Abstract
BACKGROUND
Tacrolimus is one of the commonly used immunosuppressive drugs for pediatric heart transplants. Large variation exists in pharmacokinetics during the direct post-transplant period, resulting in an increased risk of adverse events. Limited data are available on the interaction of age, CYP3A5 and ABCB1 genotype, and disease severity on the variation in disposition and outcome in pediatric heart transplant recipients.
METHOD
We studied the relationship between age and CYP3A5 and ABCB1 genotype and the Pediatric Risk of Mortality (PRISM) score on tacrolimus dose (mg/kg), steady-state trough concentrations, and concentration/dose ratio, as well as rejection and renal function for 14 days after heart transplant in children.
RESULTS
Tacrolimus was administered to 39 children (median age, 6.0 years) after transplant. A correlation was found between the age at the time of transplant and the tacrolimus dosing requirements (rs = −0.447, p = 0.004) and the concentration/dose ratio (rs = 0.351, p = 0.029). CYP3A5 expressors required median (interquartile range) higher doses of tacrolimus (0.14 [0.09] vs 0.06 [0.04] mg/kg/12 hours, p = 0.001), and had lower concentration/dose ratios (45.34 [44.54] vs 177.78 [145.38] ng/ml per mg/kg/12 hours, p < 0.0001). This relationship was not seen with the ABCB1 genotype. Age and CYP3A5 genotype predicted the tacrolimus dosing requirements as well as the concentration/dose ratio (R2 = 0.351, p = 0.001 and R2 = 0.521, p < 0.001). No relationship was found between any of the CYP3A5 or ABCB1 genotypes and the estimated glomerular filtration rate.
CONCLUSION
Younger age and CYP3A5 expressor genotype were independently associated with higher dosing requirements and lower tacrolimus concentration/dose ratios.
Keywords: pharmacogenetics, tacrolimus, heart transplantation, children
After its introduction into clinical use in 1997, tacrolimus became one of the most commonly used drugs for immunosuppressive treatment of solid-organ transplant recipients. In heart transplant recipients, it is often preferred to cyclosporin1 for its lower incidence of hypertension, dyslipidemia, and fewer cosmetic adverse effects, such as hirsutism and gingival hypertrophy.1–3 A narrow therapeutic window complicates tacrolimus dosing, however.
The first weeks after transplantation are generally marked by the highest risk for organ rejection. During this period, considerable variability in drug concentrations and pharmacokinetics can contribute to rejection risk with underdosing and drug toxicity (eg, nephrotoxicity, neurotoxicity) with overdosing. Sub-therapeutic tacrolimus concentrations confer a risk for biopsy-proven rejection in adult and pediatric heart transplant recipients.4–6 Furthermore, in 112 adult cardiac transplant recipients, early renal insufficiency, defined as 10% rise in serum creatinine and a serum creatinine above 1.5 mg/dl on Day 3 after transplantation, was associated with tacrolimus levels.7
The pharmacokinetics of tacrolimus have been extensively studied in adults.8,9 However, limited data exist on the sources of large interindividual and intraindividual variability of tacrolimus pharmacokinetics in children. Faster tacrolimus clearance rate in children aged younger than 6 years and higher tacrolimus doses per kilogram of body weight to achieve the target concentrations in this age group have been reported in liver, renal, and hematopoietic stem cell transplant recipients.10–13 The causes for these differences are presently unknown but may be due to age-related changes in CYP3A activity and to the large size of the liver allograft relative to body size in children aged younger than 6 years.14
The relationship between the CYP3A5 genotype and higher tacrolimus clearance has been well established in adult cardiac transplant patients9,15 but only limited data are available in pediatric cardiac transplant recipients. A recent study16 reported a relationship in 65 pediatric patients between CYP3A5 genotype and tacrolimus clearance at 3, 6, and 12 months after transplantation. This study suggested that CYP3A5 expressors (CYP3A5*1/*3) need higher drug doses to maintain the same blood concentration at 3, 6 and 12 months after transplant. However, the effects of mutations in ABCB1 genotypes on tacrolimus disposition in liver and kidney transplant recipients were inconsistent, with reports both supporting17–20 or refuting such an association.17,20,21 The study in pediatric heart transplant recipients demonstrated an association between ABCB1 C3435T and G2677T/A and tacrolimus dosing requirements at 6 and 12 months after transplantation.16
These studies did not evaluate the influence of genotype in relation to other clinical factors, such as age and comorbidity, on dose requirements in the early period after transplantation. A large variation in tacrolimus pharmacokinetics may occur in the early post-transplantation period due to critical illness-related factors, such as mechanical ventilation, altered cardiac output and consequent altered liver and kidney blood flow, body fluid, and plasma protein changes. Hence, we speculated that in patients with a higher severity of illness, as defined by the risk of mortality at intensive care unit admission by the Pediatric Risk of Mortality (PRISM) score, lower tacrolimus requirements would be observed. Our objective was to determine the effects of age, recipient CYP3A5 and ABCB1 genotypes, and PRISM score on tacrolimus disposition in the first 14 days after transplant in pediatric heart allograft recipients. In addition, we wanted to investigate the association between recipient CYP3A5 and ABCB1 genotypes and tacrolimus levels on transplant outcomes such as rejection and renal function.
Patients and methods
This study was approved by the Institutional Research Ethics Board, and informed consent was obtained from parents and/or children during enrollment. Pediatric heart transplant recipients (aged <18 years at the time of transplant) who received oral tacrolimus during the first 14 days after transplant between 1995 and 2008 at the Hospital for Sick Children, Toronto, Ontario, were eligible for study entry. DNA samples were derived from a cohort of patients prospectively enrolled in the Sickkids Heart Centre Biobank.
Immunosuppressive protocol
Induction therapy with anti-thymocyte globulin was started peri-operatively and given up to 2 to 5 days after transplantation in all patients. Tacrolimus was started at Day 2 to 3 after transplantation, with a starting dose of 0.2 mg/kg/day orally, divided twice daily. The treating physician used therapeutic drug monitoring to adjust the tacrolimus dose to a target level of 10 to 12 ng/ml. Additional immunosuppressive therapy consisted of a maintenance dose of mycophenolate mofetil and steroids.
Pharmacokinetic and pharmacodynamics outcome measures
As dependent variables, we collected tacrolimus dose (mg/kg/12 hours) and tacrolimus trough concentrations from patient health records. Dose-corrected tacrolimus concentrations were calculated by dividing the tacrolimus trough concentration by the weight-adjusted dose.
Data were collected on the occurrence of rejection and renal function in our population. Rejection was graded according to the International Society of Heart and Lung Transplantation’s (ISHLT) grading system.22 Rejection was defined as an ISHLT grade 2R or higher.
Creatinine clearance was estimated with the Schwartz formula, using the last available serum creatinine level during the study period.
Covariates
Patient sex, age, weight, the comedication received, CYP3A5 and ABCB1 genotype, and PRISM score were collected as independent variables.
Tacrolimus concentrations
Tacrolimus blood trough concentrations were determined in ethylenediaminetetraacetic acid (EDTA)-treated whole blood (0.25 ml) on the day of sampling, using liquid chromatography tandem mass spectrometry (LC-MS-MS) as previously described, as part of clinical care.23
Genotyping
Blood for genotyping was collected in EDTA-containing tubes, and DNA was extracted using a Magna-Pure LC (Roche Diangostics GmbH, Mannheim, Germany). Polymerase chain reaction (PCR)–restriction fragment length polymorphism for CYP3A5*3 and ABCB1 C3435T, G2677T, and C1236T were performed as described previously.24–26 Patients not carrying the CYP3A5*3 allele were assigned the CYP3A5*1/*1 genotype by default.
Statistical analyses
Data are presented as mean ± standard deviation or median and interquartile range (IQR) when the data were skewed. Differences were compared using the Mann-Whitney test or the Kruskal-Wallis test. The Spearman ρ test was used to test possible correlations. A linear multivariate analysis was performed to test the influence of the predictors on all dependent variables. All data analyses were performed using SPSS 16.0 software (SPSS, Chicago, IL). The Hardy-Weinberg equilibrium was calculated by using the method from Rodriguez et al.27
Results
Patient characteristics
The study comprised 39 eligible pediatric heart transplant recipients (25 boys, 14 girls) who were a median age of 6.0 (IQR, 13.75) years and a median weight of 13.1 (IQR, 25.5) kg. A detailed list of the patients’ demographics can be found in Table 1.
Table 1.
Demographics of the Population
| Variable | Median (IQR) or No. (%) |
|---|---|
| Total patients | 39 |
| Age in years | 6.0 (13.75%) |
| < 1 year | 15/39 (38.5%) |
| Sex | |
| Female | 14 |
| Male | 25 |
| Weight, kg | 13.1 (25.5) |
| Ethnicity | |
| White | 28 |
| African American | 2 |
| Asian | 4 |
| Unknown | 5 |
| Diagnosis | |
| Dilated cardiomyopathy | 22 |
| Congenital heart disease | 15 |
| Unknown | 2 |
| PRISM scorea | 9.50 (10) |
| Need for mechanical ventilation | |
| Pre-transplant | 7/39 (17.9%) |
| Post-transplant | 29/39 (74.4%) |
| Days ventilation required | 2.56 (3.6)b |
| Tacrolimus oral dose c | 0.06 (0.06) |
| Tacrolimus trough levelc | 9.6 (2.08) |
| Concentration/dose ratioc | 150.79 (173.3) |
IQR, interquartile range; PRISM, Pediatric Risk of Mortality.
The PRISM score is based on variables collected during the first 24 hours of intensive care unit admission after transplantation.
p < 0.05 expressors vs non-expressors.
The tacrolimus dose (mg/kg/12 hours), tacrolimus trough level (ng/ml), and concentration/dose ratio (ng/ml per mg/kg/12 hours) are averages of all values obtained during the 14 day post-transplant period.
Tacrolimus disposition
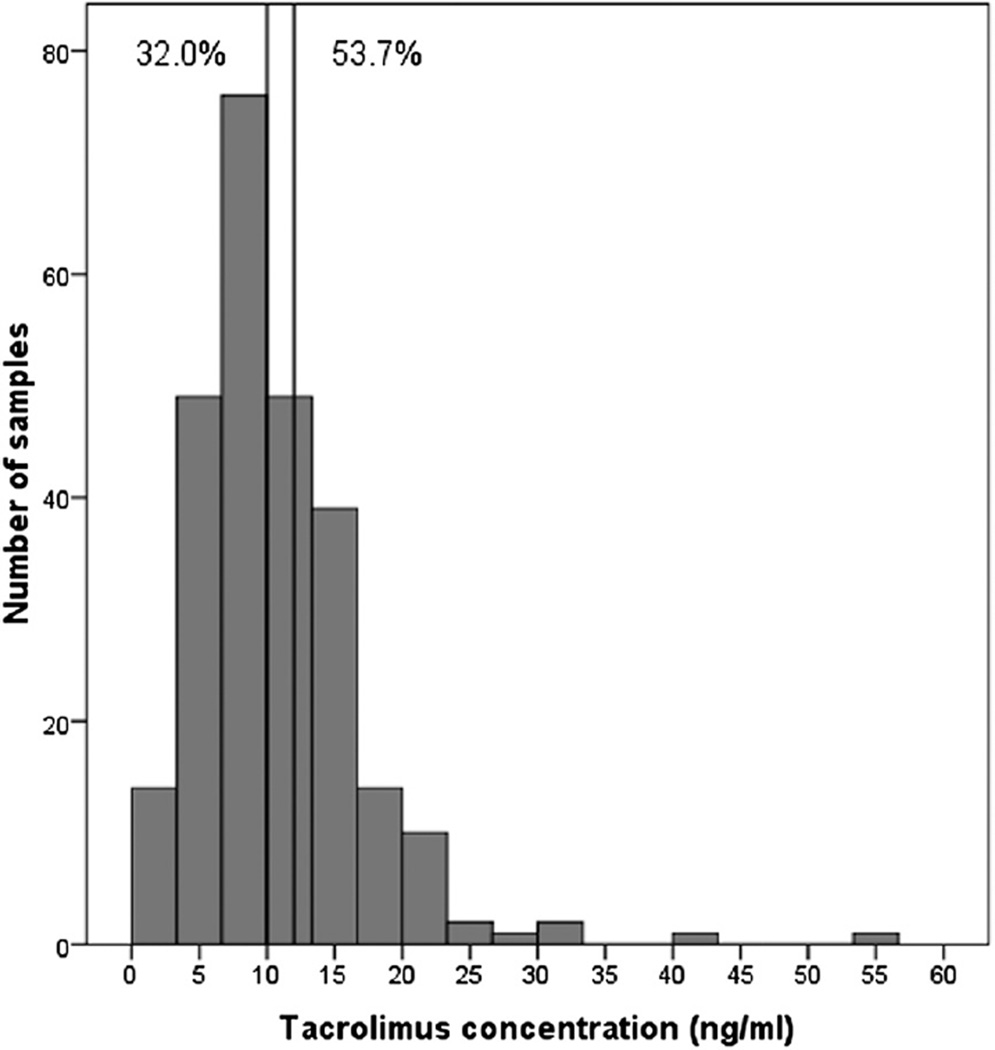
During the 2-week post-transplant period, 258 tacrolimus concentrations were available for analysis. A median of 6 concentration measurements were available for each patient during the study period. The median tacrolimus trough concentration was 9.6 (IQR, 2.08) ng/ml, and the median dose requirement was 0.06 (IQR, 0.06) mg/kg/12 hours. The median concentration/weight-adjusted dose ratio (as a surrogate for estimated clearance) was 150.79 (IQR, 173.3) ng/ml/dose. Of all analyzed concentrations, 32.0% were above the target range and 53.7% were below the target range (Figure 1). On Day 7, 28% were above the target range and 51.3% were below the target range.
Figure 1.
Histogram of tacrolimus concentrations. The vertical lines denote therapeutic range in first 2 weeks after heart transplant. The percentages designate the samples outside of therapeutic window.
Outcome
None of the patients were diagnosed with a grade 2R or higher of rejection in the first 14 days after transplantation. The median estimated glomerular filtration rate (eGFR) at the last available creatinine level was 130.29 (IQR 66.27) ml/min/1.73 m2.
Relationship with genotype
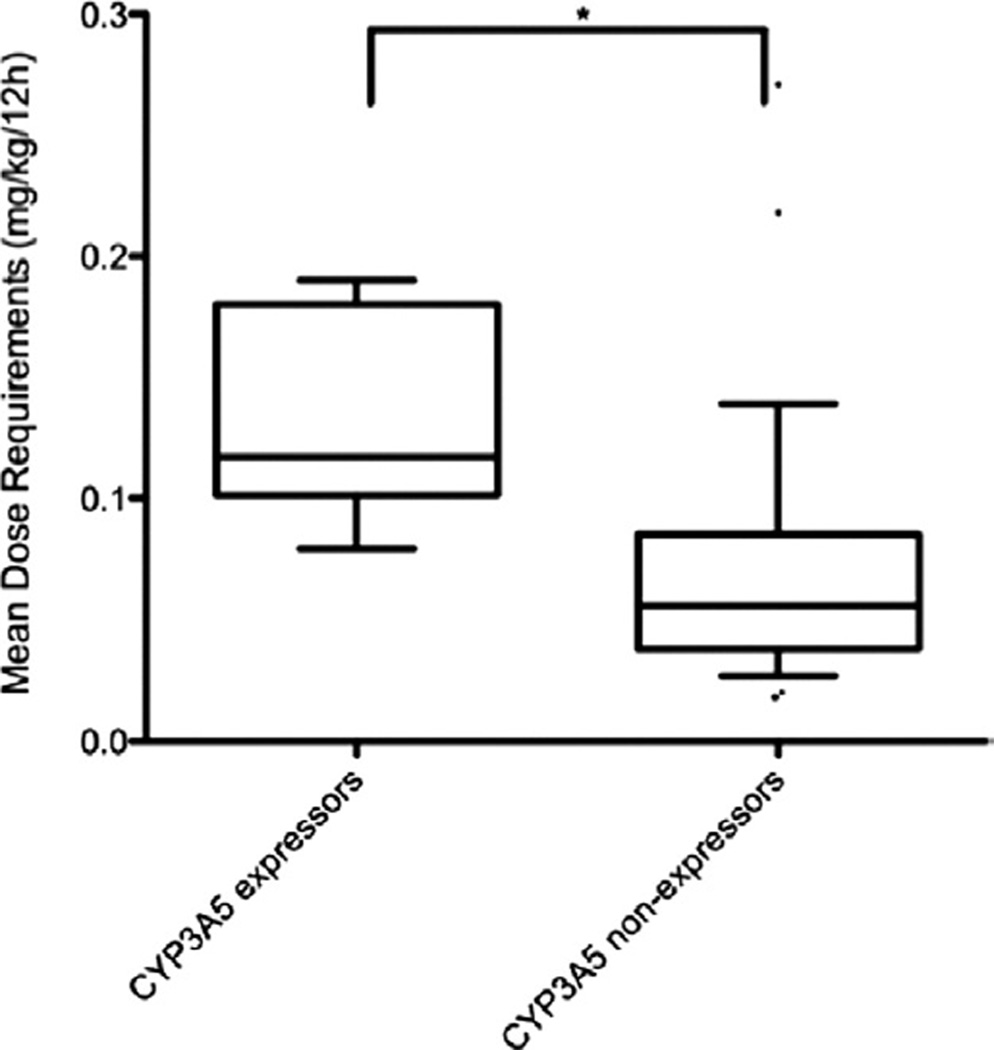
DNA for CYP3A5 genotyping was available for 37 of the 39 patients. Only 1 patient carried the CYP3A5*1/*1 genotype, 7 carried the CYP3A5*1/*3 genotype, and 29 carried the CYP3A5*3/*3 genotype. CYP3A5 genotypes did not deviate from the Hardy-Weinberg equilibrium (chi-square = 0.49, p = 0.5). CYP3A5 expressors (CYP3A5*1/*1 and CYP3A5*1/*3) required significantly higher doses of tacrolimus than the nonexpressors, at 0.14 (IQR, 0.09) vs 0.06 (IQR, 0.04) mg/kg/12 hour (p = 0.001; Figure 2). Expressors also had significantly lower tacrolimus trough concentrations, at 7.7 (IQR, 5.85) vs 9.8 (IQR 3.05) ng/ml (p = 0.032), and lower concentration/ dose ratios of 45.34 (IQR, 44.54) vs 177.78 (IQR, 145.38) ng/ml per mg/kg/12 hours (p < 0.0001; Table 2).
Figure 2.
Box and whisker plots show the relationship between CYP3A5 genotype and tacrolimus dosing requirements. The horizontal line in the middle of each box indicates the median; the top and bottom borders of the box mark the 75th and 25th percentiles, respectively, the whiskers mark the 90th and 10th percentiles, and • designates the outliers. * p < 0.05 expressors vs non-expressors.
Table 2.
Relationship of CYP3A5 Genotype With Tacrolimus Disposition
| Tacrolimus trough levels (ng/ml) |
Tacrolimus dosing requirements (mg/kg/12 hours) |
Concentration/dose ratio (ng/ml per mg/kg/12 hours) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | No. | Median (IQR) | p-value | No. | Median (IQR) | p-value | No. | Median (IQR) | p-value |
| CYP3A5 | 37 | 0.032a | 37 | 0.002a | 37 | <0.0001a | |||
| Expressors | 8 | 7.70 (5.85) | 8 | 0.139 (0.09) | 8 | 45.34 (44.54) | |||
| Non-expressors | 29 | 9.80 (3.05) | 29 | 0.055 (0.04) | 29 | 177.78 (145.38) | |||
IQR, interquartile range.
p < 0.05 expressors vs non-expressors.
DNA for ABCB1 C3435T, ABCB1 G2677T/A, and ABCB1 C1236T genotyping was available for 37 of the 39 patients. The frequencies of each of the genotypes are described in Table 3. None of the ABCB1 genotypes deviated from the Hardy-Weinberg equilibrium. No relationship was found between tacrolimus dosing requirements, tacrolimus trough concentrations, or concentration/dose ratio and ABCB1 3435, 2677, and 1236 genotypes (Table 4).
Table 3.
ABCB1 Genotype Frequencies
| Hardy-Weinberg equilibrium |
|||
|---|---|---|---|
| Genotype | Frequency (%) | Chi-square | p-value |
| ABCB1 | 1.29 | 0.2 | |
| 3435 CC | 12 (32) | ||
| 3435 CT | 15 (41) | ||
| 3435 TT | 10 (27) | ||
| ABCB1 | 0.02 | 0.9 | |
| 2677 GG | 11 (30) | ||
| 2677 GA | 1 (3) | ||
| 2677 GT | 17 (46) | ||
| 2677 TT | 8 (21) | ||
| ABCB1 | 0.29 | 0.5 | |
| 1236 CC | 10 (27) | ||
| 1236 CT | 20 (54) | ||
| 1236 TT | 7 (19) | ||
Table 4.
Relationship of ABCB1 Genotype With Tacrolimus Disposition
| Tacrolimus trough level (ng/ml) |
Tacrolimus dosing requirements (mg/kg/12 hours) |
Concentration/dose ratio (ng/ml per mg/kg/12 hours) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | No. | Median (IQR) | p-value | No. | Median (IQR) | p-value | No. | Median (IQR) | p-value |
| ABCB1 3435 | 37 | 0.466 | 37 | 0.899 | 37 | 0.823 | |||
| CC | 12 | 9.85 (5.07) | 12 | 0.062 (0.11) | 12 | 150.16 (301.16) | |||
| CT | 15 | 9.50 (2.75) | 15 | 0.055 (0.08) | 15 | 150.79 (175.33) | |||
| TT | 10 | 10.03 (1.97) | 10 | 0.073 (0.06) | 10 | 145.10 (156.82) | |||
| ABCB1 2677 | 37 | 0.318 | 37 | 0.963 | 37 | 0.531 | |||
| GG | 11 | 9.80 (5.70) | 11 | 0.067 (0.09) | 11 | 154.55 (331.46) | |||
| GA/GT | 18 | 9.50 (3.68) | 18 | 0.073 (0.08) | 18 | 139.54 (151.79) | |||
| TT | 8 | 10.17 (1.88) | 8 | 0.072 (0.05) | 8 | 174.12 (138.62) | |||
| ABCB1 1236 | 37 | 0.413 | 37 | 0.835 | 37 | 0.776 | |||
| CC | 10 | 10.20 (5.40) | 10 | 0.061 (0.06) | 10 | 179.01 (283.16) | |||
| CT | 20 | 9.50 (2.88) | 20 | 0.074 (0.08) | 20 | 139.54 (178.71) | |||
| TT | 7 | 10.20 (2.30) | 7 | 0.081 (0.04) | 7 | 170.46 (144.19) | |||
IQR, interquartile range.
Relationship with age and PRISM scores
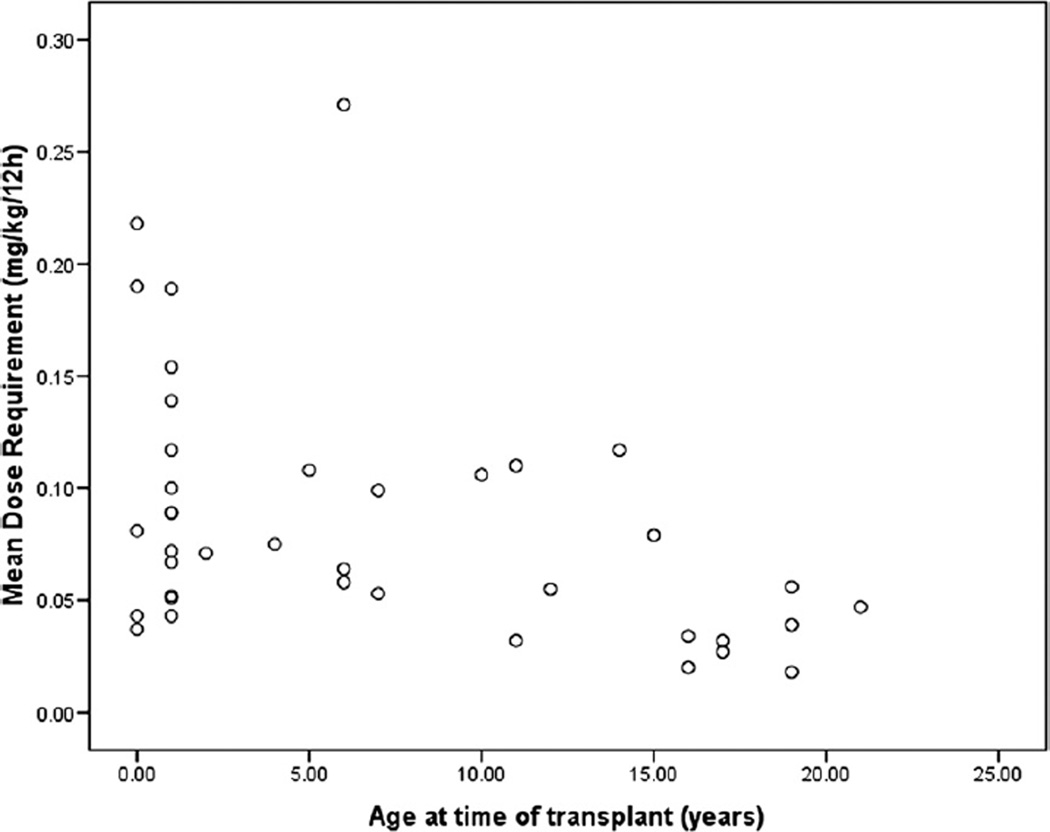
Tacrolimus dosing requirements were higher in younger than older children (rs = −0.447, p = 0.004; Figure 3). Concentration/dose ratios were lower in younger children (rs = 0.351, p = 0.029); however, the tacrolimus trough concentrations were not significantly correlated with age (rs = 0.052, p = 0.752). No significant correlation was found between the PRISM score and tacrolimus dosing requirements (rs= −0.29, p = 0.09), concentration/dose ratios (rs= 0.20, p = 0.25), or tacrolimus trough concentrations (rs= −0.150, p = 0.38).
Figure 3.
The relationship between mean tacrolimus dosing requirements and the patient’s age at the time of transplant
Interplay of age and CYP3A5 genotype
Age and CYP3A5 genotype both appeared to be associated with tacrolimus dosing requirements or the concentration/ dose ratio. The contribution of both parameters was assessed with multivariate linear regression. Age and CYP3A5 genotype were independently associated with the tacrolimus dosing requirements (R2 = 0.351, p = 0.001) and with the concentration/dose ratio (R2 = 0.521, p < 0.001). This was reflected by the observation that in CYP3A5 expressors younger than 6 years, the dosing requirements were more than 1.5 times higher than in CYP3A5 expressors older than 6 years (0.15 [IQR, 0.08] vs 0.09 [IQR, 0.04] mg/kg/12 hours). CYP3A5 non-expressors younger than 6 years also needed 1.5 times higher doses than CYP3A5 non-expressors older than 6 years (0.07 [IQR, 0.18] vs 0.047 [IQR, 0.25] mg/kg/12 hours). In addition, the dosing requirements of CYP3A5 expressors younger than 6 years were 3 times higher than CYP3A5 non-expressors older than 6 years (0.15 [IQR, 0.08] vs 0.04 [IQR, 0.25] mg/kg/12 hours).
When the analysis excluded 1 patient who received fluconazole and 2 patients who received amiodarone, which are CYP3A inhibitors, the results were similar for the relationship between CYP3A5 and ABCB1 genotype and age and tacrolimus disposition.
Relationship between genetic variation, tacrolimus levels, and outcomes
We did not find a relationship between eGFR at the last available creatinine level and median or highest tacrolimus trough level (rs = 0.128, p = 0.439; rs = −0.005, p = 0.975). We also did not find a relationship between eGFR at the last available creatinine level and CYP3A5 genotype for expressors (median eGFR, 125.37 [IQR, 56.77] ml/min/ 1.73 m2) vs non-expressors (130.43 [IQR, 72.65] ml/min/ 1.73 m2, p = 0.941).
Discussion
Our data show that less than 15% of tacrolimus trough concentrations are within the (narrow) target range in the early post-transplant period in pediatric heart transplant recipients. Age and CYP3A5 genotype, independently, both contribute to the variation in the tacrolimus dosing requirements in this cohort.
Limited data exist on pharmacogenetic influences in pediatric transplant recipients:
Zheng et al16 reported similar results at 3, 6, and 12 months after transplantation in 65 pediatric heart transplant recipients. These investigators showed a significant difference in the tacrolimus concentration/dose ratio between CYP3A5 expressors and non-expressors, with the expressors requiring higher doses to maintain the same tacrolimus blood concentration.
A lower tacrolimus oral clearance was reported by Zhao et al28 for pediatric kidney transplant recipients with the CYP3A5*3/*3 genotype compared with those with the CYP3A5*1/*3 genotype less than 2 months after transplant.
Two other studies of pediatric liver transplant recipients found no relationship between recipient CYP3A5 genotype and tacrolimus disposition; in contrast, the liver donor’s CYP3A5 genotype was a significant predictor.18,29
Our study showed a CYP3A5 recipient genotype-tacrolimus disposition relationship in the first 2 weeks after transplantation, arguably one of the most vulnerable periods. We did not find associations between ABCB1 genotype and tacrolimus dosing requirements and disposition. This is consistent with a study in children done by Zheng et al,16 which also failed to find an association at 3 months after pediatric heart transplant. In contrast, at 6 and 12 months after transplant, they found lower concentration/dose ratios in patients with the GG and CC haplotype (ABCB1 G2677T/A and C3435T, respectively). They explained this by higher cytokines concentrations in the early post-transplant period (ie, 3 months) that may have contributed to increased variability in P-glycoprotein expression. Other studies also provide conflicting data about this association, with more studies showing positive associations late after transplant rather than the early period.17,29
The effect of age on tacrolimus pharmacokinetics has been reported.30 Studies of pediatric renal31 and liver12,13,32 transplant recipients have shown that pre-pubertal children need 2 to 3 times higher doses than adults. In pediatric bone marrow transplantation, a higher clearance rate, compared with adults, was reported.33 Within the pediatric population, age-related differences between younger and older children in tacrolimus pharmacokinetics have been reported as well. Przepiorka et al10 documented a decreased tacrolimus clearance in the first 2 weeks after hematopoietic stem cell transplantation only for children aged older than 12 years. In addition, at steady state, the clearance rate was higher for those younger than 6 years than in older children. In pediatric renal transplant recipients, Kim et al11 showed that the younger children (< 5 years and 5–12 years) required 2.7 and 1.9 times higher dosages, respectively, than older children (>12 years), and that a significant inverse correlation between dose/kg and age among all age groups was present. Naesens et al34 reported that younger pediatric renal transplant recipients needed significantly higher doses to achieve comparable tacrolimus trough concentrations compared with older children. Our results show similar findings, with higher dose requirements and lower concentration/dose ratios (as surrogate marker for clearance) in younger pediatric heart transplant recipients.
Ontogeny in tacrolimus biotransformation may explain these findings. The hepatic metabolism of drugs is altered in younger children, with different ages for the different cytochromes to reach maturity, resulting in different metabolism rates14 and consequent clearance rates. For many CYP3A4/5 substrates, it is widely established that clearance is increased in the age group between 6 months and 3 years. This has been attributed to higher CYP3A4/5 activity compared with adults, but others have suggested this is due to a larger liver/body size ratio in children than in adults.14,35
No patient experienced rejection within the 14 days after transplantation. Therefore, we were unable to test a relationship of genetic variation with outcomes. However, the potential influence of the pharmacokinetic variability in the first 14 days after transplantation on the long-term rejection risk still needs to be studied. In addition, we could not establish a relationship between genetic variability, tacrolimus levels, and renal function. Possible reasons could be the relatively small sample size as well as the limited 14-day interval. As an increase in serum creatinine is only apparent with a marked decrease in renal function, the 14-day interval may not have been big enough to see an effect of the high tacrolimus levels on causing a rise in serum creatinine. Factors other than tacrolimus-induced nephrotoxicity, such as comedication and altered hemodynamics, may affect renal function directly after transplant.
Importantly, our study shows that CYP3A5 genotype and age are independently associated with tacrolimus disposition. As major changes occur in drug disposition during development, the effect of genetic variation in drug disposition should be studied in the context of this age-related variation. We observed that all CYP3A5 expressors, independent of age, had higher tacrolimus dosing requirements than non-expressors. Taking age into account further amplified the genotype effect, with younger CYP3A5 expressors needing, on average, tacrolimus doses that were 3 times higher than those needed by older CYP3A5 non-expressors.
One limitation of our study is the relatively small sample size, which affected the amount of confounders we could look at. In addition, the drug dosing data in our study were retrospectively collected, and this may have introduced unknown variation in the noted vs the actually administered dose, such as inaccuracies with drug dispensing and vomiting with repeated dosing. Although we only studied oral doses of tacrolimus, most of the children do receive tacrolimus orally. Some children, however, cannot tolerate oral tacrolimus and receive intravenous tacrolimus. These children need to be studied separately because differences exist between oral doses and intravenous doses. The systemic exposure is different with intravenous tacrolimus because the first-pass metabolism (eg, metabolism by CYP3A5 and ABCB1 transport before absorption) is bypassed, although liver metabolism would still occur.
In conclusion, we showed that in the first 14 days after heart transplantation, younger age and CYP3A5 expressor status were independently associated with higher tacrolimus dosing requirements and concentration/dose ratio (as surrogate marker for clearance). Drug dosing algorithms need to be developed to guide initial dosing that is individualized based on age and genotype, with the goal of optimizing the ability to safely and rapidly achieve therapeutic targets.
Acknowledgments
The authors thank the Sickkids Labatt Family Heart Centre Biobank Registry for access to DNA samples from study subjects and Dr Ilan Matok for his help with the statistical analysis.
This research was supported by grants from the Ter Meulen Fund of the Royal Dutch Academy of Sciences (S.N.W.), the Hospital for Sick Children Research Institute (S.N.W., I.N.) and ZonMW Clinical Fellowship (S.N.W.).
Footnotes
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Penninga L, Møller CH, Gustafsson F, Steinbrüchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66:1177–1187. doi: 10.1007/s00228-010-0902-6. [DOI] [PubMed] [Google Scholar]
- 2.Asante-Korang A, Boyle GJ, Webber SA, Miller SA, Fricker FJ. Experience of FK506 immune suppression in pediatric heart transplantation: a study of long-term adverse effects. J Heart Lung Transplant. 1996;15:415–422. [PubMed] [Google Scholar]
- 3.Crespo-Leiro MG. Tacrolimus in heart transplantation. Transplant Proc. 2003;35:1981–1983. doi: 10.1016/s0041-1345(03)00566-9. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BV, Boyle GJ, Miller SA, et al. Optimal dosing of intravenous tacrolimus following pediatric heart transplantation. J Heart Lung Transplant. 1999;18:786–791. doi: 10.1016/s1053-2498(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 5.Albornoz López R, Aumente Rubio MD, Arizón Del Prado JM, et al. Tacrolimus blood levels and incidence of graft rejection in heart transplantation. Farm Hosp. 2005;29:158–163. doi: 10.1016/s1130-6343(05)73657-6. [DOI] [PubMed] [Google Scholar]
- 6.Aidong W, Zhenjie C, Tong L, et al. Therapeutic drug monitoring of tacrolimus in early stage after heart transplantation. Transplant Proc. 2004;36:2388–2389. doi: 10.1016/j.transproceed.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Baran DA, Galin I, Sandler D, et al. Tacrolimus in cardiac transplantation: efficacy and safety of a novel dosing protocol. Transplantation. 2002;74:1136–1141. doi: 10.1097/00007890-200210270-00014. [DOI] [PubMed] [Google Scholar]
- 8.Barry A, Levine M. A systematic review of the effect of CYP3A5 genotype on the apparent oral clearance of tacrolimus in renal transplant recipients. Ther Drug Monit. 2010;32:708–714. doi: 10.1097/FTD.0b013e3181f3c063. [DOI] [PubMed] [Google Scholar]
- 9.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49:141–175. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Przepiorka D, Blamble D, Hilsenbeck S, et al. Tacrolimus clearance is age-dependent within the pediatric population. Bone Marrow Transplant. 2000;26:601–605. doi: 10.1038/sj.bmt.1702588. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti Vehaskari V. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9:162–169. doi: 10.1111/j.1399-3046.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 12.MacFarlane GD, Venkataramanan R, McDiarmid SV, et al. Therapeutic drug monitoring of tacrolimus in pediatric liver transplant patients. Pediatr Transplant. 2001;5:119–124. doi: 10.1046/j.1397-3142.2000.00000.x. [DOI] [PubMed] [Google Scholar]
- 13.Jain AB, Fung JJ, Tzakis AG, et al. Comparative study of cyclosporine and FK 506 dosage requirements in adult and pediatric orthotopic liver transplant patients. Transplant Proc. 1991;23:2763–2766. [PMC free article] [PubMed] [Google Scholar]
- 14.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 15.Kniepeiss D, Renner W, Trummer O, et al. The role of CYP3A5 genotypes in dose requirements of tacrolimus and everolimus after heart transplantation. Clin Transplant. 2011;25:146–150. doi: 10.1111/j.1399-0012.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H, Webber S, Zeevi A, et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3:477–483. doi: 10.1034/j.1600-6143.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 17.Hawwa AF, McElnay JC. Impact of ATP-binding cassette, subfamily B, member 1 pharmacogenetics on tacrolimus-associated nephrotoxicity and dosage requirements in paediatric patients with liver transplant. Expert Opin Drug Saf. 2011;10:9–22. doi: 10.1517/14740338.2010.505600. [DOI] [PubMed] [Google Scholar]
- 18.Goto M, Masuda S, Kiuchi T, et al. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics. 2004;14:471–478. doi: 10.1097/01.fpc.0000114747.08559.49. [DOI] [PubMed] [Google Scholar]
- 19.Singh R, Srivastava A, Kapoor R, K Sharma R, D Mittal R. Impact of CYP3A5 and CYP3A4 gene polymorphisms on dose requirement of calcineurin inhibitors, cyclosporine and tacrolimus, in renal allograft recipients of North India. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:169–177. doi: 10.1007/s00210-009-0415-y. [DOI] [PubMed] [Google Scholar]
- 20.Haufroid V, Mourad M, Van Kerckhove V, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–154. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Elens L, Capron A, Kerckhove VV, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17:873–883. doi: 10.1097/FPC.0b013e3282e9a533. [DOI] [PubMed] [Google Scholar]
- 22.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Volosov A, Napoli KL, Soldin SJ. Simultaneous simple and fast quantification of three major immunosuppressants by liquid chromatography—tandem mass-spectrometry. Clin Biochem. 2001;34:285–290. doi: 10.1016/s0009-9120(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 24.van Schaik RHN, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48:1668–1671. [PubMed] [Google Scholar]
- 25.Hesselink DA, van Schaik RHN, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol. Ther. 2003;74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 26.Aarnoudse ALHJ, van Schaik RHN, Dieleman J, et al. MDR1 gene polymorphisms are associated with neuropsychiatric adverse effects of mefloquine. Clin Pharmacol Ther. 2006;80:367–374. doi: 10.1016/j.clpt.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez S, Gaunt TR, Day INM. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169:505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao W, Elie V, Roussey G, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86:609–618. doi: 10.1038/clpt.2009.210. [DOI] [PubMed] [Google Scholar]
- 29.Fukudo M, Yano I, Masuda S, et al. Population pharmacokinetic and pharmacogenomic analysis of tacrolimus in pediatric living-donor liver transplant recipients. Clin Pharmacol Ther. 2006;80:331–345. doi: 10.1016/j.clpt.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 31.Shishido S, Asanuma H, Tajima E, Honda M, Nakai H. Pharmacokinetics of tacrolimus in pediatric renal transplant recipients. Transplant Proc. 2001;33:1066–1068. doi: 10.1016/s0041-1345(00)02418-0. [DOI] [PubMed] [Google Scholar]
- 32.McDiarmid SV, Colonna JO, Shaked A, et al. Differences in oral FK506 dose requirements between adult and pediatric liver transplant patients. Transplantation. 1993;55:1328–1332. doi: 10.1097/00007890-199306000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Mehta P, Beltz S, Kedar A, Graham-Pole J, Wingard JR. Increased clearance of tacrolimus in children: need for higher doses and earlier initiation prior to bone marrow transplantation. Bone Marrow Transplant. 1999;24:1323–1327. doi: 10.1038/sj.bmt.1702053. [DOI] [PubMed] [Google Scholar]
- 34.Naesens M, Salvatierra O, Li L, et al. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85:1139–1345. doi: 10.1097/TP.0b013e31816b431a. [DOI] [PubMed] [Google Scholar]
- 35.Blake MJ, Castro L, Leeder JS, Kearns GL. Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med. 2005;10:123–138. doi: 10.1016/j.siny.2004.11.001. [DOI] [PubMed] [Google Scholar]