Abstract
Objectives
To define the incidence of and explore risk factors for seizures and epilepsy in children with spontaneous intracerebral hemorrhage (ICH).
Design
Prospective cohort study.
Setting
Three tertiary care pediatric hospitals.
Participants
Seventy-three pediatric subjects with spontaneous ICH including 20 perinatal (≥37 weeks gestation to 28 days) and 53 childhood subjects (>28 days to <18 years at presentation).
Main outcome measures
Acute symptomatic seizures (clinically evident and electrographic-only within 7 days), remote symptomatic seizures, and epilepsy.
Results
Acute symptomatic seizures occurred in 35 subjects (48%). Acute symptomatic seizures as a presenting symptom of ICH occurred in 12 (60%) perinatal and 19 (36%) childhood subjects, P=.07. Acute symptomatic seizures after presentation occurred in 7 children. Electrographic-only seizures were present in 9/32 (28%) with continuous EEG monitoring. One-and two-year remote symptomatic seizure-free survival were 82% (95% CI 68%–90%) and 67% (95% CI 46%–82%), respectively. One- and two-year epilepsy-free survival were 96% (95% CI 83%–99%) and 87% (95% CI 65%–95%), respectively. Elevated intracranial pressure requiring acute intervention was a risk factor for acute seizures after presentation, remote symptomatic seizures, and epilepsy (P=.014, P=.025 and P=.0365, respectively log-rank test).
Conclusions
Presenting seizures are common in perinatal and childhood ICH. Continuous EEG may detect electrographic seizures in some subjects. Single remote symptomatic seizures occur in many, and development of epilepsy is estimated to occur in 13% at two-years. Elevated intracranial pressure requiring acute intervention is a risk factor for acute seizures after presentation, remote symptomatic seizures, and epilepsy.
Introduction
Seizures are believed to be a common presenting symptom in neonates and children with spontaneous intracerebral hemorrhage (ICH). However, few data are available regarding the epidemiology of acute symptomatic seizures or risk for later epilepsy. Estimates of seizures at presentation or in the “early” or “acute” period range from 18%–50%, but definitions of acute symptomatic seizures or early symptomatic seizures vary.1–9 Studies have reported epilepsy or recurrent seizures in 10% to 25% at follow-up, but duration of follow-up in these studies was not clearly specified.4, 8, 10 We aimed to define the incidence of acute symptomatic seizures from a large prospective pediatric ICH cohort, both as a presenting symptom and after presentation (within seven days).11 We also aimed to define the incidence of remote symptomatic seizures and epilepsy. Potential risk factors for acute symptomatic seizures, remote symptomatic seizures, and epilepsy were explored.
Methods
Study design and subjects
This is a prospective cohort study of perinatal (full-term newborns ≥37 weeks gestation to ≤28 days) and childhood subjects (>28 days of life to 18 years) presenting between 2007 and 2012 with spontaneous ICH at three tertiary care institutions at which the authors are affiliated. Consent was obtained from subjects’ parents and assent from children ≥7 years. The institutional review boards of all three institutions approved the study. Ascertainment was thought to be near-complete since the institutions have clinical protocols for ICH management that include stroke service consultation.
Definitions
Spontaneous ICH was defined as intraparenchymal hemorrhage (IPH) and/or intraventricular hemorrhage (IVH) not caused by trauma, brain tumor, hemorrhagic transformation of arterial ischemic stroke or cerebral sinus venous thrombosis. Isolated subarachnoid hemorrhages were excluded. All ICHs were confirmed on head computed tomography (HCT) or magnetic resonance imaging (MRI). Seizures were classified by occurrence time. Acute symptomatic seizures were defined as those occurring from presentation to seven days after the incident ICH.11 Presenting seizures described those occurring as the first symptom or along with other symptoms immediately at presentation. Acute symptomatic seizures after presentation were those occurring after presenting seizure(s) were controlled or after the subject had come to medical attention but still within 7 days of ICH. Status epilepticus was defined as a continuous seizure lasting >30 minutes or recurrent seizures totaling >30 minutes in any 1-hour period.12 Remote symptomatic seizures occurred >7 days from the incident ICH. Epilepsy was defined as two or more unprovoked remote symptomatic seizures more than 24 hours apart.13 Consistent with prior studies, an electrographic seizure was defined as an abnormal paroxysmal event different from the background and lasting longer than 10 seconds (or shorter if associated with clinical change), with a temporal-spatial evolution in morphology, frequency, and amplitude, and with an electrographic field.12 Clinically evident seizures and electrographic-only seizures qualified as seizures for all analyses. Elevated intracranial pressure (ICP) requiring urgent intervention was defined as the need for mannitol, 3% normal saline, drainage of cerebrospinal fluid via intraventricular catheter, urgent hematoma evacuation, or decompressive hemicraniectomy.
Clinical and radiographic data
Data were acquired both prospectively and from abstraction of hospital medical records and stroke clinic follow-up records. These data included seizure occurrence, timing of seizure relative to incident ICH, seizure semiology, and anticonvulsant administration. Radiographic information including IPH, IVH, or both, and cortical involvement of the ICH were recorded. At each follow-up stroke clinic visit, subjects were assessed for seizure occurrence. In those with seizures, seizure date, semiology, and antiseizure medication use were recorded. For this study, all emergency room visits, hospital admissions and telephone encounters after the incident ICH were reviewed to ensure complete ascertainment of seizures.
Statistical analysis
STATA version 11.1 (Stata Corporation, College Station, TX) was used for all analyses. Fisher’s exact tests were used to analyze predictors of acute seizures for categorical variables. The Wilcoxon rank-sum test was utilized to determine the difference in age of those presenting with and without seizures in the childhood subjects. The Kaplan-Meier estimate of survival was calculated to determine the remote symptomatic seizure-free survival and epilepsy-free survival of subjects who lived. Survival time was calculated from date of incident ICH. The log-rank test was used to explore whether a difference in survival functions existed between subjects with various putative risk factors for development of remote symptomatic seizures and epilepsy. A two-sided probability value of ≤.05 was considered statistically significant. Bonferroni correction for multiple comparisons was made for a priori analyses. Our a priori hypotheses were that young age at presentation, cortical involvement of ICH, acute symptomatic seizures after presentation, ICH due to vascular malformation, and elevated ICP requiring urgent intervention would predict remote symptomatic seizures and epilepsy.
Results
Population
During the study period, consent was obtained from 73 of 87 eligible subjects (84%). ICH occurred in 20 perinatal and 53 childhood subjects. For children, the median age was 9 years [interquartile range (IQR) 2–14 years]. Racial distribution was 49 white (3 Hispanic) and 24 black subjects. No subject had a history of unprovoked seizures or epilepsy, but 1 childhood subject had a history of a single febrile seizure. ICH locations and etiologies are in Table 1.
Table 1.
Intracerebral Hemorrhage Locations and Etiologies
| Perinatal (n=20) | Childhood (n=53) | |
|---|---|---|
| Location | ||
| Isolated IPH | 3 (15%) | 29 (55%) |
| IPH with IVH extension | 14 (70%) | 18 (34%) |
| Isolated IVH | 3 (15%) | 6 (11%) |
| Etiology | ||
| Aneurysm | 0 | 5a (9%) |
| Arteriovenous malformation | 1 (5%) | 20 (37%) |
| Cavernous malformation | 2 (10%) | 7 (13%) |
| Developmental venous anomaly | 0 | 1 (2%) |
| Moyamoya | 0 | 1 (2%) |
| Coagulopathy | 5 (25%) | 6 (11%) |
| Anticoagulation | 0 | 5 (9%) |
| Unknown | 12 (60%) | 9 (17%) |
Abbreviations: IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage.
, One child with an aneurysm also had coagulopathy.
Acute symptomatic seizures
Seizures at presentation
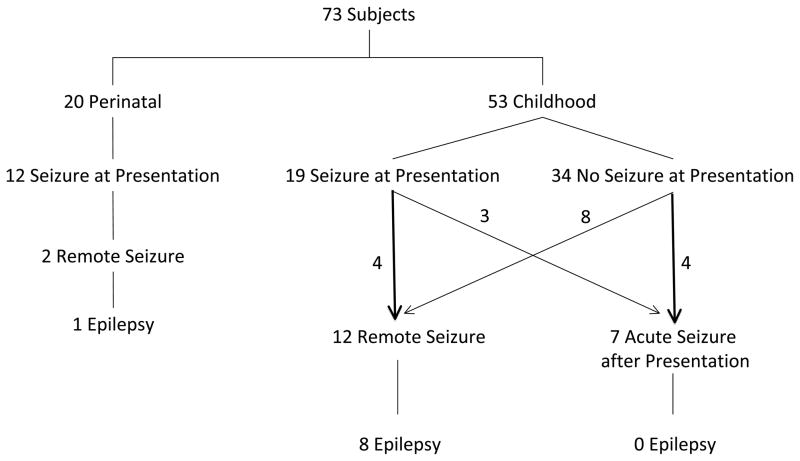
Seizures as a presenting symptom occurred in 31 subjects (42%) (Figure 1). Twelve perinatal (60%; 95% binomial CI 36%–81%) and 19 childhood subjects (36%; 95% binomial CI 23%–50%) presented with seizures (P=.07, Fisher’s exact). For children, the median age of those who presented with seizure was lower than that for those who did not present with seizure (2 years, IQR .4–9 years versus 10.8 years, IQR 6.4–15.2 years, P=.0018, Wilcoxon rank-sum). Seizure semiology was focal in 10 perinatal and 14 childhood subjects. Five children (9%) and 10 perinatal subjects (50%) presented with status epilepticus. Univariable analyses for predictors of seizures at presentation and seizure semiology are in Table 2.
Figure 1.
Seizures in the Cohort
Table 2.
Risk Factors for Acute Symptomatic Seizures at Presentation
| Risk Factor | # with Seizure at Presentation/# with Risk Factor | # with Seizure at Presentation/# without Risk Factor | P Valuea |
|---|---|---|---|
| Perinatal ICH | 12/20 (60%) | 19/53 (36%) | .07 |
| Cortical location | 15/35 (43%) | 16/38 (42%) | 1.00 |
| Vascular malformation | 13/36 (36%) | 18/37 (49%) | .35 |
Abbreviation: #, number.
, Fisher’s exact P value.
Acute symptomatic seizures after presentation
Seven childhood subjects (13%) had acute seizures after presentation but within 7 days of ICH (median 2 days, range 1–5 days). Seizure semiology was focal in 6 children. Three of these 7 also presented with seizures and 4 were on antiseizure medications at time of seizure. Three acute seizures after presentation were electrographic-only and were identified on cEEG. Univariable predictors of acute seizures after presentation are in Table 3. Only elevated ICP requiring acute intervention was associated with acute seizures after presentation. Six subjects (8%) (3 perinatal, 3 childhood) died during the acute hospitalization. One perinatal and one childhood subject who died had acute symptomatic seizures.
Table 3.
Risk Factors for Acute Symptomatic Seizures after Presentation to 7 Days
| Risk Factor | # with Acute Seizure after Presentation/# with Risk Factor | # with Acute Seizure after Presentation/# without Risk Factor | P Valuea |
|---|---|---|---|
| Perinatal ICH | 0/20 (0%) | 7/53 (13%) | .20 |
| Seizure at presentation | 3/31 (10%) | 4/42 (10%) | .64 |
| Lack of AED | 3/34 (9%) | 4/39 (10%) | 1.00 |
| Elevated ICP requiring urgent intervention | 6/28 (21%) | 1/44 (2%) | .014b |
Abbreviations: #, number; AED, antiseizure medication; ICP, intracranial pressure.
, Fisher’s exact P value.
, Statistically significant.
EEG
EEGs were performed at the discretion of the treating neurologist in 15 (75%) perinatal and 31 (58%) childhood subjects (Table 4). An EEG was performed in 30 of 35 subjects with acute symptomatic seizures and in 16 of 38 subjects without acute symptomatic seizures. Use of cEEG monitoring was more frequent in those with perinatal versus childhood ICH (13/20 versus 19/53, P=.035, Fisher’s exact) and in those with acute symptomatic seizures versus those without acute symptomatic seizure (22/35 versus 10/38, P=.002, Fisher’s exact).
Table 4.
EEG Results from Hospitalization
| Perinatal (n=20) | Childhood (n=53) | |
|---|---|---|
|
| ||
| EEG performed during acute hospitalization | 15 (75%) | 31 (58%) |
| # routine EEGs performeda | 5 (in 5 subjects) | 21 (in 18 subjects) |
| # continuous EEGs performeda | 14 (in 13 subjects) | 21 (in 19 subjects) |
|
| ||
| Epileptiform dischargesb | 9 (45%) | 14 (26%) |
|
| ||
| Electrographic-clinical seizures | 2 (10%)c | 1 (2%) |
|
| ||
| Electrographic-only seizures | 5 (25%)c | 4 (8%)d |
Abbreviation: #, number.
, Some subjects had both routine and continuous EEGs.
, Epileptiform discharges on EEG were defined as focal or generalized sharp or spike waves.
, One subject had both electrographic-clinical and electrographic-only seizures.
, One had clinical status epilepticus at presentation that was still evident on continuous EEG as electrographic-only seizures.
Five of 13 (38%) perinatal subjects who had cEEG monitoring and 4 of 19 (21%) childhood subjects who had cEEG monitoring had electrographic-only seizures. All 5 perinatal subjects and 3 of 4 childhood subjects with electrographic-only seizures had seizures at presentation of ICH and were on antiseizure medication at the time of the electrographic-only seizures. Of the childhood subjects, elevated ICP requiring acute intervention predicted use of cEEG (13/26 versus 6/27, P=.047, Fisher’s exact). Three of 4 childhood subjects with electrographic-only seizures had elevated ICP requiring urgent intervention.
Antiseizure medications
Antiseizure medication use was based on clinical practices and is described in the online supplement. Only four subjects who did not have acute symptomatic seizures were treated with and discharged on prophylactic antiseizure medications. All four had vascular malformations (2 arteriovenous malformations, 1 aneurysm, 1 developmental venous anomaly). Two of these malformations were treated (aneurysm clipped, arteriovenous malformation coiled), but the other two were untreated at time of discharge. Eight perinatal (40%) and 23 childhood subjects (43%) were never started on maintenance antiseizure medication. All 10 perinatal subjects discharged on antiseizure medication were discharged on a single agent, while 4 of 26 childhood subjects (15%) discharged on antiseizure medications were treated with dual therapy. Nine perinatal and 12 childhood subjects, 58% of all subjects discharged on antiseizure medication, were weaned off all antiseizure medications in the follow-up period. The median length of treatment in the cohort in those who were weaned in the follow-up period was 84 days (IQR, 63–130 days). One perinatal and 4 childhood subjects not discharged on antiseizure medications were started on them during the follow-up period when epilepsy developed. One perinatal and 14 childhood subjects discharged on antiseizure medications, 42% of those discharged on antiseizure medications, were still taking medication at time of last follow-up (median 885 days, IQR 221–1247 days).
First Remote Symptomatic Seizure and Epilepsy
Follow-up time was not different between perinatal and childhood subjects. The median number of days to last follow-up for perinatal subjects was 382 (IQR 233–987 days) and for childhood subjects was 345 (IQR 119–596 days), P=.23, Wilcoxon rank-sum. Fourteen of 67 surviving subjects had an unprovoked remote symptomatic seizure after seven days from the incident ICH with a one-year and two-year seizure-free survival of 82% (95% CI 68%–90%) and 67% (95% CI 46%–82%), respectively (Figure 2a). Of the 14 children who had a first unprovoked remote symptomatic seizure, 2 presented with ICH in the perinatal period and 12 in childhood. Nine were still taking antiseizure medications at the time of first remote symptomatic seizure, and 5 with remote symptomatic seizures had not been discharged on antiseizure medications. Univariable analyses for risk factors for a first unprovoked remote symptomatic seizure are in Table 5. Only elevated ICP requiring urgent intervention during the acute hospitalization (P=.025, log-rank) predicted a first unprovoked remote symptomatic seizure. Nine of 67 surviving subjects developed epilepsy with a one-year and two-year epilepsy-free survival of 96% (95% CI 83%–99%) and 87% (95% CI 65%–95%), respectively (Figure 2b). Without Bonferroni correction, both elevated ICP requiring urgent intervention during the acute hospitalization (P=.0073, log-rank) and ICH in childhood (P=.0357, log-rank) predicted epilepsy. However, after Bonferroni correction, only elevated ICP requiring urgent intervention predicted epilepsy (P=.0365, log-rank). Of the nine children with epilepsy, 5 remained on antiseizure medication after discharge. Eight had a total of ≤5 seizures, but one child developed medically refractory epilepsy with daily seizures. This child’s epilepsy was refractory to five antiseizure medications and was eventually controlled with the ketogenic diet.
Figure 2.
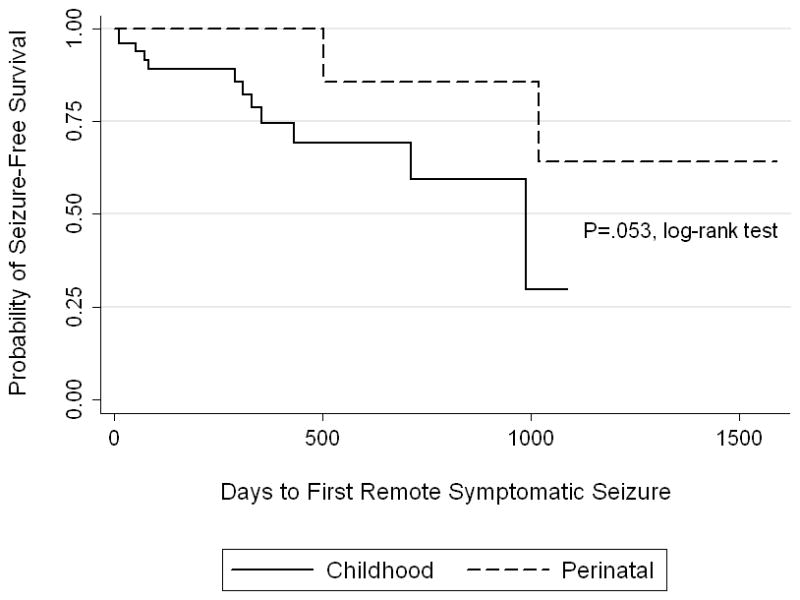
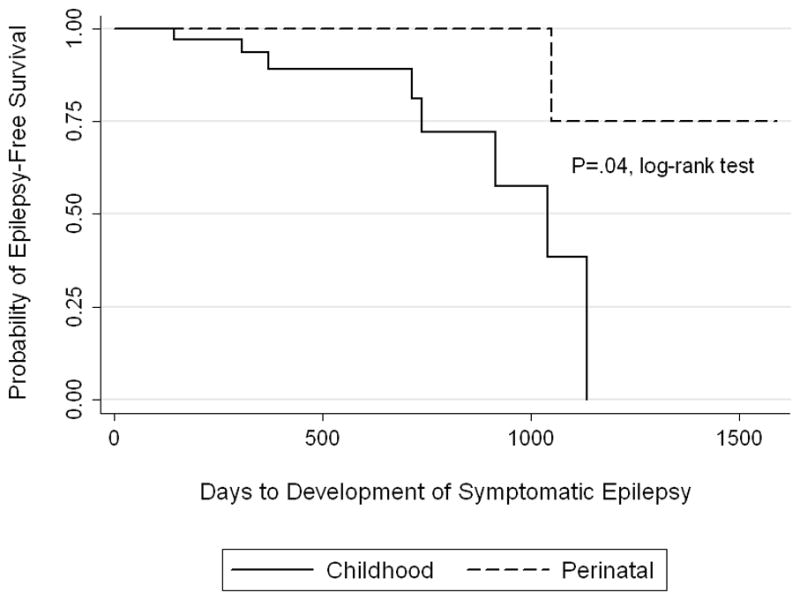
Kaplan-Meier survival curves by age group (perinatal versus childhood) demonstrating time to first remote symptomatic seizure (a) and time to development of epilepsy (b).
Table 5.
Risk Factors for First Remote Symptomatic Seizure Among Survivors (n=67), Log-rank test
| Risk Factor | # with Remote Seizure/# with Risk Factor | # with Remote Seizure/# without Risk Factor | P Value | Bonferroni corrected P Valuea |
|---|---|---|---|---|
| Seizure at presentation | 6/29 | 8/38 | .78 | |
| Acute seizure after presentation but <7 days | 0/7 | 14/60 | .32 | .96 |
| Any acute symptomatic seizure within 7 days | 6/33 | 8/34 | .63 | |
| Childhood ICH | 12/50 | 2/17 | .053 | .27 |
| Cortical involvement | 6/31 | 8/36 | .48 | 1.00 |
| Parenchymal location | 13/60 | 1/7 | .40 | |
| AED at discharge | 9/36 | 5/31 | .34 | |
| Epileptiform discharges | 4/22 | 10/45 | .90 | |
| Vascular malformation | 9/35 | 5/32 | .11 | .5 |
| Elevated ICP requiring urgent intervention | 9/26 | 5/41 | .005b | .03c |
Abbreviations: #, number; AED, antiseizure medication; ICP, intracranial pressure.
, Correction for a priori hypotheses only.
, Statistically significant prior to Boneferroni correction for multiple comparisons.
, Statistically significant after Bonferroni correction for multiple comparisons.
Comment
In one of the largest prospective studies of pediatric ICH, we found that nearly half of children experience acute symptomatic seizures, and these occurred at presentation in sixty percent of subjects with ICH during the perinatal period and in about one-third with ICH during childhood. This indicates (1) that acute symptomatic seizures are more common in children than in adults, in whom seizures are reported in 7%–31%14–16 and (2) that children with ICH present with seizures more commonly than children with arterial ischemic stroke, in whom seizures are reported as a presenting symptom in 22%.17 Among childhood subjects, younger children were more likely to present with seizure compared to older children, a finding consistent with results in pediatric arterial ischemic stroke.17 Acute symptomatic seizures after presentation but within the first seven days occurred in 13% of childhood subjects.
Continuous EEG was performed as part of clinical care in 65% of perinatal subjects and in about one-third of childhood subjects. Electrographic-only seizures were present in 28% of subjects in whom cEEG was performed (12% of cohort), and three of four childhood subjects with electrographic-only seizures on cEEG had elevated ICP. These observations are consistent with studies in critically ill children in which electrographic seizures are common in children with various types of acute encephalopathy.12 Our findings are also consistent with studies in adults with ICH, where electrographic seizures were found in 18% of 102 consecutive adults with ICH who underwent EEG monitoring.14 The potential long-term significance of our observations is highlighted by the finding that critically ill children with acute brain injuries from heterogeneous etiologies and encephalopathy with electrographic status epilepticus have worse short-term outcome compared to children with brain injuries and encephalopathy without seizures.18 Furthermore, in adult ICH, electrographic seizures are associated with hemorrhage expansion.14 Additional study is needed to define better the occurrence of electrographic seizures in children with ICH, to assess their association with outcome, and to determine whether identification and management of these seizures improves outcome.
Another important finding in this study was that elevated ICP requiring urgent intervention was associated with acute symptomatic seizures after presentation. Three of four children with electrographic-only seizures had elevated ICP requiring urgent intervention. In adults with traumatic brain injury, electrographic seizures have been associated with transient elevations in ICP19, which suggests a pathophysiologic mechanism by which seizures could further elevate ICPs or make management of elevated ICP more complex. Children with elevated ICP after ICH may derive particular benefit from cEEG monitoring and from more aggressive seizure management in the acute setting.
Surprisingly, cortical involvement of ICH, an important predictor of acute symptomatic seizures in adult ICH15, 16, was not related to acute symptomatic seizures in this pediatric cohort. Additionally, acute symptomatic seizures were not associated with remote symptomatic seizures and epilepsy. This lack of statistical significance may be related to the relatively small number of subjects.
This study used survival analysis to estimate the time to remote symptomatic seizures and epilepsy in a prospective cohort of pediatric ICH. Past reports have been limited by small numbers and lack of information on follow-up time. In this cohort, while a first remote symptomatic seizure was estimated to occur in about one-third of subjects at two years, epilepsy was less common, estimated to develop in less than 15% of the cohort at two years. Although not statistically significant, perinatal subjects seem to be at lower risk for remote symptomatic seizures and epilepsy compared to childhood subjects. As with acute symptomatic seizures after presentation, elevated ICP requiring urgent intervention was a risk factor for a first remote symptomatic seizure and for developing epilepsy, which highlights a high risk group of patients.
More than half of children were taking antiseizure medication at the time of remote symptomatic seizure and epilepsy diagnosis, which indicates that antiseizure medication use does not prevent all remote symptomatic seizures in the follow-up period. However, choice of antiseizure medication was not uniform among subjects since it was based on clinical practices of treating physicians. A randomized controlled-trial is required to address properly whether long-term use of antiseizure medications can prevent remote symptomatic seizures and epilepsy. Such a study may benefit from stratification across age group at incident ICH and across those with elevated ICP.
The present study has several limitations. First, initial Glasgow Coma Score was not documented for the majority of subjects, so no standard clinical examination of ICH severity was performed. However, elevated ICP was a risk factor for acute symptomatic seizures, remote symptomatic seizures, and epilepsy, and elevated ICP is a likely marker of more severe ICH. Second, since some subjects were lost to follow-up, the true remote symptomatic seizure-free and epilepsy-free survival may be underestimated. We believe that most pediatric patients with a remote seizure after ICH would be brought to stroke program physicians because of local referral patterns. Third, EEG monitoring was not performed in all patients, so the incidence of electrographic seizures is uncertain. Some patients with electrographic seizures may not have undergone EEG monitoring, which leads to underestimation of their frequency. However, EEG monitoring may have already targeted those most at risk.
Despite its limitations, the study has several strengths. The prospective design permits identification of ICH cases and of seizures without reliance on ICD-9 codes, which misclassify stroke diagnoses. 20, 21 The use of time-to-event analysis to evaluate remote symptomatic seizure-free and epilepsy-free survival allowed the most accurate description of the cohort.
This study provides clinicians with useful information regarding the risk of remote seizures and epilepsy for counseling parents of pediatric patients with ICH who present in the perinatal and childhood periods. Additionally, we identified a subset of subjects with elevated ICP requiring urgent intervention who appear to be at the greatest risk for acute seizures after presentation, remote symptomatic seizures, and epilepsy. Further study is needed to determine the incidence of remote symptomatic seizures and epilepsy with longer follow-up.
Supplementary Material
Table 6.
Risk Factors for Symptomatic Epilepsy Among Survivors (n=67), Log-rank test
| Risk Factor | # with Epilepsy/# with Risk Factor | # with Epilepsy/# without Risk Factor | P Value | Bonferroni corrected P Valuea |
|---|---|---|---|---|
| Seizure at presentation | 4/29 | 5/38 | .82 | |
| Acute seizure after presentation but <7 days | 0/7 | 9/60 | .58 | 1.00 |
| Any acute symptomatic seizure within 7 days | 4/33 | 5/34 | .76 | |
| Childhood ICH | 8/50 | 1/17 | .04b | .20 |
| Cortical involvement | 4/31 | 5/36 | .60 | 1.00 |
| Parenchymal location | 9/60 | 0/7 | .22 | |
| AED at discharge | 5/36 | 4/31 | .70 | |
| Epileptiform discharges | 3/22 | 6/45 | .47 | |
| Vascular malformation | 6/35 | 3/32 | .11 | .55 |
| Elevated ICP requiring urgent intervention | 6/26 | 3/41 | .007b | .04c |
Abbreviations: #, number; AED, antiseizure medication; ICP, intracranial pressure.
, Correction for a priori hypotheses only.
, Statistically significant prior to Boneferroni correction for multiple comparisons.
, Statistically significant after Bonferroni correction for multiple comparisons.
Acknowledgments
Acknowledgements and Funding
Beslow: NIH-K12-NS049453, NIH-T32-NS007413, L. Morton Morley Funds of The Philadelphia Foundation
Abend: NIH-K23-NS076550
Licht: NIH-R01-NS072338, June and Steve Wolfson Family Fund for Neurological Research
Smith: NIH-K12-NS049453
Ichord: NIH-R01-NS050488, K23-NS062110
Jordan: K23-NS062110
Funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Beslow, Abend, and Jordan. Acquisition of data: All authors. Analysis and interpretation of data: Beslow, Abend, and Jordan. Drafting of the manuscript: Beslow and Jordan. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Beslow. Administrative, technical, and material support: Gindville and Bastian. Study supervision: Jordan.
References
- 1.Lo WD, Lee J, Rusin J, Perkins E, Roach ES. Intracranial hemorrhage in children: an evolving spectrum. Arch Neurol. 2008;65(12):1629–1633. doi: 10.1001/archneurol.2008.502. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jarallah A, Al-Rifai MT, Riela AR, Roach ES. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15(5):284–289. doi: 10.1177/088307380001500503. [DOI] [PubMed] [Google Scholar]
- 3.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke. 2010;41(2):313–318. doi: 10.1161/STROKEAHA.109.568071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom I, De Schryver EL, Kappelle LJ, Rinkel GJ, Jennekens-Schinkel A, Peters AC. Prognosis of haemorrhagic stroke in childhood: a long-term follow-up study. Dev Med Child Neurol. 2003;45(4):233–239. doi: 10.1017/s001216220300046x. [DOI] [PubMed] [Google Scholar]
- 5.Jordan LC, Kleinman JT, Hillis AE. Intracerebral hemorrhage volume predicts poor neurologic outcome in children. Stroke. 2009;40(5):1666–1671. doi: 10.1161/STROKEAHA.108.541383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25(6):416–421. doi: 10.1016/s0387-7604(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 7.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48(11):1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 8.Yang JS, Park YD, Hartlage PL. Seizures associated with stroke in childhood. Pediatr Neurol. 1995;12(2):136–138. doi: 10.1016/0887-8994(94)00152-r. [DOI] [PubMed] [Google Scholar]
- 9.Chadehumbe MA, Khatri P, Khoury JC, et al. Seizures are common in the acute setting of childhood stroke: a population-based study. J Child Neurol. 2009;24(1):9–12. doi: 10.1177/0883073808320756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: the coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54(2):371–378. doi: 10.1212/wnl.54.2.371. [DOI] [PubMed] [Google Scholar]
- 11.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–675. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 12.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76(12):1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52 (Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 14.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69(13):1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 15.Beghi E, D’Alessandro R, Beretta S, et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology. 2011;77(20):1785–1793. doi: 10.1212/WNL.0b013e3182364878. [DOI] [PubMed] [Google Scholar]
- 16.De Herdt V, Dumont F, Henon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology. 2011;77(20):1794–1800. doi: 10.1212/WNL.0b013e31823648a6. [DOI] [PubMed] [Google Scholar]
- 17.Abend NS, Beslow LA, Smith SE, et al. Seizures as a presenting symptom of acute arterial ischemic stroke in childhood. J Pediatr. 2011;159(3):479–483. doi: 10.1016/j.jpeds.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topjian AA, Guteirrez-Colina AMSMS, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. doi: 10.1097/CCM.0b013e3182668035. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 20.Golomb MR, Garg BP, Saha C, Williams LS. Accuracy and yield of ICD-9 codes for identifying children with ischemic stroke. Neurology. 2006;67(11):2053–2055. doi: 10.1212/01.wnl.0000247281.98094.e2. [DOI] [PubMed] [Google Scholar]
- 21.Golomb MR, Garg BP, Williams LS. Accuracy of ICD-9 codes for identifying children with cerebral sinovenous thrombosis. J Child Neurol. 2007;22(1):45–48. doi: 10.1177/0883073807299959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.