Abstract
Objective
To establish normal, trimester-specific reference intervals for serum 17β-estradiol, progesterone (P), 17α-hydroxyprogesterone, cortisol, 11-deoxycortisol, androstenedione, DHEA, and DHEAS, measured simultaneously using isotope dilution tandem mass spectrometry.
Design
Sequential cohort study.
Patient(s)
Healthy women undergoing a normal pregnancy (age, 25–38 years; mean, 30 years) attending a prenatal well clinic at gestation weeks 12, 22, and 32 and approximately 1 year postpartum.
Main Outcome Measure(s)
Trimester-specific reference intervals of endogenous steroid hormones analyzed using an isotope dilution tandem mass spectrometer equipped with an atmospheric pressure photoionization source with deuterium-labeled internal standards.
Result(s)
Serum estradiol, P, 17α-hydroxyprogesterone, and 11-deoxycortisol increased throughout pregnancy; cortisol increased up to the second trimester and then remained steady, while androstenedione increased by 80 percent by gestation week 12, then remained constant. Serum DHEA-S decreased by 50% by the third trimester.
Conclusion(s)
Trimester-specific reference intervals are reported for eight serum steroids. The ratios of individual serum hormone concentrations during pregnancy relative to their 1-year postpartum concentrations illustrate the expected normal trends of changes in hormone concentrations during pregnancy.
Keywords: Reference intervals, estradiol, progesterone, cortisol, DHEA, DHEAS, androstenedione, steroids, hormones, isotope dilution tandem mass spectrometry
Normal pregnancy depends on pronounced adaptations in pregnancy-related hormone concentrations, characterized by elevated levels of several circulating steroid hormones, which normally increase with the progression of pregnancy (1–6). Endogenous steroid hormone exposure during pregnancy has been of interest in studies of duration of gestation, fetal size, twin pregnancies, control of labor, nausea and vomiting in pregnancy, pregnancy-induced hypertension, and other disease states (5–9). The studies have generally produced weak and inconsistent findings because the hormone levels were either not available or lacked specificity or because surrogate measures of exposure to altered steroid hormone levels were often used to estimate these hormones.
Steroid hormones are derived from cholesterol. Binding proteins facilitate their transport and increase their half-life but limit their entry into target cells, thereby regulating their biological activity. Binding proteins make precise determination of serum steroid hormone concentrations difficult by interfering with different steroid hormones immunoassays (IAs). Furthermore, the lack of specificity of IAs due to cross reactivity with structurally related molecules is a well-known phenomenon (10–12).
In contrast, isotope dilution liquid chromatography–tandem mass spectrometry (LC/MS/MS) is a specific detection method that allows the quantification of the analyte of interest. It also allows for a simpler approach to sample preparation without employing lengthy and time-consuming extraction and sample derivatization steps. This has been reported in a previous publication, and steroid hormone analysis using isotope dilution LC/MS/MS was compared with the analysis of the same samples using IA techniques (14). Generally, tandem mass spectrometry provides lower values than IA, no doubt because of improved specificity.
The reasons for the improved specificity in our tandem mass spectrometric method (14) include the use of multiple reaction mode monitoring (MRM) as very few analytes have identical parent and daughter ions; use of the atmospheric pressure photoionization (APPI) source, which selectively ionizes steroids very well but is less adept at ionization of many other compounds thereby increasing selectivity of the method; and the use of High Performance Liquid Chromatography (HPLC) together with tandem mass spectrometry, which further enhances the specificity of the method. The between-day precision data for the method are shown in Table 1.
TABLE 1.
Tandem mass spectrometry between-day precision.
| Level 1 |
Level 2 |
|||
|---|---|---|---|---|
| Steroid | Mean (ng/mL, nmol/L) | %CV | Mean (ng/mL, nmol/L) | %CV |
| E2 | 0.28, 1,033.48 (pmol/L) | 9 | 0.60, 2,214.6 (pmol/L) | 8.5 |
| P | 5.6, 17.81 | 11.4 | 13.6, 43.25 | 8.8 |
| 17α-hydroxyprogesterone | 0.87, 2.61 | 9.2 | 3.50, 10.5 | 7.1 |
| Cortisol | 192.7, 531 | 6.9 | 341.1, 940 | 6.1 |
| 11-Deoxycortisol | 0.78, 2.26 | 12.7 | 7.86, 22.79 | 8.1 |
| DHEA | 0.87, 3.02 | 9.2 | 2.86, 9.92 | 8.8 |
| Androstenedione | 0.9, 3.14 | 22 | 1.82, 6.35 | 18.1 |
| DHEAS | 415, 11.21 (µmol/L) | 13.4 | 1436, 38.77 (µmol/L) | 12.7 |
Note: Replicate: n = 20 for between-day. Quality control was chosen from BioRad Liquichek Immunoassay Plus Control or from in-house control prepared for 11-deoxycortisol to reflect the clinically reasonable ranges of steroids in pregnancy. CV indicates coefficient of variation.
The method, which uses the API-3000 (Sciex, Concord, Ontario, Canada) requires a fairly large serum volume (800 µL) to optimize sensitivity; however, we have recently been able to improve the sensitivity by using the more sensitive API-5000 (Sciex), which has enabled us to reduce the serum requirement to 250 µL and by changing the C18 column for a C4 column, the total chromatography time has been reduced from 18 to 10 minutes. While deuterated internal standards were used for each analyte, the internal standard used for DHEAS was deuterated testosterone (14). The quality control material used was Bio-Rad Liquichek Immunoassay Plus Control (Irvine, CA). Column life has been at least 1 month (or 600 injections).
Given the importance of steroid hormones in pregnancy, we set out to determine trimester-specific reference intervals in a longitudinal study of 52 women during normal pregnancy and 1 year postpartum. Simultaneous analysis and measurement of eight steroid hormones was conducted in the positive ion mode by LC/MS/MS employing MRM. Steroids and their respective deuterated internal standards were separated within 18 minutes. The normal reference intervals for eight serum steroid hormones, trimester-related and for non-pregnant women, as well as their relative changes during pregnancy are reported.
MATERIALS AND METHODS
The research was conducted at the Georgetown University Medical Center’s General Clinical Research Center. Informed consents were obtained, and the study was approved by the Institutional Review Board.
Study Population
Blood specimens were obtained from healthy women on normal diets undergoing their first pregnancy. All sera were stored frozen at −80°C before analysis. All of the women were of Caucasian descent/non-Hispanic, with a mean age of 30 years (25–38 years) attending a prenatal pregnancy well clinic at the Karolinska Institute, Stockholm, Sweden. The samples were obtained during gestation week 12 (first trimester), gestation week 22 (second trimester), gestation week 32 (third trimester), and approximately 1 year postpartum. Gestational dates were confirmed by ultrasound. All pregnancies were normal, viable, singleton pregnancies.
Hormones and Chemicals
Androstenedione (4-androstene-3,17-dione), cortisol (4-pregnen-11β,17α,21-triol-3,20-dione), 11-deoxycortisol (4-pregnen-17α,1-diol-3,20-dione), DHEA (5-androsten-3β-ol-17-one), DHEA-S (5-androsten-3β-ol-17-one sulfate, sodium salt), 17β-estradiol (1,3,5(10)-estratriene-3,17β-diol), P (4-pregnen-3,20-dione), 17α-hydroxyprogesterone (4-pregnen-17α-ol-3,20-dione), testosterone (T) (4-androsten-17-ol-3-one), ammonium acetate, and bovine albumin (96%) were purchased from Sigma-Aldrich (St. Louis). Deuterium-labeled internal standards (cortisol-9,11,12,12-d4, estradiol-2,4,16,16-d4, and testosterone-1,2-d2) were obtained from Cambridge Isotope Laboratory (Andover, MA); 4-androstene-3,17-dione-2,2,4,6,6,16,6-d7, dehydroepiandrosterone-16,16-d2, 4-pregnen-17α-ol-3,20-dione-2,2,4,6,6,21,21,21-d8, 11-deoxycortisol-21,21-d2, and P-2,2,4,6,6,17α,21,21,21-d9 were purchased from C/D/N Isotopes (Pointe-Claire, Quebec, Canada). High-performance liquid chromatography-grade water and methanol were obtained from Burdick & Jackson (Muskegon, MI). Optima-grade acetonitrile and toluene were purchased from Fisher Scientific (Fair Lawn, NJ). All chemicals (unless otherwise noted) had a purity of at least 98% according to the manufacturer.
Sample Preparation
Seven hundred sixty microliters of each standard mixture or serum sample containing the steroids of interest were placed into a 2.0-mL conical plastic centrifuge tube. One thousand one hundred forty microliters of acetonitrile containing the internal standards was added to the tube to precipitate the proteins in the sample. The tubes were capped, vortexed vigorously for at least 30 seconds, and centrifuged at 13,000 rpm for 10 minutes. The supernatant in the tubes was transferred into autosampler vials for injection into the LC/MS/MS system. Sample preparation was performed at room temperature and 1.7 mL of the supernatant was injected into the LC/MS/MS.
Isotope Dilution LC/MS/MS Analysis
The LC/MS/MS analysis was performed according to the method previously described by our laboratory (14). Briefly, steroid hormones were analyzed using the Applied Biosystems/Sciex API-3000 liquid chromatography tandem mass spectrometer equipped with APPI source with deuterium-labeled internal standards. The detection limit was defined as the concentration 2 SD above the response at zero dose. As described elsewhere (14), the measurement of E2 is precise and accurate down to 0.06 ng/mL. The sensitivity of each assay and within-run (intra-assay) and between-run (interassay) imprecision and coefficient of variation for different concentrations of steroids are presented in Table 1.
Statistical Analysis
Descriptive statistics, including upper and lower bounds of reference intervals, were calculated for each analyte separately by trimester using SPSS version 12.0 (SPSS, Cary, NC). Reference intervals are defined as the 2.5th and 97.5th percentiles.
RESULTS
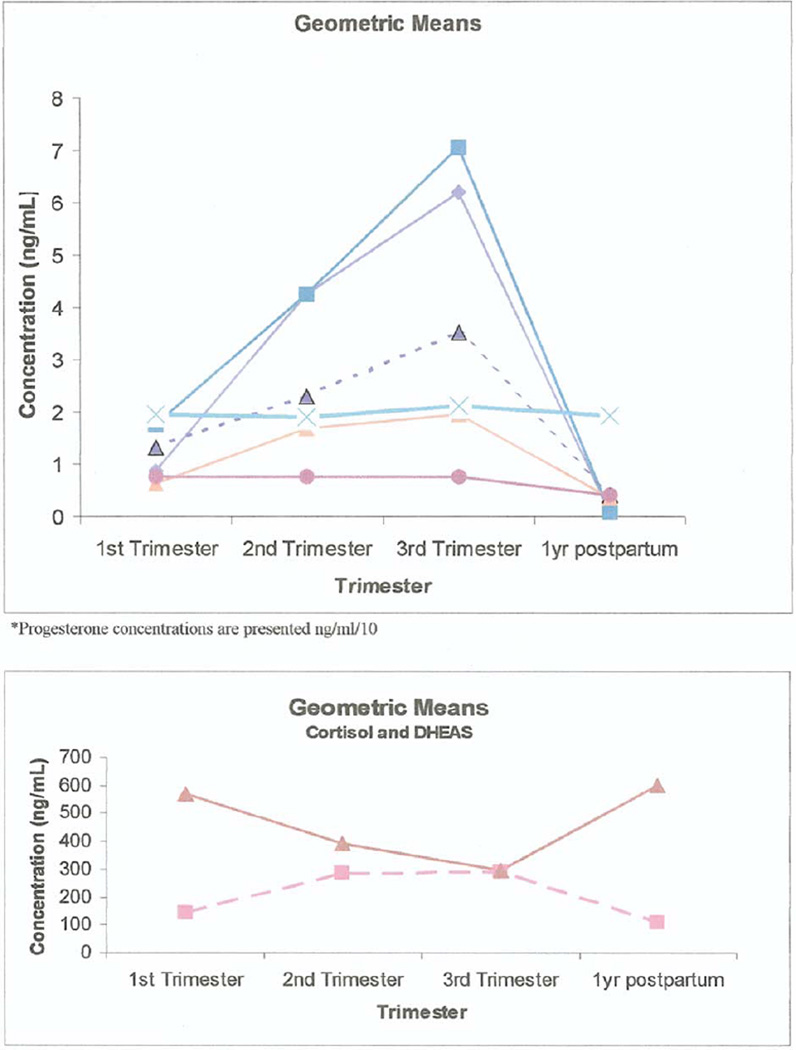
Some serum steroid hormone concentrations change dramatically during pregnancy. Longitudinal trends of seven of the eight analytes increase during pregnancy. Estradiol concentrations increase six-fold by week 32 and return to almost undetectable levels after pregnancy, and 17α-hydroxyprogesterone increases 3.5-fold while cortisol and DHEA more than double in concentration.
Figures 1 and 2 show diagrammatically the trend of the means of each of the analytes through the pregnancy starting at week 12.
FIGURE 1.
Mean steroid hormone concentrations in pregnancy.
FIGURE 2.
Ratios steroid hormone concentrations in pregnancy relative to postpartum. P concentrations are presented as ng/mL/10.
The reference intervals obtained using LC/MS/MS and means and medians are presented in Table 2. In general, serum E2, P, 17α-hydroxyprogesterone, and 11-deoxycortisol increased throughout pregnancy, cortisol increased up to the second trimester and then remained steady, and androstenedione increased by 80% by gestation week 12 and remained at that level for the remainder of the pregnancy. Serum DHEA trimester-specific medians remained constant, but the means increased slightly in the third trimester. Serum DHEAS decreased by 50% by the third trimester relative to prepregnancy concentrations (Table 2). Testosterone concentrations were less than 60 pg/mL and are therefore not reported in the tables.
TABLE 2.
Reference intervals: steroid function tests in pregnancy.
| Reference intervals |
|||||
|---|---|---|---|---|---|
| Analyte | n | Meana (±SE) | Median | 2.5th percentile | 97.5th percentile |
| E2 (ng/mL, pmol/L)b | |||||
| First trimesterc | 51 | 0.87 ± 0.07, 3,211.2 ± 253.2 | 0.87, 3,211.2 | 0.31, 1,137.8 | 3.00, 11,010 |
| Second trimester | 50 | 4.24 ± 0.26, 15,546.1 ± 961.5 | 4.29, 15,744.3 | 1.91, 7,009.7 | 10.33, 37,911.11 |
| Third trimester | 50 | 6.18 ± 0.41, 22,680.6 ± 1493.7 | 6.56, 24,075.2 | 2.17, 7,963.9 | 13.85, 50,829.5 |
| Postpartum | 50 | <0.1 ± 0.015, <367.0 ± 55.0 | <0.1, <367 | — | 1.24, 4,550.8 |
| P (ng/mL, nmol/L) | |||||
| First trimester | 51 | 17.48 ± 0.87, 55.87 ± 2.78 | 18.60, 59.15 | 7.41, 23.56 | 35.16, 111.80 |
| Second trimester | 50 | 42.30 ± 2.33, 134.51 ± 7.4 | 43.30, 137.69 | 15.78, 50.18 | 76.84, 244.35 |
| Third trimester | 50 | 70.45 ± 3.32, 224.03 ± 10.56 | 72.90, 231.82 | 34.86, 110.85 | 138.40, 440.11 |
| Postpartum | 50 | 0.86 ± 0.20, 2.73 ± 0.62 | 0.69, 2.19 | <0.1, <0.32 | 22.92, 72.88 |
| 17α-hydroxyprogesterone (ng/mL, nmol/L) | |||||
| First trimester | 51 | 1.34 ± 0.09, 4.02 ± 0.27 | 1.36, 4.08 | 0.48, 1.44 | 3.77, 11.31 |
| Second trimester | 50 | 2.30 ± 0.12, 6.90 ± 0.35 | 2.30, 6.9 | 1.13, 3.39 | 4.67, 14.01 |
| Third trimester | 50 | 3.51 ± 0.18, 10.53 ± 0.55 | 3.50, 10.5 | 1.53, 4.59 | 7.00, 21.00 |
| Postpartum | 50 | 0.40 ± 0.06, 1.19 ± 0.19 | 0.41, 1.23 | <0.1, <0.3 | 4.95, 14.85 |
| Cortisol (ng/mL, nmol/L) | |||||
| First trimester | 51 | 139.84 ± 9.16, 385.95 ± 25.28 | 143.00, 394.68 | 48.28, 133.25 | 385.10, 1065.88 |
| Second trimester | 50 | 286.21 ± 13.94, 789.94 ± 38.47 | 290.50, 801.78 | 156.65, 432.35 | 605.22, 1670.41 |
| Third trimester | 50 | 286.79 ± 13.03, 791.54 ± 35.96 | 305.50, 843.18 | 114.30, 315.47 | 477.37, 1,317.54 |
| Postpartum | 50 | 107.38 ± 6.73, 296.37 ± 18.57 | 97.60, 269.38 | 35.56, 98.15 | 293.05, 808.82 |
| 11-Deoxycortisol (ng/mL, nmol/L) | |||||
| First trimester | 50 | 0.63 ± 0.06, 1.83 ± 0.19 | 0.67, 1.94 | 0.13, 0.38 | 2.45, 7.10 |
| Second trimester | 50 | 1.69 ± 0.11, 4.89 ± 0.31 | 1.67, 4.84 | 0.71, 2.06 | 5.66, 16.41 |
| Third trimester | 50 | 1.94 ± 0.13, 5.63 ± 0.38 | 2.05, 5.94 | 0.70, 2.03 | 4.06, 11.77 |
| Postpartum | 49 | 0.35 ± 0.04, 1.02 ± 0.13 | 0.38, 1.10 | <0.1, <0.29 | 1.46, 4.23 |
| DHEA (ng/mL, nmol/L) | |||||
| First trimester | 51 | 1.93 ± 0.15, 6.71 ± 0.53 | 1.89, 6.56 | 0.57, 1.98 | 6.82, 23.67 |
| Second trimester | 50 | 1.88 ± 0.28, 6.54 ± 0.96 | 1.84, 6.38 | 0.15, 0.52 | 10.18, 35.32 |
| Third trimester | 50 | 2.11 ± 0.29, 7.32 ± 1.01 | 1.70, 5.89 | 0.24, 0.83 | 15.02, 52.12 |
| Postpartum | 50 | 1.92 ± 0.25, 6.66 ± 0.86 | 1.92, 6.66 | <0.1, <0.35 | 5.74, 19.92 |
| Androstenedione (ng/mL, nmol/L) | |||||
| First trimester | 51 | 0.77 ± 0.06, 2.68 ± 0.22 | 0.74, 2.58 | 0.17, 0.59 | 2.72, 9.49 |
| Second trimester | 50 | 0.75 ± 0.07, 2.63 ± 0.23 | 0.75, 2.62 | 0.10, 0.35 | 2.48, 8.65 |
| Third trimester | 50 | 0.76 ± 0.08, 2.65 ± 0.26 | 0.75, 2.62 | 0.08, 0.28 | 2.81, 9.81 |
| Postpartum | 50 | 0.42 ± 0.04, 1.46 ± 0.14 | 0.39, 1.36 | <0.1, <0.35 | 1.27, 4.45 |
| DHEAS (ng/mL, nmol/L) | |||||
| First trimester | 51 | 568.29 ± 63.29, 1.53 ± 0.17 | 598.00, 1.61 | 100.14, 2.70 | 2,501.00, 67.53 |
| Second trimester | 50 | 388.06 ± 43.68, 1.05 ± 0.12 | 413.50, 1.12 | 72.57, 1.96 | 1,443.25, 38.97 |
| Third trimester | 50 | 294.82 ± 35.26, 0.80 ± 0.09 | 313.50, 0.85 | 54.65, 1.47 | 1,092.05, 29.48 |
| Postpartum | 50 | 596.12 ± 62.24, 1.61 ± 0.17 | 647.00, 1.75 | 129.10, 3.48 | 2,898.75, 78.27 |
All values are geometric means ± SE.
Conversion factors from ng/mL to SI units: E2 ng/mL × 3,670 = pg/mL; P4 × 3.18 = nmol/L; 17OHP × 3 = nmol/L; cortisol × 2.76 = nmol/L; cor-11D × 2.9 = nmol/L; DHEA × 3.47 = nmol/L; androstenedione × 3.49 = nmol/L; DHEAS × 0.0027 = nmol/L.
First trimester = week 12; second trimester = week 22; third trimester = week 32.
In general, the variability or scatter tended to increase with the increase in magnitude of the measured analyte. The reference intervals for the rest of the analytes followed individual patterns. For example, for P the relative sizes of the 2.5th to 97.5th percentile interval were similar throughout pregnancy but postpartum the 97.5th percentile was over 220 times the size of the 2.5th percentile.
For DHEAS, however, the relative size of the 2.5th to 97.5th percentile interval was consistent across all time points. DHEA and androstenedione were unique; the relative sizes of the percentiles increased dramatically from the first trimester (where 6.82 is 12 times greater than 0.57 ng/mL) to the second trimester (where 10.18 is 68 times greater than 0.15 ng/mL) and maintained that level through the postpartum observation, whereas for androstenedione the relative interval sizes increased from the first to the second trimester and again from the second to the third trimester and then decreased to below the first trimester values during the postpartum period.
The reference intervals differed across analytes and within analytes across timepoints. Estradiol mean concentrations for women in their first trimester increased from 0.87 ng/mL (3,211 pmol/L) with a range of 0.31–3.00 ng/mL to a mean of 4.24 ng/mL (15,546 pmol/L) and a range of 2.17–13.85 ng/mL in the third trimester. In the postpartum period, the mean was <0.06 ng/mL (<220 pmol/L) and the median value was “undetectable” and ranged from undetectable in the 2.5th percentile to 1.24 ng/mL in the 97.5th percentile.
As described below there is at least a three-fold difference between the 2.5th percentile and 97.5th percentile. For E2 the 97.5th percentile 3.00 ng/mL (1,137.8 pmol/L) during the first trimester is ten times that of the 2.5th percentile 0.31 ng/mL (1,137 pmol/L). This range is double the range size for the second trimester and almost double that of the third trimester but only one tenth of the range for postpartum; 17α-hydroxyprogesterone followed a similar pattern.
Cortisol and 11-deoxycortisol were similar to E2 and 17α-hydroxyprogesterone with regard to the relative ranges provided in the reference interval table. In contrast to cortisol and 11-deoxycortisol, the postpartum range was as wide as the range during the first trimester.
The reference intervals for the rest of the analytes followed individual patterns. For example, for P the range of the 2.5th to 97.5th percentiles was similar throughout pregnancy but postpartum the 97.5th percentile was over 220 times the size of the 2.5th percentile. For DHEAS, however, the range of the 2.5th to 97.5th percentiles was consistent across all time points. DHEA and androstenedione were unique; the range of the percentiles increased dramatically from the first trimester (where 6.82 is 12 times greater than 0.57 ng/mL) to the second trimester (where 10.18 is 68 times greater than 0.15 ng/mL) and maintained that level through the postpartum observation. For androstenedione, the range increased from the first to the second trimester and again from the second to the third trimester and then dropped down to below the first trimester values during the postpartum period.
Table 3 presents the mean ± SEM and medians of the distributions of ratios of women’s measured steroid hormone levels at a given trimester relative to the 1-year postpartum level. The ratios of analytes measured during pregnancy relative to postpartum levels quantify the levels of change in a simple, intuitively accessible manner. On average, the first trimester value of E2 was 10.65 times higher than the 1-year postpartum (baseline) value.
TABLE 3.
Steroid concentrations by LC/MS/MS in pregnancy: mean and median ratios, relative to postpartum of eight steroids by trimester.
| E2 | P | 17α-hydroxy- progesterone |
Cortisol | 11-deoxy- cortisol |
DHEA | Androstenedione | DHEAS | |
|---|---|---|---|---|---|---|---|---|
| Trimester 1 | ||||||||
| n | 50 | 50 | 50 | 50 | 48 | 45 | 50 | 50 |
| Mean ratio | 10.65 | 20.34 | 3.34 | 1.27 | 1.67 | 1.24 | 1.82 | 0.92 |
| SEM | 1.18 | 1.26 | 1.16 | 1.08 | 1.16 | 1.16 | 1.10 | 1.05 |
| Median ratio | 16.04 | 22.60 | 3.50 | 1.44 | 1.47 | 1.10 | 1.70 | 0.89 |
| Trimester 2 | ||||||||
| n | 49 | 49 | 49 | 49 | 48 | 44 | 49 | 49 |
| Mean ratio | 51.61 | 47.99 | 5.85 | 2.66 | 4.80 | 1.06 | 1.79 | 0.65 |
| SEM | 1.19 | 1.26 | 1.16 | 1.07 | 1.14 | 1.26 | 1.13 | 1.05 |
| Median ratio | 70.44 | 62.91 | 6.08 | 2.67 | 4.02 | 0.96 | 1.82 | 0.63 |
| Trimester 3 | ||||||||
| n | 50 | 50 | 50 | 50 | 49 | 45 | 50 | 50 |
| Mean ratio | 76.02 | 82.18 | 8.83 | 2.67 | 5.41 | 1.11 | 1.82 | 0.49 |
| SEM | 1.18 | 1.24 | 1.16 | 1.07 | 1.14 | 1.26 | 1.13 | 1.05 |
| Median ratio | 100.92 | 117.33 | 9.98 | 2.86 | 5.21 | 0.61 | 1.91 | 0.49 |
The levels of E2, P, 11-deoxycortisol, and 17-hydroxyprogesterone increased steadily throughout pregnancy relative to baseline values. Cortisol levels increased from 1.27 times the baseline level at the first trimester to 2.66 times that level at the second trimester, but at the third trimester the level remained elevated at 2.67 times the baseline.
DISCUSSION
We studied the changes in serum concentrations of estradiol, progesterone, and those of other steroid hormones throughout normal pregnancy and relative to one year postpartum using an isotope dilution tandem mass spectrometry. Pregnancy is associated with profound changes in maternal steroidogenesis and with the ability of the fetoplacental unit to synthesize and metabolize steroid hormones. The normal function of the hypothalamic-pituitary-gonadal axis is crucial for the normal progression of pregnancy.
Endogenous estrogens and progestins play a vital role in controlling and maintaining the course of normal pregnancy, thus their measurement provides an index of a normal fetoplacental function (15). The measurement of serum E2 forms an integral part of the assessment of female reproductive function, including studies of infertility, oligoamenorrhea, menopause, and hormone therapy, and is widely used in the monitoring of ovulation induction (16) as well as in assisted reproduction (17). Serum E2 concentrations are also indicated in the study of cardiovascular disorders and Alzheimer’s disease (18).
First trimester steroid profiles are determined by corpus luteum P and E2 biosynthesis and secretion, as well as by the time of onset and extent of P and E2 secretion by the placenta. This dual interplay is dominated by the corpus luteum placental shift, where a relative P and E2 deficiency can develop and may lead to early as well as late abortion. Measurements of serum P and E2 are essential to reveal such deficiencies and for effective treatment (19).
During pregnancy, E2 is synthesized by placental aromatization of maternal and fetal DHEAS (16). Measurements of circulating levels of the major adrenal steroids, cortisol, DHEA, and DHEAS are useful in the evaluation of hypothalamic-pituitary-adrenal function. DHEAS has been shown to increase in puberty (20), although its function is not yet fully understood.
Serum cortisol excess can indicate Cushing’s syndrome, while a deficiency can indicate Addison’s disease. Elevated levels of stress (and of cortisol) during pregnancy have been associated with preterm labor, low infant birth weight and Apgar scores, and increased use of neonatal intensive care unit services (21–23). Epidemiological data indicate an inverse relationship between serum DHEA and DHEAS concentrations and the frequency of cardiovascular diseases, immune function, progression of HIV infection, Alzheimer’s disease, and progression of age-related diseases (25–27). Elevated levels of DHEAS are found in patients with adrenal tumors and congenital adrenal hyperplasia, and slightly elevated levels are found in patients with polycystic ovary syndrome (28, 29). Serum DHEAS has less interindividual variability than serum cortisol (30), and a higher long-term stability. DHEAS may be a more suitable marker of individual function of the adrenal cortex than cortisol (31).
Recently, a number of LC/MS and LC/MS/MS-based methods using different ion sources have been reported for the determination of T (32–34), cortisol (35–40), 11-deoxycortisol (41), androstenedione (33, 34), DHEA (42), DHEAS (33, 42), P (41, 43), 17α-hydroxyprogesterone (44), estriol (45), E2 (33, 45, 46), and estrone (46).
The LC/MS/MS method used in this study, which was developed in our laboratories, is a highly sensitive method for the simultaneous measurement of eight steroids in human serum. LC/MS/MS is currently used as a second-tier congenital adrenal hyperplasia screening test with a positive predictive value of 4.7% (up from 0.5% for 17-hydroxyprogesterone by IA) by simultaneous measurements of cortisol, 17α-hydroxyprogesterone, and androstenedione (40, 47). Previous studies (using IA techniques) have shown a 5.6-fold increase in circulating sex binding globulin concentrations, and a 6.8-fold increase in E2 occurred within the first 16 weeks followed by a further 4.8 increase by term (48), which compares to our finding of an only six-fold increase by week 32.
In summary, endogenous hormone levels during pregnancy have been of interest in studies of fetal and maternal health and disease, but trimester-specific reference intervals for some hormones are difficult to find and others are questionable because of interference due to lack of specificity in IA measurement techniques. Presented here are trimester-specific reference intervals for eight serum steroids as well as the ratios of individual serum hormone concentrations during pregnancy relative to their 1-year postpartum concentrations. While other methods for the measurement of circulating concentrations of steroids using LC/MS/MS have been published (32–34, 37–41, 43–47, 49), none of them use isotope dilution tandem mass spectrometry methods without incorporating lengthy extraction and derivatization steps to provide for the simultaneous quantitation of eight steroids.
Acknowledgments
This study was supported in part by NIH GCRC grant no. 5-MO1-RR-13297-S1 and National Cancer Institute grant 5RO1 CA-89950-03, National Institutes of Health, USA, Sciex, Canada, and by grants from the Swedish Cancer Society.
Footnotes
Presented in part at the Endocrine Society’s 87th Annual Meeting “ENDO” 2005, San Diego, California, June 4–7, 2005.
REFERENCES
- 1.Tulchinsky D, Hobel CH, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- 2.Buster JE, Abraham GE. The applications of steroid hormone radioimmunoassays to clinical obstetrics. Obstet Gynecol. 1975;46:489–499. [PubMed] [Google Scholar]
- 3.Lindberg BS, Johansson EDB, Nilsson BA. Plasma levels of nonconjugated estrone, estradiol, 17β, and estriol during uncomplicated pregnancy. Acta Obstet Gynecol Scand. 1974;32(Suppl):21–36. doi: 10.3109/00016347409156390. [DOI] [PubMed] [Google Scholar]
- 4.Johansson ENB. Plasma levels of progesterone in pregnancy measured by a rapid competitive protein binding technique. Acta Endocrinol. 1969;61:607–617. doi: 10.1530/acta.0.0610607. [DOI] [PubMed] [Google Scholar]
- 5.Yen SSC, Jaffe RB. Reproductive endocrinology: physiology, pathophysiology and clinical management. 3d ed. Philadelphia: W. B. Saunders Company; 1991. [Google Scholar]
- 6.Tulchinsky D, Little AB. Maternal-fetal endocrinology. 2d ed. Philadelphia: W. B. Saunders Company, PA; 1994. [Google Scholar]
- 7.Jaffe RB. Fetoplacental endocrine and metabolic physiology. Clin Perinatol. 1983;10:669–693. [PubMed] [Google Scholar]
- 8.Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med. 1990;341:660–666. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- 9.Berstein LM. Newborn macrosomy and cancer. Adv Cancer Res. 1988;50:231–278. doi: 10.1016/s0065-230x(08)60439-x. [DOI] [PubMed] [Google Scholar]
- 10.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45:942–956. [PubMed] [Google Scholar]
- 11.Rotmensch S, Cole LA. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet. 2000;355:712–715. doi: 10.1016/S0140-6736(00)01324-6. [DOI] [PubMed] [Google Scholar]
- 12.Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124:921–923. doi: 10.5858/2000-124-0921-HAMA. [DOI] [PubMed] [Google Scholar]
- 13.Marks V. False-positive immunoassay results: a multicenter survey of erroneous immunoassay results from assays of 74 analytes in 10 donors from 66 laboratories in seven countries. Clin Chem. 2002;48:2008–2016. [PubMed] [Google Scholar]
- 14.Guo T, Chan YM, Soldin SJ. Steroid profiles using liquid chromatography tandem mass spectrometry with atmospheric pressure photoionization source. Arch Path Lab Med. 2004;128:469–475. doi: 10.5858/2004-128-469-SPULCM. [DOI] [PubMed] [Google Scholar]
- 15.Tulchinsky D, Korenman SG. The plasma estradiol as an index of fetoplacental function. J Clin Invest. 1971;50:1490–1497. doi: 10.1172/JCI106634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valbuena D, Jasper M, Remohi J, Pellicer A, Simon C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 17.Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76:1185–1190. doi: 10.1016/s0015-0282(01)02893-x. [DOI] [PubMed] [Google Scholar]
- 18.Hogervorst E, Williams J, Combrinck M, David Smith A. Measuring serum oestradiol in women with Alzheimer’s disease: the importance of the sensitivity of the assay method. Eur J Endocrinol. 2003;148:67–72. doi: 10.1530/eje.0.1480067. [DOI] [PubMed] [Google Scholar]
- 19.Siiteri PK, MacDonald PC. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966;26:751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- 20.Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone binding globulin (SHBG), dehydroepiandrostendione sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002;40(11):1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- 21.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 22.Austin MP, Leader L. Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Aust N Z J Obstet Gynaecol. 2000;40:331–337. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 23.Urizar GG, Jr, Milazzo M, Le HN, Delucchi K, Sotelo R, Munoz RF. Impact of stress reduction instructions on stress and cortisol levels during pregnancy. Biol Psychol. 2004;67:275–282. doi: 10.1016/j.biopsycho.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Leowattana W. DHEA(S): the fountain of youth. J Med Assoc Thai. 2001;84(Suppl 2):S605–S612. [PubMed] [Google Scholar]
- 25.Celec P, Starka L. Dehydroepiandrosterone—is the fountain of youth drying out? Physiol Res. 2003;52:397–407. [PubMed] [Google Scholar]
- 26.Ceda GP, Dall’Aglio E, Salimbeni I, Rocci A, Mazzoni S, Corradi F, et al. Pituitary function in chronic heart failure in the elderly. J Endocrinol Invest. 2002;25(10 Suppl):24–28. [PubMed] [Google Scholar]
- 27.Carvalhaes-Neto N, Huayllas MK, Ramos LR, Cendoroglo MS, Kater CE. Cortisol, DHEAS and aging: resistance to cortisol suppression in frail institutionalized elderly. J Endocrinol Invest. 2003;26:17–22. doi: 10.1007/BF03345117. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez F, Chang L, Horab T, Lobo RA. Evidence for heterogeneous etiologies of adrenal dysfunction in polycystic ovary syndrome. Fertil Steril. 1996;66:354–361. [PubMed] [Google Scholar]
- 29.Chang PL, Lindheim SR, Lowre C, Ferin M, Gonzalez F, Berglund L, et al. Normal ovulatory women with polycystic ovaries have hyperandrogenic pituitary-ovarian responses to gonadotropin-releasing hormone-agonist testing. J Clin Endocrinol Metab. 2000;85:995–1000. doi: 10.1210/jcem.85.3.6452. [DOI] [PubMed] [Google Scholar]
- 30.Thomas G, Frenoy N, Legrain S, Sebag-Lanoe R, Baulieu EE, Debuire B. Serum dehydroepiandrosterone sulfate levels as an individual marker. J Clin Endocrinol Metab. 1994;79:1273–1276. doi: 10.1210/jcem.79.5.7962319. [DOI] [PubMed] [Google Scholar]
- 31.Leowattana W. DHEAS as a new diagnostic tool. Clin Chim Acta. 2004;341:1–15. doi: 10.1016/j.cccn.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Joos PE, Ryckeghem MV. Liquid chromatography-tandem mass spectrometry of some anabolic steroids. Anal Chem. 1999;71:4701–4710. doi: 10.1021/ac981073s. [DOI] [PubMed] [Google Scholar]
- 33.Dorgan JF, Fears TR, McMahon RP, Aronson Friedman L, Patterson BH, Greenhut SF. Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids. 2002;67:151–158. doi: 10.1016/s0039-128x(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 34.Chang YC, Li CM, Li LA, Jong SB, Liao PC, Chang LW. Quantitative measurement of male steroid hormones using automated on-line solid phase extraction-liquid chromatography-tandem mass spectrometry and comparison with radioimmunoassay. Analyst. 2003;128:363–368. doi: 10.1039/b210111b. [DOI] [PubMed] [Google Scholar]
- 35.Ohno M, Yamaguchi I, Saiki K, Yamamoto I, Azuma J. Specific determination of urinary 6b-hydroxycortisol and cortisol by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Chromatogr B. 2000;746:95–101. doi: 10.1016/s0378-4347(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 36.Tang PW, Law WC, Wan TSM. Analysis of corticosteroids in equine urine by liquid chromatography–mass spectrometry. J Chromatogr B. 2001;754:229–244. doi: 10.1016/s0378-4347(00)00613-7. [DOI] [PubMed] [Google Scholar]
- 37.Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography–tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2002;48:1511–1519. [PubMed] [Google Scholar]
- 38.Jönsson BAG, Malmberg B, Amilon Å, Garde AH, Øraek P. Determination of cortisol in human saliva using liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr B. 2003;784:63–68. doi: 10.1016/s1570-0232(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 39.Kushnir MM, Rockwood AL, Nelson GJ, Terry AH, Meikle AW. Liquid chromatography–tandem mass spectrometry analysis of urinary free cortisol. Clin Chem. 2003;49:965–967. doi: 10.1373/49.6.965. [DOI] [PubMed] [Google Scholar]
- 40.Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem. 2004;50:621–625. doi: 10.1373/clinchem.2003.027193. [DOI] [PubMed] [Google Scholar]
- 41.Kao PC, Machacek DA, Magera MJ, Lacey JM, Rinaldo P. Diagnosis of adrenal cortical dysfunction by liquid chromatography-tandem mass spectrometry. Ann Clin Lab Sci. 2001;31:199–204. [PubMed] [Google Scholar]
- 42.Chatman K, Hollenbeck T, Hagey L, Vallee M, Purdy R, Weiss F, et al. Nanoelectrospray mass spectrometry and precursor ion monitoring for quantitative steroid analysis and attomole sensitivity. Anal Chem. 1999;71:2358–2363. doi: 10.1021/ac9806411. [DOI] [PubMed] [Google Scholar]
- 43.Wu ZP, Zhang C, Yang CD, Zhang XR, Wu E. Simultaneous quantitative determination of norgestrel and progesterone in human serum by high-performance liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization. Analyst. 2000;125:2201–2205. doi: 10.1039/b005631f. [DOI] [PubMed] [Google Scholar]
- 44.Wudy SA, Hartmann M, Svoboda M. Determination of 17-hydroxyprogesterone in plasma by stable isotope dilution/benchtop liquid chromatography-tandem mass spectrometry. Horm Res. 2000;53:68–71. doi: 10.1159/000023516. [DOI] [PubMed] [Google Scholar]
- 45.Isobe T, Shiraishi H, Yasuda M, Shinoda A, Suzuki H, Morita M. Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003;984:195–202. doi: 10.1016/s0021-9673(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 46.Nelson RE, Grebe SK, O’Kane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 47.Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2004;89:3687–3693. doi: 10.1210/jc.2003-032235. [DOI] [PubMed] [Google Scholar]
- 48.O’Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem. 1991;37:667–672. [PubMed] [Google Scholar]
- 49.Thienpont LM, Van Nieuwenhove B, Stockl D, Reinauer H, De Leenheer AP. Determination of reference method values by isotope dilution–gas chromatography/mass spectrometry: a five years’ experience of two European Reference Laboratories. Eur J Clin Chem Clin Biochem. 1996;34:853–860. [PubMed] [Google Scholar]