Abstract
Objective
The transition from screen-film to digital mammography may have altered diagnostic evaluation of women following a positive screening examination. This study compared use and timeliness of diagnostic imaging and biopsy for women screened with screen-film or digital mammography.
Materials and Methods
Data were from 35,321 positive screening mammograms on 32,087 women aged 40–89 years, from 22 Breast Cancer Surveillance Consortium facilities in 2005–2008. Diagnostic pathways were classified by their inclusion of diagnostic mammography, ultrasound, magnetic resonance imaging (MRI), and biopsy. We compared time to resolution and frequency of diagnostic pathways by patient characteristics, screening exam modality, and radiology facility. Between-facility differences were evaluated by computing the proportion of mammograms receiving follow-up with a particular pathway for each facility and examining variation in these proportions across facilities. Multinomial logistic regression adjusting for age, calendar year, and facility compared odds of follow-up with each pathway.
Results
The median time to resolution of a positive screening mammogram was 10 days. Compared to screen-film mammograms, digital mammograms were more frequently followed by only a single diagnostic mammogram (46% vs. 36%). Pathways following digital screening mammography were also less likely to include biopsy (16% vs. 20%). However, in adjusted analyses most differences were not statistically significant (p = 0.857 for mammography only; p = 0.03 for biopsy). Substantial variability in diagnostic pathway frequency was seen across facilities. For instance, the frequency of evaluation with diagnostic mammography alone ranged from 23% to 55% across facilities.
Conclusion
Differences in evaluation of positive digital and screen-film screening mammograms were minor, and appeared to be largely attributable to substantial variation between radiology facilities. To guide health systems in their efforts to eliminate practices that do not contribute to effective care, we need further research to identify the causes of this variation and the best evidence-based approach for follow-up.
Keywords: breast cancer, diagnostic evaluation, digital mammography, mammography, screening
Introduction
Mammography is the best tool for early detection of breast cancer [1–3]. However, due to the modest positive predictive value of screening mammography, many women who will not ultimately be diagnosed with cancer undergo additional diagnostic evaluation [4]. These unnecessary diagnostic evaluations are a burden on women and the healthcare system. We could reduce this burden by improving the specificity of screening mammography and by decreasing the number of tests, particularly invasive procedures, required to reach a diagnosis.
Digital mammography has rapidly replaced screen-film as the dominant modality for both screening and diagnostic mammography. As of February 1, 2013, 89.6% of licensed mammography machines in the United States were full-field digital machines [5]. Digital mammography holds the potential to improve detection of breast cancer; it performs better than screen-film for younger women and those with dense breasts [6,7]. If this improvement in performance is attributable to clearer imaging of lesions for young women or those with dense breasts, then digital mammography holds the promise of more rapid resolution of suspected breast cancer for these women. However, to fully understand the implications for women and the healthcare system of transitioning to this new screening technology, we need to compare the efficiency of diagnostic evaluations following digital mammography to those following screen-film mammography.
The cost of diagnostic evaluation of abnormal mammograms is significant. Lee et al. [8] estimated that the annual cost to Medicare of diagnostic work-up of suspected breast cancer is $679 million. Based on an analysis of data from a nonprofit healthcare system, Chubak et al. [9] estimated that each false-positive mammogram costs the payer an average of $527. A cost-effectiveness analysis previously compared the cost of screening with screen-film versus digital mammography [10]. However, that analysis assumed common costs of abnormal mammography for the two modalities. Although the positive predictive values of screen-film and digital screening mammography are similar, at 4.0% and 3.8% for screen-film and digital, respectively [11], we do not know if the number or timeliness of diagnostic evaluations after a positive digital or screen-film mammogram are different. Any differences in diagnostic evaluations would affect the cost-effectiveness of digital mammography.
A few previous investigations have studied the use of diagnostic imaging following screening mammography [12–14], but these studies pre-date the introduction of digital mammography. In this study we characterize the use and timeliness of diagnostic evaluation following screen-film and digital screening mammography using data from a large cohort within the Breast Cancer Surveillance Consortium (BCSC).
Materials and Methods
Study Setting, Data Sources, and Subjects
Data were from five mammography registries within the BCSC: Carolina Mammography Registry, Group Health Registry in Washington State, New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System (http://breastscreening.cancer.gov). Registries collected data from community radiology facilities including patient characteristics and clinical information at each mammogram. Radiologists’ assessments and recommendations were based on the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS®) [15]. Breast cancer diagnoses were obtained by linking BCSC data to pathology databases, regional Surveillance, Epidemiology, and End Results (SEER) programs, and state tumor registries. Data were pooled at a central Statistical Coordinating Center. Registries and the Coordinating Center received Institutional Review Board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analysis. All procedures were Health Insurance Portability and Accountability Act compliant, and registries and the Coordinating Center received a Federal Certificate of Confidentiality and other protections for the identities of women, physicians, and facilities.
Women who receive screening mammograms at BCSC facilities might receive some follow-up care at facilities outside of the BCSC. For the four BCSC registries, excluding Group Health, included in this study, we identified a subset of BCSC facilities for which the majority of diagnostic evaluations were captured in the BCSC database by comparing follow-up procedures captured by the BCSC to a linked database of Medicare claims. We used data from Medicare claims files (the Carrier Claims and Outpatient files) and the Medicare denominator file, which provides demographic, enrollment, and vital status data. For the four registries, 87% of women age 65 years and older were successfully matched to Medicare claims [16]. Inability to match women to Medicare claims was most often due to missing social security numbers.
For the four non-Group Health registries, we evaluated capture of diagnostic evaluation following an abnormal screening mammogram by identifying all BCSC mammograms with a radiologist’s abnormal interpretation among women who were aged 65 or older on mammography examination dates from January 1, 2005 to December 31, 2006, the most recent year for which Medicare claims data were available. We then searched Medicare claims data for 270 days after the mammography date to identify subsequent breast imaging and biopsy captured by Medicare claims. We chose this 270-day window to conservatively capture all subsequent evaluations because previous research indicated that the majority of evaluations are completed within 6 months or less [17]. For each diagnostic imaging or biopsy event found in claims data we looked for corresponding diagnostic procedures in BCSC data. Diagnostic procedures were categorized as mammography, ultrasound, and fine-needle aspiration (FNA)/biopsy based on Healthcare Common Procedure Coding System procedure codes. For each BCSC facility we computed the proportion of diagnostic procedures found in Medicare claims that were also captured in BCSC records for each class of diagnostic procedure. We included facilities in our study if this assessment indicated at least 80% capture by the BCSC of diagnostic mammography, breast ultrasound, and FNA/biopsy.
We were unable to use this method to validate capture of diagnostic evaluations for Group Health facilities because, as a managed care organization, Group Health is not required to submit itemized claims to the Center for Medicare and Medicaid Services. However, for Group Health, complete capture of follow-up procedures was likely because Group Health is an integrated health plan and care system. Women were likely to receive follow-up care inside the system, which would be captured in our database. In addition, Group Health claims data provided information on women who received care outside the system. Therefore, all Group Health facilities were included in our analysis.
Thus, our study used data on women screened at all Group Health facilities along with BCSC facilities from the four other registries with at least 80% capture of diagnostic evaluations, for a total of 22 facilities contributing data. We analyzed diagnostic evaluation pathways following a positive digital or screen-film screening examination. We included all abnormal screening mammograms captured by the BCSC for women aged 40–89 years performed in 2005–2008 at an included facility. A screening mammogram was considered to be positive according to the standard BCSC definition (http://breastscreening.cancer.gov/data/bcsc_data_definitions.pdf): BI-RADS assessment of 0 (needs additional imaging evaluation); 4 (suspicious abnormality); 5 (highly suggestive of malignancy); or 3 (probably benign finding) with a recommendation for additional imaging, clinical examination, or biopsy, FNA, or surgical consultation [15].
Definitions
Screening mammograms were classified as digital or screen-film based on information provided by the radiology facility. Diagnostic evaluation pathways were defined as the series of procedures that women underwent following a positive screening mammography. Pathways were established using imaging and FNA/biopsy data captured by the BCSC. For each screening mammogram, we looked ahead in 90-day increments from the date of the initial screening examination to identify diagnostic imaging (digital or screen-film mammography, ultrasound, or magnetic resonance imaging [MRI]) and biopsy or FNA that the woman received. The woman’s pathway was extended if any diagnostic evaluation was performed within 90 days of the previous evaluation. The pathway continued until no further imaging or biopsy was performed within 90 days after the last evaluation. Pathways were then defined on the basis of the sequence of imaging examinations and biopsy/FNA observed in the BCSC database. Each element included in a pathway represents a distinct visit for an additional diagnostic evaluation. For example, “mammography, ultrasound, biopsy” represents a screening mammogram followed by a diagnostic mammogram, an ultrasound, and a biopsy or FNA. Pathways could include repeated instances of the same imaging modality. For example, “mammography, mammography, ultrasound” represents a pathway in which a screening mammogram was followed by two mammography visits followed by an ultrasound prior to resolution of diagnostic evaluation.
Patient characteristics were collected by questionnaire at each examination. Mammograms were considered screening exams if the radiologist indicated routine screening. To avoid misclassifying diagnostic mammograms as screening exams, we classified mammograms as diagnostic if a breast-imaging exam occurred within the prior nine months.
Statistical analysis
We summarized characteristics of women and mammograms overall and stratified by digital or screen-film screening examination using descriptive statistics (counts and proportions). We described the distribution of the most common diagnostic evaluation pathways for digital and screen-film screening mammography. For common diagnostic pathways, we computed the distribution of characteristics of women, mammograms, and cancers diagnosed in the following year using counts and percentages. We summarized the time to resolution of diagnostic evaluation by computing the number of days between the screening mammogram and the last diagnostic procedure in the pathway and report the median and interquartile range (IQR).
To investigate variability in diagnostic pathways across radiology facilities, we computed the frequency of common diagnostic pathways and any pathway including biopsy following a BI-RADS 0 screening examination for each facility. We restricted this analysis to mammograms with an initial screening assessment of BI-RADS 0 to limit variability attributable to differences in findings on screening mammography. This analysis was also limited to facilities with at least 100 BI-RADS 0 screening mammograms to reduce apparent variability across facilities due to instability of estimates.
We compared diagnostic evaluation pathways after digital versus screen-film screening examinations adjusting for between-facility differences using multinomial logistic regression to estimate adjusted relative risk ratios (RRRs). The RRR estimated how receipt of digital versus screen-film screening mammography changed the relative risk that a woman would proceed through a particular pathway compared to diagnostic mammography alone. The RRR was estimated as the ratio of the relative risk of a digital screening mammogram leading to each of the common diagnostic pathways compared to diagnostic mammography alone divided by the relative risk of a screen-film screening mammogram leading to each of the common diagnostic pathways compared to diagnostic mammography alone. For each pathway we also used linear regression to estimate the adjusted difference in the mean time to resolution after digital versus screen-film screening mammograms. We used log-linear regression to estimate the relative risk of a digital versus screen-film screening mammogram being followed by a pathway that included biopsy. All regression models were adjusted for age (categorized in 10-year increments), examination year (categorized in 1-year increments), and facility.
Statistical significance was evaluated at the two-sided alpha = 0.05 level. All statistical analyses were conducted using R 2.14.0 (R Development Core Team, Vienna, Austria).
Results
Study sample
We identified a total of 22 facilities meeting inclusion criteria; these were Group Health facilities and those with >80% capture of diagnostic imaging and biopsy/FNA. From these facilities, 35,321 positive screening mammograms from 32,087 women met inclusion criteria. We found that 95.4% of positive screening mammograms were followed by diagnostic evaluation of some kind within 90 days.
Use of digital mammography increased notably over the study period from 28% of screening mammograms in 2005 to 60% in 2008. Overall, demographic characteristics of women receiving screen-film and digital screening mammograms were similar (Table 1). However, women receiving digital mammograms were more likely to be younger (40% vs. 30% aged 40–49 years for digital vs. screen-film) and to have less dense breasts (10% vs. 3% BI-RADS 1 breast density for digital vs. screen-film).
Table 1.
Characteristics of women and screening mammograms included in analyses of diagnostic evaluation following positive screening mammography.
| Overall (N=35,321) | Digital index mammogram (N = 16,801) | Screen-film index mammogram (N = 18,520) | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Screening examination year | |||
| 2005 | 8276 (23.4) | 2354 (14.0) | 5922 (32.0) |
| 2006 | 9385 (26.6) | 4718 (28.1) | 4667 (25.2) |
| 2007 | 9058 (25.6) | 4588 (27.3) | 4470 (24.1) |
| 2008 | 8602 (24.4) | 5141 (30.6) | 3461 (18.7) |
| Age (years) | |||
| 40–49 | 12129 (34.3) | 6659 (39.6) | 5470 (29.5) |
| 50–59 | 11785 (33.4) | 5374 (32.0) | 6411 (34.6) |
| 60–69 | 6698 (19.0) | 2908 (17.3) | 3790 (20.5) |
| 70–79 | 3397 (9.6) | 1373 (8.2) | 2024 (10.9) |
| 80–89 | 1312 (3.7) | 487 (2.9) | 825 (4.5) |
| Race/ethnicity† | |||
| Non-Hispanic white | 27739 (83.9) | 13617 (84.8) | 14122 (83.1) |
| Non-Hispanic black | 1145 (3.5) | 394 (2.5) | 751 (4.4) |
| Hispanic | 1812 (5.5) | 1036 (6.5) | 776 (4.6) |
| Asian/Pacific Islander | 2256 (6.8) | 977 (6.1) | 1279 (7.5) |
| Native American/Alaska Native | 107 (0.3) | 34 (0.2) | 73 (0.4) |
| Unknown/Other | 2262 (6.4) | 743 (4.4) | 1519 (8.2) |
| Family history of breast cancer† | |||
| Yes | 5690 (19.8) | 2798 (18.2) | 2892 (21.5) |
| No | 23094 (80.2) | 12539 (81.8) | 10555 (78.5) |
| Unknown | 6537 (18.5) | 1464 (8.7) | 5073 (27.4) |
| BI-RADS* breast density† | |||
| 1 Almost entirely fat | 1970 (6.1) | 1542 (9.9) | 428 (2.6) |
| 2 Scattered fibroglandular densities | 10933 (33.8) | 6015 (38.6) | 4918 (29.3) |
| 3 Heterogeneously dense | 16537 (51.1) | 6782 (43.5) | 9755 (58.1) |
| 4 Extremely dense | 2922 (9.0) | 1236 (7.9) | 1686 (10.0) |
| Unknown | 2959 (8.4) | 1226 (7.3) | 1733 (9.4) |
| Initial BI-RADS screening assessment | |||
| 3 probably benign finding | 34 (0.1) | 13 (0.1) | 21 (0.1) |
| 0 needs additional imaging evaluation | 35208 (99.7) | 16758 (99.7) | 18450 (99.6) |
| 4 suspicious abnormality | 51 (0.1) | 25 (0.2) | 26 (0.1) |
| 5 highly suggestive of malignancy | 28 (0.1) | 5 (0.0) | 23 (0.1) |
BI-RADS = Breast Imaging Reporting and Data System
Proportions computed from among women with non-missing values
Diagnostic evaluation following screening mammography
Diagnostic evaluation pathways after both digital and screen-film screening mammography were resolved in a median of 10 days (IQR 6–18 days for digital, 6–17 days for screen-film) (Table 2). The most common evaluation following a positive screen-film or digital screening mammogram was a single diagnostic mammogram (Table 2). The next most common diagnostic pathway was evaluation with a diagnostic mammogram and ultrasound. Two pathways, “mammography, mammography, ultrasound” and “mammography, mammography, ultrasound, biopsy” were more than 10 times more likely to be used following screen-film screening mammograms compared to digital. These two pathways were used almost exclusively by two facilities that performed only screen-film mammography, resulting in this substantial difference. The diagnostic work-up included biopsy for 20% of screen-film screening mammograms and 16% of digital screening mammograms. In our data only 222 mammograms were evaluated using MRI.
Table 2.
Time to resolution and frequency of the most common diagnostic pathways, no follow-up, or other diagnostic evaluation for positive digital or screen-film screening mammogram.
| Digital index mammogram (N = 16,801) | Screen-film index mammogram (N = 18,520) | |
|---|---|---|
| Median (IQR*) | Median (IQR) | |
| Time to resolution (days) | 10 (6, 18) | 10 (6, 17) |
| N (%) | N (%) | |
| Mammography only | 7681 (45.7) | 6698 (36.2) |
| Mammography, ultrasound | 4739 (28.2) | 4062 (21.9) |
| Mammography, biopsy | 1100 (6.6) | 1045 (5.6) |
| Mammography, ultrasound, biopsy | 979 (5.8) | 617 (3.3) |
| Ultrasound only | 736 (4.4) | 723 (3.9) |
| Mammography, ultrasound, biopsy, biopsy | 123 (0.7) | 379 (2.1) |
| Ultrasound, biopsy | 115 (0.7) | 183 (1.0) |
| Mammography, mammography, ultrasound | 42 (0.3) | 2256 (12.2) |
| Mammography, mammography, ultrasound, biopsy | 11 (0.1) | 335 (1.8) |
| Other† | 480 (2.9) | 1386 (6.5) |
| No follow-up | 795 (4.7) | 836 (4.5) |
IQR = Interquartile range
All other diagnostic pathways are included in this category
We stratified the most common diagnostic pathways following a positive screening mammogram into those that included only diagnostic imaging (Table 3) and those that included both imaging and biopsy (Table 4). Women aged 40–49 years were more likely to receive a pathway including ultrasound. Among diagnostic pathways ending in biopsy, substantial differences were observed in the age distribution of women (Table 4). The “mammography, biopsy” and “mammography, ultrasound, biopsy, biopsy” pathways were less common among women aged 40–49. The two longest pathways (“mammography, ultrasound, biopsy, biopsy” and “mammography, mammography, ultrasound, biopsy”) were more common following screen-film mammography than digital. The proportion of true-positive results was lowest (6%) for the pathway including multiple biopsies (“mammography, ultrasound, biopsy, biopsy”). The “mammography, biopsy” pathway also diagnosed a much higher proportion of Stage 0 cancers (71%) compared to all other pathways where Stage 0 constituted 19% or less of cancer diagnoses.
Table 3.
Time to resolution and distribution of screening examination modality and age for the most common work-up pathways not ending in biopsy.
| Mammography | Mammography, Ultrasound | Mammography, Mammography, Ultrasound | Ultrasound | |
|---|---|---|---|---|
| Median (IQR*) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Time to resolution (days) | 8 (5, 14) | 10 (7, 16) | 7.5 (6, 12) | 7 (3, 14) |
| N (%) | N (%) | N (%) | N (%) | |
| Modality of screening examination | ||||
| Digital | 7681 (53.4) | 4739 (53.8) | 42 (1.8) | 736 (50.4) |
| Screen-film | 6698 (46.6) | 4062 (46.2) | 2256 (98.2) | 723 (49.6) |
| Age (years) | ||||
| 40–49 | 4797 (33.4) | 3346 (38.0) | 904 (39.3) | 631 (43.2) |
| 50–59 | 4769 (33.2) | 2855 (32.4) | 794 (34.6) | 461 (31.6) |
| 60–69 | 2805 (19.5) | 1562 (17.7) | 385 (16.8) | 205 (14.1) |
| 70–79 | 1441 (10.0) | 764 (8.7) | 160 (7.0) | 115 (7.9) |
| 80–89 | 567 (3.9) | 274 (3.1) | 55 (2.4) | 47 (3.2) |
IQR = Interquartile range
Table 4.
Time to resolution and distribution of screening examination modality, age, screening mammography result, and cancer stage at diagnosis for common work-up pathways ending in biopsy.
| Mammography, Biopsy | Mammography, Ultrasound, Biopsy | Mammography, Ultrasound, Biopsy, Biopsy | Mammography, Mammography, Ultrasound, Biopsy | Ultrasound, Biopsy | |
|---|---|---|---|---|---|
| Median (IQR*) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Time to resolution (days) | 15 (9, 24) | 20 (13, 30) | 13 (7, 22) | 16.5 (13, 24.5) | 14 (7, 27) |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Modality of screening examination | |||||
| Digital | 1100 (51.3) | 979 (61.3) | 123 (24.5) | 11 (3.2) | 115 (38.6) |
| Screen-film | 1045 (48.7) | 617 (38.7) | 379 (75.5) | 335 (96.8) | 183 (61.4) |
| Age (years) | |||||
| 40–49 | 498 (23.2) | 541 (33.9) | 100 (19.9) | 119 (34.4) | 101 (33.9) |
| 50–59 | 780 (36.4) | 515 (32.3) | 185 (36.9) | 108 (31.2) | 93 (31.2) |
| 60–69 | 491 (22.9) | 309 (19.4) | 125 (24.9) | 64 (18.5) | 64 (21.5) |
| 70–79 | 253 (11.8) | 161 (10.1) | 76 (15.1) | 47 (13.6) | 27 (9.1) |
| 80–89 | 123 (5.7) | 70 (4.4) | 16 (3.2) | 8 (2.3) | 13 (4.4) |
| Result | |||||
| False-positive | 1888 (88.0) | 1114 (69.8) | 470 (93.6) | 240 (69.4) | 268 (89.9) |
| True-positive | 257 (12.0) | 482 (30.2) | 32 (6.4) | 106 (30.6) | 30 (10.1) |
| Cancer stage at diagnosis† | |||||
| Stage 0 | 182 (70.8) | 63 (13.1) | 6 (18.8) | 17 (16.0) | 0 (0) |
| Stage I | 52 (20.2) | 287 (59.5) | 15 (46.9) | 54 (50.9) | 17 (56.7) |
| Stage IIa | 9 (3.5) | 86 (17.8) | 3 (9.4) | 19 (17.9) | 4 (13.3) |
| Stage IIb | 2 (0.8) | 14 (2.9) | 4 (12.5) | 3 (2.8) | 6 (20.0) |
| Stage III | 7 (2.7) | 18 (3.7) | 2 (6.2) | 9 (8.5) | 3 (10.0) |
| Stage IV | 0 (0) | 2 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 5 (1.9) | 12 (2.5) | 2 (6.2) | 4 (3.8) | 0 (0) |
IQR = Interquartile range
Proportions for cancer stage at diagnosis computed from among women with a cancer diagnosis within one year of the screening mammogram
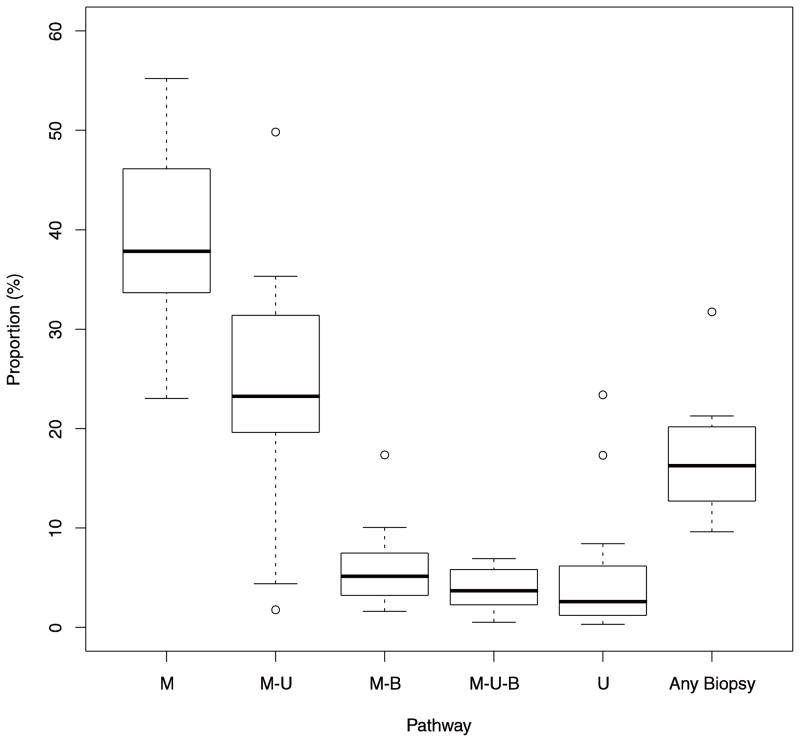
Our analysis showed wide variability across facilities in diagnostic evaluation of BI-RADS 0 screening mammograms (Figure 1). Evaluation with diagnostic mammography alone ranged from 23% of mammograms at the facility that used this pathway the least, to 55% of mammograms at the facility that used this pathway most often. The proportion of BI-RADS 0 screening mammograms that were followed by a pathway including biopsy ranged from 10% to 32%.
Fig. 1. Variability in use of diagnostic pathways across Breast Cancer Surveillance Consortium facilities.
The upper and lower boundaries of each box represent the 75th and 25th percentiles of the distribution of the proportion of BI-RADS 0 screening mammograms receiving a particular work-up pathway. Heavy lines at the center of the box represent the median of the distribution. Whiskers represent the most extreme outliers within 1.5 times the interquartile range of the boundaries of the box. Individual points are facilities lying outside this range. M = mammogram, U = ultrasound, B = biopsy
To determine the association between receipt of digital vs. screen-film screening mammography and diagnostic evaluation pathways adjusting for between-facility differences, we used RRRs. The RRR is the ratio of the adjusted relative risk of a diagnostic pathway after digital vs. screen-film screening mammography relative to diagnostic mammography alone (Table 5). Two common pathways (“mammography, mammography, ultrasound” and “mammography, mammography, ultrasound, biopsy”) were not included in this analysis because very few digital screening mammograms were followed by these pathways. After adjustment, the RRR of ultrasound alone compared to digital mammography alone was significantly lower following digital screening mammography compared to screen-film (RRR 0.39, 95% CI [0.29, 0.54]) (Table 5). Digital screening mammograms were also significantly less likely than screen-film mammograms to be followed by the “ultrasound, biopsy” pathway compared to diagnostic mammography alone (RRR 0.29, 95% CI [0.17, 0.51]). No significant differences were observed in mean time to resolution for any pathway. Digital screening mammograms were less likely to be followed by a pathway including biopsy compared to screen-film screening mammograms (relative risk = 0.89, 95% CI [0.8, 1.0], p = 0.034).
Table 5.
Adjusted relative risk ratio compared to evaluation with diagnostic mammography only and adjusted mean difference in time to resolution for digital screening mammography compared to screen-film.
| Use of pathway | Time to resolution | |||
|---|---|---|---|---|
| Pathway | RRR (95% CI)*† | p | Difference in time to resolution (days) (95% CI) | p |
| Mammography only | Ref | Ref | −0.29 (−1.16, 0.58) | 0.512 |
| Mammography, ultrasound | 1.02 (0.86, 1.20) | 0.857 | 0.76 (−0.55, 2.06) | 0.257 |
| Mammography, biopsy | 1.03 (0.83, 1.28) | 0.807 | 2.6 (−0.38, 5.58) | 0.088 |
| Mammography, ultrasound, biopsy | 0.86 (0.64, 1.16) | 0.328 | 1.88 (−2.72, 6.47) | 0.424 |
| Ultrasound only | 0.39 (0.29, 0.54) | <0.001 | −2.94 (−5.91, 0.03) | 0.052 |
| Mammography, ultrasound, biopsy, biopsy | 0.66 (0.44, 1.00) | 0.051 | 3.62 (−3.27, 10.51) | 0.303 |
| Ultrasound, biopsy | 0.29 (0.17, 0.51) | <0.001 | −1.92 (−10.27, 6.44) | 0.654 |
RRR = relative risk ratio; CI = confidence interval
All estimates are adjusted for age, examination year, and mammography facility
Discussion
We compared the use and timing of diagnostic pathways following screen-film and digital mammograms in a sample of over 30,000 positive screening mammograms from the BCSC. Overall, diagnostic follow-up pathways were similar following the two screening modalities. Positive digital screening mammograms were resolved following a single diagnostic mammogram in 46% of cases compared to 36% following screen-film mammography. Similarly, compared to screen-film mammograms, digital mammograms were followed less often by pathways including biopsy.
Substantial variability was observed between facilities’ in their use of diagnostic pathways in the evaluation of BI-RADS 0 mammograms. Some of this variability may be due to differences in the patient populations served by these facilities. Previous research has identified between-facility variability in interpretive performance of screening mammography [18]. Differences in interpretive performance may translate into differences in diagnostic evaluation. For instance, facilities with higher recall rates may have lower thresholds for recalling poor quality images and hence may have more positive mammograms that can be resolved with only an additional mammogram. Alternatively, differences in facility organization or practice patterns could translate into differences in diagnostic evaluation. One previous study found that facilities that conducted immediate interpretation had higher recall rates than facilities where mammograms were batch interpreted [19]. This type of difference could affect both timeliness and use of subsequent diagnostic imaging.
Differences in evaluation following screen-film and digital mammograms may be attributable to a learning curve effect. During the period in our study, many facilities included in our analysis had recently introduced digital mammography. Previous research found that, early in the implementation period, use of diagnostic imaging after digital mammography was higher than use after screen-film mammography, but this decreased over time [20]. No change was found in the frequency of biopsy following the introduction of digital mammography. This is consistent with our observation that digital screening mammograms were more likely to be followed by diagnostic mammography alone. This may represent a transient increase in recalls that can be resolved by a single additional mammogram following the implementation of a new imaging modality.
Women receiving positive digital screening mammograms tended to be younger than those receiving screen-film mammograms. Previous studies have shown that digital performs somewhat better in younger women [7,11]. These results might suggest that younger women are either choosing digital screening or being triaged to this modality. Breast density also appeared to be lower for digital screening mammograms compared to screen-film. This is consistent with prior research that showed that interpreted breast density tends to be lower for digital mammograms [21].
In a previous study of Medicare beneficiaries from 1995, the median time to follow-up mammography was found to be 20 days [14]. Across all diagnostic pathways, we found a median time to resolution of only 10 days, possibly indicating that timeliness of follow-up has improved over the previous decade. However, other BCSC research has shown variation across facilities in timeliness of follow-up, suggesting that even our excellent resolution times may be obscuring delays at individual facilities or for specific patient populations [22,17].
This study has some limitations. We used Medicare claims to identify a subset of BCSC mammography facilities that had good capture of diagnostic procedures following a positive mammogram. However, some procedures might have been missed so our observed diagnostic pathways might be missing some imaging events or biopsies. Additionally, this study is based on a subset of BCSC facilities with the most complete capture of follow-up imaging and invasive procedures. Although the BCSC has previously been shown to be representative of radiology facilities in community practice in the United States [23], the subset of facilities used in this analysis may not be a representative sample.
We found only modest differences between diagnostic evaluations following digital and screen-film screening mammograms, indicating that the transition to digital mammography has not introduced notable new burdens on women or their healthcare systems. However, we found substantial variation between radiology facilities. This indicates that the number and type of follow-up studies a woman receives after an abnormal mammogram depends on where she gets her care. As healthcare systems seek to eliminate variation that does not contribute to effective care, they will need evidence from further research that identifies the causes of this variation and the best evidence-based approaches to follow-up.
Acknowledgments
This work was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C). Work by Dr. Hubbard and Ms. Zhu was also supported in part by a grant from GE Healthcare. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the U.S. For a full description of these sources, please see: http://www.breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/.
Footnotes
Disclosures
Mr. Horbyluk, Dr. Lee, and Dr. Sweet are employees of GE Healthcare. The authors declare they have no conflicts of interest.
References
- 1.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2002;137:347– 360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737. W237–742. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiologic Clinics of North America. 2004;42:793– 806. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Newman L. IOM report sets policy priorities for improving breast cancer screening. J Natl Cancer Inst. 2001;93 (8):574–575. doi: 10.1093/jnci/93.8.574. [DOI] [PubMed] [Google Scholar]
- 5.FDA. [Accessed February 11, 2013.];MQSA National Statistics. Available from: http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm.
- 6.van Ravesteyn NT, Miglioretti DL, Stout NK, Lee SJ, Schechter CB, Buist DS, Huang H, Heijnsdijk EA, Trentham-Dietz A, Alagoz O, Near AM, Kerlikowske K, Nelson HD, Mandelblatt JS, de Koning HJ. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Intern Med. 2012;156 (9):609–617. doi: 10.1059/0003-4819-156-9-201205010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisano ED, Hendrick RE, Yaffe MJ, Baum JK, Acharyya S, Cormack JB, Hanna LA, Conant EF, Fajardo LL, Bassett LW, D’Orsi CJ, Jong RA, Rebner M, Tosteson AN, Gatsonis CA. Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST. Radiology. 2008;246 (2):376–383. doi: 10.1148/radiol.2461070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Stang PE, Goldberg GA, Haberman M. Resource Use and Cost of Diagnostic Workup of Women with Suspected Breast Cancer. Breast Journal. 2009;15 (1):85–92. doi: 10.1111/j.1524-4741.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 9.Chubak J, Boudreau DM, Fishman PA, Elmore JG. Cost of Breast-Related Care in the Year Following False Positive Screening Mammograms. Medical Care. 2010;48 (9):815–820. doi: 10.1097/MLR.0b013e3181e57918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosteson AN, Stout NK, Fryback DG, Acharyya S, Herman BA, Hannah LG, Pisano ED. Cost-effectiveness of digital mammography breast cancer screening. Ann Intern Med. 2008;148 (1):1–10. doi: 10.7326/0003-4819-148-1-200801010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD, Taplin SH, Sickles EA. Comparative-effectiveness of digital vs. film-screen mammography in community practice in the United States. Ann Intern Med. 2011;155 (8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carney PA, Abraham LA, Miglioretti DL, Yabroff KR, Sickles EA, Buist DSM, Kasales CJ, Geller BM, Rosenberg RD, Dignan MB, Weaver DL, Kerlikowske K. Factors associated with imaging and procedural events used to detect breast cancer after screening mammography. American Journal of Roentgenology. 2007;188 (2):385–392. doi: 10.2214/AJR.05.1718. [DOI] [PubMed] [Google Scholar]
- 13.Carney PA, Kasales CJ, Tosteson ANA, Weiss JE, Goodrich ME, Poplack SP, Wells WS, Titus-Ernstoff L. Likelihood of additional work-up among women undergoing routine screening mammography: the impact of age, breast density, and hormone therapy use. Preventive Medicine. 2004;39 (1):48–55. doi: 10.1016/j.ypmed.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Welch HG, Fisher ES. Diagnostic testing following screening mammography in the elderly. Journal of the National Cancer Institute. 1998;90 (18):1389–1392. doi: 10.1093/jnci/90.18.1389. [DOI] [PubMed] [Google Scholar]
- 15.American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS) Breast Imaging Atlas. American College of Radiology; Reston, VA: 2003. [Google Scholar]
- 16.Henderson LM, Hubbard RA, Onega TL, Zhu W, Buist DS, Fishman P, Tosteson AN. Assessing Health Care Use and Cost Consequences of a New Screening Modality: The Case of Digital Mammography. Med Care. 2012;50(12):1045–52. doi: 10.1097/MLR.0b013e318269e0d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg RD, Haneuse SJ, Geller BM, Buist DS, Miglioretti DL, Brenner RJ, Smith-Bindman R, Taplin SH. Timeliness of follow-up after abnormal screening mammogram: variability of facilities. Radiology. 2011;261 (2):404–413. doi: 10.1148/radiol.11102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taplin S, Abraham L, Barlow WE, Fenton JJ, Berns EA, Carney PA, Cutter GR, Sickles EA, D’Orsi C, Elmore JG. Mammography facility characteristics associated with interpretive accuracy of screening mammography. Journal of the National Cancer Institute. 2008;100 (12):876–887. doi: 10.1093/jnci/djn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghate SV, Soo MS, Baker JA, Walsh R, Gimenez EI, Rosen EL. Comparison of recall and cancer detection rates for immediate versus batch interpretation of screening mammograms. Radiology. 2005;235 (1):31–35. doi: 10.1148/radiol.2351040699. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard R, Zhu W, Onega T, Fishman P, Henderson L, Tosteson A, Buist D. Changes in breast-related healthcare utilization following the introduction of digital mammography. Med Care. 2012;50(12):1053–1059. doi: 10.1097/MLR.0b013e318269e9c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey JA. Quantitative assessment of percent breast density: analog versus digital acquisition. Technol Cancer Res Treat. 2004;3 (6):611–616. doi: 10.1177/153303460400300611. [DOI] [PubMed] [Google Scholar]
- 22.Karliner LS, Ma L, Hofmann M, Kerlikowske K. Language barriers, location of care, and delays in follow-up of abnormal mammograms. Med Care. 2012;50 (2):171–178. doi: 10.1097/MLR.0b013e31822dcf2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sickles EA, Miglioretti DL, Ballard-Barbash R, Geller BM, Leung JW, Rosenberg RD, Smith-Bindman R, Yankaskas BC. Performance benchmarks for diagnostic mammography. Radiology. 2005;235 (3):775–790. doi: 10.1148/radiol.2353040738. [DOI] [PubMed] [Google Scholar]