thiocyanate and nitrate (31). The inhibition constant, Ki, is estimated as 0.4 µmol to 24 µmol. At therapeutic dosage levels this competitive inhibition decreases the entrance of iodide into the thyroid, resulting in less available iodide for hormone synthesis and, therefore, a decrease in T3 and T4 synthesis (42).
TABLE 1.
| Technetate | TcO4− |
| Rhenate | RnO4−− |
| Perchlorate | ClO4− |
| Thiocyanate | SCN− |
| [Iodide] | I− |
| Nitrate | NO3− |
| Bromide | Br− |
| Chloride | Cl− |
Group VII in the Periodic Table.
Given in order of decreasing potency.
Unlike other antithyroid agents, such as methimazole and propylthiouracil, perchlorate does not block the synthesis of thyroid hormones and is unlikely to affect thyroid hormone levels in geographic areas with iodine in-take similar to that in the United States (approximately 150 µg/d (32)). The thyroid can usually maintain normal thyroid hormone levels unless the iodine supply is less than approximately 50 µg/d, or iodine uptake by the thyroid is decreased for a prolonged period of time resulting in hypertrophy and hyperplasia of the thyroid (33,43). Perchlorate could inhibit iodide accumulation enough to cause goiter and hypothyroidism when ambient iodine intake is low or iodide uptake is sufficiently inhibited. It has also been suggested that perchlorate may cause inhibition of organic binding of iodine within the thyroid gland by affecting thyroid peroxidase, but animal models have failed to demonstrate this inhibition (44,45).
Medical Use of Perchlorate
Hyperthyroidism
In 1952, Stanbury and Wyngaarden (4) reported that the thyroid uptake of tracer I131 in patients with hyperthyroidism was markedly inhibited for as long as 6 hours when patients were pretreated with 100 mg potassium perchlorate 1 hour before administration of the tracer. A dose of 100 mg potassium perchlorate also caused a nearly complete release of accumulated I131 in a 1-meth-yl-2-mercaptoimidazole (MMI)–blocked thyroid, whereas smaller doses of perchlorate (3, 10, and 50 mg) did not. Based on these results and similar results in normal rats (43), physicians began treating hyperthyroidism patients with orally administered potassium perchlorate. The duration of perchlorate exposure in these patients ranged from a single dose to several weeks of treatment (4,46). One case study reported treating a single patient with perchlorate for 22 years (47). Morgans and Trotter (48) reported that treatment of hyperthyroidism with potassium perchlorate at doses of 400 mg/d for several weeks had adverse effects such as GI irritation, skin rash, and lymphadenopathy but cited no serious complications. In 1960, both Crooks and Wayne (49) and Morgans and Trotter (46) reported that the incidence of adverse effects was much greater with a perchlorate dose of 1,500 to 2,000 mg/d than with a dose of 600 to 1000 mg/d. Consequently, the two teams recommended treatment doses of 1,000 mg/d and 800 mg/d, respectively (46,49). Wenzel and Lente (50) used 900 mg/d followed by 40 to 120 mg/d perchlorate in the treatment of Graves’ hyperthyroidism for more than 1 year and did not report any serious adverse effects.
Hyperthyroidism in Pregnancy
In 1960, Crooks and Wayne (49) reported that 12 women with hyperthyroidism during pregnancy were successfully treated with potassium perchlorate at doses of 600 to 1000 mg/d. The only observed adverse effect was that one of the infants had a very slight enlargement of the thyroid gland, which returned to normal size within 6 weeks postpartum. The other newborns in this study showed no abnormalities.
Bone Marrow Toxicity
The use of perchlorate for treatment of hyperthyroidism became limited in the mid-1960s because of reports of agranulocytosis and fatal aplastic anemia occurring in patients treated with 400 to 1,000 mg perchlorate per day (Table 2) (51–56). The first case of agranulocytosis in a patient receiving potassium perchlorate (a young woman treated with 1500 mg potassium perchlorate for 3 weeks) was reported by Crooks and Wayne in 1960 (49). The first death from aplastic anemia in a patient receiving potassium perchlorate was reported by Hobson in 1961 (51). After several deaths from aplastic anemia in patients treated with perchlorate at doses of 400 to 1000 mg/d for 8 to 33 weeks in the mid-1960s, the use of perchlorate for treatment of hyperthyroidism was generally discontinued in the United States. The last report of fatal bone marrow toxicity related to treatment with potassium perchlorate was in the 1960s. Table 2 summarizes the therapeutic dosages associated with human fatalities according to published case reports.
TABLE 2.
Treatment history for reported deaths from bone marrow toxicity among perchlorate-treated thyrotoxicosis patients
| Study | Daily dosage (mg/d) |
Body weight–adjusted daily dosage (mg/kg per day) |
Length of treatment for each case |
Effects |
|---|---|---|---|---|
| Hobson 196151 | 800 | 11 | 14 w | Fatal aplastic anemia |
| 600 | 9 | 20 w | ||
| Johnson and Moore 196152 | 1000 | 14 | 3 mo | Fatal aplastic anemia |
| 600 | 9 | 1 mo | ||
| Fawcett and Clark 196153 | 600 | 9 | 5 mo | Fatal aplastic anemia |
| 400 | 6 | 1–2 mo | ||
| Krevans et al. 196254 | 800 | 11 | 2w | Fatal aplastic anemia |
| 600 | 9 | 2 mo | ||
| 450 | 6 | 2 mo | ||
| Gjemdal 196355 | 600 | 9 | 3 mo | Fatal aplastic anemia |
| 400 | 6 | 1 mo | ||
| Barzilai and Sheinfeld 196656 | 1000 | 14 | 2 mo | Fatal aplastic anemia |
| 1000 | 14 | Few mo | Fatal agranulocytosis |
No deaths from aplastic anemia or agranulocytosis have been reported for perchlorate doses of less than 400 mg/d. In 1968, Rokke and Vogt (57) reported that 96 of 118 hyperthyroid patients were effectively treated with a combination of 400 mg/d potassium perchlorate and 400 mg/d propylthiouracil for an average duration of 7 weeks. Three of the patients had granulocytopenia that was attributed to the propylthiouracil. Further, in 1976 Evered (58) recommended perchlorate doses of 800 mg/d for the therapy of hyperthyroid patients who had reactions to thionamides (e.g., carbimazole, propylthiouracil, methimazole). In 1981, a case report described a patient with Graves’ hyperthyroidism treated for 22 years with 200 mg/d perchlorate without any apparent complications (47). This woman’s hyperthyroidism recurred 4 weeks after discontinuing potassium perchlorate treatment and was subsequently brought back under control with reinstitution of perchlorate treatment. The pathophysiology of aplastic anemia and agranulocytosis in patients treated with thyroid inhibitors such as perchlorate is not known (59,60).
Amiodarone-Induced Thyrotoxicosis
The anti-arrhythmic drug amiodarone is primarily used to treat patients with resistant tachyarrhythmias (61). It is an iodinated benzofuran derivative containing approximately 40% iodine by weight, and it can therefore induce hypothyroidism or thyrotoxicosis in some patients by causing an iodine overload. The thyrotoxicosis in a given patient is caused by either the iodine excess, an amiodarone-induced thyroiditis, or a combination of both. Numerous studies have reported the effective treatment of iodine-associated amiodarone-induced thyrotoxicosis with potassium perchlorate beginning at a dose of 800 to 1000 mg/d and continuing for 1 to 6 months at lower doses.
Current Therapeutic and Diagnostic Uses
In addition to its therapeutic use in cases of amiodarone iodine-induced thyrotoxicosis, perchlorate is occasionally used for medical diagnostic purposes. The perchlorate discharge test is used to detect iodide organification defects in the thyroid (62,63). In this test, a tracer dose of radioiodine is administered with or without 500 µg stable iodide and a thyroid radioiodine uptake is determined 3 hours later. This is immediately followed by the administration of 1 g potassium or sodium perchlorate and a repeat radioiodine uptake carried out 1 hour later. A decrease in the thyroid iodine uptake at 4 hours compared with 3 hours of greater than 15% of the 3-hour uptake constitutes a positive test indicating an iodide organification defect.
In former years, radiologic studies with pertechnetate (Tc 99m) were carried out to image the brain or the blood pool or to localize the placenta. Pretreatment with a dose of 200 to 400 mg potassium perchlorate was administered to minimize the accumulation of pertechnetate in the thyroid and salivary glands and in the choroid plexus. Perchlorate was also used to block the gastric uptake of Tc 99m in the investigation of GI bleeding (64).
Perchlorate Animal Studies
The potential toxicity of perchlorate has been studied in animal models with particular attention to dose and duration in adult animals, in newborn animals, and at various vulnerable stages of development. Animal models, which can conform to experimental conditions such as dose, frequency, duration and timing, can be used to determine species-specific threshold values and to strengthen the causal inference regarding perchlorate action. These values, helpful as they are, must be cautiously weighed with respect to species differences in physiology and kinetics. For example, the pharmacody-namics of thyroid hormones in rats differs from that in humans because rats do not have the major human thyroid hormone plasma binding protein (TBG) but do have transthyretin, a less potent thyroid hormone binding protein. It is also not known whether the rat symporter is more or less sensitive than the human symporter to the competitive inhibition of iodide uptake by perchlorate.
There are many published animal studies and a number that are still under study or prepublication (25,44,65–69). Because reduced thyroid function has been associated with developmental effects in animal testing and in humans, some of the current studies focus on reproductive, developmental, and neurobehavioral developmental toxicity and are conducted with pregnant rats, lactating rats, fetuses, and nursing pups. Certain studies, such as studies in which radioactive or potentially toxic substances are given to pregnant females, can be carried out in rats but cannot be conducted in humans. For example, rats fed 1.2% (120,000 ppm) sodium perchlorate for 11 days were smaller with larger thyroids and lower T4 but not T3 levels. Rat fetuses exposed to radioactive iodine and given potassium perchlorate are protected by the perchlorate from radioactive iodine uptake (70). A two-generation reproduction study in rats given perchlorate in drinking water evaluated the potential for perchlorate to cause reproductive defects. In a developmental study in rats, the potential for perchlorate to cause teratogenicity is being assessed along with any changes in TSH, T4, and T3 in developing pups and in dams during pregnancy and lactation. Neurohistologic and thyroid effects are measured at the same time points and correlated with hormone concentration changes at different developmental stages.
From a considerable number of rodent and human studies, it is clear that the essential toxicology of perchlorate is based on the consequences of its competitive inhibition of the uptake of iodide by the thyroid. Despite some uncertainties there is considerable information available in the existing toxicologic database to address the potential human health effects of low-level exposure to perchlorate in drinking water.
HUMAN STUDIES
Epidemiologic studies contribute to the identification of specific features evident in a human population as a consequence of human exposure to chemicals such as perchlorate. Three types of human studies of perchlorate exposure have been performed—occupational studies, environmental studies, and volunteer exposure studies. The environmental studies have concentrated mostly on newborns; there is a concern that newborns may be the most vulnerable subpopulation because normal thyroid function is necessary for normal neurologic development. Epidemiologic studies using neonatal screening data from state health departments in California, Nevada, and Arizona, and other studies using human data are summarized below.
OCCUPATIONAL PERCHLORATE EXPOSURE
Occupational exposure of worker populations during the commercial production or use of ammonium perchlorate is much higher than potential environmental exposure. Exposure to perchlorate can be through inhalation, ingestion, or dermal contact. Exposure to ammonium perchlorate dust is mostly through ingestion or inhalation via respiratory and oral routes, because significant absorption of perchlorate through intact skin is unlikely. Significant systemic absorption of the inhaled perchlorate through mucous membranes in the respiratory and GI tracts is likely because of the high aqueous solubility of perchlorate at body temperature. Fine particles may be absorbed through the lungs; others may be absorbed in the GI tract.
Besides determining the exposure levels and associated potential health effects of ammonium perchlorate exposure on workers, the occupational studies were designed to estimate a safe working level of perchlorate. Neither the Occupational Safety and Health Administration (OSHA) in the United States nor any regulatory body in any other country has a specific occupational standard for perchlorate. OSHA regulates perchlorate as a nuisance dust, with a permissible exposure limit of 15 mg/m3 (time-weighted average). However, because ammonium perchlorate has considerable explosive potential, safety concerns outweigh pharmacologic concerns. The following studies have demonstrated that occupational exposure to perchlorate has not been hazardous to the thyroid health status of the workers studied at these plants nor to the health of the other examined organ systems.
Two occupational health studies have been published: one by Gibbs et al. (71), the other by Lamm et al (72). If occupational exposures to perchlorate suppress the thyroid by blocking iodine uptake, the expected observation would be that TSH levels increase whereas the free T4 index (FTI), the thyroid hormone binding ratio (THBR), and concentrations of T4 and T3 decrease. However, neither study found such a pattern.
Gibbs et al. (71) screened employees at an ammonium perchlorate production facility in Nevada and compared findings in those subjects to findings in a large control population from the same chemical complex. Both cumulative and single-shift exposures were assessed. The calculated single-shift doses of airborne perchlorate particles ranged from 0.2 to 436 µg/kg (average 36 µg/kg). The average working-lifetime cumulative dose in the higher exposure group was estimated to be 38 mg/kg. The study demonstrated no adverse effect on thyroid function (nor any on kidney, liver, or bone marrow function) among perchlorate manufacturing employees, either across the work shift in the short term or across the working-life cumulative exposure in the long term.
A study at a different site assessed the relationship between workers’ perchlorate exposure and absorption and their thyroid function (72). A cross-sectional health study examined two similar worker populations: a group of ammonium perchlorate workers in three exposure groups and a comparison group of other workers from the same industrial complex who were not exposed to perchlorate. The study compared postshift measurements of thyroid function in 58 workers at a Utah perchlorate manufacturing plant. More than 40% of the workers had been working with perchlorate for more than 5 years. The perchlorate production workers were classified into three categories of presumptive perchlorate exposures (low, medium, and high) based on the visible dust generated.
Perchlorate exposures were assessed by three measures—inhaled and respirable particles during a 12-hour work shift, changes in urinary perchlorate concentration across the shift, and serum perchlorate levels at the end of the shift. Group airborne perchlorate exposures were determined from particulate sampling data. Individual perchlorate absorption doses were calculated from pre-shift and postshift urinary perchlorate measurements and were independent of the airborne exposure measurements. Group mean perchlorate absorption dosages ranged from 0.9 to 34 mg/shift with group mean absorbed dosages of 1, 4, 11, and 34 mg perchlorate per day. Mean serum perchlorate levels were 0.11 ppm, 0.41, and 1.6 ppm for the three perchlorate groups and 0.00 ppm for the comparison group with a laboratory limit of detection of 0.005 ppm. No perchlorate was detected in the serum of any comparison group workers. The exposure groups were clearly distinguishable with no overlap of serum levels between the groups. Thyroid function was assessed by serum levels of TSH, free T4 index (FTI), T4, T3, thyroid hormone-binding ratio (THBR), and thyroid peroxidase (TPO) antibodies (to identify workers with underlying Hashimoto thyroiditis), and by clinical examination. Complete blood counts and blood chemistries were obtained and iodine status was assessed. Analyses were conducted using the four exposure groups.
This study was the first to measure and assess urinary perchlorate concentrations in workers exposed to perchlorate. Perchlorate in the urine reflects body burden and was measured at the beginning and end of the exposure period. Using these measures and the measured perchlorate airborne concentrations to which the workers were exposed, the amount of perchlorate that had been absorbed by each individual during the shift was calculated. The study included two measures of perchlorate exposure (total and respirable particles) and one measure of perchlorate absorption (urinary perchlorate). The two measures of perchlorate exposure correlated very strongly with each other and with the perchlorate absorption measure. Urinary perchlorate measurements demonstrated that exposure to the airborne particulate perchlorate resulted in systemic absorption (range 0 to 70 mg/d). If the occupational exposure to perchlorate were suppressing the thyroid by blocking iodine uptake, the expected observation would be that the TSH levels would increase and the FTI and T4 concentrations would decrease, as would T3 and THBR. However, no relationship was found between thyroid function and the absorbed dose of perchlorate in either continuous or categorical analyses. No hematotoxicity and no clinical chemical abnormalities were found at any of these levels of exposure. There were also no significant changes in the levels of thyroid hormones (median TSH levels of 2.8 µU/mL and 2.58 µU/mL, and median FTI levels of 5.3 and 5.6) across the exposure spectrum. These data indicate a No Observed Adverse Effect Level (NOAEL) in humans of ≥34 mg/d.
Crump (73) applied a benchmark analysis to the data from the study published by Lamm et al. (72) as an aide to determining an appropriate reference dose (RfD) for perchlorate. A benchmark dose (BMD) is a dose corresponding to a specific increase in risk. It is calculated by fitting a mathematical model to dose-responsive data. A lower statistical confidence bound on the benchmark dose (BMDL) is used by EPA as a replacement for the No Observed Adverse Effect Limit (NOAEL) in the calculation of a reference dose, and is stated in mg/shift units (74). In this analysis a 0.1 change in response was used to define BMDL. The advantage of a benchmark analysis is that it can be calculated from a “negative” study that shows no positive findings (i.e., no effect from perchlorate). The BMDL represents the dose below which a 0.1 increase in the probability of a response is not statistically consistent with the (negative) data. In the analysis of the occupational study data, changes in TSH and FTI levels were the endpoints considered.
The calculations were made from data arranged both as quantal responses and as a continuous variable. The analyses of the quantal responses are less informative because collapsing the data into strata reduces the amount of information. The calculated BMDL values for perchlorate as a quantal variable and using a Weibull model were 18 mg/shift based on FTI values and 28 mg/shift based on TSH values. The calculated BMDL values for perchlorate as a continuous variable using either a Weibull model or a K-power model were 44 or 46 mg/shift based on FTI values and 57 or 58 mg/shift based on TSH values. An exposure of 50 mg perchlorate during a 12-hour shift is a reasonable estimate of the BMDL for perchlorate. Table 3 summarizes the exposure range for various medical, occupational, and environmental perchlorate levels.
TABLE 3.
Perchlorate dose-response in humans exposed therapeutically, occupationally, in clinical studies, or environmentally via drinking water
| Effect/endpoint | Daily dose | Body-weight adjusted daily dose* |
|---|---|---|
| Fatal hemotoxicity (aplastic anemia) | 1000–2000 mg | 15–30 mg/kg |
| Nonfatal hemotoxicity (blood dyscrasias, including agranulocytosis) |
600–1000 mg | 8.5–14 mg/kg |
| 400 mg agranulocytosis | 5.7 mg/kg | |
| Therapeutic effect range for amiodarone treatment | 1000 mg start followed by 100 mg | 12.8 mg/kg then 1.4 mg/kg |
| Pharmacological effect range (normalization of tyroid function in hyperthyroid patients) |
200–1000 mg | 2.8–14 mg/kg |
| Calculated safe occupational average (BMDL) | 50 mg | 0.7 mg/kg |
| Demonstrated safe occupational average† | Per shift average | Per shift average |
| 2.5 mg1 | 0.036 mg/kg1 | |
| 34 mg2 | 0.48 mg/kg2 | |
| No-effect level for TSH elevation in newborns‡ (environmental level 5–25 ppb) |
Amount in 2L drinking water | 2.9 µg/kg3 |
| 200 µg3 | 0.29 µg/kg4 | |
| 20 µg4 |
Based on a 70-kg adult.
No-effect level for tests of thyroid function in occupationally exposed.
Exposed in utero via maternal consumption of drinking water. TSH, thyroid-stimulating hormone.
ENVIRONMENTAL EPIDEMIOLOGY STUDIES
Permanent adverse health effects, including neurologic damage, can occur if T4 levels are less than normal during fetal development. Other possible health out-comes that might be related to the presence of perchlorate in the drinking water include congenital hypothyroidism, transient neonatal hypothyroidism, pediatric hypothyroidism, goiter, and thyroid disease. In every state in the United States, newborns are screened for metabolic diseases in mandatory state-run programs.
Neonatal Studies
The fetal thyroid does not develop until 10 weeks of age. Therefore fetuses are completely dependent on their mother for thyroid hormone during the first trimester of pregnancy. Fetal and maternal thyroid physiology differ. The thyroid system of the fetus develops independently of the mother’s. The systems interact by means of the placenta and amniotic fluid, which modulate the transfer of iodine and small but important amounts of the thyroid hormone T4 from mother to fetus (75–77). In the absence of a functioning fetal thyroid, the maternal T4 that crosses the placenta is usually able to sustain a sufficient fetal T4 blood level of 40 to 60 nmol/L (78,79). The usual manifestations of intrauterine hypothyroidism are increased serum TSH concentrations and low or low-normal serum concentrations of T4 (80,81). Because untreated congenital hypothyroidism in infants can lead to irreversible mental retardation, every newborn in the United States is supposed to be screened within the first days of life for congenital hypothyroidism. Neonatal screening is routinely performed in most of the developed world. Children’s IQs are generally normal if the newborn’s T4 level is 43 nmol/L or higher, and if the postnatal treatment (6 to 11 µg L-thyroxine/kg per day) is sufficient to restore serum TSH to normal levels (82,83). Studies have demonstrated that neonatal hypothyroidism that is rapidly diagnosed and appropriately treated is associated with normal childhood intelligence. Even children born without a thyroid (athyreotic) have normal intellect if L-thyroxine is administered early (81). However, there are reported cases of subtle residual deficits in cognitive function, language, hearing, and vestibular function (84,85). Untreated maternal hypothyroidism and subclinical hypothyroidism in the second trimester have been reported to be associated with a slightly lower childhood IQ levels in the offspring compared with children born of mothers with normal thyroid function (86–88). Studies on children born to mothers who were treated with anti-thyroid drugs during pregnancy show that their children have IQs that are no different from a comparison group (89,90).
In 1997 perchlorate was found in the drinking water of six southern California counties at 5 to 8 (µg/L and in one southern Nevada county at levels as high as 14 µg/L. The congenital hypothyroidism data from the neonatal screening programs in each of these states for 1996 and 1997 were examined, and no increase in congenital hypothyroidism was observed in the exposure areas. The overall risk ratio was 1.0 (91).
In the Nevada screening program, all newborns are screened initially using a neonatal blood T4 level to diagnose congenital hypothyroidism. Those with abnormally low T4 levels or with values in the lowest laboratory decile for that day are screened again using a blood TSH level on the initial blood sample. Those with elevated TSH levels are then tracked in the state program to ensure that they get an appropriate diagnostic follow-up for congenital hypothyroidism. Newborns whose blood thyroxine and thyrotropin levels return to normal may be diagnosed as having had transient neonatal hypothyroidism. The metabolic screening data set provides an opportunity to monitor neonatal hypothyroxemia and hyperthyrotropinemia to assess whether the occurrence of either has been affected by the environmental perchlorate in the drinking water.
The monthly mean T4 levels of neonates from Las Vegas (an area with perchlorate-containing drinking water) were compared with those of neonates from Reno (an area with no detectable perchlorate in its drinking water) for the 15-month period April 1998 to June 1999. Perchlorate was detected in the drinking water of Las Vegas water at levels of 9 to 15 ppb µg/L) for 8 of those months and was undetectable (i.e., < 4 ppb) for 7 months. The neonatal blood T4 levels of the 23,305 neonates born in either Las Vegas or Reno during this period and sampled within the first 5 days of life were studied (92). The analysis found that whereas the variables sex, birth weight, and age at the time of sample collection significantly affected the mean blood T4 levels, the perchlorate levels in the Las Vegas drinking water did not affect the neonatal T4 levels. Furthermore, the cumulative in utero perchlorate exposure did not affect the neonatal T4 level.
Subsequently, an analysis of the neonatal TSH levels of newborns from Las Vegas and Reno was conducted for those born from December 1998 through October 1999 with a birth weight of 2,500 to 4,500 g and sampled within the first month of life (93). Similarly, analysis found that at the times of sample collection (2 to 7 days versus 8 to 30 days) the mean blood TSH levels were not different in Las Vegas versus Reno. The TSH analysis samples those neonates who form the “sensitive subpopulation” with respect to elevation of TSH levels. Male infants predominated in this subpopulation in both cities studied. This study of neonatal TSH levels in the first month of life found no effect from living in the areas with environmental perchlorate exposures as high as 15 µg/L (P = 0.97).
The highest known exposures to perchlorate in drinking water in the world occur in northern Chile, where water percolating through the geologic strata causes natural contamination of the water supply with nitrates and perchlorate. This area has been a basin for nitrate mining for more than 200 years. A study from northern Chile compared the neonatal findings for three communities with drinking water levels of 100 to 120 ppb (Taltal), 5 to 7 ppb (Chañaral), and undetectable levels (i.e., < 4 ppb) (Antofagasta) (94), respectively. Neonatal TSH levels were obtained on a total of 9,784 newborns from the three communities. Like the Nevada analysis, sex (male) and age (older than 3 days) affected the analysis. There was no difference between Chañaral and Antofagasta (5 to 7 ppb versus undetectable levels), and the TSH levels in Taltal (100 to 120 ppb) were paradoxically lower.
The third ecologic study reviewing thyroid hormone levels in children born in an area with perchlorate in the drinking water was by Brechner et al. (95) in Arizona. They compared the neonatal screening data of newborns of mothers from Yuma, Arizona (6 ppb of perchlorate in the drinking water) with those from Flagstaff, Arizona (no perchlorate). These two towns account for only 4% of the Arizona births. They found no difference in the race- and ethnicity-adjusted neonatal T4 values of Yuma and Flagstaff, but they did report a difference in neonatal TSH levels. Race and ethnicity were not found to be significant variables in their TSH analysis. The two areas differed markedly in their age at sampling distributions, with a median of day 1 for the Yuma births and a median of day 5 for the Flagstaff births. Whereas the age variable was statistically significant (as expected), the age adjustment may not have been sufficient to control for the extreme difference in age distribution between the two areas. Furthermore, as the sex variable has not yet been examined, the analysis is as yet incomplete.
Pediatric Studies
Children and adolescents are the age groups at greatest risk from low iodine intake (96). The team in Chile examined school-aged children in the three villages for their thyroid status. Crump et al. (94) conducted a study on 162 school-aged children in the three communities with different concentrations of perchlorate in their drinking water: Taltal (100 to 120 µg/L); Chañaral (5 to 7 µg/L); and Antofagasta (undetectable, i.e., < 4 µg/L). The primary findings were that there were no differences in TSH levels, free T4 levels, or goiter prevalence in the different areas despite the observation that more children in Taltal reported a family history of thyroid disease. The findings do not support the hypothesis that perchlorate in drinking water at concentrations as high as 100 to 120 µg/L suppresses thyroid function in school-aged children.
Adult Studies
The prevalence of thyroid disease in Nevada has been examined for an effect associated with perchlorate in the drinking water in a study design analogous to the Las Vegas versus Reno comparison for neonatal thyroid diseases. The Nevada Medicaid databases for 1997 and 1998 were examined. The prevalence rates among Medicaid-eligible residents were compared for Clark County (which has perchlorate in its drinking water) and both Washoe County and the rest of the state (which do not). Clark County is the county including Las Vegas. Ninety-six percent of the residents in Clark County drink perchlorate-containing water. No other parts of the state do. Washoe County encompasses the city of Reno. A cohort study compared the prevalence of thyroid diseases (ICD-9 193 and 240–246) in the Nevada counties with and without perchlorate in their drinking water using the Medicaid database (1/97 to 12/98) (97). The prevalence rates of simple goiter, nodular goiter, thyrotoxicosis, congenital hypothyroidism, acquired hypothyroidism, thyroid cancer, or other thyroid diseases were no greater in Clark County than in either of the other two areas.
Acquired hypothyroidism was the diagnosis thought a priori to be potentially related to perchlorate exposure. Acquired hypothyroidism, with county prevalence rates of 1.2% to 1.6%, is the most prevalent of the thyroid diseases. The prevalence of acquired hypothyroidism in Clark County was compared with that in other Nevada counties. Clark County did not have an increased prevalence of acquired hypothyroidism. This analysis finds no evidence that perchlorate-containing drinking water at the given level (as much as 16 µg/L) increased the prevalence of acquired hypothyroidism or any other thyroid condition.
Cancer
Three measures can be used to assess whether there is an increased risk of thyroid cancer in the areas with perchlorate-containing water: thyroid cancer prevalence, mortality, and incidence. The above study contained information on thyroid cancer prevalence in a perchlorate area in comparison to nonperchlorate areas (97). The prevalence rates were low, at 0.02%, 0.03%, and 0.03% for Clark County, Washoe County, and the rest of the state, respectively. Review of the National Cancer Institute’s Atlas of Cancer Mortality for California and Nevada shows that, for both males and females, the areas with perchlorate in the drinking water have lower thyroid cancer mortality rates than do the areas without perchlorate (98). The Nevada Central Cancer Registry data show no difference in the frequency of reported thyroid cancer incidence in Clark County from Washoe County or the rest of the state (Chang S, Lamm SH, unpublished data, 2001). Thus all three thyroid cancer measures show no association with perchlorate exposure.
Rodent studies have occasionally found benign thyroid tumors in rats given perchlorate at 10 mg/kg per day or more. These tumors are not a direct effect of perchlorate exposure, because perchlorate is nonmutagenic. They are a consequence of excessive stimulation of the thyroid gland from the elevated serum TSH because of perchlorate-induced hypothyroidism (secondary effect), resultant cell proliferation, and subsequent neoplasia. Thus, the animal data do not suggest a carcinogenic risk.
PROSPECTIVE EXPOSURE STUDIES IN VOLUNTEERS
The first exposure study of perchlorate in humans was that of Eichler (30), who in 1929 demonstrated that 50% of a 1- to 2-g dose was excreted in the urine within 6 to 8 hours and that 95% was excreted within 4 days. In 1938, Durand (99) reported that 50% of an 800-mg oral dose of perchlorate was excreted within 5 to 9 hours. Burgi et al. (100) in 1974 showed that 600 mg/d perchlorate (9.7 mg/kg per day) for 1 week was insufficient to totally deplete the thyroid gland of iodine. Similarly, Brabant et al. (101) in 1992 showed that 900 mg/d perchlorate for 4 weeks was also insufficient to totally deplete the thyroid gland of iodine, although it decreased the free T4 levels. Inexplicably, however, the TSH levels decreased rather than increased. Brabant is reported to have conducted a 5-week replicate of that study. Although the replicate is unpublished, reportedly mild goiters were observed in some subjects.
To ascertain the exposure levels at which perchlorate inhibits iodine uptake, studies have been conducted in which volunteers consumed perchlorate-containing water on a daily basis for 2 weeks. Thyroid function studies and iodine-uptake studies were conducted before the 2-week exposure, during the 2-week exposure, and 2 weeks after the 2-week exposure. Lawrence et al. reported no effect on thyroid function studies (T4, T3, free thyroxine index, thyroid hormone binding ratio, and TSH) with either daily doses of 3 mg (102) or 10 mg (103). The serum perchlorate level was 0.6 µg/mL (6 µmol) during the 10 mg/d dosage and below the detection limit during the 3 mg/d dosage. A 38% inhibition of iodine uptake was observed during the 10 mg/d perchlorate study. Two weeks after exposure, the iodine uptake was 25% greater than baseline. At the 3 mg/d dosage, a nonsignificant 10% decrease from baseline was observed. Linear-log extrapolation of the iodine uptake inhibition data would predict a no-effect level of approximately 2 mg/d.
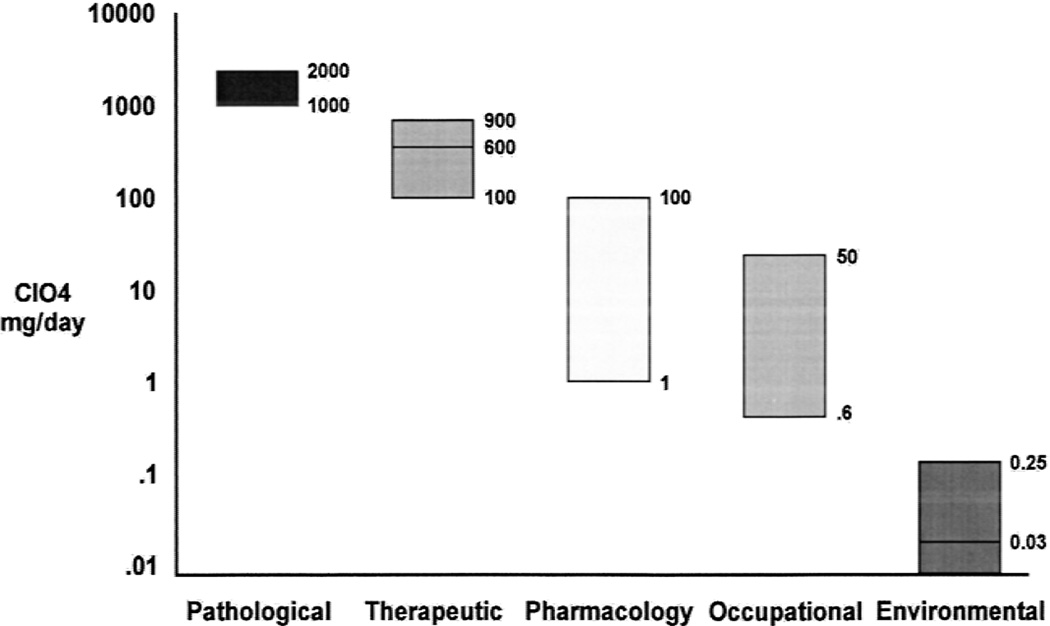
A similar study was conducted by Greer et al. (104). Perchlorate dose groups of 35 mg/d, 7 mg/d, and 1.4 mg/d were studied. At each dose level (8 volunteers in each group), a significant inhibition of iodine uptake was observed. A linear-log regression relationship of the data predicted a no-effect level of 0.5 mg/d. Four volunteers were tested at 0.5 mg/d and no effect on iodine uptake was observed. The data indicated a no-effect on iodine uptake level equivalent to an environmental drinking water perchlorate level of 250 µg/L. Figure 2 summarizes the relationship of human health effects and exposure to perchlorate.
FIG. 2.
Model human health and perchlorate exposure ranges.
Reference Dose
In the United States, both the federal government and state governments are attempting to establish safe levels of perchlorate in drinking water and to provide guidance to water purveyors. The US EPA circulated for peer-review an analysis of literature entitled “Review Draft (12/31/98) on Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization Based on Emerging Information.” They identified the mode of action of perchlorate “as an inhibitor of iodide uptake that results in disturbances of the hypothalamic–pituitary–thyroid axis” (E-4, L29) and then reviewed both published and unpublished literature to identify the study with the highest no-observed-adverse-effect-level (NOAEL) based on that mode of action. Based on reported thyroid histopathology in 5-day-old pups (PND5) in the 0.1 mg/kg per day group in a reviewed but unpublished rat neurodevelopment study and a composite uncertainty factor of 100, they determined a reference dose of 0.0009 mg/kg per day, (105) which is equivalent to a water level of 32 µg/L (106).
The EPA proposal was not accepted by its peer-review panel, which considered it likely to be conservative. The EPA is currently reassessing its work and considering new study findings in preparation for a resubmittal to its peer-review panel. At the state level, the State of California Department of Health Services set a provisional advisory action level at 18 µg/L in 1997, based on EPA analyses at that time, with a request that water containing ≥40 µg/L be removed from service.
The California guidance advisory action levels are generally being used, with perchlorate levels in Southern California being steady at 5 to 8 ppb and in Clark County, Nevada fluctuating between undetected (i.e., < 4 ppb) and 24 ppb, depending on hydrologic changes in Lake Mead. Monitoring of drinking water for perchlorate occurs throughout the United States, as required by the EPA for unregulated water contaminants, while both the EPA and state agencies reconsider their guidance and what level to establish as a minimal contaminant level (MCL).
SUMMARY AND CONCLUSIONS
A sensitive analytic method developed in 1997 enabled detection of perchlorate in water with a much lower limit of detection of (4 µg/L, or 4 ppb) than was previously possible. This led to increased detection of perchlorate in surface water and groundwater in several states, primarily California and Nevada. There are at least 14 states with confirmed perchlorate in ground or surface water, usually from sites of perchlorate manufacture or use and possibly from agriculture (107). Concerns about low doses of perchlorate in drinking water and the resurgence of the clinical use of perchlorate to control amiodarone-induced hyperthyroidism suggest a need to examine the actions of perchlorate and the safety of various exposure levels. Many studies on the therapeutic and diagnostic use of perchlorate have been published in the scientific literature. Most of the available human data comes from case studies of patients with thyroid disease from the 1950s and 1960s who were treated with potassium perchlorate for hyperthyroidism. Other published studies have investigated the effects of ammonium perchlorate in animal models.
The thyroid is considered the critical effect organ of perchlorate toxicity, and it seems that all of the adverse effects produced by perchlorate are associated with the initial inhibition of iodide uptake by the thyroid gland. In humans this begins somewhere near the 1-mg/d dosage range. Sustained doses as high as approximately 30 mg/d have not been found to affect thyroid hormone levels, and occupational exposures as high as approximately 50 mg/shift may also not affect thyroid hormone levels. Under appropriate clinical circumstances doses as high as approximately 1,000 mg/d are used to bring thyroid hormone levels under control. Bone marrow toxicity has been reported with doses in the 1,000 to 2,000-mg/d range, with an occasional case at the mid-100-mg/d dose. Because of concern about the potential adverse effects of perchlorate, toxicologic assessments and new evaluations of human and ecologic perchlorate exposure have been undertaken or are under way. This review focused on recent human studies that examined the health effects of exposure to perchlorate on adults, children, and neonates.
What current levels are considered safe or not is a decision for the regulatory agencies. However, assuming a daily intake of 2 L water per day, the highest known level of perchlorate in drinking water (24 µg/L) would yield a daily exposure of less than 50 µg/d. This seems a wide margin of safety, being tenfold lower than the 500 µg/d level that Greer et al. (100) considered the no-effect level in humans for the inhibition by perchlorate of iodine uptake by the thyroid, the mode of action held to be the initial and essential pharmacologic effect of perchlorate. It is also 1000-fold lower than the 50 mg/d level at which an effect on thyroid hormone levels in humans may be expected. The absence of an observed effect on neonatal thyroid, thyroidal diseases, or thyroidal cancer in this area is epidemiologically consistent with the human toxicologic and pharmacologic observations.
The medical and epidemiologic studies reviewed here have assessed both the safety and risk from environmental exposures to perchlorate. Integration of these findings with the results of studies on laboratory animals can expand our understanding of potential effects and of constraints to those effects. Laboratory animal studies, such as those at higher than environmental exposure levels during pregnancy, may make a further contribution. Finally, the standardization of methods for the measurement of perchlorate in urine, serum, solid matrix, and soil will allow a better analysis and interpretation of data.
Acknowledgments
The authors thank Dr. G. Goodman and L. Cummings for careful criticisms of the manuscript, and Drs. J. Wolff and K.S. Crump for their helpful comments. The authors also thank Ed Kern for his comments and support.
REFERENCES
- 1.Okamoto HS, Rishi DK, Steeper WR, et al. Using ion chromatography to detect perchlorate. J Am Water Works Assoc. 1999;91:73–84. [Google Scholar]
- 2.US Environmental Protection Agency. Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization Based on Emerging Information. Washington, DC: US Environmental Protection Agency; 1998. Publication NCEA-1-0503: 5–32—5–38. [Google Scholar]
- 3.Southern Nevada Water Authority. Feb 22; [Google Scholar]
- 4.Stanbury JB, Wyngaarden JB. Effect of perchlorate on the human thyroid gland. Metab Clin Exp. 1952;1:533–539. [PubMed] [Google Scholar]
- 5.Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 6.Kaminsky SM, Levy O, Salvador C, et al. The Na+/I− symporter of the thyroid gland. Soc Gen Physiol Ser. 1993;48:251–262. [PubMed] [Google Scholar]
- 7.Eskandari S, Loo DD, Dai G, et al. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 8.Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- 9.Klaassen C. Summary of Findings: Peer-Review Summary of Perchlorate. Washington, D.C: U.S. Environmental Protection Agency, Office of Solid Waste; 1999. Perchlorate Peer Review Workshop Report. [Google Scholar]
- 10.Schumacher J. Perchlorates: Their properties, manufacture and uses. Amer Chem Society Monograph. 1960:146. [Google Scholar]
- 11.Noble LF, Mansfield GR, Gale HS, et al. Nitrate deposits in the Amargosa region. Vol. 724. U.S Geol Surv; 1922. pp. 1–99. [Google Scholar]
- 12.Susarla S, Collette TW, Garrison AW, et al. Perchlorate identification in fertilizers. Environ Sci Technol. 1999;33:3468–3472. [Google Scholar]
- 13.Urbansky ET, Magnuson ML, Kelty CA, et al. Comments on ‘Perchlorate identification in fertilizers’ and the subsequent addition/correction. Environ Sci Tech. 2000;34:4452–4453. [Google Scholar]
- 14.Crozier RD. Chilean nitrate mining. Mining Magazine. 1981 Sep;:160. [Google Scholar]
- 15.Schilt AA. Perchlorate acid and perchlorates. Frederick Smith Chemical Company. 1979;79:630–668. [Google Scholar]
- 16.Erickson GE. Geology and origin of the Chilean nitrate deposits. Vol. 1188. U S Geol Surv; 1981. pp. 1–37. [Google Scholar]
- 17.Kirk-Othmer . Encyclopedia of Chemical Technology 1991-present. 4th ed. Vol. 18. New York, NY: John Wiley and Sons; 1991. p. 164. [Google Scholar]
- 18.Urbansky ET. Perchlorate chemistry implications for analysis and remediation. Bioremed J. 1998;2:81–95. [Google Scholar]
- 19.Urbansky ET, Schock MR. Issues in managing risk associated with perchlorate in drinking water. J Environ Manage. 1999;56:79–95. [Google Scholar]
- 20.Jackson PE, Laikhtman M, Rohrer JS. Determination of trace level perchlorate in drinking water and ground water by ion chromatography. J Chromatogr A. 1999;850:131–135. doi: 10.1016/s0021-9673(99)00026-6. [DOI] [PubMed] [Google Scholar]
- 21.Jackson PE, Gokhale S, Streib T, et al. Improved method for the determination of trace perchlorate in ground and drinking waters by ion chromatography. J Chromatogr A. 2000;888:151–158. doi: 10.1016/s0021-9673(00)00557-4. [DOI] [PubMed] [Google Scholar]
- 22.Magnuson ML, Urbansky ET, Kelty CA. Determination of perchlorate at trace levels in drinking water by ion- pair extraction with electrospray ionization mass spectrometry. Anal Chem. 2000;72:25–29. doi: 10.1021/ac9909204. [DOI] [PubMed] [Google Scholar]
- 23.Browner CM. Federal registry, Part II. 40 CFR parts 9, 141 and 142. Revisions to unregulated contaminant monitoring regulation for public water systems; final rule. Vol. 64. Washington, D.C: Environmental Protection Agency; 1999. pp. 50555–50620. [Google Scholar]
- 24.Richman KW, Howearth G, Lamm SH. Quantitative determination of perchlorate ion concentrations in urine. Abstract presented at: American Industrial Hygiene Conference and Exposition; June 7, 1999; Toronto, Ontario, Canada: [Google Scholar]
- 25.Fisher J, Todd P, Mattie D, et al. Preliminary development of a physiological model for perchlorate in the adult male rat: a frame-work for further studies. Drug Chem Toxicol. 2000;23:243–258. doi: 10.1081/dct-100100113. [DOI] [PubMed] [Google Scholar]
- 26.Koester CJ, Beller HR, Halden RU. Analysis of perchlorate in groundwater by electrospray ionization mass spectrometry/mass spectrometry. Environ Sci Technol. 2000;34:1862–1864. [Google Scholar]
- 27.Susarla S, Wolfe NL, McCutcheon SC. Perchlorate uptake in lettuce seedlings. American Chemical Society Annual Meeting, New Orleans LA, August 1999. Reprints of Extended Abstracts. Vol. 39. Washington, DC: American Chemical Society, Division of Environmental Chemistry; 1990. pp. 66–68. [Google Scholar]
- 28.Renner R. Study finding perchlorate in fertilizer rattles industry. Environ Sci & Tech. 1999;33:364A–365A. doi: 10.1021/es993030e. [DOI] [PubMed] [Google Scholar]
- 29.Urbansky ET, Magnuson ML, Kelty CA, et al. Perchlorate uptake by salt cedar (Tamarix ramosissima) in the Las Vegas wash riparian ecosystem. Sci Total Environ. 2000;256:227–232. doi: 10.1016/s0048-9697(00)00489-7. [DOI] [PubMed] [Google Scholar]
- 30.Eichler O. On the pharmacology of perchlorate. Arch Exp Pathol Pharmacol. 1929;144:251–260. [Google Scholar]
- 31.Wyngaarden JB. The effect of iodide, perchlorate, thiocyanate, and nitrate administration upon the iodide concentrating mechanism of the rat thyroid. Endocrinology. 1953;52:568–574. doi: 10.1210/endo-52-5-568. [DOI] [PubMed] [Google Scholar]
- 32.Buchinger W, Lorenz-Wawschinek O, Semlitsch G, et al. Thyrotropin and thyroglobulin as an index of optimal iodine intake: correlation with iodine excretion of 39,913 euthyroid patients. Thyroid. 1997;7:593–597. doi: 10.1089/thy.1997.7.593. [DOI] [PubMed] [Google Scholar]
- 33.Alexander WD, Wolff J. Thyroidal iodide transport. VIII Relation between transport, goitrogenic and antigoitrogenic properties of certain anions. Endocrinology. 1966;78:581–590. doi: 10.1210/endo-78-3-581. [DOI] [PubMed] [Google Scholar]
- 34.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 35.Carrasco N. Thyroid iodide transport: The Na+/I− symporter (NIS) In: Braverman L, Utiger R, editors. Werner and Ingbar’s The Thyroid. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 52–61. [Google Scholar]
- 36.Taurog A. Hormone synthesis: Thyroid iodine metabolism. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s The Thyroid. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 61–85. [Google Scholar]
- 37.Dunn JT, Dunn AD. Thyroglobulin: Chemistry biosynthesis and proteolysis. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s The Thyroid. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 91–104. [Google Scholar]
- 38.De La Vieja A, Dohan O, Levy O, et al. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 2000;80:1083–1105. doi: 10.1152/physrev.2000.80.3.1083. [DOI] [PubMed] [Google Scholar]
- 39.Eskandari S, Loo DD, Dai G, et al. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida A, Sasaki N, Mori A, et al. Different electrophysiological character of I-, ClO4-, and SCN- in the transport by Na+/I−symporter. Biochem Biophys Res Commun. 1997;231:731–734. doi: 10.1006/bbrc.1997.6178. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida A, Sasaki N, Mori A, et al. Differences in the electrophysiological response to I- and the inhibitory anions SCN- and ClO-4, studied in FRTL-5 cells. Biochim Biophys Acta. 1998;1414:231–237. doi: 10.1016/s0005-2736(98)00169-2. [DOI] [PubMed] [Google Scholar]
- 42.Anbar M, Guttmann S, Lewitus Z. The mode of action of perchlorate ions on the iodine uptake of the thyroid gland. Int J Appl Radiat Isot. 1959;7:87–96. doi: 10.1016/0020-708x(59)90153-x. [DOI] [PubMed] [Google Scholar]
- 43.Wyngaarden J, Wright BM, Ways P. The effect of certain anions upon the accumulation and retention of iodide by the thyroid gland. Endocrinology. 1952;50:537–549. doi: 10.1210/endo-50-5-537. [DOI] [PubMed] [Google Scholar]
- 44.Greer MA, Stott AK, Milne KA. Effects of thiocyanate, perchlorate and other anions on thyroidal iodine metabolism. Endocrinology. 1966;79:237–247. doi: 10.1210/endo-79-2-237. [DOI] [PubMed] [Google Scholar]
- 45.Michot JL, Osty J, Nunez J. Regulatory effects of iodide and thiocyanate on tyrosine oxidation catalyzed by thyroid peroxidase. Eur J Biochem. 1980;107:297–301. doi: 10.1111/j.1432-1033.1980.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 46.Morgans M, Trotter W. Potassium perchlorate in thyrotoxicosis. Br Med J. 1960;2:1086–1087. [Google Scholar]
- 47.Connell JMC. Long-term use of potassium perchlorate. Postgrad Med J. 1981;57:515–517. doi: 10.1136/pgmj.57.670.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgans M, Trotter W. Treatment of Thyrotoxicosis with potassium perchlorate. Lancet. 1954 doi: 10.1016/s0140-6736(54)92714-2. [DOI] [PubMed] [Google Scholar]
- 49.Crooks J, Wayne E. A comparison of potassium perchlorate, methylthiouracil and carbimazole in the treatment of thyrotoxicosis. Lancet. 1960 doi: 10.1016/s0140-6736(60)90335-4. [DOI] [PubMed] [Google Scholar]
- 50.Wenzel KW, Lente JR. Similar effects of thionamide drugs and perchlorate on thyroid- stimulating immunoglobulins in Graves’ disease: evidence against an immunosuppressive action of thionamide drugs. J Clin Endocrinol Metab. 1984;58:62–69. doi: 10.1210/jcem-58-1-62. [DOI] [PubMed] [Google Scholar]
- 51.Hobson Q. Aplastic anemia due to treatment with potassium perchlorate. BMJ. 1961;1:1368–1369. doi: 10.1136/bmj.1.5236.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson R, Moore W. Fatal aplastic anemia after treatment of thyrotoxicosis with potassium perchlorate. BMJ. 1961;1:1369–1371. doi: 10.1136/bmj.1.5236.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fawcett J, Clark C. Aplastic anemia due to potassium perchlorate. BMJ. 1961;1:1537. [Google Scholar]
- 54.Krevans J, Asper S, Rienhoff W. Fatal aplastic anemia following use of potassium perchlorate in thyrotoxicosis. JAMA. 1962;181:162–164. doi: 10.1001/jama.1962.03050280092013c. [DOI] [PubMed] [Google Scholar]
- 55.Gjemdal N. Aplastic anemia following use of potassium perchlorate in thyrotoxicosis. Acta Med Scand. 1963;174:129–131. doi: 10.1111/j.0954-6820.1963.tb07902.x. [DOI] [PubMed] [Google Scholar]
- 56.Barzilai D, Sheinfeld M. Fatal complications following use of potassium perchlorate in thyrotoxicosis Report of two cases and a review of the literature. Isr J Med Sci. 1966;2:453–456. [PubMed] [Google Scholar]
- 57.Rokke KE, Vogt JH. Combination of potassium perchlorate and propylthiouracil in the treatment of thyrotoxicosis. Acta Endocrinol (Copenh) 1968;57:565–577. doi: 10.1530/acta.0.0570565. [DOI] [PubMed] [Google Scholar]
- 58.Evered DC. Today’s treatment; treatment of thyroid disease. BMJ. 1976;1:264–266. doi: 10.1136/bmj.1.6004.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Klauw MM, Goudsmit R, Halie MR, et al. A population-based case-cohort study of drug-associated agranulocytosis. Arch Intern Med. 1999;159:369–374. doi: 10.1001/archinte.159.4.369. [DOI] [PubMed] [Google Scholar]
- 60.Young NS. Introduction: acquired aplastic anemia. Semin Hematol. 2000;37:2. doi: 10.1016/s0037-1963(00)90025-8. [DOI] [PubMed] [Google Scholar]
- 61.Martino E, Bartalena L, Bogazzi F, et al. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22:240–254. doi: 10.1210/edrv.22.2.0427. [DOI] [PubMed] [Google Scholar]
- 62.Inada M, Nishikawa M, Kawai I. Hypothyroidism associated with positive results of the perchlorate discharge test in elderly patients. Am J Med. 1983;74:1010–1015. doi: 10.1016/0002-9343(83)90803-3. [DOI] [PubMed] [Google Scholar]
- 63.Reardon W, Coffey R, Chowdhury T, et al. Prevalence, age of onset, and natural history of thyroid disease in Pendred syndrome. J Med Genet. 1999;36:595–598. [PMC free article] [PubMed] [Google Scholar]
- 64.Hilditch TE, Birnie GG, Sik MJ, et al. Use of perchlorate to block gastric uptake of free 99Tcm in the investigation of gastrointestinal bleeding. Nucl Med Commun. 1985;6:701–706. doi: 10.1097/00006231-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Harper AG, Friedman BI, Avis KE, et al. Perchlorate blocking of 99m TcO 4 -uptake by simultaneous intravenous injection in dogs. J Nucl Med. 1972;13:363–366. [PubMed] [Google Scholar]
- 66.Gupta GS, Chopra VK. Biological damage in spleen by iodine-125 in potassium perchlorate blocked thyroid of rats. Strahlentherapie. 1980;156:579–582. [PubMed] [Google Scholar]
- 67.Hildebrandt JD, Halmi NS. Intrathyroidally generated iodide: the role of transport in its utilization. Endocrinology. 1981;108:842–849. doi: 10.1210/endo-108-3-842. [DOI] [PubMed] [Google Scholar]
- 68.Hiasa Y, Kitahori Y, Kato Y, et al. Potassium perchlorate, potassium iodide, and propylthiouracil: promoting effect on the development of thyroid tumors in rats treated with N-bis(2-hydroxypropyl)-nitrosamine. Jpn J Cancer Res. 1987;78:1335–1340. [PubMed] [Google Scholar]
- 69.Ribela MT, Marone MM, Bartolini P. Use of radioiodine urinalysis for effective thyroid blocking in the first few hours post exposure. Health Phys. 1999;76:11–16. doi: 10.1097/00004032-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Sztanyik LB, Turai I. Modification of radioiodine incorporation into the fetuses and newborn rats by thyroid blocking agents. Acta Physiol Hung. 1988;72:343–354. [PubMed] [Google Scholar]
- 71.Gibbs JP, Ahmad R, Crump KS, et al. Evaluation of a population with occupational exposure to airborne ammonium perchlorate for possible acute or chronic effects on thyroid function. J Occup Environ Med. 1998;40:1072–1082. doi: 10.1097/00043764-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 72.Lamm SH, Braverman LE, Li FX, et al. Thyroid health status of ammonium perchlorate workers: a cross- sectional occupational health study. J Occup Environ Med. 1999;41:248–260. doi: 10.1097/00043764-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Crump K. Benchmark doses for perchlorate obtained from Lamm et al.(1999) study of thyroid function in perchlorate workers. Presented at EPA Expert Review Panel on Perchlorate; February 12, 1999; San Bernardino, CA: [Google Scholar]
- 74.US Environmental Protection Agency. The use of the benchmark dose approach in health risk assessment. Washington, D.C: Office of Research and Development; 1995. EPA/630/R-94/007. [Google Scholar]
- 75.Fisher DA, Burrow GN, Dussault JH, et al. Recommendations for screening programs for congenital hypothyroidism. Report of a committee of the American Thyroid Association. Am J Med. 1976;61:932–934. doi: 10.1016/0002-9343(76)90417-4. [DOI] [PubMed] [Google Scholar]
- 76.Roti E, Gnudi A, Braverman LE. The Placental transport, synthesis and metabolism of hormones and drugs which affect thyroid function. Endocr Rev. 1983;4:131–149. doi: 10.1210/edrv-4-2-131. [DOI] [PubMed] [Google Scholar]
- 77.Burrow GN. Thyroid status in normal pregnancy. J Clin Endocrinol Metab. 1990;71:274–275. doi: 10.1210/jcem-71-2-274. [DOI] [PubMed] [Google Scholar]
- 78.Larsen PR. Maternal thyroxine and congenital hypothyroidism. N Engl J Med. 1989;321:44–46. doi: 10.1056/NEJM198907063210108. [DOI] [PubMed] [Google Scholar]
- 79.Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321:13–16. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]
- 80.Klein RZ, Haddow JE, Faix JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35:41–46. doi: 10.1111/j.1365-2265.1991.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 81.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 82.Ilicki A, Larsson A. Psychological development at 7 years of age in children with congenital hypothyroidism Timing and dosage of initial treatment. Acta Paediatr Scand. 1991;80:199–204. doi: 10.1111/j.1651-2227.1991.tb11834.x. [DOI] [PubMed] [Google Scholar]
- 83.Tillotson SL, Fuggle PW, Smith I, et al. Relation between biochemical severity and intelligence in early treated congenital hypothyroidism: a threshold effect. BMJ. 1994;309:440–445. doi: 10.1136/bmj.309.6952.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grant DB, Fuggle P, Tokaar S, et al. Psychomotor development in infants with congenital hypothyroidism diagnosed by neonatal screening. Acta Med Austriaca. 1992;19(Suppl 1):54–56. [PubMed] [Google Scholar]
- 85.Glorieux J, Dussault JH, Van Vliet G. Intellectual development at age 12 years of children with congenital hypothyroidism diagnosed by neonatal screening. J Pediatr. 1992;121:581–584. doi: 10.1016/s0022-3476(05)81150-3. [DOI] [PubMed] [Google Scholar]
- 86.Rovet JF, Ehrlich RM. Long-term effects of L-thyroxine therapy for congenital hypothyroidism. J Pediatr. 1995;126:380–386. doi: 10.1016/s0022-3476(95)70452-3. [DOI] [PubMed] [Google Scholar]
- 87.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 88.Pop VJ, Kuijpenst JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 89.Burrow GN, Klatskin EH, Genel M. Intellectual development in children whose mothers received propylthiouracil during pregnancy. Yale J Biol Med. 1978;51:151–156. [PMC free article] [PubMed] [Google Scholar]
- 90.Messer PM, Hauffa BP, Olbricht T, et al. Antithyroid drug treatment of Graves’ disease in pregnancy: long-term effects on somatic growth, intellectual development and thyroid function of the offspring. Acta Endocrinol (Copenh) 1990;123:311–316. doi: 10.1530/acta.0.1230311. [DOI] [PubMed] [Google Scholar]
- 91.Lamm SH, Doemland M. Has perchlorate in drinking water increased the rate of congenital hypothyroidism? J Occup Environ Med. 1999;41:409–411. doi: 10.1097/00043764-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 92.Li Z, Li FX, Byrd D, et al. Neonatal thyroxine level and perchlorate in drinking water. J Occup Environ Med. 2000;42:200–205. doi: 10.1097/00043764-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 93.Li FX, Byrd D, Deyhle GM, et al. Neonatal thyroid stimulating hormone level and perchlorate in drinking water. Teratology. 2000;92:429–431. doi: 10.1002/1096-9926(200012)62:6<429::AID-TERA10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 94.Crump C, Michaud P, Tellez R, et al. Does perchlorate in drinking water affect thyroid function in newborns or school-age children? J Occup Environ Med. 2000;42:603–612. doi: 10.1097/00043764-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Brechner RJ, Parkhurst GD, Humble WO, et al. Ammonium perchlorate contamination of Colorado River drinking water is associated with abnormal thyroid function in newborns in Arizona. J Occup Environ Med. 2000;42:777–782. doi: 10.1097/00043764-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Gruters A, Delange F, Giovannelli G, et al. Guidelines for neonatal screening programms for congenital hypothyroidism. Working group on congenital hypothyroidism of the European Society for Paediatric Endocrinology. Eur J Pediatr. 1993;152:974–975. doi: 10.1007/BF01957217. [DOI] [PubMed] [Google Scholar]
- 97.Li FX, Squartsoff L, Lamm SH. The prevalences of thyroid diseases in Nevada counties with respect to perchlorate in drinking water. J Occ Environ Med. 2001 doi: 10.1097/00043764-200107000-00010. (Under review) [DOI] [PubMed] [Google Scholar]
- 98.National Cancer Institute. National Institutes of Health National Cancer Institute, Atlas of Cancer Mortality in the United States. 1999:276–279. NIH publication No. 99–4564.
- 99.Durand MJ. Recherches sur l’elimination des perchlorates, sur leur repartition dans les organes et sir leur toxicite. Bull Soc Chim Ciol (Paris) 1938;20:428–435. [Google Scholar]
- 100.Burgi H, Benguerel M, Knopp J, et al. Influence of perchlorate on the secretion on non-thyroxine iodine by the normal human thyroid gland. Eur J Clin Invest. 1974;4:65–69. doi: 10.1111/j.1365-2362.1974.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 101.Brabant G, Bergmann P, Kirsch CM, et al. Early adaptation of thyrotropin and thyroglobulin secretion to experimentally decreased iodine supply in man. Metabolism. 1992;41:1093–1096. doi: 10.1016/0026-0495(92)90291-h. [DOI] [PubMed] [Google Scholar]
- 102.Lawrence JE, Lamm SH, Braverman LE. Letter to the Editor. Thyroid. 2001 May [Google Scholar]
- 103.Lawrence JE, Lamm SH, Pino S, et al. The effect of short-term low-dose perchlorate on various aspects of thyroid function. Thyroid. 2000;10:659–663. doi: 10.1089/10507250050137734. [DOI] [PubMed] [Google Scholar]
- 104.Greer MA, Goodman G, Pleus R, et al. Does environmental perchlorate exposure alter human thyroid function? Determination of the dose-response for inhibition of radioiodine uptake. Endocrine J. 2000;47:146. [Google Scholar]
- 105.U.S. Environmental Protection Agency. Review Draft (12/31/98) on Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization Based on Emerging Information EPA/NCEA. 1998. [Google Scholar]
- 106.York RG, Parker RM, Mattie DR, et al. A neurobehavioral developmental study of ammonium perchlorate administered orally in drinking water to rats. Toxicologist. 1999;19:1. [Google Scholar]
- 107.Renner R. Perchlorate-tainted wells spur government action. Envir Sci Technol. 1998;32:210A. doi: 10.1021/es983489t. [DOI] [PubMed] [Google Scholar]