Abstract
Purpose
Open reduction and internal fixation of distal radius fractures often necessitates release of the brachioradialis from the radial styloid. However, this common procedure has the potential to decrease elbow flexion strength. To determine the potential morbidity associated with brachioradialis release, we measured the change in elbow torque as a function of incremental release of the brachioradialis insertion footprint.
Methods
In 5 upper extremity cadaveric specimens, the brachioradialis tendon was systematically released from the radius, and the resultant effect on brachioradialis elbow flexion torque was measured. Release distance was defined as the distance between the release point and the tip of the radial styloid.
Results
Brachioradialis elbow flexion torque dropped to 95%, 90% and 86% of its original value at release distances of 27mm, 46mm, and 52mm, respectively. Importantly, brachioradialis torque remained above 80% of its original value at release distances up to 7 centimeters.
Conclusions
Our data demonstrate that release of the brachioradialis tendon from its insertion has minor effects on its ability to transmit force to the distal radius.
Clinical Relevance
These data may imply that release of the distal brachioradialis tendon during distal radius open reduction internal fixation can be performed without meaningful functional consequences to elbow flexion torque. Even at large release distances, overall elbow flexion torque loss after brachioradialis release would be expected to be less than 5% due to the much larger contributions of the biceps and brachialis. Use of the brachioradialis as a tendon transfer donor should not be limited by concerns of elbow flexion loss, and the tendon could be considered as an autograft donor.
Keywords: Brachioradialis, distal radius fracture, elbow torque
1. Introduction
The brachioradialis (BR) muscle is an elbow flexor that originates on the lateral supracondylar ridge and inserts just proximal to the radial styloid. It is most effective when the elbow is in a flexed position, as both biceps and brachialis generally have decreasing moment arms at flexion angles greater than 90° (1). Due to the placement of its insertion on the radius it also participates in pronation and supination, depending on forearm rotation. Its insertion forms the floor of the first dorsal compartment and takes the shape of a teardrop or a heart, with its point oriented proximally. The insertion is 15mm in length, 11mm in width, and extends distally to a point that is 17mm proximal to the tip of the radial styloid (2).
Previous work suggested that the BR could be responsible for a commonly seen pattern of dorsal, proximal, and radial displacement of distal radius fracture fragments (3), and Henry’s approach may be modified to include release of the insertion of the BR to facilitate exposure during open reduction of these injuries (4,5). As such, multiple authors recommend releasing the BR insertion during open reduction and internal fixation of distal radius fractures (2,6–8). However, this common procedure potentially compromises elbow flexion torque, and these recommendations appear to be made without reference to any work quantifying the functional effect of BR release. While the BR is the smallest of the 3 major elbow flexors, its moment arm over most of the elbow range of motion is greater than that of biceps or brachialis (1). Thus, the purpose of this study was therefore to measure the change in elbow torque as a function of BR release and report the results and implications.
2. Materials and Methods
Sample Preparation
This experiment used 5 fresh-frozen upper limbs amputated through the mid-humerus. The BR muscle-tendon unit was identified from origin to insertion, and specimens were prepared by removing all other muscles of the arm and forearm. Muscular origins and elbow insertions were preserved so as to not disrupt the stability provided by the joint capsule. The hands were removed at the level of the wrist joint with care taken to preserve all distal radial ligaments to allow direct visualization of the distal end of the radius (Fig. 1).
Figure 1.

Limb dissection with all muscles except BR removed. Note the fibrous connections between the BR and the radius that can transmit force to the radius at release distances proximal to the tendinous insertion. Inset: Close-up view of the Krackow stitch used to secure the BR tendon.
Two self-tapping 5 mm Schanz screws (Synthes, West Chester, PA) were drilled transversely through the distal humerus. One self-tapping 4 mm Schanz screw was inserted into the distal articular surface of the radius and advanced proximally in line with the shaft. With the forearm in neutral rotation, a 1.6 mm Kirschner wire was placed through the midshaft radius and ulna to prevent pronation and supination during testing. Care was taken to place wires dorsal or volar to the BR path, and screws were cut short to avoid interference with the muscle or tendon during testing (Fig. 2). The 5 mm Schanz screws were used to fix the humerus to a frame constructed from carbon fiber rods (11 mm diameter) using self-holding clamps. Screws, carbon fiber rods, and clamps were obtained from a Synthes (West Chester, PA) external fixation system. The 4 mm Schanz screw at the distal articular surface was affixed to a load cell with 6 degrees of freedom (Model MC3A-6-500, Advanced Mechanical Technology Inc., Watertown, MA) with the forearm in neutral rotation and with the elbow in 90 degrees of flexion.
Figure 2.
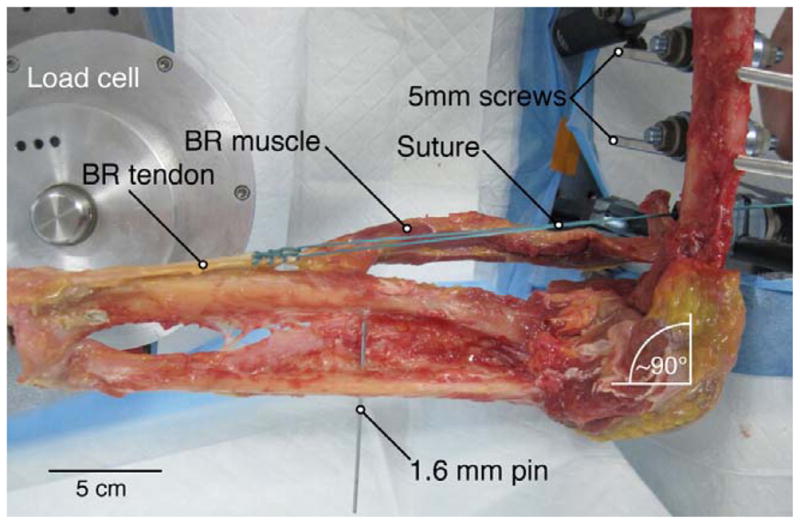
Experimental setup for biomechanical testing of BR torque production. The elbow was placed at 90 degrees of flexion and the forearm was placed in neutral rotation. This orientation was fixed with Schanz screws and a Kirschner wire to prevent elbow rotation and forearm pronation/supination.
Suture (Size #5 Ethibond Excel, Ethicon, San Angelo, TX) was secured to the proximal BR tendon, just distal to the muscle-tendon junction, using a Krackow stitch with at least 6 locking loops (Fig. 2). The free end of the suture was clamped to a dual-mode servo-motor (Model 310; Aurora Scientific, Inc., Aurora, Ontario, Canada). The limb was oriented so that the suture followed the natural path of the BR tendon and muscle, crossing the humerus at a point 5–6 cm proximal to the lateral epicondyle, consistent with previously measured BR moment arm values at 90 degrees of flexion (1,9).
Biomechanical Testing
Testing was performed by loading the suture via the servo-motor with a 20N force for 1000 ms while simultaneously recording all 3 force and torque components (using the 6-axis load cell) at the distal radius with a sampling rate of 1000 Hz. The 20 N force was chosen based on the fact that the maximum predicted tension generated in the BR, as determined by physiologic cross sectional area (1.9 cm2 (10)) and specific tension (22 N/cm2 (11)), is 44N. Thus, 20N was chosen to represent a reasonable physiological force exerted by the BR while performing daily activities (about 50% maximal force). After measurements were completed with the BR insertion intact, the BR tendon was released from the radius in 2–5 mm increments by transecting the tendon and soft tissues down through the periosteum (Fig. 3). Release distances were defined as the distance between the point of each transection and the tip of the radial styloid. After each release the tendon was pulled as described above. This release method differs from surgical release of the BR, in which a knife blade is passed under the tendon from proximal to distal, releasing the tendon from its insertion on the fracture fragment. The method of release used in this study was chosen because it allowed sequential testing of the same specimen at different release distances; transections were long enough (volar to dorsal) to ensure that tendon distal to the incision was completely released. Due to the progression from firm tendinous insertion to soft fibrous attachments, placement of releases at exact 2mm intervals was not always possible; the variability in release increments reflects the difference in material properties at different locations. This successive incremental release and force testing was performed until the fibrous attachments of BR tendon tore away from the bone or until the suture at the proximal end of the tendon was reached.
Figure 3.
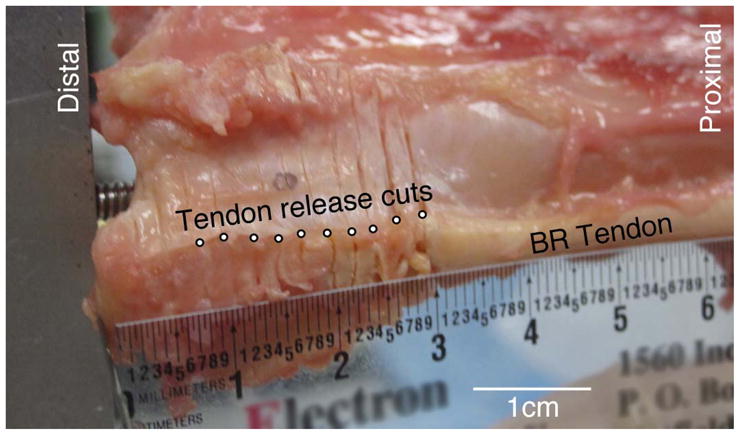
Close-up view of BR insertion. Successive BR releases progressing proximally from the radial styloid can be seen as individual incisions. Releases were performed as shown by transecting the tendon and soft tissue through the periosteum.
Data Analysis
Raw data collected include motor excursion distance, motor force applied, and force transmitted to the distal radius. All were collected at 1000 Hz. From these data stiffness and predicted torque loss were calculated. Acquired data were smoothed using a moving average function with a 21 ms time interval. Maximum force measured after smoothing was taken as the force (applied or transmitted) at a given release distance. Stiffness was calculated as the quotient of the force applied and the excursion of the motor arm necessary to produce such force. Release distance values were normalized to an average arm based on the distance from the lateral epicondyle to the radial styloid. Distances reported here are for an average arm with a distance of 259 mm from epicondyle to styloid. Because the release distances varied among experiments, data were binned into groups of 5 sequential data points and averaged to allow comparison among all arms. Data are reported as mean ± standard error unless otherwise noted.
3. Results
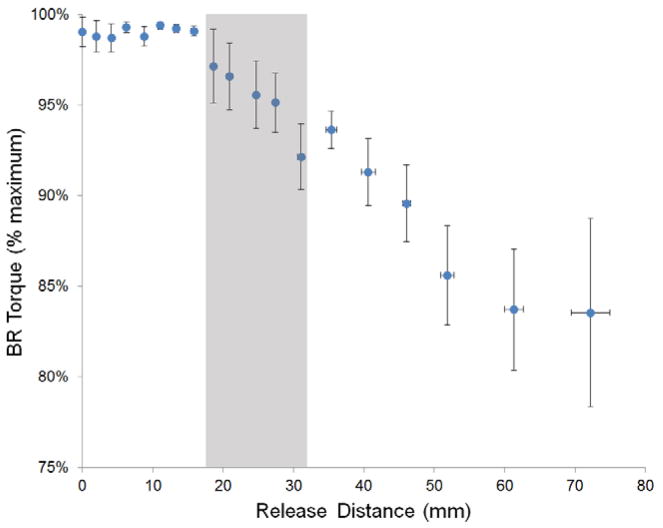
The 5 specimens (4 females, 1 male) had a mean age of 78 years (range: 59–88 ys) and a mean radial styloid to lateral epicondyle distance of 259 ± 5 mm. The mean percentage of total torque generated by the 20 N simulated BR force was recorded as a function of release distance (Fig. 4). As expected, as the BR was released, resultant elbow flexion torque decreased. However, the magnitude of this decrease was modest. Torque dropped to 95±2%, 90±2%, and 86±3% of its original value at release distances of 27±4mm, 46±6mm, and 52±10mm, respectively. Even at the greatest release distance (72 cm), the resultant torque did not drop below 80% of its native value.
Figure 4.
Graphical representation of mean torque as a function of release distance (n=5 specimens). Torque is expressed relative to the maximum torque generated by placing a 20 N load on the BR tendon. It can be seen that release distances up to 15mm result in virtually no loss of torque production. Note that, even at large release distances, BR force production would be expected to decrease by less than 20%. The shaded region corresponds to the BR tendon insertion footprint, as previously described (2). Note that the vertical axis origin extends to 75% maximum.
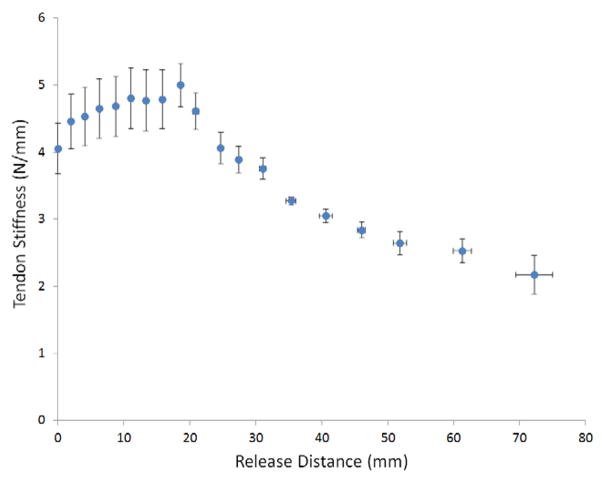
To gain insights into the biomechanical consequences of the release, mean tendon stiffness was calculated by measuring the excursion of the motor arm necessary to generate the 20N of force (Fig. 5). Mean stiffness rose until a release distance of 18mm, after which it monotonically decreased with larger release distances.
Figure 5.
Mean BR tendon stiffness as a function of release distance (n=5 specimens). This graph summarizes the overall stiffness change of the BR tendon with at different release distances. Stiffness increased at small release distances, reflecting a decrease in tendon length without loss in force production capacity.
4. Discussion
Our findings demonstrated that distal BR tendon insertion release of up to 7 cm results in less than a 20% decrease in BR-induced elbow flexion torque. Relative contributions of the 3 major elbow flexors (biceps brachii, brachialis, BR) can be estimated by the relative products of physiological cross sectional area and moment arm. The physiological cross sectional area for biceps brachii, brachialis, and BR are 5.1 cm2, 5.4 cm2, and 1.9 cm2, respectively (10,11). At 90° flexion, moment arms are 3.8 cm, 2.9 cm, and 5.2 cm for males; values are 3.9 cm, 2.4 cm, and 4.6 cm for females (1). Using these values, we calculate that BR is responsible for 21–22% of elbow torque at 90° flexion for both males and females. Therefore, a 20% loss in BR-induced elbow flexion torque would correspond to about 4–5% loss of overall elbow flexion torque. Thus, we believe that BR release can be performed without noteworthy functional consequences. Release distances that nearly reached the musculotendinous junction resulted in torque losses of less than 20%, suggesting that more than 80% of BR elbow flexion torque is retained through intermuscular fascial connections along the muscle belly.
Extensive Properties: Stiffness
Measurement of tendon stiffness provided insights into the biomechanical consequences of BR release (Fig. 5). Mean stiffness increased for short release distances (up to 19mm), a result that would be expected in shortening the length of a homogenously stiff material such as the distal footprint of the BR tendon. However, the tendinous insertion of the BR is more complicated than a single isolated insertion site - there are fibrous connections between the BR tendon and the radius that have previously been identified (2). The decrease in tendon stiffness beyond 18 mm represented releasing the tendon beyond the proximal limit of the BR insertion footprint, as force transmission to the radius occurs primarily through these more compliant fibrous connections. These fibers were thin and easily pierced by blunt dissection (Fig. 1), but were surprisingly effective at force transmission to the distal radius.
Clinical Implications
The results of our mechanical data are relevant to clinical scenarios when tempered by a sensible consideration of the limitations inherent to our experimental approach. Release of the BR insertion, as routinely performed during distal radius open reduction internal fixation, can likely be performed without adversely affecting elbow flexion torque. A previous radiographic study of distal radius fractures found that the average distance from the radial styloid to the radial limit of the fracture line was 28 mm (SD 6 mm) (2). Based on our results, the mean functional loss of BR-induced torque at this level should be less than 10% (Fig. 4). Our results also demonstrated that the insertion may be released from distal to proximal for 7 cm with less than a 20% decrease in BR-induced torque production. Prior studies demonstrate that at 90° of elbow flexion, the BR is predicted to contribute 18–19% of elbow torque, as calculated from magnetic resonance imaging data (9) or physiological cross sectional area (10,11) and moment arm data (1). Therefore, even at long release distances, total loss of elbow flexion torque after BR release is expected to be less than 5%.
In addition, in its normal anatomic configuration, there are connections between the BR and surrounding muscles. Our experimental approach considered the BR muscle-tendon unit in isolation, ignored these connections, and assumed that all force was transmitted to the radius via the tendinous insertion. Surrounding connections likely transmit force independent of the actual insertion point so torque loss from tendon release would likely be mitigated due to the presence of these alternative paths of force transmission. In addition, post-surgical healing and scarring of the transected tendon may recapitulate part of its ability to transmit force distally. Our experiment therefore represents a worst-case scenario of BR release effects.
BR-to-flexor pollicis longus transfer is commonly performed in tetraplegia surgery (12–14) to restore lateral key pinch. When performing this procedure, it is implicit that restoration of pinch is more important than the potential concomitant decrease in elbow flexion strength that might occur as a result of changes to the BR’s native anatomic configuration. This work supports and strengthens this assumption. However, it is important to note that a prior study demonstrated that large release distances are necessary to obtain sufficient fiber excursion for a BR-to-flexor pollicis longus transfer to be successful (10); the distances suggested (as much as 9 cm proximal to the insertion to obtain minimal additional excursion) are larger than those measured in the current study. Because we have concluded that the majority of BR elbow flexion torque is retained through intermuscular fascial connections along the muscle belly, the limited decrease in elbow flexion torque observed with tendon release may actually be greater in BR-to-flexor pollicis longus transfers.
Another implication of this study is the potential use of the BR tendon as autograft donor in the distal forearm. Currently the most common graft choices are palmaris longus or flexor carpi radialis and are used for procedures such as interposition arthroplasty (15), tendon grafts, or pulley reconstruction (16,17). However, the small size (3.1mm2 (18)) and inconsistent presence (19) of the palmaris longus tendon diminishes its utility (20), and flexor carpi radialis harvest may result in uneven and altered wrist function (21). The distal BR tendon, however, is larger than the palmaris longus, is always present, and we have shown that it has several cm of length available for harvest without adversely affecting elbow flexion torque.
It is important to note that this study is purely mechanical and represents an isolated system; thus, the conclusions drawn must be interpreted in this context. Our experiment did not consider changes in moment arm due to pronation/supination or flexion/extension and only reflected the consequence of BR release at a single joint configuration; greater flexion angles result in larger moment arms (8) and loss of BR function could be enhanced at these angles. However, the BR’s relative contribution to flexion at these angles is approximately 24–26% (for males and females, respectively); elbow torque loss would still be less than 5%, suggesting that our findings are broadly applicable.
Our approach does not account for differential activation of the BR. It is traditionally considered a weak elbow flexor and also has a much smaller physiological cross sectional area than biceps or brachialis. However, it is possible that its relative recruitment and activation may change with forearm position. Our experimental approach was not designed to detect differences in torque loss due to changing levels of muscle activation.
Another limitation is that the values reported here represent release distances based on average anatomy. The BR tendon dimensions observed during dissection were sufficiently variable to warrant further investigation. We caution that tendons should be not removed or released with impunity. Rather the results of this study can form the basis of an informed decision when considering release of the BR tendon.
Acknowledgments
Funding for this study was provided by a grant from the American Foundation for Surgery of the Hand, the Department of Veterans Affairs, and NIH grant R24HD050837. The authors thank Shannon Bremner for technical assistance with device development. The authors also wish to thank the individuals who donated their bodies and tissues for the advancement of education and research. We also thank Dr. Wendy Murray (Northwestern University) for providing valuable BR modeling data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray WM, Delp SL, Buchanan TS. Variation of muscle moment arms with elbow and forearm position. J Biomech. 1995;28(5):513–525. doi: 10.1016/0021-9290(94)00114-j. [DOI] [PubMed] [Google Scholar]
- 2.Koh S, Andersen CR, Buford WL, Patterson RM, Viegas SF. Anatomy of the distal brachioradialis and its potential relationship to distal radius fracture. J Hand Surg. 2006;31(1):2–8. doi: 10.1016/j.jhsa.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Sarmiento A. The brachioradialis as a deforming force in Colles’ fractures. Clin Orthop Relat Res. 1965;38:86–92. [PubMed] [Google Scholar]
- 4.Orbay JL, Badia A, Indriago IR, Infante A, Khouri RK, Gonzalez E, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204–211. doi: 10.1097/00130911-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Orbay JL, Fernandez DL. Volar fixation for dorsally displaced fractures of the distal radius: a preliminary report. J Hand Surg. 2002;27(2):205–215. doi: 10.1053/jhsu.2002.32081. [DOI] [PubMed] [Google Scholar]
- 6.Badia A, Khanchandani P. Volar Plate Fixation. In: Slutsky DJ, Osterman AL, editors. Fractures and Injuries of the Distal Radius and Carpus. Philadelphia: Elsevier; 2009. pp. 149–156. [Google Scholar]
- 7.Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958–965. doi: 10.1016/j.jhsa.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Wulf CA, Ackerman DB, Rizzo M. Contemporary evaluation and treatment of distal radius fractures. Hand Clin. 2007;23(2):209–226. vi. doi: 10.1016/j.hcl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami Y, Nakazawa K, Fujimoto T, Nozaki D, Miyashita M, Fukunaga T. Specific tension of elbow flexor and extensor muscles based on magnetic resonance imaging. Eur J Appl Physiol Occup Physiol. 1994;68(2):139–147. doi: 10.1007/BF00244027. [DOI] [PubMed] [Google Scholar]
- 10.Fridén J, Albrecht D, Lieber RL. Biomechanical analysis of the brachioradialis as a donor in tendon transfer. Clin Orthop Relat Res. 2001;(383):152–161. doi: 10.1097/00003086-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Lieber RL. Skeletal Muscle Structure, Function, and Plasticity. 3. Baltimore: Lippincott Willims & Wilkins; 2010. [Google Scholar]
- 12.Fridén J, Reinholdt C, Gohritz A, Peace WJ, Ward SR, Lieber RL. Simultaneous powering of forearm pronation and key pinch in tetraplegia using a single muscle-tendon unit. J Hand Surg Eur Vol. 2012;37(4):323–328. doi: 10.1177/1753193411423894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogk JPM, Johanson ME, Hentz VR, Saul KR, Murray WM. A simulation analysis of the combined effects of muscle strength and surgical tensioning on lateral pinch force following brachioradialis to flexor pollicis longus transfer. J Biomech. 2011;44(4):669–675. doi: 10.1016/j.jbiomech.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray WM, Hentz VR, Fridén J, Lieber RL. Variability in surgical technique for brachioradialis tendon transfer. Evidence and implications. J Bone Joint Surg Am. 2006;88(9):2009–2016. doi: 10.2106/JBJS.E.00973. [DOI] [PubMed] [Google Scholar]
- 15.Burton RI, Pellegrini VD. Surgical management of basal joint arthritis of the thumb. Part II. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg. 1986;11(3):324–332. doi: 10.1016/s0363-5023(86)80137-x. [DOI] [PubMed] [Google Scholar]
- 16.Clark TA, Skeete K, Amadio PC. Flexor tendon pulley reconstruction. J Hand Surg. 2010;35(10):1685–1689. doi: 10.1016/j.jhsa.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann RA, Pacek CA. Pulley reconstruction using palmaris longus autograft after repeat trigger release. J Hand Surg Br. 2006;31(3):285–287. doi: 10.1016/j.jhsb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GD, Botte MJ, Josephs MS, Newton PO, Davis JL, Woo SL. Morphologic and biomechanical comparison of tendons used as free grafts. J Hand Surg. 1993;18(1):76–82. doi: 10.1016/0363-5023(93)90249-3. [DOI] [PubMed] [Google Scholar]
- 19.Seiler JG. Flexor Tendon Injury. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, editors. Green’s Operative Hand Surgery. Philadelphia: Elsevier; 2011. pp. 189–238. [Google Scholar]
- 20.Jakubietz MG, Jakubietz DF, Gruenert JG, Zahn R, Meffert RH, Jakubietz RG. Adequacy of palmaris longus and plantaris tendons for tendon grafting. J Hand Surg. 2011;36(4):695–698. doi: 10.1016/j.jhsa.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Naidu SH, Poole J, Horne A. Entire flexor carpi radialis tendon harvest for thumb carpometacarpal arthroplasty alters wrist kinetics. J Hand Surg. 2006;31(7):1171–1175. doi: 10.1016/j.jhsa.2006.05.005. [DOI] [PubMed] [Google Scholar]