Abstract
The dinoflagellate Dinophysis spp. is responsible for diarrhetic shellfish poisoning (DSP). In the bivalves exposed to the toxic bloom of the dinoflagellate, dinophysistoxin 3 (DTX3), the 7-OH acylated form of either okadaic acid (OA) or DTX1, is produced. We demonstrated in vitro acylation of OA with palmitoyl CoA in the presence of protein extract from the digestive gland, but not other tissues of the bivalve Mizuhopecten yessoensis. The yield of 7-O-palmitoyl OA reached its maximum within 2 h, was the highest at 37 °C followed by 28 °C, 16 °C and 4 °C and was the highest at pH 8 in comparison with the yields at pH 6 and pH 4. The transformation also proceeded when the protein extract was prepared from the bivalves Corbicula japonica and Crassostrea gigas. The OA binding protein OABP2 identified in the sponge Halichondria okadai was not detected in the bivalve M. yessoensis, the bivalve Mytilus galloprovincialis and the ascidian Halocynthia roretzi, though they are known to accumulate diarrhetic shellfish poisoning toxins. Since DTX3 does not bind to protein phosphatases 1 and 2A, the physiological target for OA and DTXs in mammalian cells, the acylation of DSP toxins would be related to a detoxification mechanism for the bivalve species.
Keywords: okadaic acid, dinophysistoxin, Halichondria okadai, okadaic acid binding protein, diarrhetic shellfish poisoning, acyl coenzyme A transferase, detoxification
1. Introduction
Diarrhetic shellfish poisoning (DSP) was first recognized in Japan along the coast of the Tohoku Area in the 1970s [1], where the dinoflagellate Dinophysis fortii was dominantly identified in the toxic bloom [2]. Okadaic acid (OA) and dinophysistoxins (DTXs) were isolated from the digestive glands of shellfish [3,4] and were deduced to be DSP toxins (Figure 1). The structure of OA, DTX1 and DTX2 were determined in 1981 [5], 1982 [3] and 1992 [6], respectively. Since DSP causes gastrointestinal dysfunction, which accompanies diarrhea, vomiting and abdominal pains, the local governments periodically monitor the appearance of Dinophysis spp. and quantitate toxin concentration in shellfish in order to determine whether to suspend shipments during the period when the toxin level was beyond 0.05 mouse unit/g. DTX3, named for 7-O-acylated derivatives of OA or DTX1, was not synthesized by the dinoflagellate, in spite of being present in bivalves [7]. The ratio of acylated forms to non-acylated forms detected in digestive glands of DSP-exposed (toxic) bivalves depended on the bivalve species. It was reported that Japanese scallops possess a higher capability to convert DTX1 to DTX3 in comparison with mussels [8]. In vitro transformation reaction reported by two research groups [9,10] suggested that bivalves are considered as the platform for the transformation reaction, ruling out the bacterial contribution [11] in this transformation.
Figure 1.
Structures of okadaic acid (OA), dinophysistoxin 1 (DTX1), dinophysistoxin 2 (DTX2) and dinophysistoxin 3 (DTX3).
Shellfish poisoning used to be classified in four groups depending on the symptoms; Diarrhetic shellfish poisoning (DSP), paralytic shellfish poisoning (PSP) [12], neurotoxic shellfish poisoning (NSP) [13] and amnesic shellfish poisoning (ASP) [14]. Now, the classification is based on the chemical characteristics of shellfish poisoning toxins groups [15]. The eight groups are: the azaspiracid group, brevetoxin group, cyclic imine group, domoic acid group, okadaic acid group, pectenotoxin group, saxitoxin group and yessotoxin group. For all these groups, the producers and structures of responsible toxins have already been revealed. The transformation of some of these toxins occurring in bivalves as observed in DSP toxin-carrying shellfish has not been investigated for all the toxins. In bivalves that accumulate PSP toxins, multiple products with various levels of toxicity were produced after the transformation reaction [16]. For DSP toxins, besides the acylation at C7-OH as described earlier, the diol esters of OA [17] were hydrolyzed to OA [18]. In addition, DTX4 [19] and DTX5 [20], produced by the dinoflagellate Prorocentrum spp., have not been reported in bivalves to date. Hydrolysis of diol esters of OA or DTXs 4 and 5 leads to an increase of toxicity by restoring binding ability to serine/threonine protein phosphatases 1 (PP1) and 2A (PP2A), the primary target for OA in mammalian tissues [21]. Since DTX3 lacks binding ability to PP1 and PP2A [22], acylation would decrease fatal risk to the animals accumulating the DSP toxins. An intriguing observation, however, was made by Yanagi et al. that DTX3 retained its ability to cause diarrhea and exhibit cytotoxicity [23]. Therefore, the physiological significance of acylation of DSP toxins to bivalve species was not clearly understood until now.
OA was originally isolated from the marine sponge Halichondria okadai [5]. OA specifically bound to PP1 and PP2A with 150 nM and 30 pM of dissociation constant [22], which led to an exhibition of tumor promotion [24]. Since then, OA has been widely used for basic research. X-ray crystal structure revealed the well-folded structure of OA in the catalytic subunit of PP2A, showing the presence of an intramolecular hydrogen bond between the carboxyl group and C24-OH [5,25]. PP1 and PP2A are two of the fundamental proteins in all the living species, and their sequences are highly conserved. OA binding proteins OABP1 and OABP2 were isolated from H. okadai [26]. The amino acid sequence of OABP1, partially deduced from the cDNA sequence, was 88% homologous to rabbit PP2A. On the other hand, OABP2 was not homologous to any protein phosphatases reported in the literature. Since a homology search did not let us infer the function of OABP2, curiosity was raised on the physiological roles of this protein. OABP2 was composed of three homologues: OABP2.1, 2.2 and 2.3. OABP2.1 was 98% and 66% homologous to OABP2.2 and OABP2.3, respectively. Among them, OABP2.1 and 2.3 were stably expressed as recombinant proteins in E. coli [27]. Since OA bound to the recombinant OABP2.1 and 2.3 with 1.30 ± 0.56 nM and 1.54 ± 0.35 nM of dissociation constant, the binding site of OA would be located in sequences that OABP2.1 and 2.3 have in common. OABP2 was not identified in crude extracts of the sponge H. japonica, which is often seen in the same habitat as that of H. okadai. Thus, the presence of OABP2 seemed to be physiologically important to H. okadai, though the presence of OABP2 in toxic bivalves has not been investigated so far.
We herein investigated toxic and non-toxic bivalves by Western blotting using an anti-OABP2 serum in order to assess the expression of the protein in the bivalve species. Next, we compared in vitro transformation of OA into DTX3 in the presence of crude extracts from different tissues of the scallop Mizuhopecten yessoensis to identify the location of the transformation reaction. We also tested two other bivalve species, Corbicula japonica and Crassostrea gigas, to determine whether they were able to transform OA into DTX3 and to address the generality of the transforming ability.
2. Results and Discussion
2.1. Okadaic Acid Binding Proteins in Shellfish
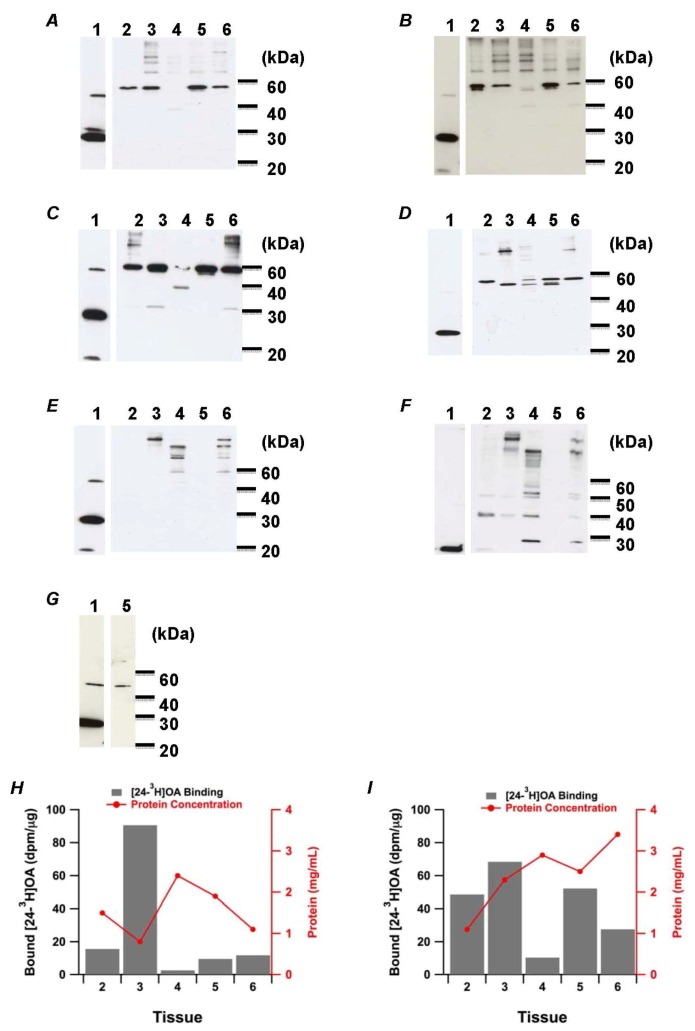
Western blotting analysis of the crude extracts was carried out to identify the OA binding protein OABP2.1 in the nontoxic and toxic shellfish extracts. From the scallop Mizuhopecten yessoensis and the mussel Mytilus galloprovincialis, the gills, gonad, adductor muscle, digestive gland and mantle were subjected to Western blotting. Since it was difficult to separate each tissue from the ascidian H. roretzi, only the digestive gland was used for preparing the extract. The results indicated that none of the extract from non-toxic M. yessoensis (Figure 2A,B), toxic M. yessoensis (Figure 2C,D), M. galloprovincialis (Figure 2E,F) and the ascidian H. roretzi (Figure 2G) appeared to contain OABP2.1, whereas the antiserum detected an intense amount of recombinant OABP2.1 around 22 kDa (lane 1). In fact, the anti-OABP2.1 serum detected some different-sized proteins. The antiserum primarily detected ca. 60 kDa-sized proteins in every tissue, except the adductor muscle from nontoxic and toxic M. yessoensis (lanes 2, 3, 5 and 6, Figure 2A–D). On the other hand, the antiserum did not exhibit distinctive labeling in the digestive gland from M. galloprovincialis (Figure 2E,F) and H. roretzi (Figure 2G), while it labeled multiple proteins in the adductor muscle (lane 4) and mantle (lane 6) from M. galloprovincialis (Figure 2E,F).
Figure 2.
Western blotting analysis of shellfish extracts with anti-OABP2.1 serum. (A–E) Extracts were prepared from the nontoxic scallop Mizuhopecten yessoensis (A, B), the toxic scallop M. yessoensis (C, D), the toxic Mytilus galloprovincialis (E, F) and toxic ascidian Halocynthia roretzi (G). The pellet fractions were A, C and E. The supernatant fractions were B, D and F. The whole homogenate was used for H. roretzi. Lanes 1–6 correspond to recombinant OABP2.1, gills, gonad, adductor muscle, digestive gland and mantle, respectively. (H, I) Binding of [24-3H]OA to the pellet fraction (H) and supernatant fraction (I) of nontoxic M. yessoensis (grey bars). Protein concentration of each fraction was represented with red lines. Lane description was the same as described for A–G.
OA binding proteins were searched for in each tissue extract of nontoxic M. yessoensis by examining [24-3H]OA binding (Figure 2H,I). In the results, the pellet fraction from the gonad (lane 3, Figure 2H) showed the highest binding of the radioligand, and the supernatant fraction from the gills (lane 2), digestive gland (lane 5) and mantle (lane 6) showed moderate binding. Both of the pellet and supernatant fractions from the adductor muscle (lane 4) had relatively weak binding.
Phosphorylation and dephosphorylation are the fundamental signal transduction processes and are targeted by many natural toxins [28,29]. OA specifically inhibits protein phosphatases 1 and 2A with 150 nM and 30 pM of the dissociation constant, respectively [22], which leads to the appearance of cytotoxicity [30,31] and cancer promotion [24,32]. Secondary metabolites, such as OA, isolated from marine sponges, are not considered as sponge metabolites, but as metabolites of a symbiotic species [33,34]. Due to difficulty in the purification of such symbiotic species from the sponges, research studies have been aimed towards the cloning of secondary metabolite-producing genes from metagenome [35]. However, the metagenomic approach has not been successful with all the targeted compounds. In order to purify the secondary metabolite-producing species, it would be important to understand the symbiotic relationship by identifying the clue substance(s) that affect the host species, as well as the symbiotic species.
OABP2, the OA binding protein isolated from the sponge H. okadai, was postulated as such a key substance that would regulate the concentration of OA in the sponge to reduce self-toxicity and unleash OA to exhibit cytotoxicity when undesired species come into the sponge. We hypothesized that other OA-accumulating species might possess OABP2. In this study, however, OABP2 was not identified in bivalves that accumulate DSP toxins when the toxic bloom occurs (Figure 2). The anti-OABP2 serum almost equally labeled a protein around 60 kDa in each tissue extract from M. yessoensis, while it labeled different sized protein(s) from extracts prepared from other species. It was unlikely that the proteins detected by anti-OABP2.1 serum in each tissue were the OA binding proteins, since the [24-3H]OA binding profile did not support the Western blotting results. The results comprised the presence of multiple binding proteins for OA in the M. yessoensis, which was compatible with the observation made by Rossignoli et al., where various sized proteins were bound to OA more or less nonspecifically [36].
2.2. In Vitro Transformation of OA into 7-O-Palmitoyl OA (DTX3) in M. yessoensis
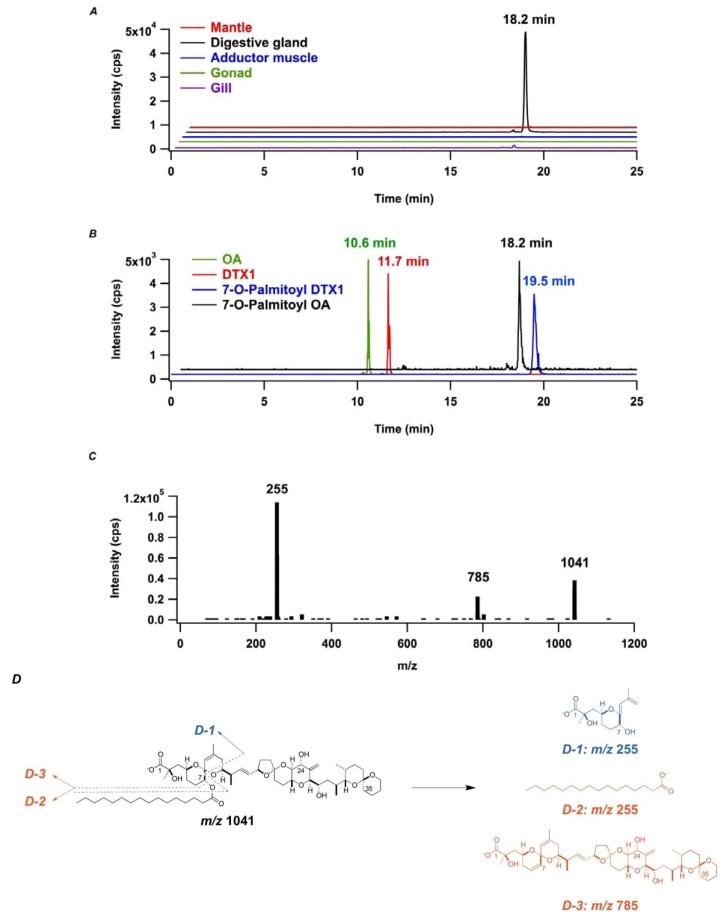
In vitro transformation reaction of OA was performed in the microsomal fraction and the mitochondrial fraction prepared from the digestive gland of nontoxic M. yessoensis. Both of the fractions turned out to show OA-transforming ability as described later, which was compatible with the observation by Rossignoli et al. [10]. We then decided to investigate the transformation reaction in the microsomal fraction [37]. After 2 h of the reaction, quantitation of unreacted OA and 7-O-palmitoyl OA in the AcOEt layer was carried out with LC-MS/MS with [M − H]− as the precursor ion and m/z 255 as the product ion [38]. The multiple reaction monitoring (MRM) peak with m/z 803 > 255 (Q1 > Q3) detected at 10.6 min was assigned to that of unreacted OA. Under this condition, however, the MRM peak with m/z 1041 > 255, corresponding to that for 7-O-palmitoyl OA, was not detected.
When OA was incubated with the microsomal fraction in the presence of palmitoyl CoA and ATP, the MRM peak clearly appeared at 18.2 min (Figure 3A). In fact, the DSP toxins standard contained OA, DTX1 and lipid adducts to DTX1 (7-O-acyl DTX1), but not the lipid adduct to OA (7-O-acyl OA), and it was necessary to confirm that the signal could be assigned to that for 7-O-palmitoyl OA. Due to the structural difference between OA and DTX1 at the substituent at C-35 (H or CH3), we assumed that the relative retention time for DTX1 to OA would be similar to that for 7-O-palmitoyl DTX1 to 7-O-palmitoyl OA. The retention times for DTX1 and OA was 11.7 min and 10.6 min, respectively (Figure 3B). Likewise, since 7-O-palmitoyl DTX1 was eluted at 19.5 min, the MRM peak with 1041 > 255 at 18.2 min was acceptable to 7-O-palmitoyl OA. To validate our methodology for identifying 7-O-palmitoyl OA, we performed LC-MS/MS on enhanced product ion (EPI) scan mode (Figure 3C) with 1041 m/z as the precursor ion. At the retention time of 18.2 min where the MRM peak with m/z 1041 > 255 was detected (Figure 3A), the MS/MS spectrum revealed two product ions at m/z 785 and m/z 255, which presumably accounted for the ion [OA − H2O − H]− (D-3) due to loss of palmitic acid and OA fragment usually selected as the product ion (D-1) or [Palmitic acid − H]− (D-2), respectively (Figure 3D). All of these speculations on the LC-MS/MS analysis clarified production of the 7-O-palmitoyl OA in the transformation reaction.
Figure 3.
Identification of 7-O-palmitoyl OA in in vitro transformation reaction mixture. (A) In vitro transformation reactions were performed in the presence of microsomal fractions prepared from different tissues of Mizuhopecten yessoensis. From the front side to the backside, the microsomal fraction was prepared from the gill (purple), gonad (green), adductor muscle (blue), digestive gland (black) and mantle (red) and was subjected to in vitro acylation reactions. The production of 7-O-palmitoyl OA was confirmed by LC-MS/MS analysis on multiple reaction monitoring (MRM) negative ion mode with m/z 1041 > 255 as precursor ion/product ion; (B) Mass chromatograms for m/z 1041 > 255 from sample (black) and three mass chromatograms for m/z 803 > 255 (green, OA), m/z 817 > 255 (red, DTX1) and m/z 1055 > 255 (blue, 7-O-Palmitoyl DTX1) from diarrhetic shellfish poisoning (DSP) standard were obtained from LC-MS/MS on MRM negative ion mode. The latter three chromatograms were overlapped and shown in the front side; (C) The MS/MS spectrum for 7-O-palmitoyl OA. The peak at 18.2 min in the mass chromatogram in (A) was analyzed with LC-MS/MS on enhanced product ion (EPI) scan mode. (D) The estimated structures for the three product ions m/z 1041, m/z 785 (D-3) and m/z 255 (D-1, D-2) observed in the MS/MS spectra in (C).
Stoichiometry of the transformation reaction was examined by quantitating the area of the MRM peak for the 7-O-palmitoyl OA in reference to that for 7-O-palmitoyl DTX1 in the standard mixture. The yield of 7-O-palmitoyl OA was 0.5% based on OA applied in the reaction (Figure 3A). Since the ratio of DTX3 to DTX1 in the toxic M. yessoensis was 10–14 [38], the observed transformation rate was extremely low. On the analysis with LC-MS/MS on MRM negative ion mode, we searched for other lipid adducts to OA that were produced by a reaction with endogenous lipid molecules in the protein extract, though none of the other adducts were identified. In order to assess production of DTX3 in other tissues in the M. yessoensis, the microsomal fractions prepared from the adductor muscle, gill, mantle and gonad were compared for the transformation ability of OA. The highest yield was obtained from the digestive gland, and a marginal yield was obtained from other tissues (Figure 3A). We thus speculated that optimization of the condition might be required to reproduce the naturally occurring transformation in the M. yessoensis.
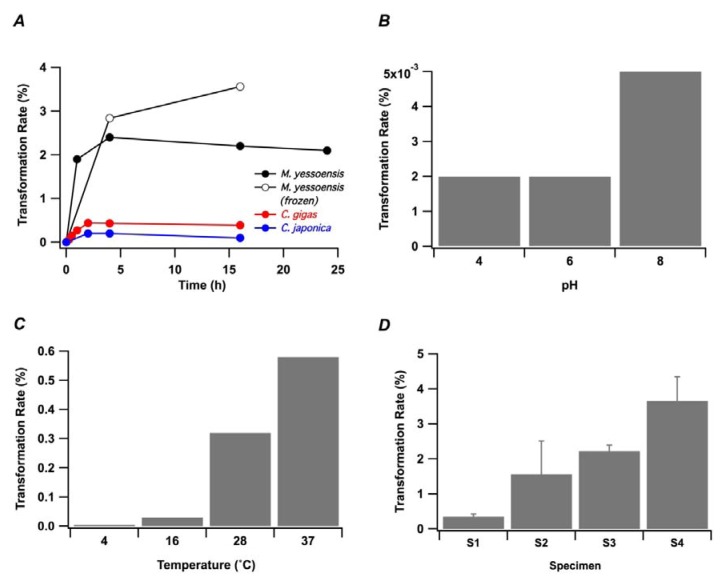
A time course of the reaction was examined in Figure 4A. OA was reacted with palmitoyl CoA in the presence of a microsomal fraction of the digestive gland from live M. yessoensis for 15 min, 30 min, 60 min, 2 h, 4 h and 16 h. The production of 7-O-palmitoyl OA was increased during the initial two hours and ceased down thereafter. The transformation reaction was also observed in a similar manner when the microsomal fraction was prepared from the frozen M. yessoensis. 7-O-palmitoyl DTX1 was subjected to the same reaction condition, though it was almost 100% recovered (data not shown), suggesting that a hydrolysis of the transformation product did not occur. We then attempted to examine the effect of pH and temperature on the transformation reaction. A similar transformation rate was obtained at pH 4 and at pH 6, while it was more than 2-fold at pH 8 (Figure 4B). Also, when the transformation reactions were performed at 4 °C, 16 °C, 28 °C and 37 °C, a higher transformation rate was obtained at a higher temperature (Figure 4C).
Figure 4.
Dependence of in vitro transformation of OA on (A) shellfish species, (B) pH, (C) temperature and (D) specimens. (A) Time course of the transformation reaction at pH 6/37 °C in the presence of digestive gland extracts from M. yessoensis (closed circles), the frozen M. yessoensis (open circles), C. gigas (red) and C. japonica (blue) was monitored. (B–D) Transformation reaction was carried out at different pH/37 °C in (B), at pH 6/different temperature in (C), at pH 6/37 °C with 4 specimens in (D). S1, S2, S3 or S4 in (D) was the code name for each specimen.
Suzuki et al. first obtained direct evidence on the transformation of OA into DTX3 in 1999 [9]. Non-toxic M. yessoensis were fed with the toxic dinoflagellate Dinophysis fortii. In the digestive gland of M. yessoensis, most of the OA was converted to 7-O-palmitoyl OA.
Rossignoli et al. injected agar capsules containing OA into live Mytilus edulis and continued injecting it for 12 days [10]. They found that the amount of OA and DTX3 (7-O-acyl OA) accumulated in the digestive gland of M. edulis gradually increased and that the lipid conjugated to 7-OH was not only palmitic acid (C16:0), but also C14:0, C15:0, C16:1, C17:0, C18:1, C18:2, C18:4, C20:5 and so on. They also demonstrated the first in vitro transformation of OA in the presence of the microsomal fraction of the M. edulis. The significance of our work was that we provided more evidence for in vitro transformation in the M. yessoensis extract with the assistance of acyl CoA transferase. Furthermore, kinetics and stoichiometry of the reaction were revealed. The transformation reaction from OA to 7-O-palmitoyl OA got saturated within 2 h and did not proceed even when a longer incubation period was applied. The transformation rate varied from 0.01% to 3%, and the rest of the input OA was recovered. Hydrolysis of 7-O-palmitoyl DTX1 to regenerate DTX1 did not occur, though the crude extract should have contained esterase, as exemplified by the hydrolysis of the diol esters of OA to OA in the digestive gland of Greenshell mussel Perna canaliculus [18]. The ratio of DTX3/DTX1 accumulated in naturally intoxicated M. yessoensis was more than 10, which explained the low efficacy of the in vitro transformation [38]. Since the difference between in vivo and in vitro experiments should not be ignored, the transformation could be hampered by other factors. A degradation of the enzyme or depletion of palmitoyl CoA applied to the reaction might have occurred. Otherwise, the dissociation of 7-O-palmitoyl OA from the enzyme could not be properly regulated in the in vitro system.
The LC-MS/MS analysis on an MRM negative ion mode implied substrate specificity for the OA/DTX-transforming enzyme (Figure 3B). The relative retention time of the m/z 1041 > 255 peak (18.2 min) to 7-O-palmitoyl DTX1 (19.5 min) was identical to the relative retention time of OA (10.6 min) to DTX1 (11.7 min). No other peak appeared in the m/z 1041 > 255 mass chromatogram, suggesting that the acylation proceeded regioselectively at C7-OH in spite of the fact that there were four hydroxyl substituents in OA.
As for another acyl-CoA transferase to the hydroxyl group, the acyl-CoA:steroid acyltransferase from the digestive gland of the oyster Crassostrea virginica was identified, which did not only catalyze acylation at 3β-OH, but also at 17β-OH of the steroid hormones [37], indicating that the enzyme cannot distinguish minute differences in the substrate. In contrast to the OA/DTX-transforming enzyme, the optimum pH of the acyl-CoA:steroid acyltransferase from C. virginica was around 6.0. In a similar fashion to the OA/DTX-transforming enzyme, the reaction yield became higher as the temperature was increased. The rat liver microsome also had enzymatic activity that promoted acylation to the hydroxyl group of retinol [39,40]. Contrary to the in vitro transformation reaction of OA observed in this study, the enzymatic activity turned out to be the most intense during pH 6–7. Furthermore, the transformation proceeded even in the absence of acyl-CoA, and the yield was markedly increased in the presence of acyl-CoA.
2.3. In Vitro Transformation of OA into 7-O-Palmitoyl OA (DTX3) in Other Bivalve Species
In order to compare the in vitro transformation reaction between different bivalve species, the bivalves C. japonica and C. gigas were chosen. Since a sufficient amount of protein was not obtained from the digestive gland of a single C. japonica, multiple specimens were treated. It turned out that the two bivalves’ extract transformed OA to 7-O-palmitoyl OA at the lower rate than the M. yessoensis extract (Figure 4A). It is important to address that the transformation reaction was observed in the extracts from three bivalve species M. yessoensis, C. gigas and C. japonica. The first two species were the subject of monitoring DSP toxins, while C. japonica has not been reported for intoxication of DSP. Without being scientifically provided, the reason why C. japonica have not been the vector of the diarrhetic shellfish poisoning would be speculated as follows: (1) C. japonica exists in brackish-water region that may not be accessible to Dinophysis spp.; (2) The depth range of habitat is different between the two species; and (3) The locations for the C. japonica industry is limited and the incidents of human intoxication could occur at much less frequency compared to the incidents derived from scallop. We then assessed the difference among specimens on transformation ability, and four M. yessoensis specimens were tested for the reaction. As a consequence, an 8-fold difference at maximum was observed among those specimens (Figure 4D), which made it difficult to compare the transformation efficacy among different species shown in Figure 4A. However, the enzymatic activity recognized in C. japonica let us propose that the transformation reaction could be a fundamental system that commonly exists in the bivalve species.
The absence of OABP2 in bivalves postulated uniqueness of the protein in the H. okadai. In addition, the result raised a question why the DSP toxin-containing bivalves were tolerant to DSP-toxins, since depuration of DSP toxins depends on bivalve species and usually takes 2–3 weeks [41,42]. Various transformation reactions were reported for shellfish toxins in the digestive gland of host species. Pectenotoxin, which used to be classified as DSP toxin, was synthesized by the Dinophysis spp. and was subjected to hydrolysis to its seco acid in the Greenshell™ mussel Perna canaliculus [18,43]. These transformations could be programmed in each species to reduce risk of self-intoxication.
2.4. Characterization of OA-Transforming Enzyme with an Attempt of the Purification
We then attempted to characterize the transformation enzyme by a simple separation technique. The microsomal fraction was sonicated in the presence of EDTA to obtain the soluble fraction. Both the supernatant and pellet were subjected to the transformation reaction. Enzymatic activity was not observed in the supernatant and remained in the pellet, suggesting that the enzyme did not become soluble under the mild condition. Other acyl transferases reported in literature did not necessarily share this intrinsic nature. The enzymatic activity contributing pregnenolone esterification, considered as a potential detoxification mechanism in Saccharomycess cerevisiae, was extracted to the soluble fraction without using surfactant [44]. Likewise, acyltransferase identified in Ascaris suum also resided in the soluble fraction [45]. On the other hand, the membrane-associated property of acyl-CoA:cholesterol O-acyltransferase was addressed by Spector et al. in 1979, where the enzyme fractions were extracted with triton X-100 [46]. Further separation of the OA/DTX-transforming enzyme would be required to address the membrane-intrinsic nature.
3. Experimental Section
3.1. Materials
Due to outbreak of the dinoflagellate Dinophysis fortii, shellfishing was prohibited from July 7 to July 27 in 2010 for the scallop Mizuhopecten yessoensis and from July 8 to September 2 in 2010 for the ascidian Halocynthia roretzi. Toxic specimens of M. yessoensis, the Mytilus galloprovincialis and H. roretzi were collected in Yamada Bay, Shimohei District, Iwate Prefecture, Japan on 26 July 2010. Live nontoxic bivalves M. yessoensis, Crassostrea gigas and Corbicula japonica were purchased from a fish market and transported to the laboratory on ice. The National Research Institute of Fisheries Science provided the DSP toxin standard cocktail [47]. OA, palmitic acid and palmitoyl coenzyme A were purchased from Wako Pure Chemicals. ATP was purchased from Oriental Yeast Co., Ltd. A part of the OA was purified from the sponge H. okadai. The purity of the OA was 73% and that of the DTX1 was 12%, which were determined by LC-MS/MS in reference to the purchased OA. [24-3H]OA and 7-O-palmitoyl DTX1 (DTX3) were kindly provided by Dr. Takeshi Yasumoto of Japan Food Research Laboratories.
3.2. Preparation of the Microsomal and Mitochondrial Fractions from Bivalves
The fresh specimen was dissected into five tissues of gill, gonad, adductor muscle, digestive gland, and mantle by scissors and homogenized with Ultra Turrax T25 (IKA Japan K.K., Osaka, Japan) in 4 volumes of a buffer containing 10 mM Tris/HCl (pH 7.6), 150 mM KCl, 500 mM sucrose and Protease Inhibitor Cocktail for Use with Mammalian Cell and Tissue Extracts (Nacalai Tesque, Kyoto, Japan). The crude suspension was successively centrifuged at 800× g for 10 min, 11,000× g for 15 min and 20,500× g for 15 min. In the first two centrifugations, the supernatant was taken for the next step. In the final centrifugation, the supernatant and pellet were stored as the microsomal and mitochondrial fractions, respectively [37]. The protein concentration of these fractions were determined with Protein Assay CBB Solution (Nacalai Tesque, Kyoto, Japan) and used for the following transformation reaction.
3.3. In Vitro Transformation Reaction
1.0 μg (1.2 nmol) of OA, 26 μg (26 nmol) of palmitoyl coenzyme A and 1.3 mg (2.6 μmol) of ATP were incubated with 2.0 mg of the microsomal fraction. The reaction mixture was extracted three times with ethyl acetate (1 mL). The organic phase was concentrated under a flow of nitrogen gas and was dissolved in methanol. LC-MS/MS analysis was carried out as described in literature. Briefly, the LC-MS/MS analysis on MRM negative ion mode on acyl OA analogues was performed using the following ion channels, with a dwell time of 22 ms, a declustering potential of −150 V, an entrance potential of −10.5 V and a collision energy of −84 V for each analogue; 14:0-OA; 1013 > 227, 15:0-OA; 1027 > 241, 16:0-OA; 1041 > 255, 17:0-OA; 1055 > 269, 18:0-OA; 1069 > 283, 20:0-OA; 1097 > 311, 22:0-OA; 1125 > 339, 14:1-OA; 1011 > 225, 16:1-OA; 1039 > 253, 18:1-OA; 1067 > 281, 20:1-OA; 1095 > 309, 18:2-OA; 1065 > 279, 18:3-OA; 1063 > 277, 18:4-OA; 1061 > 275, 20:4-OA; 1089 > 303, 18:5-OA; 1059 > 273, 20:5-OA; 1087 > 301, 22:6-OA; 1113 > 327. The toxin calibrants of OA, DTX1 and DTX3 (7-O-palmitoyl-OA) from toxin standards distribution project in Japan were used [47].
3.4. Binding Assay
2.5 nM of [24-3H]OA was incubated on ice for 2 h with 5.0 μg of protein extract obtained from the bivalve species in 100 μL of a binding buffer consisting of 20 mM Tris/HCl, 150 mM NaCl, 1.0 mM EDTA and 0.01% Tween 20 (pH 7.4). The experiments were performed in the absence and presence of 100 nM of non-radioactive OA to estimate nonspecific binding of the ligand. The reaction mixture was directly added to the Nick column (GE Health Care Japan, Tokyo, Japan), and the protein fraction was collected with 750 μL of the binding buffer. The radioactivity was measured with a liquid scintillation counter to determine the specific binding of [24-3H]OA.
4. Conclusions
The absence of OABP2 in shellfish emphasized uniqueness of the protein in the H. okadai. The acylation of OA into DTX3 only underwent in the presence of the digestive gland extract of shellfish under assistance of palmitoyl CoA and proceeded regioselectively. We postulated that the acylation was a fundamental mechanism for the shellfish.
Acknowledgments
The authors would like to thank Joseph Anthony Stavoy of Center for the Advancement of Higher Education of Tohoku University for English editing and Fukushi Kourou of Sanrikuyamada Fisheries Co-operative Association for investigating diarrhetic shellfish poisoning in Yamada Bay, Shimohei District, Iwate Prefecture. The authors are grateful to Takeshi Yasumoto of Japan Food Research Laboratories for providing [24-3H]OA and DTX3 standard and to Yasukatsu Oshima for critical comments on the manuscript. This research was supported by JSPS KAKENHI Grant Number 21603003, 24510291 (K.K.), NOVARTIS Foundation (Japan) for the Promotion of Science (K.K.) and JSPS for Funding Program for the Next Generation World-Leading Researchers (LS012) (M.Y.Y.).
Footnotes
Samples Availability: Available from the authors.
References
- 1.Yasumoto T., Oshima Y., Yamaguchi M. Occurrence of a new type of shellfish poisoning in Tohoku District. Bull. Jpn. Soc. Sci. Fish. 1978;44:1249–1255. [Google Scholar]
- 2.Yasumoto T., Oshima Y., Sugawara W., Fukuyo Y., Oguri H., Igarashi T., Fujita N. Identification of Dinophysis fortii as the causative organism of diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1980;46:1405–1411. doi: 10.2331/suisan.46.1405. [DOI] [Google Scholar]
- 3.Murata M., Shimatani M., Sugitani H., Oshima Y., Yasumoto T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1982;48:549–552. doi: 10.2331/suisan.48.549. [DOI] [Google Scholar]
- 4.Kumagai M., Yanagi T., Murata M., Yasumoto T., Kat M., Lassus P., Rodriguezvazquez J.A. Okadaic acid as the causative toxin of diarrhetic shellfish poisoning in Europe. Agric. Biol. Chem. 1986;50:2853–2857. doi: 10.1271/bbb1961.50.2853. [DOI] [Google Scholar]
- 5.Tachibana K., Scheuer P.J., Tsukitani Y., Kikuchi H., Vanengen D., Clardy J., Gopichand Y., Schmitz F.J. Okadaic acid, a cytotoxic polyether from marine sponges of the genus Halichondria. J. Am. Chem. Soc. 1981;103:2469–2471. doi: 10.1021/ja00399a082. [DOI] [Google Scholar]
- 6.Hu T.M., Doyle J., Jackson D., Marr J., Nixon E., Pleasance S., Quilliam M.A., Walter J.A., Wright J.L.C. Isolation of a new diarrhetic shellfish poison from Irish mussels. J. Chem. Soc. Chem. Commun. 1992:39–41. doi: 10.1039/C39920000039. [DOI] [Google Scholar]
- 7.Yasumoto T., Murata M., Oshima Y., Sano M., Matsumoto G.K., Clardy J. Diarrhetic shellfish toxins. Tetrahedron. 1985;41:1019–1025. doi: 10.1016/S0040-4020(01)96469-5. [DOI] [Google Scholar]
- 8.Suzuki T., Mitsuya T. Comparison of dinophysistoxin-1 and esterified dinophysistoxin-1 (dinophysistoxin-3) contents in the scallop Patinopecten yessoensis and the mussel Mytilus galloprovincialis. Toxicon. 2001;39:905–908. doi: 10.1016/S0041-0101(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T., Ota H., Yamasaki M. Direct evidence of transformation of dinophysistoxin-1 to 7-O-Acyl-dinophysistoxin-1 (dinophysistoxin-3) in the scallop Patinopecten yessoensis. Toxicon. 1999;37:187–198. doi: 10.1016/S0041-0101(98)00182-2. [DOI] [PubMed] [Google Scholar]
- 10.Rossignoli A.E., Fernandez D., Regueiro J., Marino C., Blanco J. Esterification of okadaic acid in the mussel Mytilus galloprovincialis. Toxicon. 2011;57:712–720. doi: 10.1016/j.toxicon.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Vale P. Profiles of fatty acids and 7-O-Acyl okadaic acid esters in bivalves: Can bacteria be involved in acyl esterification of okadaic acid? Comp. Biochem. Physiol. C. 2010;151:18–24. doi: 10.1016/j.cbpc.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Schantz E.J., Ghazarossian V.E., Schnoes H.K., Strong F.M., Springer J.P., Pezzanite J.O., Clardy J. Structure of saxitoxin. J. Am. Chem. Soc. 1975;97:1238–1239. doi: 10.1021/ja00838a045. [DOI] [PubMed] [Google Scholar]
- 13.McFarren E.F., Tanabe H., Silva F.J., Wilson W.B., Campbell J.E., Lewis K.H. The occurrence of a ciguatera-like poison in oysters, clams and Gymnodinium breve Cultures. Toxicon. 1965;3:111–123. doi: 10.1016/0041-0101(65)90005-X. [DOI] [PubMed] [Google Scholar]
- 14.Wright J.L.C., Boyd R.K., Defreitas A.S.W., Falk M., Foxall R.A., Jamieson W.D., Laycock M.V., McCulloch A.W., McInnes A.G., Odense P., et al. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from Eastern Prince Edward Island. Can. J. Chem. 1989;67:481–490. doi: 10.1139/v89-075. [DOI] [Google Scholar]
- 15.Toyofuku H. Joint FAO/WHO/IOC activities to provide scientific advice on marine biotoxins (research report) Mar. Pollut. Bull. 2006;52:1735–1745. doi: 10.1016/j.marpolbul.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Fast M.D., Cembella A.D., Ross N.W. In vitro transformation of paralytic shellfish toxins in the clams Mya arenaria and Protothaca staminea. Harmful Algae. 2006;5:79–90. doi: 10.1016/j.hal.2005.05.005. [DOI] [Google Scholar]
- 17.Hu T.M., Marr J., Defreitas A.S.W., Quilliam M.A., Walter J.A., Wright J.L.C., Pleasance S. New diol esters isolated from cultures of the dinoflagellates Prorocentrum lima and Prorocentrum concavum. J. Nat. Prod. 1992;55:1631–1637. doi: 10.1021/np50089a011. [DOI] [Google Scholar]
- 18.MacKenzie L.A., Selwood A.I., Marshall C. Isolation and characterization of an enzyme from the Greenshell™ mussel Perna canaliculus that hydrolyses pectenotoxins and esters of okadaic acid. Toxicon. 2012;60:406–419. doi: 10.1016/j.toxicon.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Hu T.M., Curtis J.M., Walter J.A., Wright J.L.C. Identification of DTX4, a new water-soluble phosphatase inhibitor from the toxic dinoflagellate Prorocentrum lima. J. Chem. Soc. Chem. Commun. 1995:597–599. doi: 10.1039/C39950000597. [DOI] [Google Scholar]
- 20.Hu T.M., Curtis J.M., Walter J.A., McLachlan J.L., Wright J.L.C. Two new water-soluble DSP toxin derivatives from the dinoflagellate Prorocentrum maculosum: Possible storage and excretion products. Tetrahedron Lett. 1995;36:9273–9276. doi: 10.1016/0040-4039(95)02010-M. [DOI] [Google Scholar]
- 21.Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases—Specificity and kinetics. Biochem. J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takai A., Murata M., Torigoe K., Isobe M., Mieskes G., Yasumoto T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992;284:539–544. doi: 10.1042/bj2840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagi T., Murata M., Torigoe K., Yasumoto T. Biological-activities of semisynthetic analogs of dinophysistoxin-3, the major diarrhetic shellfish toxin. Agric. Biol. Chem. 1989;53:525–529. [Google Scholar]
- 24.Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: An additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl. Acad. Sci. USA. 1988;85:1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing Y., Xu Y., Chen Y., Jeffrey P.D., Chao Y., Lin Z., Li Z., Strack S., Stock J.B., Shi Y. Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell. 2006;127:341–353. doi: 10.1016/j.cell.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama N., Konoki K., Tachibana K. Isolation and characterization of okadaic acid binding proteins from the marine sponge Halichondria okadai. Biochemistry. 2007;46:11410–11420. doi: 10.1021/bi700490n. [DOI] [PubMed] [Google Scholar]
- 27.Konoki K., Saito K., Matsuura H., Sugiyama N., Cho Y., Yotsu-Yamashita M., Tachibana K. Binding of diarrheic shellfish poisoning toxins to okadaic acid binding proteins purified from the sponge Halichondria okadai. Bioorg. Med. Chem. 2010;18:7607–7610. doi: 10.1016/j.bmc.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Marston A. Natural products as a source of protein kinase activators and inhibitors. Curr. Top. Med. Chem. 2011;11:1333–1339. doi: 10.2174/156802611795589575. [DOI] [PubMed] [Google Scholar]
- 29.McCluskey A., Sim A.T.R., Sakoff J.A. Serine-threonine protein phosphatase inhibitors: Development of potential therapeutic strategies. J. Med. Chem. 2002;45:1151–1175. doi: 10.1021/jm010066k. [DOI] [PubMed] [Google Scholar]
- 30.Amzil Z., Pouchus Y.F., Leboterff J., Roussakis C., Verbist J.F., Marcailloulebaut C., Masselin P. Short-time cytotoxicity of Mussel extracts—A new bioassay for okadaic acid detection. Toxicon. 1992;30:1419–1425. doi: 10.1016/0041-0101(92)90517-9. [DOI] [PubMed] [Google Scholar]
- 31.Tubaro A., Florio C., Luxich E., Vertua R., DellaLoggia R., Yasumoto T. Suitability of the MTT-based cytotoxicity assay to detect okadaic acid contamination of mussels. Toxicon. 1996;34:965–974. doi: 10.1016/0041-0101(96)00073-6. [DOI] [PubMed] [Google Scholar]
- 32.Fujiki H., Suganuma M., Suguri H., Yoshizawa S., Takagi K., Uda N., Wakamatsu K., Yamada K., Murata M., Yasumoto T., et al. Diarrhetic shellfish toxin, dinophysistoxin-1, is a potent tumor promoter on mouse skin. Jpn. J. Cancer Res. 1988;79:1089–1093. doi: 10.1111/j.1349-7006.1988.tb01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor M.W., Radax R., Steger D., Wagner M. Sponge-associated microorganisms: Evolution, ecology and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007;71:295–347. doi: 10.1128/MMBR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy J., Codling C.E., Jones B.V., Dobson A.D.W., Marchesi J.R. Diversity of microbes associated with the marine sponge, Haliclona simulans, isolated from Irish waters and identification of polyketide synthase genes from the sponge metagenome. Environ. Microbiol. 2008;10:1888–1902. doi: 10.1111/j.1462-2920.2008.01614.x. [DOI] [PubMed] [Google Scholar]
- 35.Piel J. A Polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl. Acad. Sci. USA. 2002;99:14002–14007. doi: 10.1073/pnas.222481399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossignoli A.E., Blanco J. Subcellular distribution of okadaic acid in the digestive gland of Mytilus galloprovincialis: First evidences of lipoprotein binding to okadaic acid. Toxicon. 2010;55:221–226. doi: 10.1016/j.toxicon.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Janer G., Mesia-Vela S., Porte C., Kauffman F.C. Esterification of vertebrate-type steroids in the eastern oyster (Crassostrea virginica) Steroids. 2004;69:129–136. doi: 10.1016/j.steroids.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T., Kamiyama T., Okumura Y., Ishihara K., Matsushima R., Kaneniwa M. Liquid-chromatographic hybrid triple-quadrupole linear-ion-trap MS/MS analysis of fatty-acid esters of dinophysistoxin-1 in bivalves and toxic dinoflagellates in Japan. Fish. Sci. 2009;75:1039–1048. doi: 10.1007/s12562-009-0111-3. [DOI] [Google Scholar]
- 39.Ross A.C. Retinol esterification by rat liver microsomes—Evidence for a fatty acyl coenzyme A—Retinol acyltransferase. J. Biol. Chem. 1982;257:2453–2459. [PubMed] [Google Scholar]
- 40.Yost R.W., Harrison E.H., Ross A.C. Esterification by rat-liver microsomes of retinol bound to cellular retinol-binding protein. J. Biol. Chem. 1988;263:18693–18701. [PubMed] [Google Scholar]
- 41.Torgersen T., Lindegarth S., Ungfors A., Sandvik M. Profiles and levels of fatty acid esters of okadaic acid group toxins and pectenotoxins during toxin depuration, Part I: Brown crab (Cancer pagurus) Toxicon. 2008;52:407–417. doi: 10.1016/j.toxicon.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Torgersen T., Sandvik M., Lundve B., Lindegarth S. Profiles and levels of fatty acid esters of okadaic acid group toxins and pectenotoxins during toxin depuration. Part II: Blue mussels (Mytilus edulis) and flat oyster (Ostrea edulis) Toxicon. 2008;52:418–427. doi: 10.1016/j.toxicon.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T., Mackenzie L., Stirling D., Adamson J. Pectenotoxin-2 seco acid: A toxin converted from pectenotoxin-2 by the New Zealand Greenshell mussel, Perna canaliculus. Toxicon. 2001;39:507–514. doi: 10.1016/S0041-0101(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 44.Cauet G., Degryse E., Ledoux C., Spagnoli R., Achstetter T. Pregnenolone esterification in Saccharomyces cerevisiae—A potential detoxification mechanism. Eur. J. Biochem. 1999;261:317–324. doi: 10.1046/j.1432-1327.1999.00282.x. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin G.L., Saz H.J., Debruyn B.S. Purification and properties of an acyl CoA transferase from Ascaris suum muscle mitochondria. Comp. Biochem. Physiol. B. 1986;83:523–527. doi: 10.1016/0305-0491(86)90290-7. [DOI] [PubMed] [Google Scholar]
- 46.Spector A.A., Mathur S.N., Kaduce T.L. Role of acylcoenzyme A: Cholesterol O-acyltransferase in cholesterol metabolism. Prog. Lipid Res. 1979;18:31–53. doi: 10.1016/0163-7827(79)90003-1. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T., Watanabe R. Shellfish Toxin Monitoring System in Japan and Some Asian Countries. In: Cabado A.G., Vieites J.M., editors. New Trends in Marine and Freshwater Toxins, Food and Safety Concerns. NOVA; New York, NY, USA: 2012. pp. 305–314. [Google Scholar]