Abstract
The changes in distribution and concentration of neuropeptides, gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin, and gonadotropin-releasing hormone receptor (GnRH-R) were evaluated and compared with reproductive parameters, such as cytochrome P450 side-chain cleavage (P450 SCC) enzyme activity, androgen receptors (AR) in the testis and serum testosterone levels, from birth to senescence in mice. The results showed the localization of these molecules mainly in the interstitial and germ cells as well as showed significant variations in immunostatining from birth to senescence. It was found that increased staining of testicular GnRH-R coincided with increased steroidogenic activity during pubertal and adult stages, whereas decreased staining coincides with decreased steroidogenic activity during senescence. Similar changes in immunostaining were confirmed by Western/slot blot analysis. Thus, these results suggest a putative role of GnRH during testicular pubertal development and senescence. Treatment with a GnRH agonist ([DTrp6, Pro9-NEt] GnRH) to mice from prepubertal to pubertal period showed a significant increase in steroidogenic activity of the mouse testis and provided further support to the role of GnRH in testicular pubertal maturation. The significant decline in GnRH-R during senescence may be due to a significant increase in GnIH synthesis during senescence causing the decrease in GnRH-R expression. It is considered that significant changes in the levels of GnRH-R may be responsible for changes in steroidogenesis that causes either pubertal activation or senescence in testis of mice. Furthermore, changes in the levels of GnRH-R may be modulated by interactions among GnRH, GnIH, and kisspeptin in the testis.
The reproductive system undergoes dramatic changes throughout the postnatal period, from the neonatal and infantile period to puberty onset, adulthood, and finally senescence (Fink, 2000; Tena-Sempere and Huhtaniemi, 2003). Changes in such intricate functional and developmental features suggest a multi-faceted nature of the regulatory system responsible for attainment, maintenance, and changes in reproductive capacity of mammals. A recent study on human and other mammalian species suggests reproductive senescence is a complex process that involves altered functions of neuropeptides that control the hypothalamic–pituitary–gonadal axis that respond to estradiol (Downs and Wise, 2009). Gonadotropin-releasing hormone (GnRH) is the most important neuropeptide that provides the primary driving force upon the reproductive axis. In the male rat, senescence-associated reproductive decline was shown to be due to diminished hypothalamic GnRH secretion (Gruenewald et al., ‘94). It has been further reported that hypothalamic GnRH gene expression is decreased in senescent male rats (Gruenewald and Matsumoto, ‘91), which suggests that a reduction in GnRH synthetic capacity may contribute to the senescence-associated decline in gonadotropin secretion and gonadal activity.
Although it was generally believed that GnRH is the only hypothalamic regulator of pituitary gonadotropin synthesis and release, recent studies now suggest that the relationship of GnRH with other neuropeptides is crucial to the synthesis and secretion of GnRH (Herbison, 2006). Signals of various neuropetides participate in the dynamic control of GnRH biosynthesis and release in the hypothalamus. In 2000, Tsutsui and co-workers discovered a novel hypothalamic neuropeptide that, in contrast to GnRH, actively inhibits gonadotropin release in quail and termed it GnIH (Tsutsui et al., 2000). From the past 10 years of research, we now know that GnIH exists in several avian species, including quail, chickens, sparrows, and starlings, and regulates avian reproduction by decreasing gonadotropin release and synthesis via action on the GnRH system and the anterior pituitary gland, mediated via GPR147 (Review, Tsutsui, 2009; Tsutsui et al., 2010a, b). Subsequently, GnIH orthologs were found in various mammals (Review, Tsutsui, 2009; Tsutsui et al., 2010a, b). GnIH and its mammalian orthologs, RF-amide related peptides (RFRPs), belong to the group with the LPXRF-amide carboxyl peptide consensus sequence (X= L or Q) (Review, Tsutsui, 2009; Tsutsui et al., 2010a, b). Mammalian GnIH orthologs that are encoded by the RFRP gene have also been identified in the brains of various mammalian species (Fukusumi et al., 2001, Yoshida et al., 2003; Ukena et al., 2002; Ubuka et al., 2009). For example, either central or peripheral administration of GnIH or RFRP-3, a mammalian GnIH ortholog, dose-dependently inhibited secretion of LH in hamsters (Kriegsfeld et al., 2006), rats (Johnson et al., 2007; Murakami et al., 2008), and sheep (Clark et al., 2008). Another newly identified excitatory neuropeptide, kisspeptin, was shown to be a critical mediator of pubertal transition and ovarian steroid induced LH surge (Lederman et al., 2010). It was shown that the decrease in kisspeptin neurotransmission may contribute to age-related LH surge abnormalities (Lederman et al., 2010). Kisspeptin is the most potent excitatory neuropeptide for GnRH neurons (Han et al., 2005). Kisspeptin neurons may relay signals from periphery to GnRH neuron (Oakley et al., 2009). The discovery of GnIH and kisspeptin and their close relationships with GnRH has opened up a new approach in our understanding of neuroendocrine mechanisms for the control of reproduction (Roa et al., 2009). Most of the recent studies on GnRH, GnIH, and kisspeptin have focused on puberty and regulation of reproduction in young adults (Ebling, 2005; Pineda et al., 2010; Roseweir and Millar, 2009) with only a few studies addressing the importance of these neuropeptides in reproductive aging (Lederman et al., 2010).
Gonads are also the sites of synthesis and binding of many neuropeptides, including GnRH, GnIH, and kisspeptin (McGuire and Bentley, 2010). The significance of these neuropeptides in the gonad has been extensively studied in isolation and mostly during puberty and adult condition (Clarkson and Herbison, 2006). Whether these neuropeptides interact with each other at gonadal level has not yet been investigated. The primary aim of the present study was to evaluate, simultaneously, the changes in the distribution and concentration of three closely related neuropeptides (GnRH, GnIH, and kisspeptin) and GnRH-R in the testis and compare with the changes in cytochrome P450 side-chain cleavage enzyme (P450 SCC), androgen receptors (AR) in the testis and serum testosterone levels, from birth to senescence in mice in order to understand the physiological significance of these molecules in the testis. The result of this study suggests a possible role for GnRH during testicular pubertal development. In order to confirm this finding, an in vivo study was performed to determine whether a GnRH agonist ([DTrp6, Pro9-NEt] GnRH) treatment during the prepubertal period affects testicular steroidogenic enzyme (P450 SCC) and whether this effect is mediated through increase in GnRH-R levels. In order to understand the role of GnIH in senescence, an in vitro study was performed to determine whether GnIH affects testicular GnRH-R.
MATERIALS AND METHODS
Animals
All experiments were conducted in accordance with principles and procedures of the 2002 Animal Act, India, and approved by the Department of Zoology Research Committee, Banaras Hindu University. Male Parkes strain mice of different age groups were used from the inbred colony maintained in our animal house for the present study. Animals were housed under standard laboratory conditions (light:dark 12 hr:12 hr), and were provided with pelleted food and water ad libitum.
Sample Collection
Mice were classified into following age groups: (a) Birth (day 1); (b) prepubertal (4 weeks); (c) pubertal (6 weeks); (d) reproductively active (15 weeks); and (e) senescent (65 weeks). The day of the birth was designated as day 1. Mice were weighed and sacrificed at their respective age groups (n = 7) by decapitation under mild dose of anesthetic ether. Serum was collected from blood and kept at −20° C for testosterone assay. Testis of one side of each animal was kept at −20°C for immunoblot and the other side of the testis was fixed in Bouin’s fluid for histology and immunohistochemistry. Body mass of each mouse was recorded before killing. Both testes were excised out and weighed, and the gonadosomatic index (GSI = weight of testes × 100/weight of mice) was calculated and expressed as weight of the testes in mg/100 g body weight.
Testosterone Assay
ELISA kit for testosterone assay was purchased from Dia Metra, Giustozzi, FOLIGNO (PG) ITALY (LOT No: DKO002) and is validated by spiking control. Twenty-five microliters of standard, control, or sample was added to each well of the ELISA plate. Subsequently, 100 μL of the diluted conjugate solution was added to each of these wells. The ELISA plate was then incubated with mild shaking at 37° C temperature for 1 hr. The wells were then aspirated and washed several times with distilled water. Then 100 μL of the tetramethyl benzidine (TMB) chromagen substrate was added to each well and the plate was incubated at room temperature for 15 min in the dark. Finally, 100 μL of stop solution was added and absorbance was taken at 450 nm using a microplate reader. The standard curve ranged from 0.2 to 16 ng/mL. Unknowns were run within the narrow range representing the most linear portion of the standard curve. The coefficient of intra-assay variation was 5.4% and inter-assay variation was 15%, respectively. The lowest detectable concentration of testosterone that can be distinguished from the zero standard is 0.07 ng/mL at the 95% confidence limit. The cross-reactivity of the antibody with testosterone is 100%, with androstenedione 0.8%, with 5α dihydrotestosterone 16% and with other steroids showed no cross-reactivity.
Histology
Testes were embedded in paraffin wax and serially sectioned of 6 μm; five sets of slides were prepared. One set was used for hematoxylin and eosin staining and other sets were used for immunohistochemistry.
Immunohistochemistry
Testes of mice of different age groups (birth (day 1), prepubertal, pubertal, reproductively active and senescent) were paraffin embedded, and 6 μm sections were analyzed by immunohistochemistry, for GnIH, kisspeptin, GnRH, and GnRH-R. Briefly after deparaffinization and hydration, endogenous peroxidase was quenched with 0.3% H2O2, equilibrated in 0.05 M Tris–HCl, 0.15 M NaCl (TBS, pH 7.4). Background blocking was performed with normal goat and horse serum, respectively. The tissue sections were incubated with primary antibody (see Table 1 for details) in PBS for 1 hr at room temperature in TBS. ABC staining kit was used for amplification. The peroxidase activity was revealed in 0.03% 3,3′-diaminobenzidine tetra-dihydrochloride (DAB; Sigma, St. Louis, MO) in 0.01 M Tris–HCl (pH 7.6) and 0.1% H2O2. Nucleus was counterstained with Ehrlich’s hematoxylin. Slides were then dehydrated and mounted and viewed under a light microscope (Nikon, Tokyo, Japan) and photographed. For control experiments, the antibodies (GnRH, GnIH, and kisspeptin-10) were adsorbed by preincubation with peptides (200 ng of GnRH per mL; 1μg of GnIH per mL; 1 μg of kisspeptin-10 per mL) for 1 hr at room temperature. For preabsorption, the antigens were added to diluted antisera, incubated overnight at 4°C, centrifuged and then the supernatant was used.
Table 1.
Details of antibodies used for immunohistochemistry and Slot/Western blot.
| Antibody | Target species | Source | Concentration (use for IHC) | Concentration (used for Western/slot blot) | Control |
|---|---|---|---|---|---|
| GnRH | Human | Peninsula Lab, Inc., San Carlos, CA, USA (Cat No. IHC-72011) | 1:2,000 | 1:5,000 | Preabsorbed |
| GnRH-R | Human | Santa Cruz Biotechnology, Inc. (Cat No. N-20, SC 8682) | 1:100 | 1:500 | Negative |
| GnIH | Quail | Kazuyushi Tsutsui, Tokyo, Japan | 1:5,000 | 1:8,000 | Preabsorbed |
| Kisspeptin | Mouse | Alain Caraty, Nouzilly, France | 1:5,000 | 1:8,000 | Preabsorbed |
| Cytochrome P450 SCC | Rat | Tamecula, California, Millipore, USA (Cat No. AB1244) | 1:500 | ||
| Androgen Receptor | Human | Santa Cruz Biotech, USA (Cat No. N-20, SC 816) | 1:200 | ||
| Actin | β-Actin | Sigma A2228, 128K4813 | 1:500 |
Immunoblot
The testes were pooled from different age groups to produce 10% (w/v) homogenate and testicular protein was estimated according to Chanda et al. (2003). Equal amounts of proteins (50 μg) determined by Lowry’s method were loaded on SDS–PAGE (10%) for electrophoresis. Thereafter, proteins were transferred electrophoretically to PVDF membrane (Sigma Aldrich, St. Louis, USA) overnight at 4°C. The samples with equal amounts of protein were adjusted to equal volume with PBS and samples were loaded on polyvinylidene fluoride (PVDF) membrane using Millipore slot blot apparatus for slot blot analysis. The transfer efficiency was checked by Ponceau-S staining. PVDF membrane was then blocked for 60 min with TBS (pH 7.6) containing 5% fat dry milk and then incubated with primary antiserum (see Table 1 for details) for 1 hr at room temperature or overnight at 4°C. Then membranes were washed thrice for 10 min each in TBS–Tween 20. Immunodetection was performed with anti-rabbit IgG conjugated horseradish peroxidase (1:1,000) for 4 hr and then washed in TBS for 10 min (three times). Signals were detected using an ECL kit (Bio-Rad, Hercules, CA). Blot for each protein was repeated for three times. The densitometric analysis of the immunoblots was performed by scanning and quantifying the bands for relative integrated density value (RIDV) by using computer-assisted image analysis (Image J 1,38x, NIH, USA). The data were normalized to beta actin levels and expressed relative to control as % RIDV.
Treatment
In Vivo Study
Mice were daily injected, intraperitoneally, with different doses of GnRH agonist ([DTrp6, Pro9-NEt] GnRH) (low = 200 ng/day/body weight, and high = 2 μg/day/body weight) dissolved in normal saline solution for 15 days (n = 8–10 per group). Mice in the control group (n = 10) received vehicle only. The dose of GnRH agonist ([DTrp6, Pro9-NEt] GnRH) was selected based on previous studies with minor alterations (Singh and Krishna, 2010). The treatment started on week 4 postpartum to maintain uniformity in all of the mice in the various groups. The animals were sacrificed by decapitation under mild dose of anesthetic ether within 30 min after the last injection and blood was collected. Body mass of each mouse was recorded before killing. Testes were excised out, cleaned, and weighed. The testis from one side from each animal was frozen and kept at −20°C for immunoblot of cytochrome P450 SCC and GnRH-R protein expression.
In Vitro Study
To study the effect of GnIH on GnRH-R expression, an in vitro study was performed with adult testes for 24 hr were carried out with different doses of GnIH. The dose of GnIH was selected based on previous studies with minor alterations (Ubuka et al., 2006). Adult male mice (n = 3) were sacrificed by decapitation under mild dose of anesthetic ether as soon as they were brought to the laboratory. Their testes were quickly taken out and cleaned of any adhered fat tissue in Dulbecco’s modified Eagle’s medium (DMEM; Himedia, Mumbai, India) containing 250 U/mL penicillin and 250 μg/mL streptomycin sulfate. Testes were cut into equal pieces (approximately 10 mg in weight per piece) and cultured by the method as described previously (Banerjee et al., 2011). Culture medium was a mixture of DMEM (with sodium pyruvate and L-glutamine) and Ham’s F-12 (1:1; v:v) (Himedia) containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.1% BSA (Sigma Aldrich, St. Louis, USA). After initial incubation for 2 hr at 37°C, culture medium was discarded and testes (one per tube) were finally cultured in 1 mL medium in a humidified atmosphere with 95% air and 5% CO2 to maintain pH 7.4 for 24 hr at 37°C with two doses of GnIH low dose (1 ng/mL) and high dose (10 ng/mL). Each treatment group was run in triplicate. Testes cultured under these conditions appear healthy and do not show any sign of necrosis. Testes were collected at the end of culture, washed several times with PBS and kept frozen at − 20°C for immunoblot study.
Statistical Analyses
The densitometric data were presented as the mean of the percentage relative integrated density value ± SEM. The bands obtained from immunoblots were normalized to β-actin (Sigma Aldrich, St. Louis, USA) bands. The significance of the differences in testosterone levels and GnIH, GnRH, GnRH-R, and kisspeptin intensity between groups was determined by one-way analysis of variance (ANOVA) followed by Neuman–Keul’s test. Correlation studies were performed by using SPSS software 12 for window (Apache software foundation) to compare the data from different groups. The data were considered significant if P < 0.05.
RESULTS
Changes in Testicular Mass and Gonadosomatic Index During Different Stages of Aging in Mice
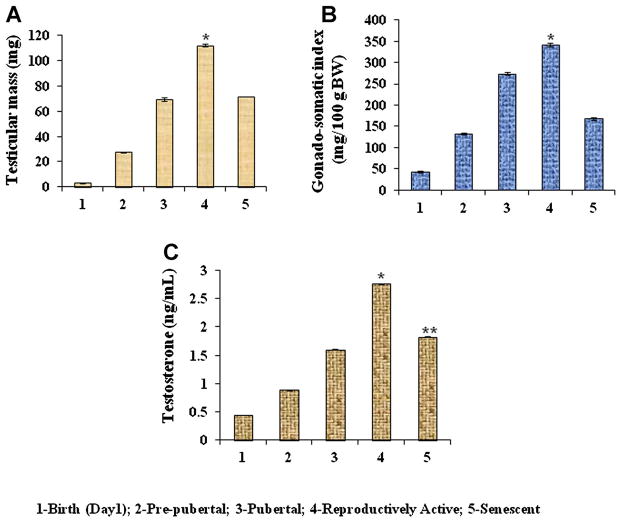
Changes in the testicular mass (mg) and GSI during different stages of aging from birth to senescence of mice are shown in Figure 1A and B. The result showed a gradual increase in body mass from birth to reproductively active state. Both the GSI and testicular mass increased gradually from birth to reproductively active state, but declined significantly (P < 0.05) during senescence in mice (Fig. 1A and B).
Figure 1.
Age-dependent variation in (A) testicular mass and (B) gonadosomatic index (GSI), in mice. Values are represented as mean ± SEM (N = 8–10). *Values are significantly higher (P < 0.05) during reproductively active period vs. other age groups (birth, prepubertal, pubertal and senescent) of mice. C: Changes in serum testosterone levels in mice during development and senescence. Values are mean ± SEM. *Values are significantly higher during reproductively active period (P < 0.05) vs. birth, prepubertal, pubertal and senescence stages. **Values are significantly (P < 0.05) low during senescence versus reproductively active stage of mice. Birth (day 1); prepubertal (1 week); pubertal (6 weeks); reproductively active (15 weeks); and senescent (65 weeks).
Changes in Testosterone Concentrations During Different Stages of Aging in Mice
Changes in the testosterone concentration (ng/mL) during different stages of aging from birth to senescence of mice are shown in Figure 1C. Testosterone concentrations increased gradually from birth to reproductively active state, but declined significantly (P < 0.05) during senescence in mice (Fig. 1C).
Changes in GnRH and GnRH-R Expressions in the Testis During Different Stages of Aging in Mice
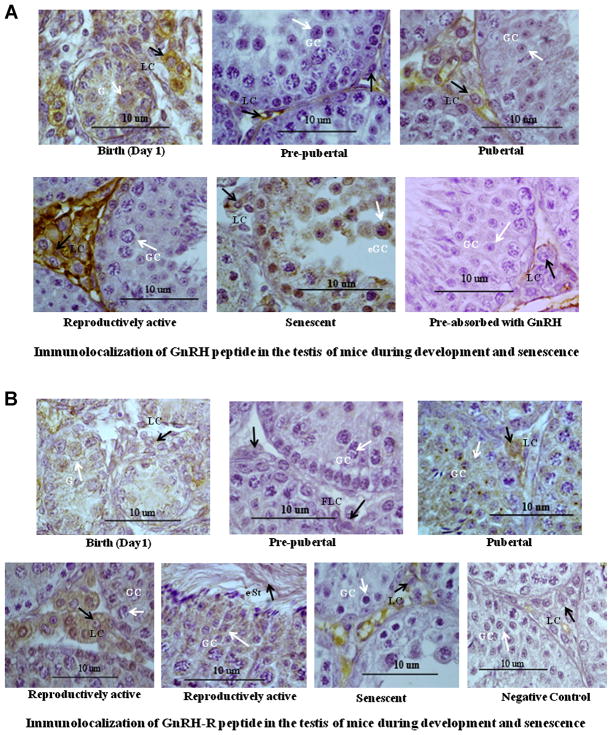
Immunocytochemical localization of GnRH in the testis of mice showed marked variation from birth to senescence (Fig. 2A). The GnRH positive staining was mainly localized in the interstitial cells during different stage of aging, except during the senescence when staining was seen in the germ cells. At birth, moderate immunostaining of GnRH was found mainly in the Leydig cells. Immunostaining of GnRH decreased markedly in the Leydig cells during prepubertal period. Immunostaining of GnRH increased markedly in the Leydig cells during pubertal period. The immunostaining of GnRH increased further in the interstitial cells during reproductively active stage. The moderate immunostaining of GnRH was found mainly in the germ cells during senescence as well as with mild staining in interstitial cells.
Figure 2.
A: Immunolocalization of GnRH peptide in the testis of mice during development and senescence. Immunoreactivity of GnRH increased in the interstitium during development. Strong immunostaining of GnRH was observed in the Leydig cell (LC) in the birth, pubertal, and reproductive active stages, whereas moderate immunostaining in germ cell (GC) of disorganized seminiferous tubule during senescence. Black arrow head showing immunostaining in Leydig cell (LC) whereas white arrow head showing immunostaining in gonocyte (G), germ cell (GC), and exfoliated germ cell (e GC) during birth and senescent, respectively. All figures are shown in 100 × magnification. B: Immunolocalization of GnRH receptor (GnRH-R) in the testis of mice during development and senescence. Immunoreactivity of GnRH-R increased in the interstitium during development. Strong immunostaining of GnRH-R was observed in the Leydig cell, germ cell as well as in elongated spermatids during pubertal and reproductive active stages. Mild immunostaining in Leydig cell and no immunoreactivity in germ cell in senescent. Black arrow head showing immunostaining in Leydig cell (LC) whereas white arrow head showing immunostaining in gonocyte (G), germ cell (GC) during birth, pubertal, reproductively active period and no immunostaining in germ cell (GC) of atrophied seminiferous tubule and senescent, respectively. All figures are shown in 100 × magnification. [Color figure can be seen in the online version of this article, available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1932-5231]
Immunocytochemical localization of GnRH-R in the testis of mice showed marked variation from birth to senescence (Fig. 2B). The GnRH-R positive staining was localized in both the interstitial cells and germ cells during different stage of aging. At birth, moderate immunostaining of GnRH-R was found both in the Leydig cells and germ cells. Immunostaining of GnRH-R decreased markedly both in the Leydig cells and germ cells during prepubertal period. Immunostaining of GnRH-R increased markedly in the Leydig cells and germ cells during pubertal period and continued in the interstitial and germ cells as well as in elongated spermatids during reproductively active stage. The mild immunostaining of GnRH-R was found mainly in the interstitial cells during senescence.
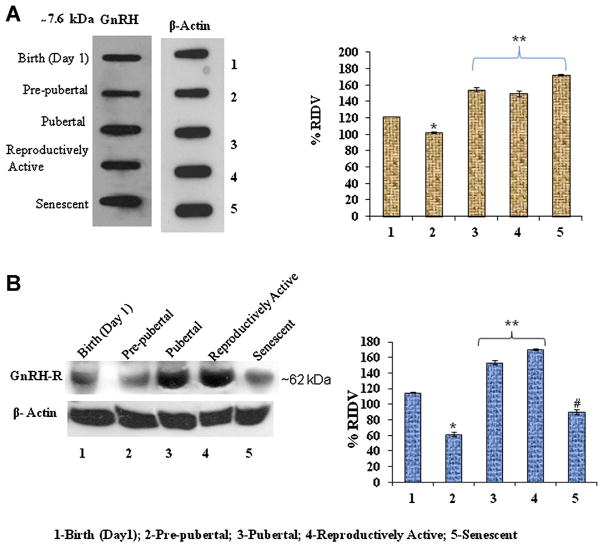
The densitometric analysis of testicular GnRH and GnRH-R immunoblots from birth to senescence showed significant variation. The immunoreactivity of GnRH and GnRH-R decreased significantly (P < 0.05) from during birth to prepubertal testis. The immunoreactivity of GnRH and GnRH-R increased significantly (P < 0.05) during pubertal and reproductively active stages (Fig. 3A and B). However, the immunoreactivity of GnRH significantly increased (P < 0.05), but immunoreactivity of GnRH-R significantly decreased (P < 0.05) during senescence (Fig. 3A and B).
Figure 3.
A: Slot blots analysis of GnRH peptide in the testis of Parkes strain mice of different age groups. *Values are significantly (P < 0.05) decreased in prepubertal period versus birth. **Values are significantly (P < 0.05) increased during puberty, reproductively active, and senescence versus birth and prepubertal stage of mice. B: Immunoblot analysis of GnRH-R protein expression in the testis of mice of different age groups. *Values are significantly (P < 0.05) decreased in prepubertal period versus birth. **Values are significantly (P < 0.05) increased during pubertal and reproductively active stages versus birth, prepubertal, and senescent mice. #Values are significantly (P < 0.05) decreased in senescence versus birth, pubertal, and reproductively active stages of mice. [Color figure can be seen in the online version of this article, available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1932-5231]
Changes in GnIH and Kisspeptin Expressions in the Testis During Different Stages of Aging in Mice
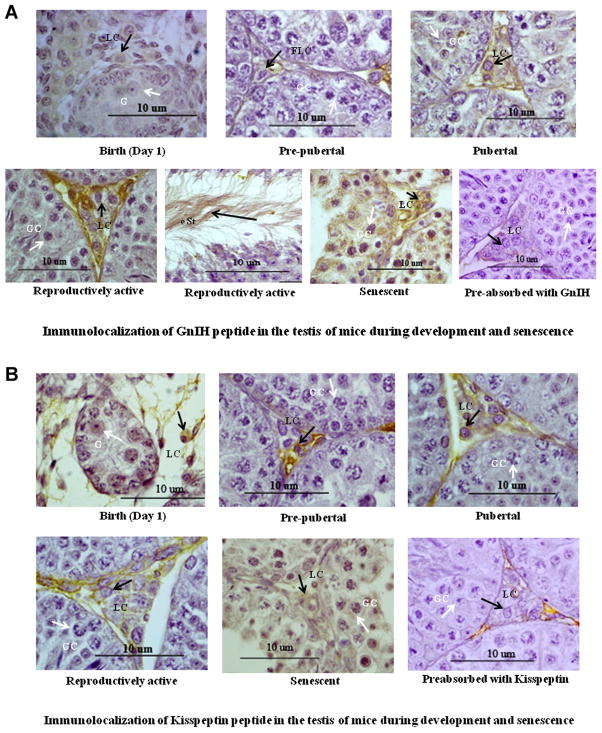
Immunocytochemical localization of GnIH and kisspeptin in the testis of mice also showed marked variation from birth to senescence. The GnIH and kisspeptin positive staining was localized in both the interstitial cells during different stage of aging. At birth, moderate immunostaining of GnIH and kisspeptin was found in the Leydig cells and in the primordial germ cells of testis. Immunostaining of GnIH and kisspeptin decreased marginally both in the Leydig cells during prepubertal period. Immunostaining of GnIH and kisspeptin increased in the Leydig cells during pubertal period. The strong immunostaining of GnIH were found in Leydig cell and elongated spermatids and moderate immunostaining of kisspeptin were found mainly in the interstitial cells during reproductively active stage and strong immunoreactivity of GnIH and kisspeptin was observed in degenerated germ cell and the Leydig cell in the testis of senescent mice (Fig. 4A and B).
Figure 4.
A: Immunolocalization of GnIH in the testis of mice during development and senescence. Immunoreactivity of GnIH showed mild staining in Leydig cell (LC) from birth to prepubertal stages, no staining in gonocyte (G) and strong staining in Leydig cell (LC) in the testis during pubertal and reproductively active period and in germ cells as well as in elongated spermatids during senescence of mice. Black arrow head showing immunostaining in Leydig cell (LC) whereas white arrow head showing immunostaining in germ cell (GC) during senescent. All figures are shown in 100 × magnification. B: Immunolocalization of kisspeptin in the testis of mice during development and senescence. Immunoreactivity of kisspeptin protein was mainly localized in the interstitium and Leydig cell in the testis. Immunoreactivity of kisspeptin showed mild to moderate staining in Leydig cell (LC) from Birth to prepubertal stages and strong staining in the Leydig cell (LC) during pubertal, reproductively active and in germ cells (GC) during senescent mice. Black arrow head showing immunostaining in Leydig cell (LC) whereas white arrow head showing immunostaining in germ cell (GC) during senescent. All figures are shown in 100 × magnification. [Color figure can be seen in the online version of this article, available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1932-5231]
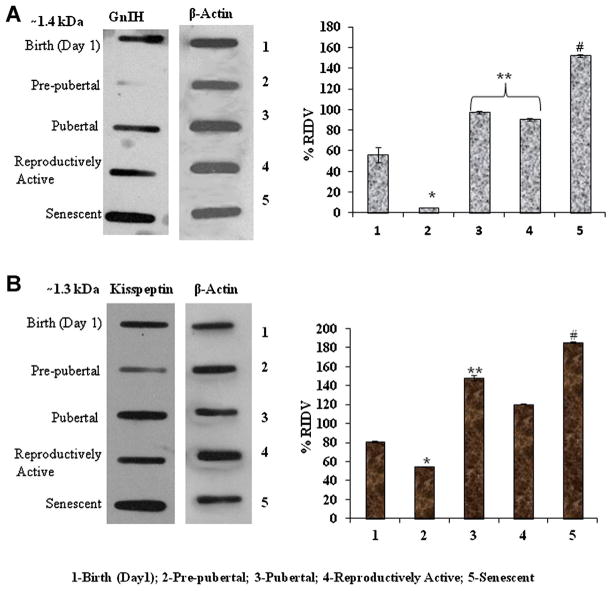
The densitometric analysis of testicular GnIH and kisspeptin immunoblots showed significant variation from birth to senescence. The immunoreactivity of GnIH and kisspeptin decreased significantly (P < 0.05) from birth to prepubertal testis. The immunoreactivity of GnIH increased significantly (P < 0.05) during pubertal and reproductively active stages (Fig. 5A) and kisspeptin subsequently increased significantly (P < 0.05) during pubertal stage (Fig. 5B).
Figure 5.
A: Slot blots analysis of GnIH peptide expression in the testis of Parkes strain mice of different age groups. *Values are significantly decreased (P < 0.05) in prepubertal period vs. birth of mice. **Values are significantly increased (P < 0.05) during puberty, reproductively active vs. birth and prepubertal stage of mice. #Values are highly significant (P < 0.01) increased in senescence versus pubertal and reproductively active stage of mice. B: Slot blots analysis of kisspeptin protein expression in the testis of Parkes strain mice of different age groups. *Values are significantly decreased (P < 0.05) in prepubertal period versus birth of mice. **Values are significantly increased (P < 0.05) in pubertal versus reproductively active stage of mice. #Values are highly significant (P < 0.01) increased in senescence versus pubertal and reproductively active stage of mice. [Color figure can be seen in the online version of this article, available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1932-5231]
The immunoreactivity of GnIH and kisspeptin further increased (P < 0.05) during senescence (Fig. 5A and B).
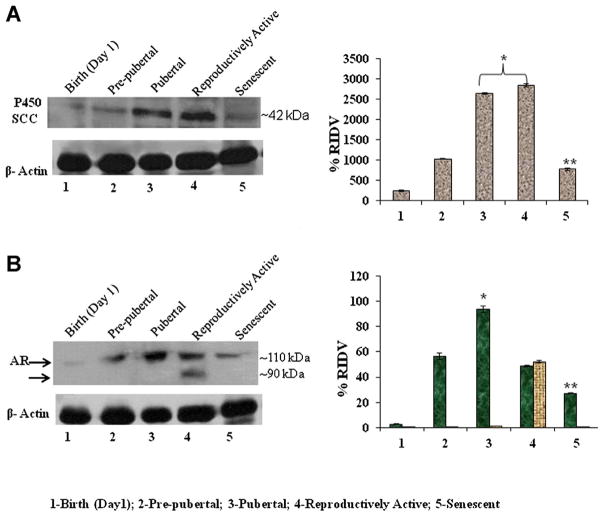
Changes in Testicular Cytochrome P450 SCC and Androgen Receptor (AR) Protein Levels During Different Stages of Aging in Mice
The changes in cytochrome P450 SCC and androgen receptor protein levels in the testis were studied by immunoblot followed by densitometric analysis during different stages of aging, from birth to senescence (Fig. 6A). The immunoblot of cytochrome P450 SCC protein showed a single immunoreactive band at about 42 kDa and androgen receptor protein showed double immunoreactive bands at about 90 and 110 kDa, respectively. The result showed marked changes in protein levels, as measured by immunoreactivity, of both cytochrome P450 SCC and androgen receptor in the testis of mice during different stages from birth to senescence. The intensity of immunoblot of cytochrome P450 SCC increased significantly (P < 0.05) from birth to pubertal reproductively active states and then declined significantly (P < 0.05) during senescence (Fig. 6A). The protein level, as measured by immunoreactivity, of androgen receptor increased significantly (P < 0.05) from prepubertal period to pubertal period of mice. Double immunoreactive bands at about 90 and 110 kDa were clearly observed in the reproductively active testis and then declined significantly (P < 0.05) during senescence (Fig. 6B).
Figure 6.
A: Immunoblot analysis of P450 SCC and AR expression in the testis of mice in different age groups. *Values are significantly increased (P < 0.05) during pubertal and reproductively active stages versus birth and prepubertal stage of mice. **Values are significantly declined (P < 0.05) in senescence versus prepubertal, pubertal, and reproductively active stage of mice. B: The immunoblot analysis of AR was detected two immunoreactive molecules against AR antibody in the reproductively active mice testes. *Values are significantly (P < 0.05) increased during pubertal stage versus birth, prepubertal, and reproductively active stages of mice. **Values are highly significant (P < 0.01) decreased in senescence versus prepubertal, pubertal, and reproductively active stages of mice. [Color figure can be seen in the online version of this article, available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1932-5231]
Correlation Between Testicular GnRH, GnIH, Kisspeptin, and GnRH-R With Testicular P450 SCC and Serum Testosterone Concentrations of Mice During Different Stages of Aging
The results showed a significant correlation between changes in GnRH with GnIH and kisspeptin concentrations in the testis of mice from birth to senescence. Changes in the expression of testicular GnRH-R showed a significant correlation with testicular cytochrome P450 SCC and serum testosterone concentrations in mice (Table 2).
Table 2.
Correlations between testicular GnRH and GnRH-R with testicular GnIH, kisspeptin, P450 SCC and serum testosterone concentrations of mice during different stages of aging (birth to senescence).
| GnIH | KP-10 | Testosterone | Cytochrome P450 SCC | |
|---|---|---|---|---|
| GnRH | r = 0.924; P < 0.05 | r = 0.940; P < 0.05 | NS | NS |
| GnRH-R | NS | NS | r = 0.7783; P < 0.05 | r = 0.7529; P < 0.05 |
NS—non significant, values are significantly different at P < 0.05.
Effects of In Vivo Treatment of GnRH-R Agonist to Prepubertal Mice on Testicular Expressions of Cytochrome P450 SCC and GnRH-R Protein
Densitometric analysis of immunoblot showed that a marked variation of the percentage relative integrated density value (% RIDV) of GnRH-R and cytochrome P450 SCC proteins in the testis of mice treated with different doses (200 ng and 2 μg/day/body weight) of GnRH-agonist ([DTrp6, Pro9-NEt] GnRH) (Fig. 7A and B). The low dose of GnRH-agonist produced no significant variation, whereas the higher dose produced a significant increase (P < 0.05) in the expressions of GnRH-R protein in the treated mice as compared with control (Fig. 7A). GnRH-agonist produced dose-dependent significant increases (P < 0.05) in the expressions of cytochrome P450 SCC protein in the testis of treated mice as compare to control (Fig. 7B).
Figure 7.
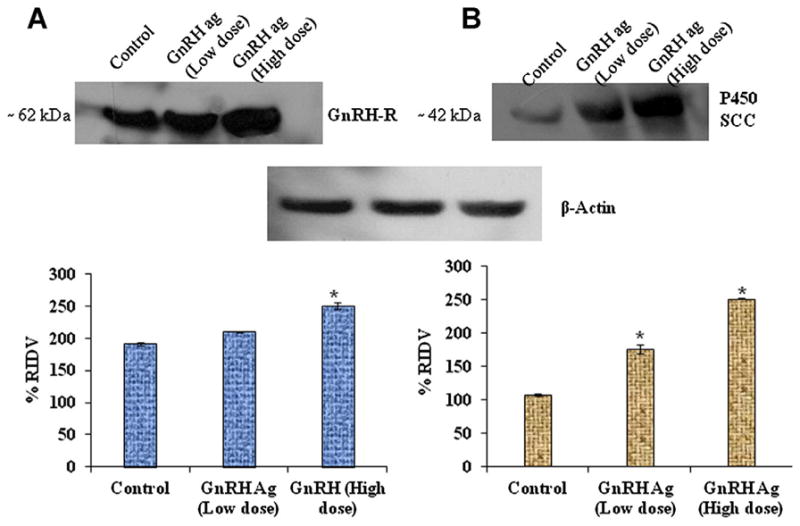
Effect of GnRH-agonist ([DTrp6, Pro9-NEt] GnRH) on GnRH-R and P450 SCC expressions. Different doses of GnRH treatment during prepubertal stage showed significant (P < 0.05) increased in expression of testicular GnRH-R in 2 μg/body weight versus control (A). *Values are significant (P < 0.05) increased in expression of testicular P450 SCC in both doses of GnRH (200 ng/day and 2 μg/day) versus control (B). [Color figure can be seen in the online version of this article, available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1932-5231]
Effects of In Vitro GnIH Treatment on Testicular Expression of GnRH-R Protein
Densitometric analysis of immunoblot showed a significant variation of the % RIDV of GnRH-R protein in the testis of mice treated with different doses of GnIH (low dose = 1 ng/mL and high dose = 10 ng/mL). GnIH treatment produced dose dependent significant decreased (P < 0.05) expression of GnRH-R protein in the testis as compared with the control mice (Fig. 8).
Figure 8.
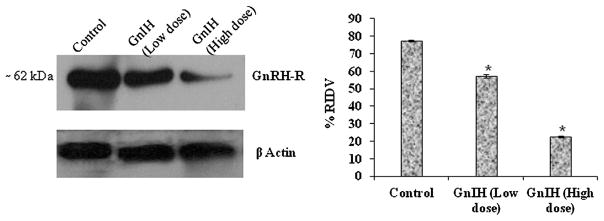
Effect of GnIH on GnRH receptor (GnRH-R) protein expression in the testis. The in vitro study revealed that 1 and 10 ng/mL of GnIH significantly (P < 0.05) decreased the expression of testicular GnRH-R versus control. *Values are significant (P < 0.05) versus control.
DISCUSSION
The present results showed a significant variation in the expressions of GnRH, GnIH, kisspeptin, and GnRH-R in the testis of mice from birth to senescence. Our study showed the presence of three important neuropeptides (GnRH, GnIH, kisspeptin) and GnRH-R in the testis of mice at the time of birth. This is in agreement with earlier studies showing the presence of GnRH and GnRH-R in the testes of newborn and mature rats (Huhtaniemi et al., ‘85). Fetal expressions of the GnRH and GnRH-R genes were also reported in the rat testis (Botte et al., ‘98). Our study, thus, supports earlier findings that GnRH may affect development and function of the testis as early as perinatal stage (Ugrumov et al., ‘85). The immunostaining of these neuropeptides (GnRH, GnIH, and kisspeptin) and GnRH-R increased markedly from prepubertal to pubertal period and paralleled with the increase in cythochrome P450 SCC levels in the testis of mice. This increase in GnRH during pubertal period may be responsible for activation of the H–P–G axis and responsible for elevation in testosterone levels, which is required for completion of the inguinoscrotal phase of testicular descent (Pitteloud et al., 2002). The presence of GnRH and GnRH-R has been demonstrated in the adult testis of several mammals (Sharpe and Fraser, ‘80; Bhasin et al., ‘83; Sharp and Cooper, ‘87; Bull et al., 2000; Adams, 2005; Ramakrishnappa et al., 2005). In mature rats, as well as adult humans, GnRH-R mRNA was found in the interstitial cells (Bahk et al., ‘95) and GnRH-R mRNA was demonstrated in testicular germ cells of the rat and mouse (Adams, 2005). The present immunohistochemical study showed marked increases in the immunostainings of GnRH, GnIH, kisspeptin, and GnRH-R particularly in Leydig cells during pubertal period. GnRH-R and GnIH immunostaining also appeared in the sperm during reproductively active period. This study suggests the role of these neuropeptides (GnRH, kisspeptin, and GnIH) and GnRH-R in the regulation of testicular steroid synthesis and/or sperm maturation. Recently, the expressions of GnIH and GnIH-R have also been shown in the gonads of avian species (Bentley et al., 2008). GnIH may also be involved in the regulation of germ cell differentiation as both GnIH and GnIH-R were expressed in germ cells (spermatocytes and spermatids) in the testis (Bentley et al., 2008). These studies suggest direct action of GnIH on the Leydig cell via GnIH-R in avian gonads (Bentley et al., 2008). The expression of kisspeptin and its receptor, GPR-54, genes have also been demonstrated in human and rodent gonads (Kotani et al., 2001; Ohtaki et al., 2001; Funes et al., 2003; Tena-Sempere, 2006; Castellano et al., 2006; Shahab et al., 2005). This study interestingly showed a shift in GnRH immunostaining mainly from Leydig cells during reproductively active period to mostly in the germ cells in the testis of senescent mice. This could be one of the reasons for the increase in GnRH concentration in the testis of senescent mice despite the sharp decline in GnRH immunostaining in the Leydig cells. Whether this shift in immunoreactivity is due to disorganization of the aged testis needs further study. The present observation together with earlier studies, thus suggests that testicular GnRH and GnIH may play an autocrine or paracrine regulation of gonadal development and functions, including testicular steroidogenesis and sperm maturation (Uzbekova et al., 2001; Zhao et al., 2010). Localization of GnRH, particularly in the germ cells of rats and mice (Ramakrishnappa et al., 2005) and GnIH in birds (Bentley et al., 2008; McGuire and Bentley, 2010) and hamsters (Uzbekova et al., 2001), suggests possible roles of GnRH and GnIH in spermatogenesis.
Whether the effect of GnRH is modulated by GnIH and kisspeptin in the testis during various phases of growth and development has not so far been studied. Our study showed a positive correlation between testicular GnRH, GnIH, and kisspeptin from birth to senescence. It had earlier been shown that GnIH inhibits, whereas kisspeptin stimulates, GnRH secretion and action (Han et al., 2005; Tsutsui et al., 2010a, b). The present in vitro treatment with GnIH caused a marked decline in the concentration of GnRH-R in the mouse testis. Thus, it is hypothesized that action of GnIH on GnRH may be responsible for modulating expression of GnRH-R in the testis.
One of the important observations of this study was the significant increase in the expression of GnRH, GnIH, kisspeptin, and GnRH-R in the testis from prepubertal to pubertal period. The changes in these parameters correlated significantly with the increase in testicular activity as shown by increases in the testicular weight and circulating testosterone concentration. This observation suggests that increased expressions of these neuropeptides (GnRH, GnIH, and kisspeptin) and GnRH-R may be essential for the increase in steroidogenesis in the testis responsible for pubertal development. Earlier study on bull immunized with GnRH showed suppressed testicular development, including suppressed circulating testosterone concentrations (Cook et al., 2000). It is well-recognized that increased stimulation of GnRH neurons within the hypothalamus of juvenile mammals results in the onset of puberty followed by full activation of the H–P–G axis (Chandolia et al., ‘97). In the present study, the treatment with GnRH-agonist ([DTrp6, Pro9-NEt] GnRH) to prepubertal mice for 15 days, showed significant increases in the expression of the steroidogenic enzyme cythochrome P450 SCC and GnRH-R protein in the testis, which suggests that testicular GnRH-R concentration induces progonadotropic changes in prepubertal stage. This finding suggests that GnRH promotes pubertal activation by stimulating testicular steroidogenesis. Earlier study noted that testicular growth is enhanced and spermatogenesis and Sertoli cell number increased in testicular tissues of yearling bull receiving supplemental GnRH (Zirkin and Chen, 2000).
The other important observation of this study is the significant decline in the concentration of GnRH-R in the testis during senescence. The decreased GnRH-R concentration coincides with the significant decrease in circulating testosterone levels in mice during senescence. It is well known that serum testosterone levels decrease with aging as shown earlier in rats (Wang and Stocco, 2005). The capacity of Leydig cells to produce testosterone is higher in adult than in old rats. Earlier studies reported the decreased StAR level in the Leydig cells of old mice suggesting that there may be deficits in the transport of cholesterol to the inner mitochondrial membrane during aging (Chen et al., ‘96). The present study showed a significant decrease in the concentration of cytochrome P450 SCC enzyme in the senescent mice as compared with adult mice. It had earlier been shown in the brown Norway rat that the cause of age-related reduction in steroidogenesis is not due to the decline in LH levels (Gruendelwald et al., 2000). In humans, a decrease in serum testosterone levels typically begins in the fifth decades in males (Bélanger et al., ‘94; Zwart et al., ‘96). Such a decrease in testosterone levels is accompanied by either increased or unchanging levels of LH (Zwart et al., ‘96). These observations do not rule out age-related deficits of the H–P axis during human aging, suggesting a primary testicular deficit. In this study, the expressions of testicular GnRH, GnIH, and kisspeptin increased significantly during the senescence period, whereas the testicular expression of GnRH-R protein declined significantly during this period. The decreased GnRH-R correlated closely with the significant decline in testosterone synthesis. The reason for such differential regulation of GnRH and GnRH-R during the senescence is not clear from the present study. However, our present observation is in agreement with earlier findings in the male brown Norway rat showing that the age related reproductive decline is thought to be, in part, due to decreased GnRH action (Gruendelwald et al., 2000). The decreased expression of GnRH-R in the testis could be due to increased synthesis of GnIH during senescence. Our in vitro study clearly showed down-regulation of GnRH-R in the testis of mice treated with GnIH as in senescence.
Data presented in this study showed biphasic regulation of GnRH-R in the testis during different reproductive phases. The increased concentration of GnRH caused down-regulation of GnRH-R from birth to senescence, except the prepubertal to pubertal period, where the increase in GnRH concentrations caused up-regulation of GnRH-R. The significant increase in GnRH-R expression coincided with the increased rate of steroidogenesis during pubertal period, whereas the decreased GnRH-R during senescence coincided with the decreased rate of steroidogenesis in the testis. Therefore, the changes in GnRH-R expression may be responsible for pubertal activation and senescence in the testis of mice. What causes the variation in GnRH-R expression is not clear from the present study. Further study is required to determine whether aged testes are capable of responding to exogenous GnRH agonist. It is likely that the cumulative effects of interaction of GnRH with GnIH and kisspeptin may regulate testicular expression of GnRH-R and may be responsible for the biphasic effects observed in the present study. More detailed studies are required to confirm this hypothesis.
In brief, the present study showed significant variations in the concentrations of GnRH, GnIH, kisspeptin, and GnRH-R in the testis of mice from birth to senescence and showed a significant correlation with the changes in testicular steroidogenic factors and circulating testosterone concentrations. Immunohistochemical analyses showed localization of testicular GnRH, GnIH, kisspeptin, and GnRH-R mainly in the interstitial and germ cells. Our results suggest that GnRH, GnIH, and kisspeptin may act as autocrine or paracrine factors for the regulation of gonadal development and function. Treatment with GnRH-agonist ([DTrp6, Pro9-NEt] GnRH) to mice during prepubertal periods produced significant increases in the expressions of steroidogenic enzyme P450 scc and GnRH-R in the testis. This observation suggests possible roles of GnRH in testicular maturation. The significant decline in GnRH-R coincided with the decrease in testosterone synthesis during senescence. The decreased GnRH-R expression may be due to the increase in GnIH synthesis during senescence. The GnRH-R expression may be modulated by the interactions among GnRH, GnIH, and kisspeptin at the level of the testes. Further study is needed to substantiate this hypothesis.
Acknowledgments
We thank Dr. Alain Caraty at the Institut National de la Recherche Agronomique de Tours for the generous gift of KP-10 antibody. This work was supported in part by grant-in-aid from UGC, New Delhi to A.K.; scientific research from the Ministry of Education, Science and Culture, Japan (18107002, 22132004, and 22227002) to K.T. and grants from NIH, USA (HD41749, HD52155, and RR03034) to R.S. and S.A. highly acknowledges the financial assistance from the UGC, New Delhi.
LITERATURE CITED
- Adams TE. Using gonadotropin-releasing hormone (GnRH) and GnRH analogs to modulate testis function and enhance the productivity of domestic animals. Anim Reprod Sci. 2005;88:127–139. doi: 10.1016/j.anireprosci.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth Defect Res B. 2011;92:195–205. doi: 10.1002/bdrb.20295. [DOI] [PubMed] [Google Scholar]
- Bélanger A, Candas B, Dupont A, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Heber D, Peterson M, Swerdloff R. Partial isolation and characterization of testicular GnRH-like factors. Endocrinology. 1983;112:1144–1146. doi: 10.1210/endo-112-3-1144. [DOI] [PubMed] [Google Scholar]
- Botte MC, Chamagne AM, Carre MC, Counis R, Kottler ML. Fetal expression of GnRH and GnRH receptor genes in rat testis and ovary. J Endocrinol. 1998;159:179–189. doi: 10.1677/joe.0.1590179. [DOI] [PubMed] [Google Scholar]
- Bahk JY, Hyun JS, Chung SH. Stage specific identification of the expression of GnRH mRNA and localization of the GnRH receptor in mature rat and adult human testis. J Urol. 1995;154:1958–1961. [PubMed] [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, et al. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol. 2008;156:34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Bull P, Morales P, Huyser C, Socías T, Castellón EA. Expression of GnRH receptor in mouse and rat testicular germ cells. Mol Hum Reprod. 2000;6:582–612. doi: 10.1093/molehr/6.7.582. [DOI] [PubMed] [Google Scholar]
- Clark IJ, Sari IP, Qi Y, et al. Potent action of RFamide-related peotide-3 on pituitary gonadotropes indicative of a hypophysio-tropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda D, Ahbilasha, Krishna A. Seasonal adiposity and delayed ovulation in a vespertilionid bat, Scotophilus heathi: role of tumor necrosis factor-α. Physiol Biochem Zool. 2003;76:71–80. doi: 10.1086/367941. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147:4852–4862. doi: 10.1210/en.2006-0117. [DOI] [PubMed] [Google Scholar]
- Cook RB, Popp JD, Kastelic JP, Robbins S, Harland R. The effects of active immunization against GnRH on testicular development, feedlot performance, and carcass characteristics of beef bulls. J Anim Sci. 2000;78:2778–2783. doi: 10.2527/2000.78112778x. [DOI] [PubMed] [Google Scholar]
- Chandolia RK, Honaramooz A, Bartlewski PM, Beard AP, Rawlings NC. Effects of treatment with LH releasing hormone before the early increase in LH secretion on endocrine and reproductive development in bull calves. J Reprod Fertil. 1997;111:41–50. doi: 10.1530/jrf.0.1110041. [DOI] [PubMed] [Google Scholar]
- Chen HL, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJP. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–683. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- Fink G. Neuroendocrine regulation of pituitary function: general principles. In: Conn PM, Freeman ME, editors. Neuroendocrinology in physiology and medicine. New Jersey: Humana Press; 2000. pp. 107–134. [Google Scholar]
- Fukusumi S, Habata Y, Yoshida H, et al. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochem Biophys Acta. 2001;1540:221–232. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Hess DL, Matsumoto AM. The Brown Norway rat as a model for male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol Biol Sci Ser B. 1994;49:42–50. doi: 10.1093/geronj/49.2.b42. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Age-related decreases in serum gonadotropin levels and gonadotropin-releasing hormone gene expression in the medial preoptic area of the male rat are dependent upon testicular feedback. Endocrinology. 1991;129:2442–2450. doi: 10.1210/endo-129-5-2442. [DOI] [PubMed] [Google Scholar]
- Gruendelwald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalmic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male brown Norway rat. J Androl. 2000;21:72–84. [PubMed] [Google Scholar]
- Herbison AE. In Knobil and Neill’s physiology of reproduction. In: Neill JD, editor. Physiology of the GnRH neuronal network. Vol. 3. San Diego: Academic press; 2006. pp. 1415–1482. [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi IT, Catt KJ, Clayton RN. Newborn and immature rat testes contain gonadotropin-releasing hormone (GnRH) receptors, and their testosterone production is stimulated by a GnRH agonist in vitro. Mol Cell Endocrinol. 1985;40:41–44. doi: 10.1016/0303-7207(85)90156-x. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide related peptide-3 stimulates GH secretion, inhibits LH secretion, and has a variable effect on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, et al. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Lederman MA, Lebesgue D, Gonzalez VV, et al. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology. 2010;58:314–320. doi: 10.1016/j.neuropharm.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, et al. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- McGuire NL, Bentley GE. Neuropeptides in the gonads: from evolution to pharmacology. Front Pharmacol. 2010;1:114–126. doi: 10.3389/fphar.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Pineda R, Garcia-Galiano D, Roseweir A, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151:722–730. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Hayes FJ, Boepple PA, et al. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metabol. 2002;87:152–160. doi: 10.1210/jcem.87.1.8131. [DOI] [PubMed] [Google Scholar]
- Roa J, Castellano JM, Navarro VM, et al. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides. 2009;30:57–66. doi: 10.1016/j.peptides.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Millar RP. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update. 2009;15:203–212. doi: 10.1093/humupd/dmn058. [DOI] [PubMed] [Google Scholar]
- Ramakrishnappa N, Rajamahendran R, Lin YM, Leung PCK. GnRH in non-hypothalamic reproductive tissues. Anim Reprod Sci. 2005;88:95–113. doi: 10.1016/j.anireprosci.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Singh P, Krishna A. Effects of GnRH agonist treatment on steroidogenesis and folliculogenesis in the ovary of cyclic mice. J Ovarian Res. 2010;3:26–38. doi: 10.1186/1757-2215-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Fraser HM. Leydig cell receptors for luteinizing hormone releasing hormone and its agonists and their modulation by administration or deprivation of the releasing hormone. Biochem Biophys Res Commun. 1980;95:256–262. doi: 10.1016/0006-291x(80)90732-9. [DOI] [PubMed] [Google Scholar]
- Sharp RM, Cooper I. Comparison of the effects on purified Leydig cells of four hormones (oxytocin, vasopressin, opiates and LHRH) with suggested Paracrine roles in the testis. J Endocrinol. 1987;113:89–96. doi: 10.1677/joe.0.1130089. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena-Sempere M, Huhtaniemi I. Gonadotropins and gonadotropin receptors. In: Fauser BCJM, editor. Reproductive medicine—Molecular, cellular and genetic fundamentals. New York: Parthenon Publishing; 2003. pp. 225–244. [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. Review: a new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): biosynthesis, mode of action and functional significance. Prog Neurobiol. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Bedecarrats G, et al. Review: gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010a;31:284–295. doi: 10.1016/j.yfrne.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12:631–639. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Kriegsfeld LJ, et al. Discovery and evolutionary history of gonadotropin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol. 2010b;22:716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512:255–258. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinolgy. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Morgan K, Pawson AJ. Identification of human gonadotropin-inhibitory hormone homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE. 2009;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugrumov MV, Ivanova IP, Mitskevich MS, et al. Axovascular relationships in developing median eminence of perinatal rats with special reference to luteinizing hormone-releasing hormone projections. Neuroscience. 1985;16:897–906. doi: 10.1016/0306-4522(85)90104-6. [DOI] [PubMed] [Google Scholar]
- Uzbekova S, Ferriere F, Guiquen Y, et al. Stage-dependent and alternative splicing of sGnRH messengers in rainbow trout testis during spermatogenesis. Mol Reprod Dev. 2001;59:1–10. doi: 10.1002/mrd.1000. [DOI] [PubMed] [Google Scholar]
- Wang X, Stocco DM. The decline in testosterone biosynthesis during male aging: a consequence of multiple alterations. Mol Cell Endocrinol. 2005;238:1–7. doi: 10.1016/j.mce.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Habata Y, Hosoya M, et al. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta. 2003;1593:151–157. doi: 10.1016/s0167-4889(02)00389-0. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu E, Yang C, et al. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology. 2010;151:617–627. doi: 10.1210/en.2009-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin BR, Chen HL. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000;63:977–981. doi: 10.1095/biolreprod63.4.977. [DOI] [PubMed] [Google Scholar]
- Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and alpha-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol. 1996;135:399–406. doi: 10.1530/eje.0.1350399. [DOI] [PubMed] [Google Scholar]