Abstract
Background Breast cancer incidence is higher among black women than white women before age 40 years, but higher among white women than black women after age 40 years (black–white crossover). We used newly available population-based data to examine whether the age-specific incidences of breast cancer subtypes vary by race and ethnicity.
Methods We classified 91908 invasive breast cancers diagnosed in California between January 1, 2006, and December 31, 2009, by subtype based on tumor expression of estrogen receptor (ER) and progesterone receptor (PR)—together referred to as hormone receptor (HR)—and human epidermal growth factor receptor 2 (HER2). Breast cancer subtypes were classified as ER or PR positive and HER2 negative (HR+/HER2−), ER or PR positive and HER2 positive (HR+/HER2+), ER and PR negative and HER2 positive (HR−/HER2+), and ER, PR, and HER2 negative (triple-negative). We calculated and compared age-specific incidence rates, incidence rate ratios, and 95% confidence intervals by subtype and race (black, white, Hispanic, and Asian). All P values are two-sided.
Results We did not observe an age-related black–white crossover in incidence for any molecular subtype of breast cancer. Compared with white women, black women had statistically significantly higher rates of triple-negative breast cancer at all ages but statistically significantly lower rates of HR+/HER2− breast cancers after age 35 years (all P < .05). The age-specific incidence of HR+/HER2+ and HR−/HER2+ subtypes did not vary markedly between white and black women.
Conclusions The black–white crossover in breast cancer incidence occurs only when all breast cancer subtypes are combined and relates largely to higher rates of triple-negative breast cancers and lower rates of HR+/HER2− breast cancers in black vs white women.
Among women younger than 40 years in the United States, rates of invasive breast cancer are higher among black women than among white women, but among women aged 40 years or older, the rates are higher among white women than among black women (1–5). This “black–white crossover” in breast cancer incidence was first identified several decades ago and has since been interpreted as evidence of intrinsic biological differences in breast cancer between black and white women (6–10) or between early- and late-onset breast cancer (1–4) and continues to stimulate discussion of breast cancer epidemiology (11,12).
Recently, breast cancer epidemiology has also emphasized the heterogeneity of molecularly defined subtypes that are categorized by tumor expression of estrogen receptor (ER) and progesterone receptor (PR) (together referred to as hormone receptor [HR]) as well human epidermal growth factor receptor 2 (HER2/neu and ERbB-2, hereafter referred to as HER2). Currently recognized breast cancer subtypes include ER or PR positive and HER2 negative (HR+/HER2−), ER or PR positive and HER2 positive (HR+/HER2+), ER and PR negative and HER2 positive (HR−/HER2+), and ER, PR, and HER2 negative (triple-negative breast cancer) (13,14). These subtypes are known to differ in their prognosis, response to treatment (15,16), and, as suggested by preliminary epidemiological studies (17,18), risk factors. The incidence rates of breast cancer subtypes vary by race: black women have high rates of triple-negative breast cancer (19) whereas white women have high rates of ER-positive subtypes (20); however, it is unclear whether a black–white crossover (or any potentially informative age-related crossovers between any racial or ethnic groups) occurs when breast cancer molecular subtypes are considered separately. We took advantage of recently available HER2 data for breast cancers occurring in the large and diverse California population to examine recent age-specific incidence rates of breast cancer by molecular subtype and by race and ethnicity.
Methods
Study Population
We obtained data from the California Cancer Registry, which contributes approximately half of the data in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program and is estimated to include more than 99% of all invasive cancers diagnosed in the state of California. We included in our analysis all 91 908 invasive breast cancers [defined as International Classification of Disease for Oncology, 3rd edition (21), sites 50.0–50.9; all histologies other than sarcoma or lymphoma, codes 9050–9055, 9140, 9590–9989] diagnosed between January 1, 2006, and December 31, 2009, among women aged 20 years or older.
The California Cancer Registry has collected information on breast cancer expression of ER and PR since 1990 and of HER2 since 1999. The pivotal trials describing the efficacy of trastuzumab for treating HER2-overexpressing breast cancer were published in 2001 for advanced-stage cancers, and in 2006 for cancers of all stages (16,22). Between 1999 and 2006, 71% of all breast cancers had complete HER2 information; after 2006, more than 85% of all breast cancers had HER2 information recorded. Thus, we limited this analysis to 2006–2009, the most recent years for which more complete data were available.
Tumor expression of ER, PR, and HER2 was recorded in the California Cancer Registry database as positive, negative, borderline, not tested, not recorded, or unknown based on pathology or medical record information. Tumor expression of ER and PR was evaluated by pathologists using dextran-coated charcoal assays or immunohistochemistry (23); HER2 expression was tested by immunohistochemistry or fluorescence in situ hybridization (16). Patient race, ethnicity, and age at diagnosis were recorded in the California Cancer Registry database based on direct abstracting from medical records, and race and ethnicity were based largely on the patient’s self-report (24). We categorized race and ethnicity into the following mutually exclusive categories: Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic Asian or Pacific Islander (hereafter referred to as Hispanic, white, black, and Asian, respectively). Race and/or ethnicity were either not known or could not be categorized for 1159 (1.3%) of the 91 908 invasive breast cancer patients.
Classification of Breast Cancer Subtypes
On the basis of tumor expression of ER, PR, and HER2 as described in the pathology record, we classified the breast cancers into four mutually exclusive subtype categories: HR+/HER2−, HR+/HER2+, HR−/HER2+, and triple-negative breast cancer (5,13,14,19,25,26). Of the 90 749 cancers (excluding those for which patient race or ethnicity was unknown), 14 512 (16.0%) did not have information needed to assign to one of these subtypes, including 8006 cancers (8.8%) for whom only HER2 status was unknown, 660 cancers (<1%) for whom only HR status was unknown, and 5846 cancers (6.4%) for whom both HR and HER2 status were unknown. Cancers for which subtype was missing did not differ statistically significantly with respect to patient age, race, or ethnicity from those for which subtype was known (data not shown).
Statistical Analysis
We used SEER*Stat software version 7.0.5 (27) developed by the National Cancer Institute to compute age-specific incidence rates per 100 000 woman-years by age group at diagnosis (20–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and ≥85 years), incidence rate ratios, and corresponding 95% confidence intervals (CIs) based on the Poisson distribution (28). Incidence rate ratios (IRRs) conveyed the relative risk of developing a particular subtype for one population group compared with a specified reference group. Population estimates were developed by SEER based on US Census projections. Incidence rate plots were scaled semi-logarithmically to aid visual estimation of proportional rates of change (29). All analyses were conducted in accordance with the Institutional Review Board approval of the Cancer Prevention Institute of California (protocol number 2001-043). All statistical comparisons were two-sided.
Results
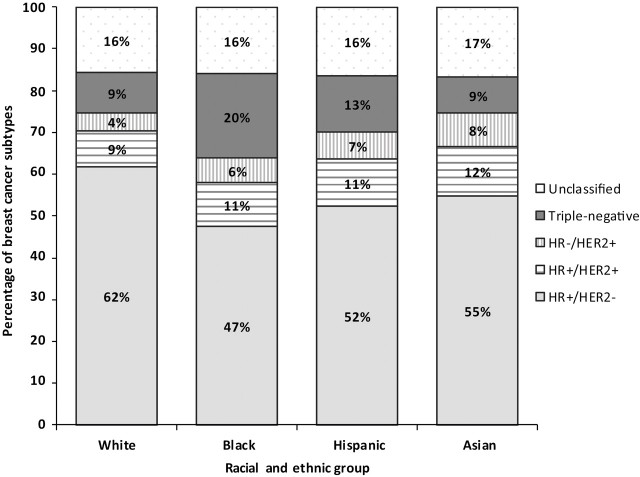
Figure 1 shows the distribution of breast cancer subtypes diagnosed among women in California according to patient race and ethnicity. Among white and Asian women, there were more than six times as many HR+/HER2− tumors as triple-negative tumors (ratio for whites: 6.9:1; ratio for Asian: 6.1:1). The ratios of HR+/ HER2− tumors to triple-negative tumors were much lower among Hispanic women (4.0:1) and black women (2.4:1). Table 1 shows the distribution of breast cancer subtypes by age group for the four racial and ethnic groups and overall, confirming substantial racial and ethnic variation in the proportions of subtypes across all age groups. However, in each racial and ethnic group, proportionately more triple-negative breast cancers were diagnosed at younger ages than at older ages.
Table 1.
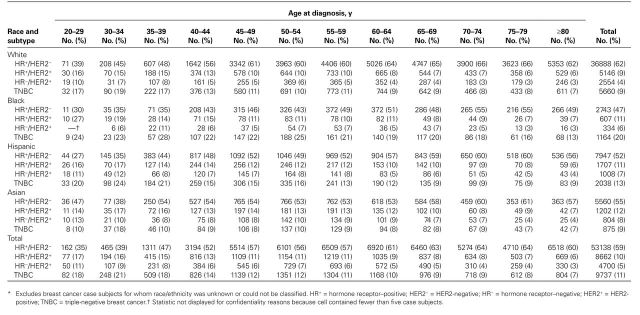
Distribution of female breast cancer patients by age, race/ethnicity, and subtype, California, 2006–2009*
Figure 1.
Distribution of breast cancer subtypes, by race and ethnicity, among California women, 2006–2009. HR+ = hormone receptor-positive; HER2– = HER2-negative; HR– = hormone receptor–negative; HER2+ = HER2-positive; TNBC = triple-negative breast cancer.
We next examined differences in the age-specific incidence of breast cancer by patient race and ethnicity (Figure 2, A) and molecular subtype (Figure 2, B). Among women who were younger than 45 years at diagnosis, breast cancer incidence rates (expressed as the number of breast cancers diagnosed per 100 000 woman-years were higher among black women than among white women (whites aged 40–44 years: 129.2 [95% CI = 124.6 to 134.0]; blacks aged 40–44 years: 133.7 [95% CI = 122.1 to 146.2]); among women who were older than 50 years at diagnosis, the rates were higher among white women than among black women (whites aged 50–54 years: 254.7 [95% CI = 248.6 to 260.9]; blacks aged 50–54 years: 234.4 [95% CI = 218.0 to 251.7]) (Figure 2, A). Rates among Hispanic women and Asian women were lower than rates among white women and black women at all ages at diagnosis. At all ages at diagnosis, the magnitudes of the differences in rates between racial and ethnic groups were small compared with the substantial differences in rates among the four breast cancer subtypes (Figure 2, B). At approximately age 50 years at diagnosis, the rate of HR+/HER2− breast cancer was almost five times higher than the rate of triple-negative breast cancer (rates among women aged 50–54 years, HR+/HER2−: 126.1 [95% CI = 123.0 to 129.3]; triple-negative: 27.8 [95% CI = 26.3 to 29.3]). At all ages, rates of HR+/HER2+ and triple-negative breast cancers were similar. HR−/HER2+ breast cancer had the lowest age-specific incidence rates.
Figure 2.
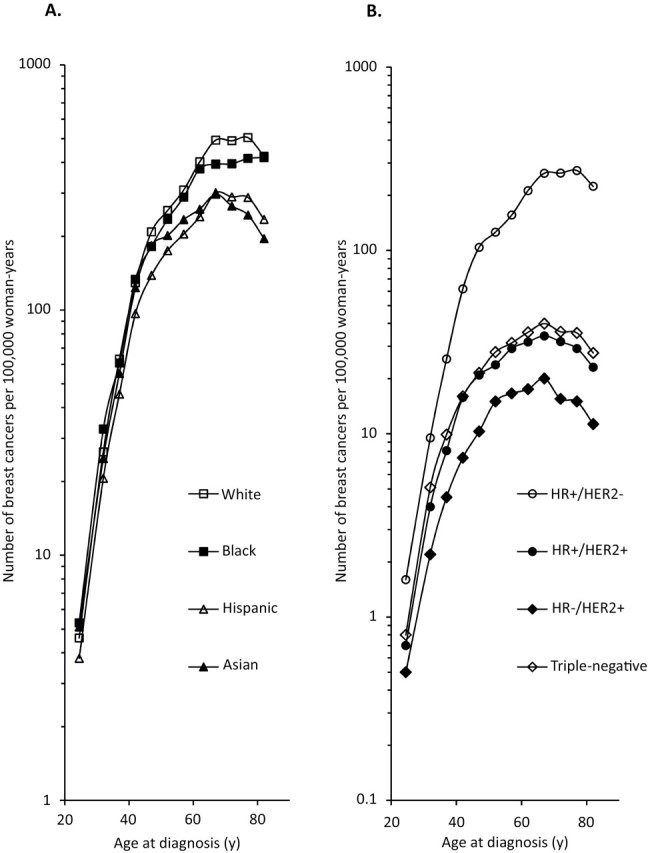
Age-specific incidence rates of breast cancer by A) race and ethnicity and by B) molecular subtype, among California women, 2006–2009. HR+ = hormone receptor-positive; HER2- = HER2-negative; HR- = hormone receptor-negative; HER2+ = HER2-positive; TNBC = triple-negative breast cancer.
Figure 3 shows age-specific incidence rates by molecular subtype for the four racial and ethnic groups. There was no age-related crossover between rates among black women vs white women for any of the subtypes. The HR+/HER2− subtype had the highest incidence rates of all subtypes at all ages of diagnosis for this subtype, and incidence rates were higher in white women than in women of the other racial and ethnic groups at all ages (rates among women aged 50–54 years, whites: 152.8 [95% CI = 148.1 to 157.6], blacks: 101.2 [95% CI = 90.5 to 112.8], Hispanics: 85.9 [95% CI = 80.7 to 91.2], Asians: 107.1 [95% CI = 99.6 to 114.9]). Black women had higher rates of triple-negative breast cancer compared with women in the other three racial and ethnic groups at all ages. The relative differences in incidence rates between racial and ethnic groups were larger for triple-negative breast cancer compared with the other breast cancer subtypes, where differences in incidence rates by race and ethnicity were more subtle. Compared with white women, black women had statistically significantly higher rates of triple-negative breast cancer at all ages but statistically significantly lower rates of HR+/HER2− breast cancers after age 35 years (all P < .05). To examine the statistical significance of the differences in age-specific rates, we calculated incidence rate ratios and 95% confidence intervals for each nonwhite racial and ethnic group compared with white women by breast cancer subtype (Supplementary Figure 1, available online). For triple-negative breast cancer, black vs white incidence rate ratios were statistically significantly greater than 1.0 after age 35 years, whereas the Asian vs white incidence rate ratios were statistically significantly less than 1.0 after age 35 years, and the Hispanic vs white incidence rate ratios were statistically significantly less than 1.0 after age 55 years (all P < .05). For HR+/HER2− breast cancer, incidence rates were statistically significantly higher among white women than other women at all ages after age 45 years (all P < .05). Asian women aged 40–64 years had a higher incidence of HR−/HER2+ breast cancer compared with white women (rates among women aged 50–54 years, Asians: 19.8 [95% CI = 16.7 to 23.4]; whites: 14.2 [95% CI = 12.8 to 15.8]). For HR+/HER2+ breast cancers, there were no obvious statistically significant racial and ethnic differences in rates.
Figure 3.
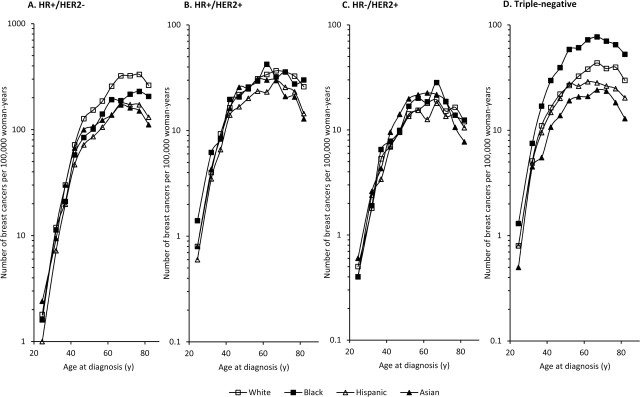
Distribution of breast cancer subtypes, by race and ethnicity, among California women, 2006–2009. HR+ = hormone receptor-positive; HER2– = HER2-negative; HR– = hormone receptor–negative; HER2+ = HER2-positive; TNBC = triple-negative breast cancer.
Discussion
Using recent data about HER2-defined breast cancer occurrence in the large, diverse population of California, we found no evidence for a black–white crossover in breast cancer incidence for any of the four major molecular subtypes of breast cancer. Compared with white women, black women had higher rates of triple-negative breast cancer at all ages and lower rates of HR+/HER2− breast cancer after age 35 years. The black–white crossover in breast cancer incidence occurred only when all breast cancer subtypes are combined. Our data also demonstrate substantial racial and ethnic variation in the age-specific occurrence of breast cancer subtypes, especially the triple-negative form.
The HER2 status of tumors has only recently become information that is routinely collected as part of breast cancer registration and surveillance because HER2 expression has only recently become a standard part of breast cancer pathological assessment (16) . Previous studies of breast cancer occurrence that differentiated tumors according to ER status revealed important clues about the contribution of tumor estrogen sensitivity to the observed deceleration of increasing age-specific incidence around the age of menopause (30). Now, with population-based data regarding HER2-defined breast cancer subtypes, we are able to expand our understanding of the divergent occurrence of breast cancer subtypes by age and by race and ethnicity.
Our findings are consistent with those of Carey et al. (14), who described higher occurrence of triple-negative breast cancer in black vs white premenopausal women, and of Chlebowski et al. (18), who reported that black women were five times more likely than white women to have high-grade, ER-negative cancers; both groups invoked possible genetic causes for these differences based on studies of black breast cancer patients in Africa (31). Our findings are also consistent with previous cancer registry–based studies of women that found higher overall incidences of triple-negative breast cancer among black women and of HR+/HER2− breast cancers among white women compared with women of other racial and ethnic groups (5,19,32,33) as well as other epidemiological and clinical suggestions that these two subtypes are etiologically and biologically distinct (34–36).
In 2008, Anderson et al. (1) used a structured quantitative approach to show in SEER data that the black–white crossover in overall breast cancer incidence persisted across time and birth cohort; they concluded that the most likely explanation for the crossover involved biological heterogeneity of subtypes between black and white women. Other authors have pointed to the role of different racial and ethnic prevalence of risk factors, including reproductive history and other factors associated with socioeconomic status (4,37–40), or ethnic variations in the magnitude of risk associated with particular exposures (eg, use of postmenopausal hormone therapy, parity, and body mass index [BMI]) (1). Still other interpretations hypothesize novel, ethnically disparate causes, such as environmental exposures or susceptibility genes (18,31). Although some ethnic disparities in both mammographic screening and BRCA1 and BRCA2 gene mutation testing have been reported (41,42), it is unlikely that these occur at levels sufficient to explain the black–white crossover in breast cancer incidence as reported in previous studies. After synthesizing the previous literature with our new observations, we suggest that the observed crossover mainly reflects racial and ethnic differences in the age-specific incidences of triple-negative breast cancer (higher in black women) and HR+/HER2− breast cancer (higher in white women, particularly those older than 35 years).
Several epidemiological studies have examined risk factors for specific breast cancer subtypes using datasets large enough to provide adequate statistical power for detecting differences among breast cancer subtypes. In a recent pooled analysis of 34 observational studies from the Breast Cancer Association Consortium (BCAC) (17), reproductive risk factors (age at menarche, parity, and age at birth of first child) and BMI were associated with the risk of ER+ or PR+ breast tumors but not with the risk of triple-negative breast cancer. In fact, a pooled analysis of the 12 eligible BCAC studies that had data on tumor HER2 status did not find an association between the risk of triple-negative breast cancer and any of the other risk factors that are typically considered when studying breast cancer as a single entity, with the exception of family history, which was positively associated with triple-negative and other breast cancer subtypes (17). However, even that large pooled analysis did not have sufficient statistical power to examine associations between BMI or parity and the risk of triple-negative breast cancer specifically in black women. In a smaller study (36), adjustment for BMI and age did not fully explain the elevated risk of triple-negative breast cancer among black women compared with white women. The consistently higher incidence of triple-negative breast cancer among black women compared with white women at all ages suggests that black women are more susceptible to triple-negative breast cancer (43) than white, Hispanic, or Asian women. Indeed, our data show that the most marked racial and ethnic differences in incidence occur for triple-negative breast cancer, making this subtype worthy of particular scrutiny in genomic or other studies. Certainly, genetic or other nonenvironmental factors remain important candidates for explaining racial and ethnic differences in the incidence of specific molecular subtypes of breast cancer. Recent studies of the BRCA1 and BRCA2 breast cancer susceptibility genes suggest that although mutation carrier prevalence is comparable among ethnic groups, there is substantial ethnic variation in the spectrum of mutations (44).
Our analysis adds to the evidence suggesting that middle-aged Asian women have higher risks of HER2-overexpressing tumors compared with white women (33,45). A previous analysis of California data showed that incidence of HER2-overexpressing tumors was higher among Korean, Filipina, Vietnamese, and Chinese women than among white women (45). A pooled analysis of 13 BCAC studies found that BMI, parity, and age at birth of first child were associated with the risk of HER2-overexpressing tumors (17), but it is uncertain whether these associations could explain higher incidence of HER2-overexpressing tumors among Asian populations. Although in this analysis we were not able to further stratify rates for Asian women by nativity or acculturation, previous analyses have shown that both factors affect overall incidence rates of breast cancer among Asian women (46,47). Future epidemiological studies of molecular subtypes of breast cancer among Asian or Hispanic women should examine occurrence separately by ethnic subgroup and nativity.
This study has several strengths. We took advantage of some of the first population-based data for which ER, PR, and HER2 status are sufficiently complete to classify the four major current molecular subtypes of breast cancer. Moreover, the California data offer a large sample size and diversity for examining age-specific patterns in racial and ethnic groups and are generally representative of data in the larger SEER program.
However, this study, like all cancer registry–based analyses, was limited by some degree of missing data on these biomarkers, by the nonstandardized assessment of biomarker subtype as reported in individual pathology reports constituting the basis for cancer registry abstraction, and by the lack of detailed individual-level information on breast cancer risk factors. Although we cannot speak to the full set of risk factors for specific subtypes, we can confirm the suspicions of previous authors (1) that the differential occurrence of etiologically distinct entities, namely triple-negative breast cancer and HR+/HER2− breast cancer, is responsible for the black–white difference in the age-specific incidence of breast cancer. For no subtype did we observe the black–white crossover in incidence that appears when breast cancer is analyzed as a single entity (ie, without regard to subtype), which suggests that the high rates of triple-negative breast cancer among black women at younger ages and the high rates of HR+/HER2– breast cancers among white women at older ages are the major contributors to the black–white crossover.
Future studies of racial and ethnic differences in breast cancer occurrence should examine molecular subtypes separately. As further molecular technological advances are made to characterize breast tumors in more meaningful ways, these discoveries should guide treatment options and, it is hoped, will lead to success in reducing the mortality and survival disparities for triple-negative and other aggressive forms of breast cancer that now disproportionately affect younger women.
Funding
This study was supported by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program under contract HHSN261201000040C awarded to the Cancer Prevention Institute of California (CPIC).The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the SEER Program under contract HHSN261201000040C awarded to CPIC (formerly the Northern California Cancer Center), contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute.
Notes
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred. No author reports any financial conflict of interest. The study sponsors did not have any role in the design of the study, the analysis or interpretation of the data, the writing of the article, or the decision to submit the article for publication.
Supplementary Material
References
- 1. Anderson WF, Rosenberg PS, Menashe I, et al. Age-related crossover in breast cancer incidence rates between black and white ethnic groups J Natl Cancer Inst 2008;100(24):1804–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton LA, Sherman ME, Carreon JD, et al. Recent trends in breast cancer among younger women in the United States J Natl Cancer Inst 2008;100(22):1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joslyn SA, Foote ML, Nasseri K, et al. Racial and ethnic disparities in breast cancer rates by age: NAACCR Breast Cancer Project Breast Cancer Res Treat 2005;92(2):97–105 [DOI] [PubMed] [Google Scholar]
- 4.Krieger N. Social class and the black/white crossover in the age-specific incidence of breast cancer: a study linking census-derived data to population-based registry records Am J Epidemiol 1990;131(5):804–814 [DOI] [PubMed] [Google Scholar]
- 5.Parise CA, Bauer KR, Brown MM, et al. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004 Breast J. 2009;15(6):593–602 [DOI] [PubMed] [Google Scholar]
- 6.Natarajan N, Nemoto T, Mettlin C, et al. Race-related differences in breast cancer patients results of the 1982 national survey of breast cancer by the American College of Surgeons Cancer 1985;56(7):1704–1709 [DOI] [PubMed] [Google Scholar]
- 7.Pegoraro RJ, Karnan V, Nirmul D, et al. Estrogen and progesterone receptors in breast cancer among women of different racial groups Cancer Res. 1986;46(4, pt 2):2117–2120 [PubMed] [Google Scholar]
- 8.Elledge RM, Clark GM, Chamness GC, et al. Tumor biologic factors and breast cancer prognosis among white, hispanic, and black women in the United States J Natl Cancer Inst. 1994;86(9):705–712 [DOI] [PubMed] [Google Scholar]
- 9.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival Breast Cancer Res Treat 2002;73(1):45–59 [DOI] [PubMed] [Google Scholar]
- 10.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients Cancer 2007;110(4):876–884 [DOI] [PubMed] [Google Scholar]
- 11.Pathak DR, Osuch JR, He J. Breast carcinoma etiology: current knowledge and new insights into the effects of reproductive and hormonal risk factors in black and white populations Cancer. 2000;88(Suppl 5):1230–1238 [DOI] [PubMed] [Google Scholar]
- 12.Anderson WF, Reiner AS, Matsuno RK, et al. Shifting breast cancer trends in the United States J Clin Oncol. 2007;25(25):3923–3929 [DOI] [PubMed] [Google Scholar]
- 13.Bernstein L, Lacey JV. Receptors, associations, and risk factor differences by breast cancer subtypes: positive or negative? J Natl Cancer Inst. 2011;103(6):451–453 [DOI] [PubMed] [Google Scholar]
- 14.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study JAMA. 2006;295(21):2492–2502 [DOI] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer. Version 2, 2011.
- 16.Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer J Clin Oncol. 2007;25(1):118–145 [DOI] [PubMed] [Google Scholar]
- 17.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies J Natl Cancer Inst. 2011;103(3):250–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome J Natl Cancer Inst. 2005;97(6):439–448 [DOI] [PubMed] [Google Scholar]
- 19.Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype Cancer. 2007;109(9):1721–1728 [DOI] [PubMed] [Google Scholar]
- 20.Hausauer AK, Keegan THM, Chang ET, et al. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype Breast Cancer Res 2007;9(6):R90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology 3rd ed Geneva: World Health Organization; 2000. [Google Scholar]
- 22.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51 [DOI] [PubMed] [Google Scholar]
- 23.Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer J Clin Oncol. 2010;28(16):2784–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez SL, Le GM, West DW, et al. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace Am J Public Health. 2003;93(10):1685–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours Nature 2000;406(6797):747–752 [DOI] [PubMed] [Google Scholar]
- 26.Lund M, Trivers K, Porter P, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA Breast Cancer Res Treat. 2009;113(2):357–370 [DOI] [PubMed] [Google Scholar]
- 27. Surveillance Research Program, National Cancer Institute. SEER*Stat software, version 7.0.5. http://www.seer.cancer.gov/seerstat. Accessed November 5, 2011.
- 28.Fay MP. Approximate confidence intervals for rate ratios from directly standardized rates
- with sparse data Commun Stat Theory. 1999;28(9):2141–2160 [Google Scholar]
- 29.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates Am J Epidemiol. 1995;141(4):300–304 [DOI] [PubMed] [Google Scholar]
- 30.Pike MC, Spicer DV, Dahmoush L, et al. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk Epidemiol Rev. 1993;15(1):17–30 [DOI] [PubMed] [Google Scholar]
- 31.Ademuyiwa FO, Olopade OI. Racial differences in genetic factors associated with breast cancer Cancer Metast Rev. 2003;22(1):47–53 [DOI] [PubMed] [Google Scholar]
- 32.Kurian A, Fish K, Shema S, et al. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups Breast Cancer Res. 2010;12(6):R99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity Crit Rev Oncol Hemat. 2010;76(1):44–52 [DOI] [PubMed] [Google Scholar]
- 34.Lund MJ, Butler EN, Hair BY, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes Cancer 2010;116(11):2549–2559 [DOI] [PubMed] [Google Scholar]
- 35.Millikan R, Newman B, Tse C-K, et al. Epidemiology of basal-like breast cancer Breast Cancer Res Treat. 2008;109(1):123–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stead L, Lash T, Sobieraj J, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index Breast Cancer Res. 2009;11(2):R18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger N, Chen J, Waterman P. Temporal trends in the black/white breast cancer case ratio for estrogen receptor status: disparities are historically contingent, not innate Cancer Cause Control. 2011;22(3):511–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinton LA, Benichou J, Gammon MD, et al. Ethnicity and variation in breast cancer incidence Int J Cancer. 1997;73(3):349–355 [DOI] [PubMed] [Google Scholar]
- 39.Trivers K, Lund M, Porter P, et al. The epidemiology of triple-negative breast cancer, including race Cancer Cause Control. 2009;20(7):1071–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin D, Morris C, Allen M, et al. Does socioeconomic disparity in cancer incidence vary across racial/ethnic groups? Cancer Cause Control. 2010;21(10):1721–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk Genet Med. 2011;13(4):349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chagpar AB, Polk HC , Jr, McMasters KM. Racial trends in mammography rates: a population-based study Surgery 2008;144(3):467–472 [DOI] [PubMed] [Google Scholar]
- 43.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer J Clin Oncol 2009;27(27):4515–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications Curr Opin Obstet Gyn. 2010;22(1):72–78. doi:10.1097/GCO.0b013e328332dca3 [DOI] [PubMed] [Google Scholar]
- 45.Telli M, Chang E, Kurian A, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry Breast Cancer Res Treat. 2011;127(2):471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keegan THM, Gomez SL, Clarke CA, et al. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California Int J Cancer. 2007;120(6):1324–1329 [DOI] [PubMed] [Google Scholar]
- 47.Gomez SL, Quach T, Horn-Ross PL, et al. Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status Am J Public Health 2010;100(S1):S125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.