Abstract
The mammalian target of rapamycin complex 1 (mTORC1) is a critical regulator of cap-dependent translation through its direct activation of ribosomal protein p70 S6 kinase (S6 kinase) and indirect activation of eukaryotic initiation factor 4E (eIF4E). We recently reported that inhibition of eIF4E expression caused apoptosis in cancer cells in the absence of serum. This was indicated by treatment with the mTORC1 inhibitor rapamycin, which suppressed both S6 kinase and 4E-BP1 phosphorylation (dephosphorylated 4E-BP1 binds and inactivates eIF4E), or by knockdown of eIF4E. We report here that knockdown of eIF4E also causes apoptosis in the presence of serum. This was unexpected because rapamycin induces G1 cell cycle arrest in the presence of serum. Upon investigation, we have found that inactivated S6 kinase prevents the apoptotic effect observed by singular knockdown of eIF4E and results in G1 cell cycle arrest. This effect is dependent on TGF-β (transforming growth factor-β) signaling which contributes to G1 cell cycle arrest. Suppression of S6 kinase phosphorylation alone is insufficient to mediate cell cycle arrest, indicating that complete G1 cell cycle arrest is due to suppression of both S6 kinase and eIF4E. These data indicate that the cytostatic effect of rapamycin is suppression of both S6 kinase and eIF4E, while the cytotoxic effects are due suppression of eIF4E in the absence of S6 kinase-dependent activation of TGF-β signals. Our findings place an importance on the evaluating the activity/expression level of S6 kinase and eIF4E as readouts for rapamycin/rapalog efficacy.
Keywords: mTOR, eIF4E, apoptosis, TGF-β, cell cycle
1. Introduction
It is widely accepted that rapamycin suppresses phosphorylation of mTOR complex 1 (mTORC1) substrate S6 kinase at low nano-molar doses. Through an allosteric mechanism, rapamycin preferentially inhibits mammalian target of rapamcyin complex 1 (mTORC1) but also inhibits mTORC2 under certain conditions [1; 2]. We recently reported that high (micro-molar) doses of rapamycin also suppressed phosphorylation of another mTORC1 substrate, eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1). The high dose induced apoptosis in cancer cells in the absence of serum. We attributed cell death to indirect inhibition of the cap-dependent translation initiator, eIF4E, because dephosphorylated 4E-BP1 binds eIF4E [3; 4]. Supportively, eIF4E ablation also caused apoptosis. This finding correlated with previous reports that the key mTORC1 substrate for cell proliferation and survival is eIF4E [5; 6]. In contrast, rapamycin induced G1 cell cycle arrest in the presence of serum that protected cells from progressing into S-phase [7], wherein the lack of eIF4E activity would otherwise cause apoptosis [7; 8]. G1 cell cycle arrest was dependent on TGF-β signaling,[7] which is suppressed by mTOR [9; 10], Low-dose rapamycin concentrations sufficient to suppress phosphorylation of S6 kinase (but not 4E-BP1) induced TGF-β signaling [10]. While low-dose rapamycin treatment impeded G1 cell cycle progression, high-dose treatment was required to completely block progression from mitosis to S-phase [8]. In sum, rapamycin induces G1 cell cycle arrest at doses that effectively suppress the phosphorylation of both S6 kinase and 4E-BP1.
In this report, we investigated the role of eIF4E ablation on cell cycle arrest in the presence of serum. As aforementioned, inhibiting S6 kinase (low-dose rapamycin) was insufficient to mediate complete G1 arrest. Here, our results indicate similarly, eIF4E ablation is insufficient to induce cell cycle arrest and instead results in apoptotic cell death. Surprisingly, suppression of S6 kinase effectively prevented the apoptotic effect of eIF4E ablation. Dual inhibition of both proteins causes a TGF-β-dependent G1 cell cycle arrest. The findings reveal complex effects of rapamycin on mTORC1 substrates with clinical implications for using translational activators S6 kinase and eIF4E as readouts for clinical efficacy.
2. Materials and Methods
2.1. Cells, cell culture conditions and cell viability
The human cancer cell lines MDA-MB-231 and BT-549 cells were obtained from the American Tissue Type Culture Collection (ATCC) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma) supplemented with 10% Fetal Bovine Serum (Sigma). Cell viability was determined 24 hr after treatment as described previously [8].
2.2. Antibodies and Reagents
The following antibodies: PARP, cleaved PARP, P-S6 kinase T389, S6 kinase, and eIF4E were from Cell Signaling; α-actin was from Sigma. Negative control siRNA (Dharmacon), siRNAs targeted against S6 kinase, eIF4E, and Smad4 were obtained from Santa Cruz Biotechnology. The neutralizing anti-TGF-β antibody was from R&D Systems. Lipofectamine RNAiMax (Invitrogen) was used for transient transfections. Rapamycin and rottlerin were obtained from Calbiochem.
2.3. Western Blot Analysis
Extraction of proteins from cultured cells and Western blot analysis of extracted proteins was performed using the ECL system (Amersham) as described previously [1].
2.4. Transient transfections
Cells were plated in 6-well plates in medium containing 10% FBS. The next day (50% confluence), transfections with siRNAs (75nM) in Lipofectamine RNAiMAX were performed. After 6 hours, reagents were replaced with fresh 10% FBS and cells were allowed to incubate for an additional 48 hours.
2.5. Flow cytometric analysis
Cell suspensions were recovered and resuspended in the following fixing solution: 20ml 1X PBS, 2% BSA, 0.1% NaN3. 9ml of 100% ethanol was added drop wise. Fixed cells were centrifuged, washed, and then resuspended in 500µl sorting buffer: 1X PBS, 0.5% BSA, 1mM EDTA, 40µg/ml Propidium Iodide, 100µg/ml RNAse A, and filtered through 40-mm diameter mesh to remove clumps of nuclei. Percentages of cells within each of the cell cycle compartments (G0–G1, S, or G2-M) were determined by flow cytometry (FACSCalibur; Becton Dickinson).
3. Results
3.1. Ablation of eIF4E expression induces apoptosis in the presence of serum in MDA-MB-231 cells
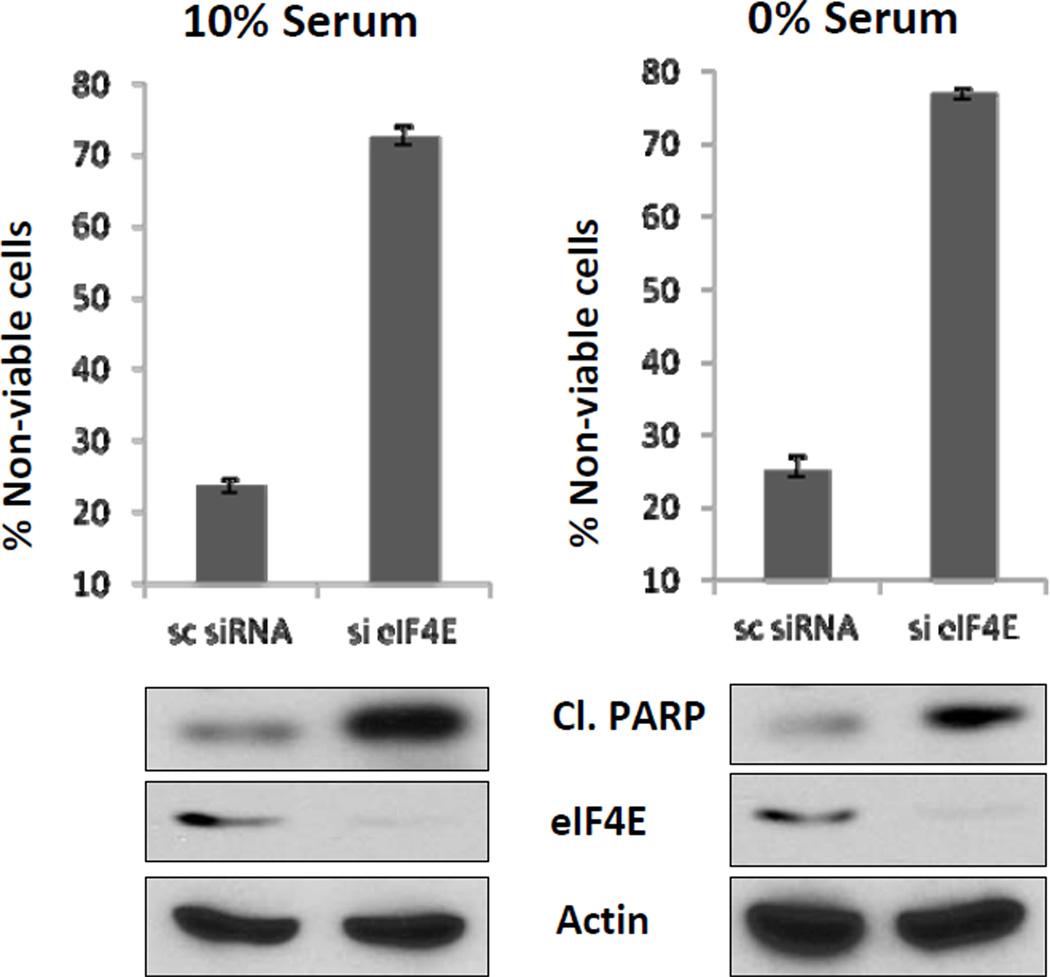
Previously, we reported that high (20 µM) rapamycin doses induced apoptosis in MDA-MB-231 as well as in other cancer cell lines in the absence of serum [7; 8; 11; 12]. The apoptotic effect was due to complete dephosphorylation of 4E-BP1, which subsequently inactivated eIF4E [3; 4]. The indirect effect of rapamycin on eIF4E correlated with knockdown of eIF4E as this also induced apoptosis. Consistent with the effect of high dose rapamycin being due to suppression of eIF4E, knockdown of 4E-BP1 (which suppresses eIF4E when dephosphorylated) reversed the apoptotic effect of rapamycin in the absence of serum [8]. In contrast, rapamycin induced cell cycle arrest in the presence of serum [8]. It is not possible to evaluate the direct effect of inhibiting eIF4E on cell cycle arrest by rapamycin treatment because the high dose required for arrest inhibits both S6 kinase along with 4E-BP1. Thus, to investigate how the absence of eIF4E affects cell cycle progression, we used siRNA against eIF4E in MDA-MB-231 cells. Surprisingly, knockdown of eIF4E induced cell death in both the presence and absence of serum (Fig. 1). The loss of cell viability was accompanied by cleavage of the caspase 3 substrate PARP, indicating apoptotic cell death (Fig. 1). Thus, whereas rapamycin induced apoptosis only in the absence of serum [8], ablation of eIF4E induced apoptosis in both the presence and absence of serum.
Figure 1.
Ablation of eIF4E expression induces apoptosis in the presence of serum in MDA-MB-231 cells. MDA-MB-231 cells were transfected at 50% confluence with negative control siRNA or siRNA for eIF4E. 24 hr later, the cells were put in fresh media with either 0% or 10% serum. After another 24, the percentage of non-viable cells was determined as described in Materials and Methods. Also at determined at this time was the level of cleaved PARP, eIF4E, and actin by Western blot analysis. The cell viability data is representative of data obtained from at least four independent experiments. The immunoblots are representative of experiments repeated at least two times.
3.2. Suppression of S6 kinase prevents the apoptotic effect of suppressed eIF4E expression in the presence of serum
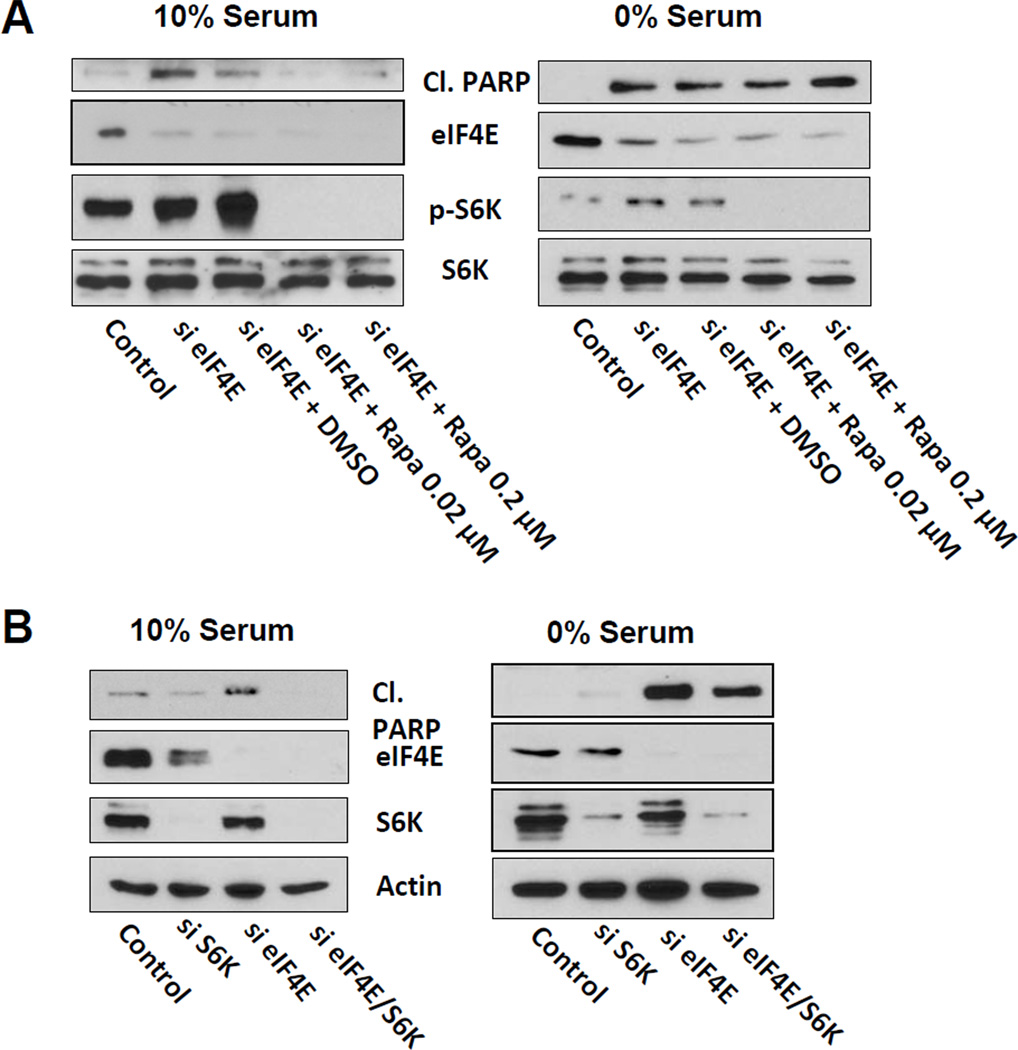
Since high-dose rapamycin treatment also inhibits S6 kinase phosphorylation, we reasoned that protection from apoptosis induced by high-dose rapamycin in serum could be due to suppression of S6 kinase. Therefore, we investigated the effect of low-dose rapamycin treatment upon eIF4E knockdown in MDA-MB-231 cells. Low-dose rapamycin treatment (20 nM) prevented the apoptotic cell death induced by singular inhibition of eIF4E expression in the presence, but not in the absence of serum (Fig. 2A). Thus, doses of rapamycin that suppress S6 kinase phosphorylation, but not 4E-BP1 phosphorylation [8], provided the cells with a means of resistance to eIF4E ablation in the presence of serum.
Figure 2.
Suppression of S6 kinase reverses the apoptotic effect of suppressed eIF4E expression. (A) MDA-MB-231 cells were transfected at 50% confluence with negative control siRNA or siRNA for eIF4E as indicated. 24 hr later, the cells were put in fresh media containing either 10% or 0% serum and the indicated concentration of rapamycin or DMSO vehicle. After another 24 hr, the level of cleaved PARP, eIF4E, P-S6K, and S6K by Western blot analysis. (B) MDA-MB-231 cells were transfected at with negative control siRNA, siRNA for eIF4E, or S6 kinase as indicated. 24 hr later, the cells were put in fresh media with 10% serum. After another 24 hr, the level of cleaved PARP, eIF4E, S6K, and actin by Western blot analysis. The data are representative of experiments repeated at least two times.
We also investigated the effect of dual knockdown of S6 kinase and eIF4E. As with low-dose rapamycin treatment, suppression of S6 kinase expression prevented the apoptotic effect of eIF4E ablation only in the presence of serum (Fig. 2B). Taken together, these data demonstrate that suppression of either S6 kinase phosphorylation or the expression of S6 kinase prevented the apoptotic effect of singularly suppressing eIF4E expression in the presence, but not in the absence, of serum.
3.3. Suppression of apoptosis by S6 kinase ablation is dependent on TGF-β signaling
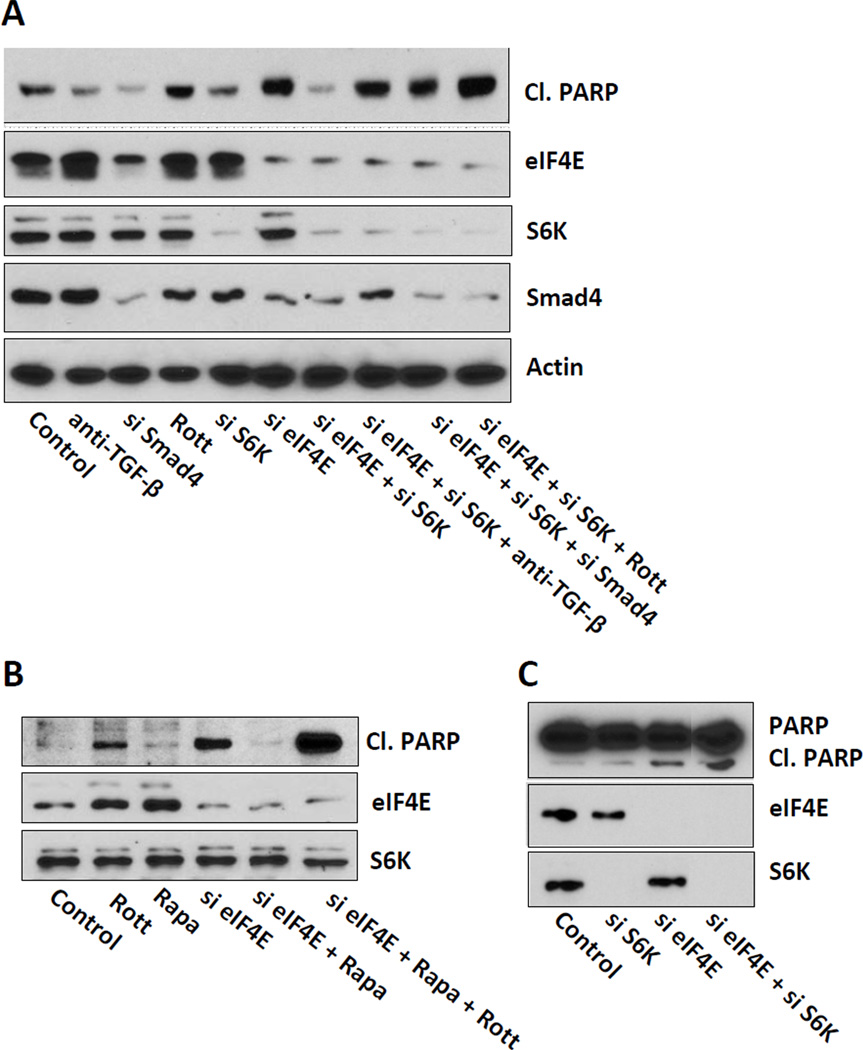
We previously reported that the protective effect of serum upon rapamycin treatment was contingent upon the TGF-β signaling, which induced a G1 cell cycle arrest [7; 8]. Therefore, we examined whether prevention of apoptosis by dual inhibition of S6 kinase and eIF4E was dependent on TGF-β signaling. MDA-MB-231 cells were co-treated with siRNAs against eIF4E and S6 kinase and either a neutralizing anti-TGF-β antibody, rottlerin – a compound that inhibits protein kinase Cδ (PKCδ) [7], which is required for TGF-β signaling [13; 14], or siRNA against Smad4, which is also required for canonical TGF-β signaling [15]. All three co-treatments resulted in induction of cleaved PARP (Fig. 3A). Similarly, we also asked if the protective effect of low-dose rapamycin treatment upon eIF4E ablation was dependent on TGF-β. In fact, rottlerin treatment prevented the protective effect of low-dose rapamycin (Fig. 3B). We also examined the effect of suppressing eIF4E and S6 kinase in BT-549 breast cancer cells because they do not express PKCδ [16]. The BT549 cells remained sensitive to eIF4E ablation upon S6 kinase knockdown (Fig. 3C). These data demonstrate that the S6 kinase suppression of the apoptotic effect of eIF4E ablation is dependent on TGF-β signals.
Figure 3.
Suppression of apoptosis by S6 kinase ablation is dependent on TGF-β signaling. (A) MDA-MB-231 cells were transfected at 50% confluence with negative control siRNA, siRNA for eIF4E, S6 kinase, or Smad4 as indicated. 24 hr later, the cells were put in fresh media with 10% serum and a neutralizing TGF-β antibody or rottlerin (Rott) (3 µM) where indicated. After another 18 hr, the level of cleaved PARP, eIF4E, S6K, Smad4, and actin by Western blot analysis. (B) MDA-MB-231 cells were transfected with negative control siRNA or siRNA for eIF4E as in (A). 24 hr later, the cells were put in fresh media with 10% serum and either 20 nM rapamycin or DMSO vehicle where indicated. Where indicated, rottlerin (Rott) (3 µM) was also provided. After another 18 hr, the level of cleaved PARP, eIF4E, and S6K by Western blot analysis. (C) BT-549 cells were prepared and transfected with negative control siRNA, siRNA for eIF4E, or S6 kinase as indicated. 24 hr later, the cells were put in fresh media with 10% serum and rapamycin (20 nM) where indicated. After another 18 hr, the level of cleaved PARP, eIF4E, and S6K by Western blot analysis. The data are representative of experiments repeated at least two times.
3.4. Suppression of S6 kinase induces G1 arrest in cells with ablated eIF4E
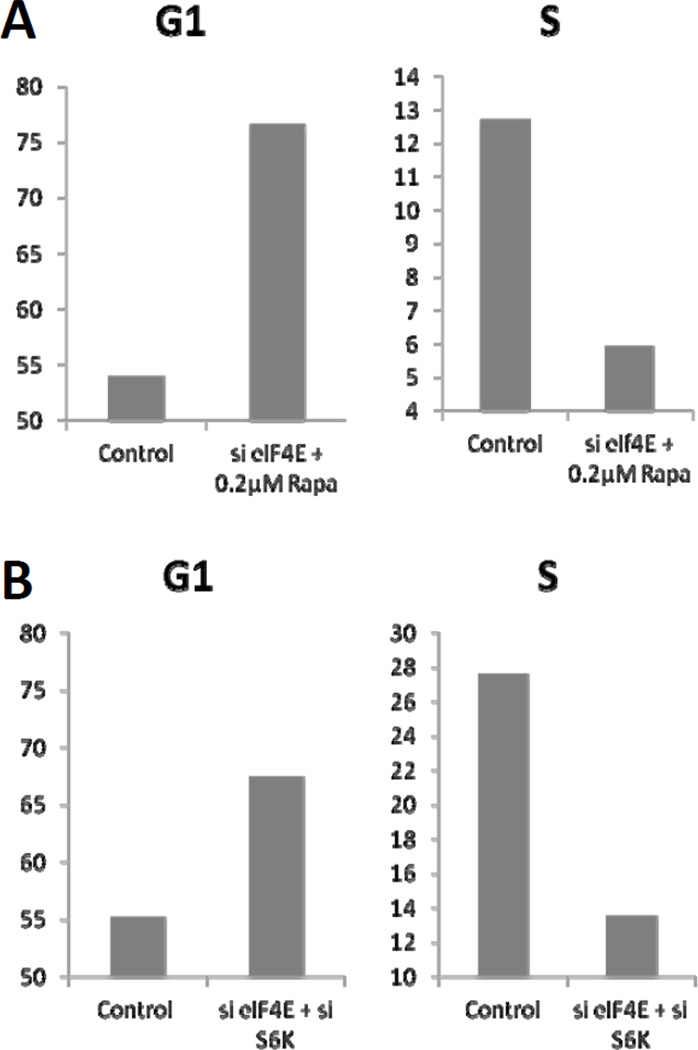
While low-dose rapamycin induced TGF-β signaling,[10] it only weakly suppressed G1 cell cycle progression in MDA-MB-231 cells [8; 17]. High-dose (20 µM) was required to completely block synchronized cells from entering S-phase [8]. Therefore, we compared the effect of low-dose rapamycin and suppression of S6 kinase expression on MDA-MB-231 cells with ablated eIF4E expression on cell cycle progression. Both low-dose rapamycin (Fig. 4A) treatment and suppression of S6 kinase expression (Fig. 4B) resulted in the accumulation of cells with G1 DNA content and corresponding reductions in the number of cells with S-phase DNA content. These data demonstrate that the TGF-β-dependent suppression of G1 cell cycle progression in the presence of serum results in G1 cell cycle arrest – consistent with the idea that blocking cell cycle progression (from G1 into S-phase) prevents apoptosis associated with the absence of eIF4E expression.
Figure 4.
Suppression of S6 kinase induces G1 arrest in cells with ablated eIF4E. (A) MDA-MB-231 cells were transfected at 50% confluence with negative control siRNA or siRNA for eIF4E. 24 hr later, the cells were put in fresh media with 10% serum and the indicated concentrations of rapamycin. (B) MDA-MB-231 cells were transfected at 50% confluence with negative control siRNA, siRNA for eIF4E, or S6 kinase. 24 hr later, the cells were put in fresh media with 10% serum. The cells in both (A) and (B) were then subjected to flow cytometric analysis 24 hr later as described in Materials and Methods. The DNA content per cell shown graphically above is from a representative experiment that was repeated three times.
4. Discussion
Previously, we reported that rapamycin caused G1 cell cycle arrest in the presence of serum [8]. We associated the effect of high-dose rapamycin on inhibition of 4E-BP1 phosphorylation with an indirect inhibition of eIF4E [8]. Here, we investigated the role of eIF4E in cell cycle arrest. Surprisingly, we observed cell death upon eIF4E knockdown in MDA-MB-231 breast cancer cells in both the presence and absence of serum. Since highdose rapamycin treatment continues to suppress phosphorylation of S6 kinase (as it does at low doses), we reasoned that cells avoid the apoptosis induced by high-dose rapamycin through suppression of S6 kinase. In fact, the MDA-MB-231 cells were insensitive to eIF4E knockdown with suppression of S6 kinase phosphorylation or its expression. Preventing apoptosis was due to a de-repression of S6 kinase-dependent inhibition of TFG-β signaling in the presence of serum that prevented G1 cell cycle progression.
Our findings have implications on the cytostatic and cytotoxic properties of rapamycin. Administered at nano-molar doses, rapamycin has generally been considered a cytostatic drug [18]. These concentrations suppress S6 kinase phosphorylation but actually only retard G1 cell cycle progression [8; 19]. Instead, micro-molar doses of rapamycin that also suppress the phosphorylation of 4E-BP1 are required to achieve complete G1 cell cycle arrest [8]. Cell cycle arrest observed with high-dose rapamycin treatment in the presence of serum is contingent upon TGF-β signaling, which is suppressed by active mTORC1 [7; 9; 10]. Cancer cells with defective TGF-β, in principle, should undergo apoptosis rather than arrest upon high-dose rapamycin treatment. Defective TGF-β signaling is common in both pancreatic [20] and colon cancer [6; 21]. Thus, these cancers may be candidates for targeting the phosphorylation of 4E-BP1 by mTORC1.
A somewhat puzzling aspect of this study is the effect of suppressing S6 kinase leading to survival of cells where eIF4E expression has been suppressed. S6 kinase is generally considered to have pro-survival effects [22; 23]. However, there is evidence to support the pro-apoptotic role of S6K1 [24]. An appealing hypothesis is that although S6 kinase is typically anti-apoptotic, the lack S6 kinase in combination with a lack of eIF4E is lethal. In other words, active S6 kinase is critical for protein synthesis and cell growth, but in the absence of eIF4E, the signal for protein synthesis is incomplete and the cell recognizes that something is wrong and undergoes apoptosis. Cells have a similar response to cell proliferation. For example the transformation of primary cells requires two oncogenes – such as Ras and Myc [25]. However, Ras or Myc by themselves induces either apoptosis or senescence [26]. Thus, when cells need cooperating signals, there is evidence to indicate that there are mechanisms for the cells to abort and undergo apoptosis – and this may be the case for loss of eIF4E when S6 kinase is still active.
With regard to therapeutically targeting mTORC1, our study stresses the importance of evaluating S6 kinase, 4E-BP1 and eIF4E with mTORC1 inhibitors. Logically, the requirement to suppress 4E-BP1 phosphorylation in order to achieve complete G1 arrest may be linked to an eIF4E-bound state. Dephosphorylation of all 4EBP1 sites in a hierarchical manner is necessary to release eIF4E [27]. eIF4E initiates cap-dependent translation of mRNAs for the translation of mRNAs of proteins involved in cell cycle progression [3; 4]. The lack of expression of proteins critical for progression through S-phase may explain why suppression of eIF4E is cytotoxic if cell death is not preempted by an arrest in G1.
Another issue with regard to targeting 4E-BP1 phosphorylation with rapamycin, is that the level needed to suppress all 4E-BP1 phosphorylation sites in cell culture exceeds the maximum tolerated dose in the clinic [28; 29]. However, sustained rapamycin treatment has been shown to suppress mTORC2 [2], which is even more resistant to rapamycin than the mTORC1 phosphorylation of 4E-BP1. The impact of rapamycin on 4EBP1 phosphorylation has been examined in clinical trials [29], however these trials examined the impact on the phosphorylation of 4E-BP1 at Thr70, which is sensitive to doses of rapamycin that suppress S6 kinase phosphorylation [8]. The key sites where the phosphorylation correlates with the cytotoxic effects are Thr37/46 and Ser65 [8]. It remains to be seen whether rapamycin can suppress phosphorylation on these sites with prolonged rapamycin treatment.
Targeting mTORC1 with rapamycin is also complicated by the observation that inhibition of mTORC1 and S6 kinase phosphorylation stimulates a feedback activation of the pro-survival mTOR complex 2 (mTORC2) in many cancer cells [30; 31]. However for this study that involves the breast cancer cell line MDA-MB-231 cells, we reported previously that this pathway is inactive in these cells and rapamycin does not stimulate mTORC2-dependent Akt phosphorylation at the mTORC2 site at Ser473 [1]. While this problem is not relevant for the cells used in this study, it could be relevant for many cancer cells. The obvious solution to this issue is the use of catalytic inhibitors that effectively target both mTOR complexes [32; 33]. Thus, the efficacy in the catalytic inhibitors may involve the ability to inhibit the phosphorylation of 4E-BP1 by mTORC1 and the phosphorylation of Akt at Ser473 by mTORC2. The toxicity of the catalytic inhibitors has not yet been fully evaluated.
Another approach would be to uncouple the effect of rapamycin on S6 kinase and 4E-BP1 phosphorylation by inhibiting the effect of suppressed S6 kinase phosphorylation – namely TGF-β signals. This could be done with compounds that inhibit the TGF-β signals that promote G1 cell cycle arrest. Thus, while targeting 4E-BP1 phosphorylation and eIF4E-dependent protein synthesis, may present challenges, understanding the limits of rapamycin-based therapies suggests novel approaches that can exploit the critical mTORC1 signaling node that promotes cell cycle progression and survival in what may be virtually all human cancers.
Acknowledgements
This work was supported by a grant from the National Cancer Institute (CA46677). Research Centers in Minority Institutions (RCMI) award RR-03037 from the National Center for Research Resources of the National Institutes of Health, which supports infrastructure and instrumentation, is also acknowledged. PY was supported by a Gene Center Fellowship from the RCMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol. Cell. Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5:519–523. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 5.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadir N, Jackson DN, Lee E, Foster DA. Defective TGF-β signaling sensitizes human cancer cells to rapamycin. Oncogene. 2008;27:1055–1062. doi: 10.1038/sj.onc.1210721. [DOI] [PubMed] [Google Scholar]
- 8.Yellen P, Saqcena M, Salloum D, Feng J, Preda A, Xu L, Rodrik-Outmezguine V, Foster DA. High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex1 and suppressing phosphorylation of 4E-BP1. Cell Cycle. 2011;10:3948–3956. doi: 10.4161/cc.10.22.18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song K, Wang H, Krebs TL, Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-β/ALK5-mediated Smad3 activation. EMBO J. 2006;25:58–69. doi: 10.1038/sj.emboj.7600917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadir N, Lee E, Garcia A, Toschi A, Foster DA. Suppression of TGF-β signaling by phospholipase D. Cell Cycle. 2007;6:2840–2845. doi: 10.4161/cc.6.22.4921. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Rodrik V, Foster DA. Alternative phospholipase D/mTOR survival signal in human breast cancer cells. Oncogene. 2005;24:672–679. doi: 10.1038/sj.onc.1208099. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22:3937–3942. doi: 10.1038/sj.onc.1206565. [DOI] [PubMed] [Google Scholar]
- 13.Perillan PR, Chen M, Potts EA, Simard JM. Transforming growth factor-β1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-δ in reactive astrocytes from adult rat brain. J. Biol. Chem. 2002;277:1974–1980. doi: 10.1074/jbc.M107984200. [DOI] [PubMed] [Google Scholar]
- 14.Runyan CE, Schnaper HW, Poncelet AC. Smad3 and PKCδ mediate TGF-β1-induced collagen I expression in human mesangial cells. Am. J. Physiol. Renal. Physiol. 2003;285:F413–F422. doi: 10.1152/ajprenal.00082.2003. [DOI] [PubMed] [Google Scholar]
- 15.Massague J. TGF-β in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI, Humphries MJ, Reyland ME, Foster DA. Suppression of cell migration by protein kinase Cδ. Oncogene. 2005;24:3067–3072. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- 17.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8:567–572. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 18.Easton JB, Houghton PJ. Therapeutic potential of target of rapamycin inhibitors. Expert Opin. Ther. Targets. 2004;8:551–564. doi: 10.1517/14728222.8.6.551. [DOI] [PubMed] [Google Scholar]
- 19.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 21.Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur. J. Cancer. 2005;41:2060–2070. doi: 10.1016/j.ejca.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R, Hess D, Krek W. S6K1-mediated disassembly of mitochondrial URI/PP1γ complexes activates a negative feedback program that counters S6K1 survival signaling. Mol. Cell. 2007;28:28–40. doi: 10.1016/j.molcel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology. 2009;50:216–229. doi: 10.1002/hep.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J. Clin. Oncology. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 30.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov. Today. 2011;16:325–331. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roulin D, Waselle L, Dormond-Meuwly A, Dufour M, Demartines N, Dormond O. Targeting renal cell carcinoma with NVP-BEZ235, a dual PI3K/mTOR inhibitor, in combination with sorafenib. Mol. Cancer. 2011;10:90. doi: 10.1186/1476-4598-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]