Abstract
Background
Ulcers are frequently visible in magnified, cross-polarized, dermoscopy images of basal cell carcinoma. An ulcer without a history of trauma, a so-called “atraumatic” ulcer, is an important sign of basal cell carcinoma, the most common skin cancer. Distinguishing such ulcers from similar features found in benign lesions is challenging. In this research, color and texture features of ulcers are analyzed to discriminate basal cell carcinoma from benign lesions.
Methods
Ulcers in polarized-light dermoscopy images of 49 biopsy-proven basal cell carcinomas were identified and manually selected. For 153 polarized-light dermoscopy images of benign lesions, those areas which most closely mimicked ulcers were similarly selected. Fifteen measures were analyzed over the areas of ulcers and ulcer mimics. Six of those measures were texture measures: energy, variance, smoothness, skewness, uniformity and entropy. Nine of those measures were color measures: relative measures of red, green and blue; chromaticity of red, green and blue; and the ratios blue-to-green, blue-to-red and green-to-red.
Results
A back-propagation artificial neural network was able to discriminate most of the basal cell carcinoma from benign lesions, with an area under the ROC curve as high as 92.46%, using color and texture features of ulcer areas.
Conclusion
Separation of basal cell carcinoma from benign cutaneous lesions by image analysis techniques applied to ulcers is feasible. As ulcers are a critical feature in malignant lesions, this finding may have application in the automatic detection of basal cell carcinoma.
Keywords: basal cell carcinoma, image analysis, ulcers, dermoscopy
Introduction
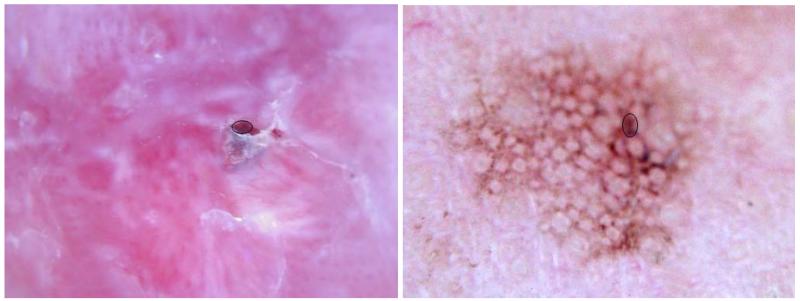
Cutaneous ulcers are eroded areas of skin. These areas have an appearance that varies with time. Small, early ulcers appear bright red with saturated color due to undiluted fresh blood (Figure 1a), progress to larger areas (Figure 1b) and finally appear dark reddish-brown and dried during the healing stage (Figure 1c, shows a darker, dried ulcer surrounded by lighter scale). Although they possess a variety of colors due to a varying admixture of serum and a variety of textures, from smooth and moist to rough and dry, these varied ulcer states have the same physiologic meaning: an eroded skin surface. In the absence of any injury (trauma) to the skin, these “atraumatic” ulcers may indicate the presence of basal cell carcinoma, the most common malignancy (1).
Fig. 1.
Ulcers in basal cell carcinoma: a. Early, patchy ulcers b. Larger ulcer c. Dried, crusted ulcer
Polarized-light dermoscopy uses light emitting diodes (LEDs) and two polarizers: one in the incident light path and one in the reflected light path, with a difference in polarizer orientation of 90 degrees. This arrangement is called cross-polarization. The use of cross-polarized light minimizes surface reflections, enabling an enhanced view of such features as cutaneous pigmentation (2). In contrast to contact dermoscopy, another form of dermoscopy that employs polarized or non-polarized light with surface contact and a medium such as gel to improve the surface interface, non-contact polarized-light dermoscopy allows semitranslucency (3) and better appreciation of various shades of red in malignancies (4).
The magnification afforded by dermoscopy enables the detection of ulcers at an early stage. At this stage, some benign lesions may have small ulcers, due to minor irritation or inflammation (Figure 2a). In addition, some areas in benign lesions closely mimic ulcers by their color and/or texture (Figure 2b). It is the purpose of this study to extract features of ulcers in polarized, non-contact dermoscopy images of basal cell carcinoma, to allow the discrimination of basal cell carcinoma from non-malignant lesions having similar features.
Fig. 2.
Ulcer mimics in benign lesions: a. Ruptured cyst with scar b. Pigmented actinic keratosis
Material and Methods
Data acquisition
For this study, 202 dermoscopy images of skin lesions were taken in the course of the study NIH SBIR R44 CA-101639-02A22, by Stoecker & Associates, Rolla, MO and Skin and Cancer Associates, Plantation, Florida. 153 of these images were selected at random from benign lesions consisting of melanocytic nevi, dysplastic nevi, lentigines, actinic keratoses, seborrheic keratoses, lichen planus-like keratoses, hemangiomas, warts, sebaceous hyperplasia and porokeratoses. The remaining 49 images were of basal cell carcinomas (BCC) which contained ulcers. All BCCs and any of the other lesions that were not clinically benign had the diagnosis confirmed by biopsy and histopathology. The Phelps County Regional Medical Center Institutional Review Board, Rolla Missouri, approved this research and each subject or subject’s parent or guardian signed a consent form for this research. All images were polarized light, non-contact, dermoscopy images taken with a Sony DSC-W70 7.2 megapixel digital camera in fine mode with a 3Gen DermLite DL2 dermoscopy attachment (3Gen LLC, San Juan Capistrano, CA). All images were reduced to 1024×768 resolution, retaining full 24-bit color, and images were checked for brightness, multiplying all pixel values by a multiplier needed to produce image brightness within acceptable limits. Borders were drawn by a student (SMS), to segment the 202 skin lesions from background, using a second-order spline curve and later checked by a dermatologist (WVS). Spots were selected within ulcers on the 49 BCCs and delineated with the same second-order spline curve technique, as shown in Figure 1. For the 153 benign lesions, the areas best resembling ulcers (smooth, red to dark-brown) were similarly selected, as shown in Figure 2. The closed curves segmenting both lesion and selected ulcer and ulcer-mimic spots were converted to binary image masks, allowing computation of color and texture features. A median 3x3 filter was applied to all images.
Determination of color features
Relative colors on the spots are defined as relR = Rspot − Rskiin, where Rspot is the average of the red pixel value on the spot and Rskin is the average red color of a small area surrounding the lesion, proportional to the lesion size. Similarly defined are relG and relB (5). Relative red chromaticity is defined as . The relative color ratios , , and are similarly defined for the spots.
Determination of texture features
The six texture measures are defined in Table 1, using the intensity histogram as a probability mass function, where the intensity at a pixel is given as , which is the average of the R, G and B values for a pixel.
Table 1.
Texture Measures (modified from reference 6)
| No. | Measure | Formula for Measure | Description |
|---|---|---|---|
| T1 |
Texture
Mean (Brightness) |
The mean is the average brightness or gray level, where ri is the ith gray level, L is the number of gray levels, p(r) is the histogram for a region, and p(ri) is the probability of occurrence of gray level ri. |
|
| T2 |
Texture
Variance (Contrast) |
The variance σ2, measures the average contrast. | |
| T3 |
Texture
Smoothness |
The smoothness index S, measures the relative smoothness of the gray level in a region. S approaches 0 for a very smooth region and 1 for a coarse region. |
|
| T4 |
Texture
Skewness |
The skewness index κ , measures the skewness of a histogram. κ = 0 is for a symmetric histogram and is positive or negative for histograms skewed right or left. |
|
| T5 |
Texture
Uniformity |
The uniformity measure U, measures uniformity and is maximum when all gray levels are equal. |
|
| T6 | Texture Entropy (Information) |
Entropy e, as defined by Claude Shannon, measures the uncertainty or information content of a message. The higher the entropy, the greater the information. |
1. Experiments performed
A three-layer neural network (Partek Predict Software System, Partek Inc., St. Louis, MO) with a varied number of nodes in the input layer, one hidden layer, and one output layer was used to determine lesions as malignant (BCC) or benign, based solely on the color and texture features, which characterize ulcerous areas of BCCs and their mimics. All neural network experimental configurations used on-line Partek, neural-network training with a leave-one-out methodology for training and testing. Four neural networks were used. The first one used the nine color features described in the abstract and methods of this paper. The second used the six texture features defined in Table 1. The third used all 15 color and texture features. However, Partek’s neural network obtained higher accuracy when the last two color features, the ratios and , were eliminated. So, the fourth neural network had higher accuracy than the others when tested using only 13 total features: 6 texture and 7 color features.
Results
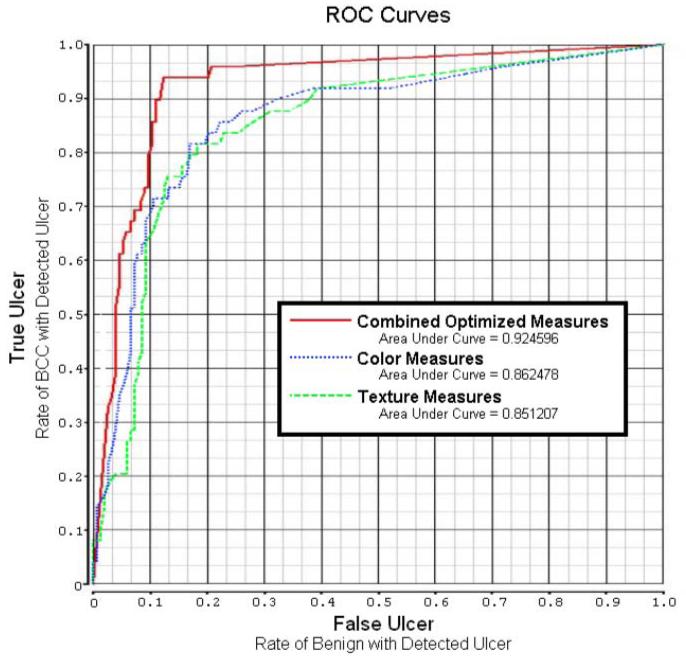
Figure 3 shows the plot of receiver operating characteristic (ROC) curves and areas under the ROC curves for the neural network results. For the different ROC curves presented, the vertical axis shows the true positive rate (sensitivity), and the horizontal axis gives the false positive rate (1-specificity). The best neural network gave an accuracy of 92.46%. After applying the best neural network to the 13 features, logistic regression analysis was performed using the SAS LOGISTIC procedure (SAS Inc., Cary, NC). The first five features chosen and their significance were: 1) relative blue, with p < 0.007; 2) relative red, with p < 0.0031; 3) texture skewness, p < 0.0015; 4) texture energy, p < 0.0029; and 5) relative green, p < 0.0324. The statistical p-values are the maximum likelihood estimates, using the Wald Chi-sq statistic. All other features in the final model lacked significance (i.e., p-values > 0.05).
Fig. 3.
ROC curve and AUC (area under curve) for color, texture, and combined, optimized neural networks.
Discussion
To our knowledge, this is the first study using a series of non-contact, polarized-light dermoscopy images to detect a malignant feature. In addition to the documented advantages noted previously for detection of semitranslucency and shades of red in malignancy, non-contact, polarized-light dermoscopy viewing is faster than contact dermoscopy, because no gel is applied to the skin.
Although digital image analysis has been used to measure the extent of ulcers (7-9), to our knowledge detection of ulcer features in BCC has not been reported. The application of ulcer features to discriminate BCC from benign lesions is more difficult than the detection and/or measurement of ulcers which are distinguishable from normal skin by simple observation. However, BCCs are not easily distinguished from mimics in other lesions by simple observation, as shown in Figures 1 and 2. Based on the AUC of the ROC curves, all neural networks were able to achieve good discrimination of BCCs from benign lesions using features from the ulcer spots alone. Based on the diagnostic results achieved using the color and texture features, and on multivariate statistical analysis of the color and texture features, color features are more useful for detection of ulcers than texture features (particularly in the high-specificity case, where few false ulcers are found). But, both are needed for the most reliable ulcer detection.
We have chosen BCC detection rather than ulcer detection as the study endpoint. Although ulcer discrimination is inherently easier, BCC detection has the potential for direct clinical application. Lesions on high-risk areas such as the head and neck can be viewed, allowing reliable assessment even for BCC less than 2 mm in diameter. To our knowledge, such tiny BCC are best detected by dermoscopy, and semi-automated methods, such as those described herein. These methods could ultimately result in automated methods that play a clinical role in skin cancer detection.
One limitation of the study was the use of relative colors to calculate color ratios. Unexpected sign changes can occur when a lesion pixel value is brighter than background color. This can yield a smaller denominator and a very large final value for chromaticity and the three color ratios , , and . The system actually achieved better performance without the final two color ratios. A better relR method for determining chromaticity is to subtract background skin chromaticity from lesion chromaticity, a method developed by Celebi et al (10). Further limitations of the study include the use of a convenience image set, with non-consecutive acquisition of images acquired as time permitted in the clinic. Lesion boundaries and ulcer boundaries were manually determined. The degree to which accurate boundaries may be obtained automatically for BCC is unknown. Further research includes automation of boundary detection and application of these color and texture methods to a larger set of lesions.
Acknowledgment
This publication was made possible by Grant Number SBIR R44 CA-101639-02A2 of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Harold S. Rabinovitz, MD of Skin and Cancer Associates, Plantation, Florida supplied images used in the image processing and analysis.
References
- 1.Stoecker WV, Stolz W. Dermoscopy and the diagnostic challenge of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144(9):1207–10. doi: 10.1001/archderm.144.9.1207. [DOI] [PubMed] [Google Scholar]
- 2.McFall K. Photography of dermatological conditions using polarized light. J Audiov Media Med. 1996;19(1):5–9. doi: 10.3109/17453059609018381. [DOI] [PubMed] [Google Scholar]
- 3.Stoecker WV, Kolm I, Rabinovitz HS, Oliviero MC, Xu J, Malters JM. Semitranslucency in dermoscopic images of basal cell carcinoma. Arch Dermatol. 2009;145(2):224. doi: 10.1001/archdermatol.2008.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benvenuto-Andrade C, Dusza SW, Agero AL, Scope A, Rajadhyaksha M, Halpern AC, Marghoob AA. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143(3):329–38. doi: 10.1001/archderm.143.3.329. [DOI] [PubMed] [Google Scholar]
- 5.Umbaugh SE, Moss RH, Stoecker WV. Automatic color segmentation of images with application to detection of variegated coloring in skin tumors. IEEE Eng Med Biol. 1989;8(4):43–52. doi: 10.1109/51.45955. [DOI] [PubMed] [Google Scholar]
- 6.Stoecker WV, Gupta K, Shrestha B, et al. Detection of basal cell carcinoma using color and histogram measures of semitranslucent areas. Skin Res Technol. 2009;15(3):283–7. doi: 10.1111/j.1600-0846.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowling FL, King L, Fadavi H, Paterson JA, Preece K, Daniel RW, Matthews DJ, Boulton AJ. An assessment of the accuracy and usability of a novel optical wound measurement system. Diabet Med. 2009 Jan;26(1):93–6. doi: 10.1111/j.1464-5491.2008.02611.x. [DOI] [PubMed] [Google Scholar]
- 8.Sebastiani M, Manfredi A, Colaci M, Giuggioli D, La Sala R, Elkhaldi N, Antonelli A, Ferri C. [Correlation of a quantitative videocapillaroscopic score with the development of digital skin ulcers in scleroderma patients] [Article in Italian] Reumatismo. 2008 Jul-Sep;60(3):199–205. doi: 10.4081/reumatismo.2008.199. [DOI] [PubMed] [Google Scholar]
- 9.Russell L, Reynolds T, Carr J, Evans A, Holmes M. A comparison of healing rates on two pressure-relieving systems. Br J Nurs. 9(22):2270–80. doi: 10.12968/bjon.2000.9.22.5414. 2000 Dec 8-2001 Jan 10. [DOI] [PubMed] [Google Scholar]
- 10.Celebi ME, Hitoshi I, Stoecker WV, Moss RH, Rabinovitz HS, Argenziano G, Soyer HP. Automatic detection of blue-white veil and related structures in dermoscopy images. Comput Med Imaging and Graphics. 2008;132(8):670–677. doi: 10.1016/j.compmedimag.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]