Abstract
Ceruloplasmin (glycosylphosphatidylinositol-linked ferroxidase associated with normal astrocytes) can also be secreted by glioma cells, where its function is unknown. Ceruloplasmin is not only present in glioma cells and in human glioma specimens but also is enriched in highly malignant glioma stem-like cells. Hyaluronan is a large extracellular glycosaminoglycan that enhances malignant glioma behaviors by interacting with CD44 receptors and by downstream activation of signaling proteins and transporters associated with malignancy. We examined the relationship between hyaluronan and ceruloplasmin expression in glioma stem-like cells. Antagonism of hyaluronan interactions with short-fragment hyaluronan oligomers decreased ceruloplasmin expression in parental and stem-like glioma cells in vivo and in cell culture, implying that hyaluronan regulates ceruloplasmin expression. Further gain and loss-of-function studies are needed to fully define the relationship between hyaluronan and ceruloplasmin, and ceruloplasmin's effect on malignant behaviors.
Keywords: ceruloplasmin, hyaluronan, glioma, stem-like cells, glycosaminoglycan
Introduction
Ceruloplasmin is a 132-kd glycoprotein produced by hepatocytes and secreted into the blood. More recently, it has been discovered within the brain, retina, lung, and testes.1–6 X-ray crystallography studies have shown that ceruloplasmin contains 6 copper sites7 that participate in the electron transfer that occurs during the oxidation of toxic ferrous iron (Fe+2) to nontoxic ferric iron (Fe+3).8–10 As an enzyme, the functions of ceruloplasmin are quite diverse, and it is interesting to note that the ceruloplasmin gene contains upstream elements that could explain a rapid response to hypoxia and inflammation.7,11,12
Serum levels of ceruloplasmin are markedly elevated in patients with breast, ovarian, renal, colon, and brain tumors.13–16 Systemic inflammation may account for elevated serum levels of ceruloplasmin produced by the liver. Alternatively, circulating cytokines may stimulate tumors to produce ceruloplasmin, as has been shown in C6 rat glioma cells in vitro.17,18 In the brain, a glycosylphosphatidylinositol-linked form of ceruloplasmin is found within astrocytes that surround blood vessels.2 However, our group was the first to show that a human brain tumor, a desmoplastic infantile ganglioglioma, secretes ceruloplasmin.19 In light of this finding, we sought to determine whether other human gliomas produce ceruloplasmin and, because the desmoplastic infantile ganglioglioma is thought to be of embryonal origin, whether ceruloplasmin is enriched in treatment-resistant subpopsulations of cells that exhibit stem-like cell characteristics.20–23
Hyaluronan is a very large glycosaminoglycan that has an instructive role in signaling via hyaluronan receptors on the cell surface.24,25 Hyaluronan interacts with several cell surface receptors, including CD44 and receptor for hyaluronic acid–mediated motility (RHAMM), which are expressed at high levels in gliomas.26,27 Hyaluronan-receptor interactions mediate at least three important physiological processes: (1) signal transduction, (2) receptor-mediated hyaluronan internalization, and (3) assembly of pericellular matrices.28,29 Each of these general functions is most likely shared by more than one receptor. CD44 and RHAMM can mediate many aspects of hyaluronan-induced signal transduction.24
In this article, we demonstrate that ceruloplasmin is present in malignant human gliomas, that it is increased in stem-like subpopulations of cells, and that antagonizing hyaluronan-receptor interactions decreases ceruloplasmin production.
Experimental Procedures
Human Brain Tumor Tissue Immunofluorescence Analysis
Frozen optimal cutting temperature embedding media (OCT)-embedded human brain tumor samples were obtained from the Medical University of South Carolina Brain Tumor Bank. Slides were kept at −80°C until the time of staining. Slides were fixed in acetone for 10 min at 4°C and then allowed to dry at room temperature. Blocking solution (5% goat serum and 3% bovine serum albumin in Tris-buffered saline) was added to the slides for 30 min. Primary antibody (ceruloplasmin, 1:200; Dako Cytomation, Carpinteria, California) was allowed to remain on the slides for 1 h. Slides were then rinsed with Tris-buffered saline with Tween 3 × 10 min, and secondary antibody (AlexaFluor 555 anti-rabbit, 1:100, Invitrogen, Carlsbad, California) was added at optimal concentrations (diluted in blocking solution) for 1 h. At the point the secondary antibody was added, slides were shielded from light. Slides were again rinsed with Tris-buffered saline with Tween 3 × 10 min, allowed to dry, and then mounted with coverslips using Gelmount mounting medium. All reagents were purchased from Fisher Scientific (Suwanee, Georgia) unless otherwise specified.
Cell Culture
Rat C6 and human U87MG glioma cells were obtained from the American Type Culture Collection (Bethesda, Maryland) and cultured in Dulbecco's Modified Eagle's Medium/Ham's F12 50:50 mix with l-glutamine (Mediatech, Fisher Scientific), 10% fetal bovine serum, and 1% penicillin/streptomycin solution. Cells were maintained in a 5% CO2 incubation chamber at 37°C and serially passaged every other day. The stem-like side population of C6 cells and the spheres of U87MG were maintained in serum-free Neurobasal-A media (Invitrogen) supplemented with B27 (Stem Cell Technologies, Seattle, Washington), the growth factors basic fibroblast growth factor and epidermal growth factor, 1% penicillin/streptomycin solution, and GlutaMAX (Invitrogen).
Isolation of Stem-like Glioma Cells
To identify and isolate C6 side population cells, we cultured the cells in either fetal bovine serum or serum-free culture medium with growth factor supplementation (basic fibroblast growth factor and epidermal growth factor). Following a well-established method for isolation of side population cells,30–32 we labeled the cells at 37°C for 90 min with 2.5 μg/mL Hoechst 33342 dye (Invitrogen) and counterstained with 1 μg/mL propidium iodide to label dead cells. Cells were analyzed and sorted in a fluorescence-activated cell sorter (MoFlo® High Performance Cell Sorter by Dako Cytomation) by using a dual-wavelength analysis (blue, 424–444 nm; red, 675 nm) after excitation with 350-nm ultraviolet light. Propidium iodide–positive dead cells (<15%) were excluded from the analysis. We collected cells from the side population, which efflux Hoechst dye, and cells from the nonside population that retain the dye.
Immunofluorescence Staining of Cultured Cells
Cells were cultured overnight in LabTEK II CC2 chamber slides and were fixed with 2% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature, blocked with Tris-buffered saline containing 3% bovine serum albumin and 0.1% Triton-X 100, and then stained with the following antibodies: ceruloplasmin (1:200, polyclonal, Dako Corporation, California), anti-III tubulin/Tuj1 (1:200; Sigma-Aldrich, St. Louis, Missouri), and anti-nestin (1:200; Chemicon, Temecula, California). The primary antibodies were detected with fluorophore-conjugated secondary antibody (Alexa Fluor 488 and 555, 1:100; Invitrogen). For double labeling, the second primary antibody was added prior to incubation with fluorescently labeled secondary antibodies. The cells were counterstained with Hoechst 33342 (Invitrogen) to visualize nuclei. Slides were mounted with GelMount mounting medium.
Hyaluronan Oligomer Treatment In Vitro
Highly purified hyaluronan oligomers donated by Anika Therapeutics Inc. (Woburn, Massachusetts) were fractionated from testicular hyaluronidase digests of hyaluronan polymer by tangential flow filtration, as described previously.33 The average molecular weight of these oligomers was approximately 2.5 × 103 (~3–10 disaccharide units). The oligomers were analyzed by high-performance liquid chromatography and capillary electrophoresis, and no contaminants were detected. Specific analyses for other glycosaminoglycans, protein, nucleic acids, and endotoxins were negative. Hyaluronan oligomers (100 μg/mL) were added 24 h before cells were harvested or analyzed.
Animal Surgeries
Female Sprague-Dawley rats (190–210 g) were obtained from Harlan (Indianapolis, Indiana) and housed under standard housing conditions as approved by the Medical University of South Carolina's Institutional Animal Care and Use Committee. On the day of surgery, animals were anesthetized with a measured dose of ketamine and xylazine. Tumor cells were engrafted into the spinal cord where they are highly invasive.34,35 A laminectomy was performed at T10 of the spinal cord, the dura mater was incised at this location, and 20 000 C6 whole or side population cells (1 μL) were injected into the spinal cord with a Hamilton 5-μL syringe with a 33-gauge needle, as previously described.34,35 The depth of injection was 1 mm into the cord, at a distance of 0.1 mm lateral of the midline of the spinal cord. After injection, the exposed area of the cord was covered with Gelfoam (Upjohn Corporation, Kalamazoo, Michigan), and the overlying muscle and connective tissue was sutured. The skin was closed with surgical staples (Stoelting Co. Wood Dale, Illinois), and buprenorphine was administered as an analgesic. Animals were routinely monitored postoperatively for any signs of neurological impairment or systemic illness. Seven days after the initial engraftment of tumor cells, a single injection of hyaluronan oligomer (1 μL) was administered directly into the tumor. Surgical techniques were the same for the hyaluronan oligomer injection as for the tumor cell injection. Seven days after hyaluronan oligomer administration, the animals were given a lethal dose of sodium pentobarbital, and the cords were extracted, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for immunofluorescence analysis.
Immunofluorescence Analysis of Rat Spinal Cord Gliomas
Longitudinal sections of fixed rat spinal cord were cut at 5 microns and analyzed by routine histological methods such as hematoxylin and eosin staining. Deparaffinized tissue sections were subjected to an antigen unmasking protocol with citrate buffer (Vector Labs, Burlingame, California). Nonspecific antibody binding was blocked by incubating sections in Tris-buffered saline containing 5% normal goat serum and 3% bovine serum albumin (blocking buffer) for 1 h. Primary antibodies were diluted in blocking buffer and applied to sections overnight at 4°C. Controls included replacement of primary antibodies with surrogate immunoglobulins or no primary antibody. Slides were washed 3 × 5 min in Tris-buffered saline with 0.05% Tween 20. Bound primary antibody was detected with fluorophore-conjugated secondary antibody (AlexaFluor 555 or 488, Invitrogen) at a concentration of 10 μg/mL diluted in blocking solution for 1 h at room temperature. Slides were washed 3 × 5 min in Tris-buffered saline with Tween. In the last wash, Hoechst nuclear stain was added, followed by an additional wash for 5 min in distilled water.
Western Blotting
Cells were cultured until 90% to 95% confluent and then harvested using trypsin. The cells were lysed with standard radioimmunoprecipitation assay buffer containing protease inhibitors. Protein concentration was determined using the bicinchoninic acid protein assay (Fisher Scientific) according to manufacturer's suggestion. Equal volumes of the protein samples were run on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and probed with the primary antibody. The membrane was washed 3 × 10 min in Tris-buffered saline with Tween, and the secondary horseradish peroxidase–conjugated antibody was added for 1.5 h. The membrane was again washed 3 × 10 min in Tris-buffered saline with Tween and then reacted with picoluminescent-enhanced chemiluminescence reagent (Fisher Scientific) and developed. Antibodies used were as follows: ceruloplasmin (Dako Corporation) 1:1000; β-actin (Ambion, Austin, Tex) 1:1000; anti-mouse horseradish peroxidase–conjugated antibody (Fisher Scientific) 1:5000; anti-rabbit horseradish peroxidase–conjugated antibody (Vector Labs) 1:1000.
Results
Malignant Human Gliomas Contain Ceruloplasmin
First, we studied fresh-frozen human glioma tissue sections to determine whether ceruloplasmin expression is widespread in human gliomas. Ceruloplasmin was expressed abundantly in primary (n = 3) and recurrent high-grade gliomas (n = 2) (Figure 1A) but not in a low-grade oligodendroglial tumor (Figure 1B). We also performed Western blots with lysates derived from several human glioblastoma xenografts that had been characterized for radiation resistance in vivo. As shown in Figure 1C, both radioresistant and radiosensitive human glioblastoma lysates contain ceruloplasmin; the amount of ceruloplasmin does not correlate with resistance to radiation (not shown).
Figure 1.
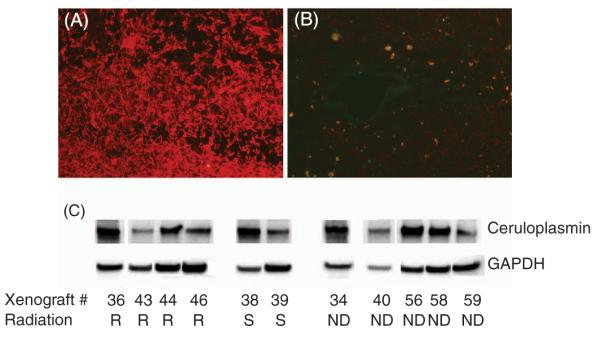
Expression of ceruloplasmin in human gliomas. (A) Human glioblastomas have large amounts of ceruloplasmin (red) compared with (B) a lower-grade oligodendroglioma. (C) Human glioblastoma xenografts contain variable amounts of ceruloplasmin, and there is no significant difference in the amount of ceruloplasmin between radiosensitive (S) and radioresistant (R) tumors (densitometry not shown).
Multipotent Stem-like Glioma Cells Are Enriched In Ceruloplasmin
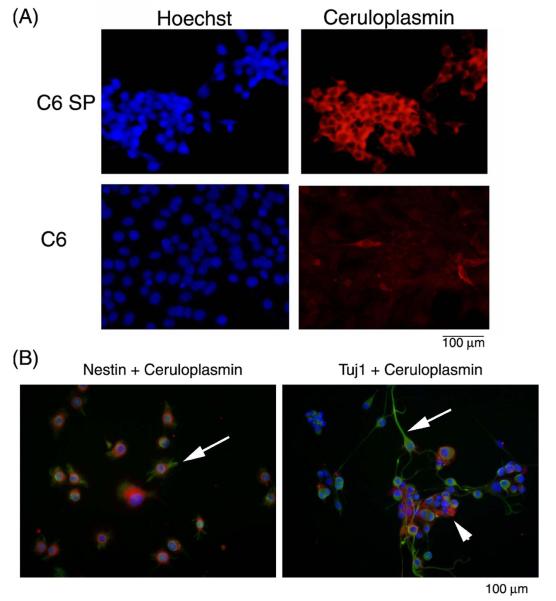
We then determined the level of expression of ceruloplasmin in the C6 glioma cell line and found it to be relatively low (Figure 2A). Because subpopulations of glioma cells with stem-like properties are more malignant and resistant to treatment,35 we determined whether such cells have more ceruloplasmin. We have previously prepared highly malignant, drug-resistant, stem-like C6 glioma cells by isolation of the side population.35 Immunofluorescence microscopy revealed that these C6 side population cells contain more ceruloplasmin than the parent C6 cell line (Figure 2A). C6 side population cells were previously shown to also express CD133 and to be multipotent, evidenced by their differentiation into cells expressing Tuj-1 and MAP2 (neuronal) and glial fibrillary acidic protein (GFAP, astrocyte marker).31,35 Nestin-positive or Tuj-1-positive C6 side population cells also stained positively for ceruloplasmin (Figure 2B).
Figure 2.
Ceruloplasmin expression in C6 side population (C6SP) and C6 whole population (C6). (A) Ceruloplasmin (red) is expressed more abundantly in the C6 side population than in the whole C6 cell line. (B) Left panel shows that ceruloplasmin (red) is co-expressed with the progenitor cell marker nestin (green). Arrow points to a cell expressing both proteins. Right panel shows that ceruloplasmin (red) is co-expressed with the neuronal marker Tuj-1 (green). Arrow points to differentiated cells expressing less ceruloplasmin (red) than more undifferentiated cluster of cells (arrow head). Nuclei are stained with Hoechst dye (blue).
Hyaluronan Oligomers Abrogate Ceruloplasmin Expression In C6 Side Population and in U87MG Neurospheres
Stem-like glioma cells can also be enriched by formation of neurospheres. Using Western blotting, we examined the levels of ceruloplasmin in both the C6 side population and the neurospheres formed from U87MG human glioma cells. Expression of ceruloplasmin was higher in both the C6 side population and the U87MG spheres (Figure 3) than in the respective parental cell lines. We antagonized hyaluronan by using small hyaluronan oligomers that competitively inhibit hyaluronan-receptor interactions. We performed Western blot analysis of ceruloplasmin expression in the C6 side population and the stem-like spheroid population of human U87MG glioma cells that had been treated with and without 100 μg/mL hyaluronan oligomers. Hyaluronan antagonism with hyaluronan oligomers abrogated ceruloplasmin production in both C6 side population and U87MG spheres (Figure 3).
Figure 3.
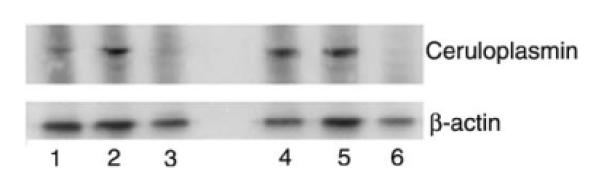
Effects of hyaluronan antagonism on glioma ceruloplasmin production in vitro. Lane (1) C6 cells (2) C6 side population (3) C6 side population plus hyaluronan oligomer (100 mg/mL, 24 h) (4) U87MG cells (5) U87MG spheres and (6) U87MG spheres plus hyaluronan oligomer (100 mg/mL, 24 h). C6 side population contain more ceruloplasmin than C6 cells. U87MG cells and U87MG spheres contain similar amounts of ceruloplasmin. However, hyaluronan oligomer significantly decreases ceruloplasmin production in regular and stem-like glioma cells.
Hyaluronan Oligomers Abrogate Glioma Ceruloplasmin Production In vivo
Because hyaluronan oligomers had a large effect on glioma cells in vitro, we studied the effects of various concentrations of hyaluronan oligomers on expression of ceruloplasmin in spinal cord tumors formed from C6 or C6 side population cells in vivo, as described previously.35 We analyzed the cords taken from rats that had been injected with 20 000 C6 cells at T10 14 days prior to tissue harvesting. The doses of hyaluronan oligomers administered were 100 and 250 ng, with phosphate-buffered saline serving as the control. Hyaluronan oligomers or phosphate-buffered saline was administered intratumo-rally 7 days after initial engraftment. The dosages of hyaluronan oligomers given were quite small considering the amount used in vitro (100 μg/mL), but the 250 ng dose previously was found to significantly inhibit tumor growth and invasion.35 Importantly, at these concentrations in vivo, and even at a concentration of 1 μg, we saw no appreciable immunogenic response or toxic effect in the animals (data not shown). Figure 4A shows that administration of 100 ng hyaluronan oligomers significantly attenuates ceruloplasmin production by tumor cells in vivo, compared with phosphate-buffered saline-injected control tumors. Additionally, the 250-ng dose decreased ceruloplasmin expression even further in the tumors, even when accounting for decreased glioma cell density. Engraftment of as few as 2000 C6 side population cells resulted in tumor formation, as detected by β-galactosidase immunostaining with high expression of ceruloplasmin, whereas engrafted C6 non-side population (non-SP) cells did not form a tumor mass and expressed very little ceruloplasmin (Figure 4B). Given that the C6 side population cells are more tumorigenic,35 we used a substantially larger dose of hyaluronan oligomers (5 μg). As shown in Figure 4C, hyaluronan oligomer treatment also significantly reduced the amount of glioma ceruloplasmin in the C6 side population tumors in vivo.
Figure 4.
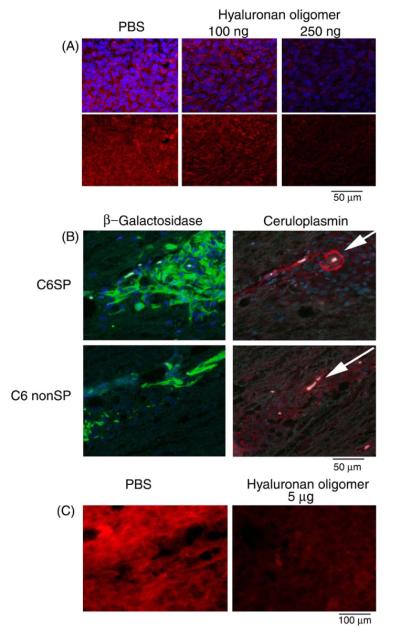
Effects of hyaluronan antagonism on glioma ceruloplasmin production in vivo. A, Gliomas that form after engrafting β-galactosidase-expressing C6 cells (green) stain heavily for ceruloplasmin (red). Hyaluronan oligomer treatment in vivo decreases ceruloplasmin production. B, Engraftment of C6 side population (C6SP) results in tumor formation (β-galactosidase, green) with high expression of ceruloplasmin, whereas engrafted C6 nonside population cells (C6 non-SP) do not express similar amounts of ceruloplasmin (red). Note high expression of ceruloplasmin (red) around tumor blood vessels in C6 side population tumor (see arrows). C, Gliomas that form after engrafting C6 side population cells also have high expression of ceruloplasmin (red) that is decreased with hyaluronan oligomer (5 μg). Nuclei are stained with Hoechst dye (blue).
Discussion
Ceruloplasmin was previously shown to be elevated in the serum of patients with brain tumors,16 but we were the first to show that a brain tumor (a single desmoplastic infantile ganglioglioma) could directly secrete ceruloplasmin.19 We have now extended this observation to a number of malignant human gliomas. Because of the presumed embryonal origin of desmoplastic infantile ganglioglioma, we isolated stem-like populations of cells from human and rodent glioma cell lines and showed that ceruloplasmin is enriched in such cells, which other studies have shown to be highly malignant and resistant to radiotherapy36 and chemotherapy.37 Additionally, we showed that hyaluronan antagonism with hyaluronan oligomers decreased expression of ceruloplasmin in both cell types. The question remains as to whether ceruloplasmin itself mediates malignancy and treatment resistance in human brain tumors.
Given that ceruloplasmin is found in astrocytes, the abundance of ceruloplasmin expression we observed in the glioma sections is likely being expressed by the cancer cells themselves. Interestingly, we also observed that compared with the high-grade glioblastomas, the low-grade oligodendroglioma expressed very little ceruloplasmin. The low expression in this oligodendroglial tumor may be due to the fact that it lacks an astrocytic component, because normal oligodendrocytes do not express ceruloplasmin. A statistically significant comparison cannot be made, however, due to the small sample size. Further evidence linking ceruloplasmin to cancer is provided by studies that identify the ceruloplasmin promoter as a cancer-specific promoter in ovarian cancer that may have utility in driving the expression of therapeutic genes.38,39 In addition, ceruloplasmin has been identified as a gene differentially expressed in an invasive and tumorigenic breast cancer model.40
Because ceruloplasmin expression was greater in the resistant and malignant stem-like side population of glioma cells, it can be hypothesized that ceruloplasmin may confer a selective advantage to these cells. Due to increased proliferation, cancer cells require more iron than noncancerous cells. Cancer cells have higher levels of the transferrin receptor and internalize iron at a higher rate than non-neoplastic cells.41 When the cell is depleted of iron, G1 arrest occurs.41,42 Therefore, one way of reducing proliferation of cancer cells is to chelate available iron.42 However, it is well established that rapidly dividing cells do not migrate quickly and vice versa. This “go or grow” model in cancer may help explain why tumors seem to appear in a part of the brain distant from the original mass.42 Perhaps the increased ceruloplasmin in the stem-like side population is not being used for rapid cell cycle progression but instead for making the cell motile. These motile cells would be the ones that are resistant to radiotherapy and chemotherapy, and that progressively invade the central nervous system. Some steps in this process may be involved in recurrence of tumors that are even more resistant to therapies than the original tumor. Brain tumors can arise following deregulation of signaling pathways such as Sonic hedgehog that are normally activated during brain development, and may derive from neural stem cells. Sonic hedgehog may also regulate ceruloplasmin,43 suggesting a link between “stemness” and ceruloplasmin in the brain. Interestingly, cyclopamine-mediated blockade of hedgehog signaling resulted in the depletion of the stem-like cancer cells in glioblastoma multiforme.44
Our lab has previously shown that hyaluronan enhances malignant properties of glioma cells, and the literature suggested that signaling molecules under hyaluronan-CD44 control might also mediate ceruloplasmin expression. By antagonizing hyaluronan-CD44 interactions in glioma cells with hyaluronan oligomers in vitro and in vivo, including their highly tumorigenic glioma stem-like cells, we decreased ceruloplasmin production. Hyaluronan oligomers disrupt the interactions of endogenous hyaluronan with its receptors, such as CD44, on the cell surface.25 This results in decreased activation of pathways such as the phosphatidylinositol-3 kinase /Akt and MAPK pathways that are widely implicated in malignant cell behaviors.45–48 Taken together, these findings suggest that ceruloplasmin production by glioma cells is regulated by hyaluronan in the glioma microenvironment.
Ceruloplasmin is thought to have a role in cancer because it is involved in angiogenesis and neovascularization. Copper is a known regulator of angiogenesis, and copper reduction inhibits experimental glioma growth and invasiveness. Copper and zinc levels have been shown to be elevated in a peritumoral zone of glioma.49 However, creating a copper deficiency in human glioblastoma multiforme patients did not show a survival benefit.50 Recently, tetrathiomolybdate (choline salt; ATN-224), a specific, high-affinity copper binder, was shown to inhibit CuZn superoxide dismutase 1 (SOD1) activity, leading to anti-angiogenic and antitumor effects.51
Because hypoxia response elements are in the promoter region of ceruloplasmin,52 and because HIF-1α has been shown to induce ceruloplasmin expression,53–55 it is likely that in cancer cells, an elevated HIF-1α level will result in increased ceruloplasmin transcription. The roles of hypoxia and HIF-1α alone are quite interesting, especially considering the data supporting the idea that HIF-1α is linked with drug-transporter expression, which may confer chemoresistance56,57 to cancer cells and is linked to radioresistance in glioma.58 In addition to hypoxia and hyaluronan levels, it is likely that an increased inflammatory response in cancer is also a key factor in upregulating ceruloplasmin. The connection between cerulopolasmin and interleukin-6 and interleukin-1β has been clearly shown in the literature.17,18,59
A correlation might exist between the level of ceruloplasmin in a glioma and its radioresistance or radiosensitivity. Because cells located in hypoxic regions are more protected from radiation-induced death, it would make sense that these cells also express more ceruloplasmin, the common factor between radioresistance and ceruloplasmin being HIF-1α. However, we found no such relationship between the level of ceruloplasmin and the radiosensitivity or radioresistance of a panel of glioma cell lysates.60 It is possible that ceruloplasmin expression is upregulated in radioresistant tumors only after exposure to radiation. Additionally, intracranial implantation may modulate ceruloplasmin expression in these xenografts. Manipulating ceruloplasmin levels in tumor cells might provide more direct evidence of its roles in human brain tumors. If ceruloplasmin's ferroxidase activity, copper-carrying function, or other mechanism of action enables human brain tumor cells and treatment-resistant glioma stem-like cells to survive, then a novel therapeutic strategy might be to pharmacologically target ceruloplasmin to treat malignant gliomas.
Acknowledgments
This work was supported by a Hollings Cancer Center/Medical University of South Carolina Department of Defense grant titled “Translational Research on Cancer Control and Related Therapy” (Subcontract GC-3319-05-4498CM) by The Malia's Cord Foundation, by a National Institutes of Health (NIH) Clinical and Translational Sciences Award (B.L.M., B.P.T.), and by NIH Grants CA073839 and CA082867 and a Charlotte Geyer Foundation Award (B.P.T.). This work was conducted in a facility constructed with support from the NIH, Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. This work also supported by NIH grant 5R13NS040925-09. Presented in part at the Neuro-biology of Disease in Children Symposium on Central Nervous System Tumors in conjunction with the 36th annual meeting of the Child Neurology Society, Quebec City, Quebec, October 10, 2007.
References
- 1.Fortna RR, Watson HA, Nyquist SE. Glycosyl phosphatidylinositolanchored ceruloplasmin is expressed by rat Sertoli cells and is concentrated in detergent-insoluble membrane fractions. Biol Reprod. 1999;61:1042–1049. doi: 10.1095/biolreprod61.4.1042. [DOI] [PubMed] [Google Scholar]
- 2.Klomp LW, Gitlin JD. Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. Hum Mol Genet. 1996;5:1989–1996. doi: 10.1093/hmg/5.12.1989. [DOI] [PubMed] [Google Scholar]
- 3.Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem. 1997;272:20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 4.Patel BN, Dunn RJ, David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem. 2000;275:4305–4310. doi: 10.1074/jbc.275.6.4305. [DOI] [PubMed] [Google Scholar]
- 5.Salzer JL, Lovejoy L, Linder MC, et al. Ran-2, a glial lineage marker, is a GPI-anchored form of ceruloplasmin. J Neurosci Res. 1998;54:147–157. doi: 10.1002/(SICI)1097-4547(19981015)54:2<147::AID-JNR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Park YS, Suzuki K, Taniguchi N, et al. Glutathione peroxidase-like activity of caeruloplasmin as an important lung antioxidant. FEBS Lett. 1999;458:133–136. doi: 10.1016/s0014-5793(99)01142-4. [DOI] [PubMed] [Google Scholar]
- 7.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 8.Messerschmidt A, Rossi A, Ladenstein R, et al. X-ray crystal structure of the blue oxidase ascorbate oxidase from zucchini. Analysis of the polypeptide fold and a model of the copper sites and ligands. J Mol Biol. 1989;206:513–529. doi: 10.1016/0022-2836(89)90498-1. [DOI] [PubMed] [Google Scholar]
- 9.Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- 10.Osaki S, Johnson DA, Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971;246:3018–3023. [PubMed] [Google Scholar]
- 11.Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a `moonlighting' protein. Cell Mol Life Sci. 2002;59:1413–1427. doi: 10.1007/s00018-002-8519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurell CB, Kullander S, Thorell J. Effect of administration of a combined estrogen-progestin contraceptive on the level of individual plasma proteins. Scand J Clin Lab Invest. 1968;21:337–343. doi: 10.3109/00365516809077003. [DOI] [PubMed] [Google Scholar]
- 13.Hough CD, Cho KR, Zonderman AB, et al. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001;61:3869–3876. [PubMed] [Google Scholar]
- 14.Kunapuli SP, Singh H, Singh P, et al. Ceruloplasmin gene expression in human cancer cells. Life Sci. 1987;40:2225–2228. doi: 10.1016/0024-3205(87)90057-9. [DOI] [PubMed] [Google Scholar]
- 15.Stassar MJ, Devitt G, Brosius M, et al. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer. 2001;85:1372–1382. doi: 10.1054/bjoc.2001.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manjula S, Aroor AR, Raja A, et al. Elevation of serum ceruloplasmin levels in brain tumours. Acta Neurol Scand. 1992;86:156–158. doi: 10.1111/j.1600-0404.1992.tb05058.x. [DOI] [PubMed] [Google Scholar]
- 17.Conley L, Geurs TL, Levin LA. Transcriptional regulation of ceruloplasmin by an IL-6 response element pathway. Brain Res Mol Brain Res. 2005;139:235–241. doi: 10.1016/j.molbrainres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 18.di Patti MC, Persichini T, Mazzone V, et al. Interleukin-1beta up-regulates iron efflux in rat C6 glioma cells through modulation of ceruloplasmin and ferroportin-1 synthesis. Neurosci Lett. 2004;363:182–186. doi: 10.1016/j.neulet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Knapp J, Olson L, Tye S, et al. Case of desmoplastic infantile ganglioglioma secreting ceruloplasmin. J Child Neurol. 2005;20:920–924. doi: 10.1177/08830738050200111201. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 21.Caussinus E, Hirth F. Asymmetric stem cell division in development and cancer. Prog Mol Subcell Biol. 2007;45:205–225. doi: 10.1007/978-3-540-69161-7_9. [DOI] [PubMed] [Google Scholar]
- 22.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 23.Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neo-plasma. 2005;52:435–440. [PubMed] [Google Scholar]
- 24.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 25.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 26.Ranuncolo SM, Ladeda V, Specterman S, et al. CD44 expression in human gliomas. J Surg Oncol. 2002;79:30–35. doi: 10.1002/jso.10045. discussion 35–36. [DOI] [PubMed] [Google Scholar]
- 27.Bouvier-Labit C, Liprandi A, Monti G, et al. CD44H is expressed by cells of the oligodendrocyte lineage and by oligodendrogliomas in humans. J Neurooncol. 2002;60:127–134. doi: 10.1023/a:1020630732625. [DOI] [PubMed] [Google Scholar]
- 28.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 29.Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002;21:15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 30.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 33.Zeng C, Toole BP, Kinney SD, et al. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer. 1998;77:396–401. doi: 10.1002/(sici)1097-0215(19980729)77:3<396::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Muir D, Johnson J, Rojiani M, et al. Assessment of laminin-mediated glioma invasion in vitro and by glioma tumors engrafted within rat spinal cord. J Neurooncol. 1996;30:199–211. doi: 10.1007/BF00177271. [DOI] [PubMed] [Google Scholar]
- 35.Gilg AG, Tye SL, Tolliver LB, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14:1804–1813. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- 36.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 37.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo HW, Day CP, Hung MC. Cancer-specific gene therapy. Adv Genet. 2005;54:235–255. doi: 10.1016/S0065-2660(05)54010-0. [DOI] [PubMed] [Google Scholar]
- 39.Lee CM, Lo HW, Shao RP, et al. Selective activation of ceruloplasmin promoter in ovarian tumors: potential use for gene therapy. Cancer Res. 2004;64:1788–1793. doi: 10.1158/0008-5472.can-03-2551. [DOI] [PubMed] [Google Scholar]
- 40.Kluger HM, Kluger Y, Gilmore-Hebert M, et al. cDNA microarray analysis of invasive and tumorigenic phenotypes in a breast cancer model. Lab Invest. 2004;84:320–331. doi: 10.1038/labinvest.3700044. [DOI] [PubMed] [Google Scholar]
- 41.Kwok JC, Richardson DR. The iron metabolism of neoplastic cells: alterations that facilitate proliferation? Crit Rev Oncol Hematol. 2002;42:65–78. doi: 10.1016/s1040-8428(01)00213-x. [DOI] [PubMed] [Google Scholar]
- 42.Elstner A, Holtkamp N, von Deimling A. Involvement of Hif-1 in desferrioxamine-induced invasion of glioblastoma cells. Clin Exp Metastasis. 2007;24:57–66. doi: 10.1007/s10585-007-9057-y. [DOI] [PubMed] [Google Scholar]
- 43.Kato M, Seki N, Sugano S, et al. Identification of sonic hedgehog-responsive genes using cDNA microarray. Biochem Biophys Res Commun. 2001;289:472–478. doi: 10.1006/bbrc.2001.5976. [DOI] [PubMed] [Google Scholar]
- 44.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007:2007–0166. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 46.Itano N, Atsumi F, Sawai T, et al. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci U S A. 2002;99:3609–3614. doi: 10.1073/pnas.052026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohara Y, Ishiguro N, Machida K, et al. Hyaluronan activates cell motility of v-Src-transformed cells via Ras-mitogen-activated protein kinase and phosphoinositide 3-kinase-Akt in a tumor-specific manner. Mol Biol Cell. 2001;12:1859–1868. doi: 10.1091/mbc.12.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward JA, Huang L, Guo H, et al. Perturbation of hyaluronan interactions inhibits malignant properties of glioma cells. Am J Pathol. 2003;162:1403–1409. doi: 10.1016/S0002-9440(10)64273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dehnhardt M, Zoriy MV, Khan Z, et al. Element distribution is altered in a zone surrounding human glioblastoma multiforme. J Trace Elem Med Biol. 2008;22:17–23. doi: 10.1016/j.jtemb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7:246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donate F, Juarez JC, Burnett ME, et al. Identification of biomarkers for the antiangiogenic and antitumour activity of the superoxide dismutase 1 (SOD1) inhibitor tetrathiomolybdate (ATN-224) Br J Cancer. 2008;98:776–783. doi: 10.1038/sj.bjc.6604226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia. 2005;10:299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 53.Mazumder B, Fox PL. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3' untranslated region. Mol Cell Biol. 1999;19:6898–6905. doi: 10.1128/mcb.19.10.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukhopadhyay CK, Mazumder B, Fox PL. Role of hypoxiainducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J Biol Chem. 2000;275:21048–21054. doi: 10.1074/jbc.M000636200. [DOI] [PubMed] [Google Scholar]
- 55.Das D, Tapryal N, Goswami SK, et al. Regulation of ceruloplasmin in human hepatic cells by redox active copper: identification of a novel AP-1 site in the ceruloplasmin gene. Biochem J. 2007;402:135–141. doi: 10.1042/BJ20060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Comerford KM, Wallace TJ, Karhausen J, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 57.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irie N, Matsuo T, Nagata I. Protocol of radiotherapy for glioblastoma according to the expression of HIF-1. Brain Tumor Pathol. 2004;21:1–6. doi: 10.1007/BF02482169. [DOI] [PubMed] [Google Scholar]
- 59.Lee KH, Yun SJ, Nam KN, et al. Activation of microglial cells by ceruloplasmin. Brain Res. 2007;1171:1–8. doi: 10.1016/j.brainres.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 60.Sarkaria JN, Carlson BL, Schroeder MA, et al. Use of an ortho-topic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]