Abstract
The medial prefrontal cortex is important for normal regulation of stress responses, and is implicated in stress-related affective disease states (e.g. depression). In the current study, we investigated the role of the prelimbic division of the prefrontal cortex in control of responses to psychogenic and systemic stressors (restraint and hypoxia, respectively). Acute stimulation of the prelimbic cortical region with bicuculline methiodide (BMI) caused significant reduction of ACTH and corticosterone responses to restraint and reduced Fos activation of paraventricular nucleus neurons, consistent with a role in central inhibition of acute psychogenic stress responses. In contrast, BMI enhanced corticosterone (but not ACTH) responses to hypoxia via a mechanism suggestive of central PVN drive and enhanced adrenal sensitivity. Acute BMI increased restraint stress-induced Fos activation in known downstream targets of the prelimbic cortex (e.g., the basolateral amygdala and central amygdaloid nuclei), suggesting a connection between modulation of amygdalar signaling and stress inhibition. In contrast, hypoxia caused robust Fos activation in the basolateral and central amygdala, which was not affected by prelimbic BMI injection. The data suggest that the prelimbic cortex stimulation is sufficient to trigger inhibition of the HPA axis to psychogenic stress, but may play a very different role in enhancing HPA responsiveness to physical threats.
Keywords: prefrontal cortex, infralimbic cortex, HPA axis, ACTH, corticosterone, paraventricular nucleus, amygdala, adrenal sensitivity
Introduction
The mammalian prefrontal cortex (PFC) is required for appropriate processing of stressful information. In humans, prefrontal cortex dysfunction is linked to stress-related diseases such as PTSD and depression [1, 2]. Prefrontal cortex complex volume is associated with glucocorticoid regulation in man [3], suggesting a functional link between the PFC and regulation of stress hormone secretion.
Recent studies reveal that the role of the PFC in stress regulation is complex, with different subregions having divergent actions on stress responses. In rodents, lesions of the dorsal divisions of the medial PFC, largely targeting the prelimbic cortex (PL), result in prolonged glucocorticoid responses to stressors of psychogenic (e.g., restraint, air puff), but not systemic (e.g., ether) origin [4–6]. Enhanced corticosterone responses are associated with increased Fos activation in hypophysiotrophic corticotrophin-releasing hormone neurons of the hypothalamic paraventricular nucleus [6, 7], consistent with enhanced drive of the neural limb of the hypothalamo-pituitary-adrenocortical (HPA) axis. Basal corticosterone release is not affected by lesions [4, 5, 8], suggesting that the PL primarily affects stress activation of the HPA axis. Notably, inhibition of the PL enhances heart-rate responses to psychological stimuli [9], suggesting that PL activation also controls autonomic stress responses. In contrast, lesions of the extreme ventromedial division of the PFC (encompassing the infralimbic cortex (IL)) result in slight attenuation of HPA axis stress responses [7, 10], and lesion or inactivation of this region reduces cardiovascular responses to psychogenic stress [9]. Thus, the dorsal and ventral divisions of the PFC (PL and IL, respectively) appear to have very different and perhaps opposing roles in stress regulation.
Neither the PL nor the IL project directly to the paraventricular nucleus of the hypothalamus (PVN) [11–13]. As is the case with other limbic HPA-regulatory structures (e.g., hippocampus, amygdala) (see [14]), the two PFC divisions act on the PVN via intermediary synapses. Targets of the PL include PVN-projecting regions such as the bed nucleus of the stria terminalis (BST) [6], lateral hypothalamus (LHA) and posterior hypothalamus. The PL also projects heaviliy to the paraventricular thalamus (PVT), which is known to play a prominent role is stress habituation and sensitization [13]. In the case of the BST, at least a proportion of the PL-PVN relays are GABAergic [15], consistent with trans-synaptic inhibitory actions of the PL on HPA axis stress responses. The IL also targets the BST and LHA, and also projects to additional potential PVN relay sites in nucleus of the solitary tract and dorsomedial hypothalamus [6, 11–13]. In addition to targeting PVN projecting regions, the PL heavily innervates the basolateral amygdala (BLA) [12], which is known to modulate HPA axis function via trans-synaptic relays [14, 16].
The current study was designed to test the sufficiency of the PL for stress circuit activation and HPA axis regulation in vivo. Microinjections of the GABA-A antagonist bicuculline methiodide (BMI) were used to acutely activate the PL. Our data support an inhibitory role for the PL in regulation of responses to acute psychogenic stress, but also suggest an opposite role in mediating responses to systemic challenge.
Methods
Animals
Male Sprague-Dawley rats (250 – 300 g; Harlan, Indianapolis, IN) were housed individually in standard rat cages and acclimated for 1 week prior to initiation of experiments. Rats were maintained in a temperature- and humidity-controlled room (lights on 06:00 to 18:00) with food and water available ad libitum. All experimental procedures and protocols were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Cannula Placement and Microinjections
Animals were anesthetized a mixture of ketamine (87 mg/kg) and xylazine (13 mg/kg). Double-barrel guide cannulae (Plastics One, Roanoke, VA) were stereotaxically implanted (+3.0 mm from bregma, 0.75 mm left and right of midline, and 3.0 mm below the skull) so that the tips of the cannulae were 0.5 mm superior to the PL. Cannulae were secured by dental cement and sealed with a double dummy cannula cut to the same dimensions. Rats were handled daily for 2 weeks and cannulae were manipulated in order to habituate animals to the injection procedure. On the day of injection, dummy cannulae were removed and replaced with internal injector cannulae under light manual restraint. Initial studies employed conjoint unilateral infusions of BMI and saline (performed simultaneously using a Harvard microsyringe pump) in unstressed rats to estimate extent of Fos activation by BMI. For restraint and hypoxia stress studies, bilateral infusions of BMI or vehicle were performed simultaneously using a Harvard microsyringe pump, with a total volume of 500 nl delivered over 60 sec. Both BMI (1 ng/μl) and saline were co-injected with Pontamine Sky Blue (1%) to mark the site of injection and approximate spread of injectate. Injections were performed immediately before initiation of restraint or hypoxia.
Stress Protocols
Stress testing was initiated between 09:00 and 10:00, during the circadian trough of CORT secretion. Different groups of injected animals were exposed to either acute restraint, serving as a psychogenic stressor, or hypoxia, serving as a systemic stressor, with plasma CORT and ACTH responses used as indices of HPA activation. A basal blood sample was taken by tail clip immediately prior to stress exposure in all rats. For restraint, animals were placed in well-ventilated Plexiglas restraint tubes for 30 min and tail clip blood samples (200 μl) were collected immediately prior to removal from the restrainer and 30 and 90 min after being returned to their home cages. For hypoxia, rats were placed in the testing chamber with a 8% oxygen/92% nitrogen mixture permeated through the chamber for 30 min. Blood was sampled by tail clip under light manual restraint 30, 60 and 120 minutes following initiation of hypoxia.
Blood Collection and Radioimmunoassay
Blood was sampled by the tail clip procedure that involves clipping the tail with a sterile scalpel blade and collecting blood in 1.5 ml microcentrifuge tubes containing 10 ul 100 mM EDTA. Tubes were then spun at 1500 g and plasma pipetted into 0.5 ml tubes and frozen at −20 °C for subsequent analysis of plasma CORT and ACTH. Plasma CORT levels were measured using a 125I RIA kit (MP Biomedicals Inc., Orangeburg, NY). Plasma ACTH concentrations were determined with an RIA that used a specific antiserum donated by Dr. William Engeland (University of Minnesota, Minneapolis, MN) at a dilution of 1: 120,000 with 125I ACTH (Amersham Biosciences, Piscataway, NJ) as a labeled tracer. For each assay, all plasma samples were run in duplicate and analyzed within the same assay.
Tissue Collection and Immunohistochemistry
Two hours after initiation of restraint or hypoxia, rats were given an overdose of sodium pentobarbital and perfused transcardially with 0.9% saline followed by 3.7% paraformaldehyde in 0.1 M PBS. Brains were removed, postfixed in 3.7% paraformaldehyde overnight at 4 °C, and then placed in 30% sucrose dissolved in PBS. Brains were serially sectioned at 25 μm on a freezing microtome and stored at −20 °C in sterile cryoprotectant solution until processing. For immunohistochemistry, sections were rinsed several times in 50 mm potassium PBS (KPBS; pH 7.4) to remove cryoprotectant. Sections were incubated in 1% H2O2 to quench endogenous peroxidase followed by washing in KPBS (5 × 5 min). Sections were subsequently blocked for 1 h with 0.2% bovine serum albumin in 50 mm KPBS with 0.2% Triton-X 10. Sections were incubated overnight at 4 °C in rabbit polyclonal anti- Fos (dilution 1: 20,000; Calbiochem, San Diego, CA, USA). The next day, sections were washed in KPBS (5 × 5 min), incubated in biotylinated goat anti-rabbit (dilution 1: 500) (Vector Laboratories, Burlingame, CA, USA) for 1 h, washed in KPBS (5 × 5 min), and reacted with avidin-biotin peroxidase (dilution 1: 800) (ABC Elite Kit; Vector Laboratories) for 1 h. Sections were washed once again in KPBS (5 × 5 min) and subsequently reacted with 0.02% diaminobenzidine/0.09% hydrogen peroxide in KPBS. Reactions were allowed to proceed for 5–10 min, at which point the reaction was stopped by sequential rinses in KPBS. Sections were mounted on gelatinized slides, allowed to dry, dehydrated with alcohol and xylene, and coverslipped.
Cell Counting
Digital images were collected for quantification of Fos positive immunoreactive nuclei in regions of interest. The number of Fos-immunoreactive cell nuclei was determined from thresholded images using Scion Image software. A uniform threshold (based on a pre-defined threshold function in Scion Image) was applied to all images in a given region of interest and the average cell count was automatically calculated. The final cell counts were expressed as the mean number of positive nuclei. The shape and size of each region of interest studied were defined according to the boundaries outlined in Paxinos and Watson (1998). A total of 2 – 6 images were analyzed for each region and averaged to produce a mean cell count for each region.
Data Analysis
Time-course data for ACTH and CORT were analyzed using two-way repeated measures ANOVA with treatment and time as factors. Treatment differences at individual time points were assessed using Fisher’s LSD post-test. Area under the curve (AUC) for ACTH and CORT responses to restraint and hypoxia were analyzed with the t-test. Adrenal sensitivity was estimated as the concentration of CORT divided by the log of the concentration of ACTH [17]. Differences in Fos cell counts between treatments were determined by t-test.
Results
Injection Sites
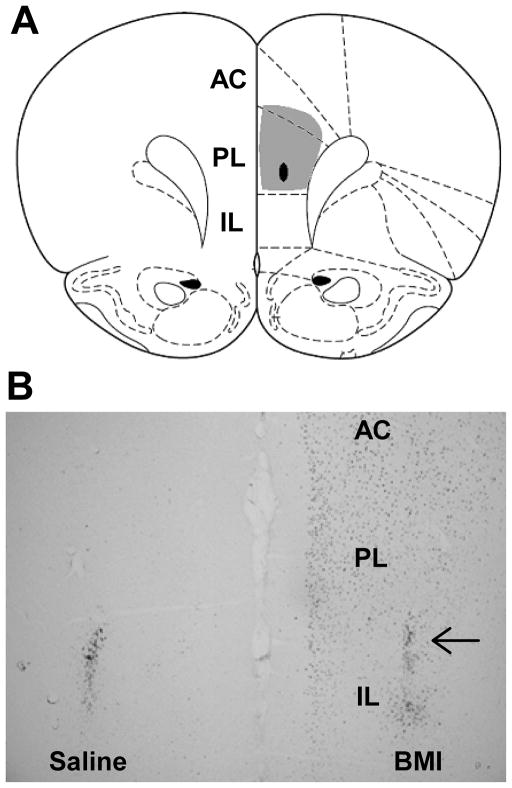
Injection of BMI in unstressed rats increased the number of c-Fos immunoreactive neurons in the PL ipsilateral to the site of injection, confirming that BMI treatment leads to disinhibition of the PL (Fig. 1A). Microinjections of saline (n = 20) or BMI (n = 20) were largely localized to the PL component of the rat PFC (Fig. 1B). Injection sites were confirmed by histological localization of injector tips in the PL (arrow) and (in BMI-injected rats) by localization of enhanced Fos staining in the PL region. The ‘hit rate’ was 75%. Typical injections encompassed most of the PL at this level, with some spread to the overlying anterior cingulate cortex and underlying infralimbic cortex. Any injections causing extensive activation of the IL were excluded from the data analysis (n’s were not sufficient to parse into an additional group for analysis). Animals with partial activation of the anterior cingulate cortex were accepted, as the connectivity of the region in rodents is inconsistent with a role in stress regulation (Ongur and Price 2000).
Figure 1.
Targeting of bicuculline (BMI) injections to the prelimbic cortex (PL). A. Atlas section mapping a prototypical injection, with injector tip indicated by the black oval and extent of maximal Fos induction indicated by the gray shaded area. B. Unilateral injections of BMI dramatically increase Fos immunoreactivity in otherwise unstimulated animals. Note the lack of Fos induction in the contralateral PL, which was injected with saline at the same time (injector tips noted by localized Fos reactivity). Note the extensive Fos induction of the PL, with some additional activation evident in the ventral anterior cingulate cortex (AC) and the dorsal infralimbic cortex (IL).
Plasma ACTH and CORT
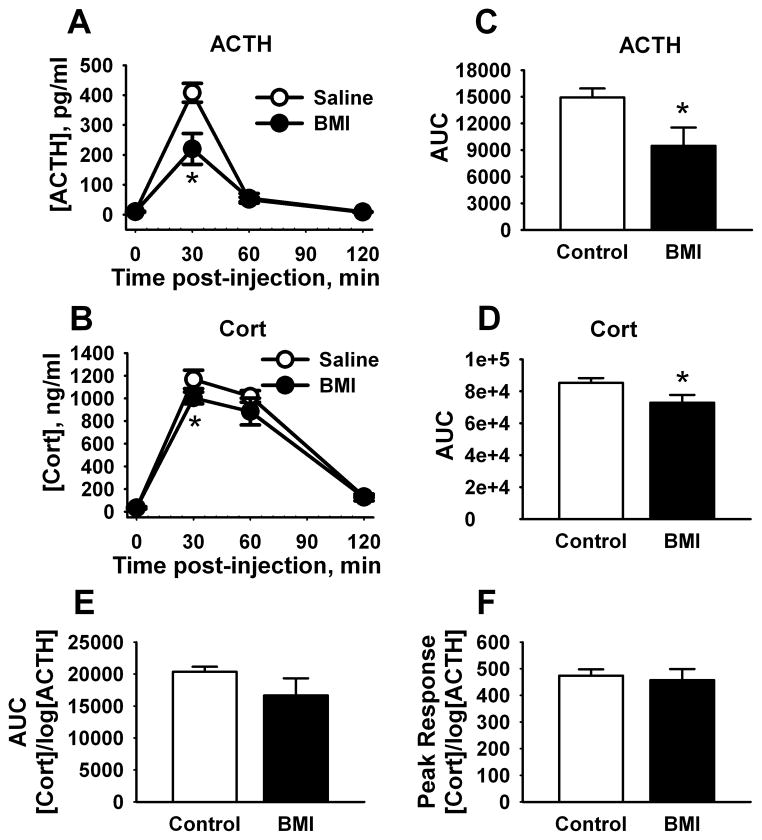
In animals exposed to acute restraint stress there was a significant main effect of treatment [F(1,57) = 10.6, p < 0.01], time [F(3,55) = 104.9, p < 0.001], and a significant treatment x time interaction [F(3,55) = 10.9, p < 0.001] for plasma ACTH. Compared to saline treatment (n = 8), BMI-injected animals (n = 7) had significantly (p < 0.001) decreased levels of ACTH 30 min after injection (Fig. 2A). There were also significant effects of treatment [F(1,58) = 4.3, p < 0.05] and time [F(3,56) = 221.6, p < 0.001] for CORT concentration, as BMI (n = 7) significantly (p < 0.05) decreased levels of plasma CORT 30 min post-injection compared to saline (n = 9) (Fig. 2B). In addition, the total AUC for the ACTH [t(13) = 2.5, p < 0.05] and CORT [t(14) = 2.3, p < 0.05] stress response was significantly lower in rats injected with BMI compared to saline (Fig. 2C–D). Finally, adrenal sensitivity, measured as CORT concentration divided by the log of ACTH concentration [17], did not differ over the entire response time-course (Fig. 2E) or at the time of peak secretion (Fig. 2F).
Figure 2.
Effects of PL BMI injection on HPA axis responses to restraint. PL-targeted injections of BMI decreased peak ACTH (A) and corticosterone (CORT) (B) responses to acute restraint, consistent with trans-synaptic inhibition. Overall ACTH (C) and CORT (D) responses were also reduced following BMI, as reflected in decreased integrated secretory responses (area under the curve (AUC)). Adrenal sensitivity was not affected by BMI (either over the entire time-course (E) or at the peak response time (F)). (n = 7 – 9/group) * = p < 0.05, Fisher’s LSD test.
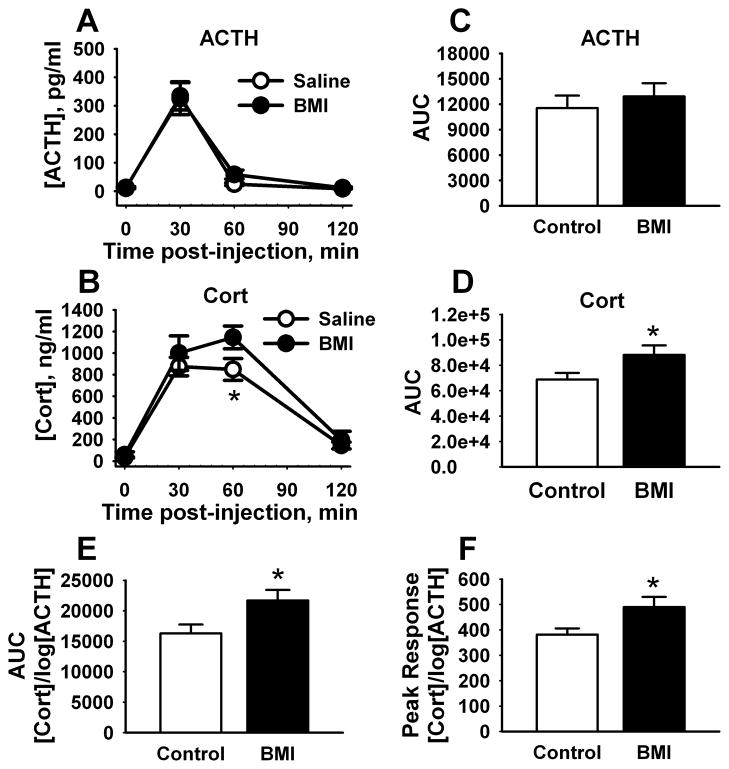
For ACTH responses to hypoxia stress, there was a significant effect of time [F(3,51) = 32.1, p < 0.001] but not treatment (Fig. 3A), indicating that BMI did not alter the magnitude of ACTH secretion following hypoxia. However, there were significant effects of treatment [F(1,47) = 5.1, p < 0.05] and time [F(3,45) = 82.7, p < 0.001] on plasma CORT levels (Fig. 3B). Animals treated with BMI (n = 5) had significantly (p < 0.01) higher CORT 60 min post-injection relative to saline (n = 8; Fig. 3B). The total AUC for CORT concentration over time was also significantly higher following BMI administration [t(11) = −2.2, p < 0.05], whereas ACTH responses were unaffected (Fig. 3C–D). Adrenal sensitivity was enhanced over the response time-course [t(13) = −2.4, p < 0.05] (Fig. 3E) and at the time of peak secretion [t(11) = −2.5, p < 0.05] (Fig. 3F), indicating that enhanced CORT release is likely due to BMI-mediated modulation of adrenal sensitivity.
Figure 3.
Effects of PL BMI injection on HPA axis responses to hypoxia.PL-targeted injections of BMI did not affect ACTH secretion following hypoxia (A). However, corticosterone responses were significantly increased (B). Integrated ACTH responses were not affected by BMI (C), whereas CORT responses were significantly increased (D). Adrenal sensitivity was significantly increased by BMI injection across the entire stress time-course (E) and at the peak response time (F). (n = 5 – 8/group) * = p < 0.05, Fisher’s LSD test.
Fos Immunoreactivity
Hypophysiotrophic (medial parvocellular) and preautonomic (dorsal parvocellular) divisions of the PVN were activated by both restraint and hypoxia (Fig. 4A–B). In general, Fos induction in the medial parvocellular zone was less pronounced after hypoxia than after restraint [t(14) = 3.5, p < 0.01]. Activation of the PL region by BMI did not significantly decrease the number of Fos immunoreactive neurons in the medial parvocellular PVN (however, p = 0.1) (Fig. 4C). In contrast, BMI increased [t(11) = −3.0, p < 0.01] the number of medial parvocellular PVN Fos immunoreactive neurons after exposure to hypoxia (Fig. 4D), consistent with enhanced central activation of the PVN. BMI reduced [t(13) = 3.1, p < 0.01] the number of restraint stress-induced Fos-immunoreactive neurons in the dorsal parvocellular PVN (Fig. 4E). However, the number of Fos immunoreactive neurons in the dorsal parvocellular PVN was not significantly altered by BMI following hypoxia (Fig. 4F).
Figure 4.
Post-stress Fos induction in the PVN after PL BMI injection. Fos immunostaining revealed robust cellular response in the PVN following restraint, which was reduced in the BMI group (A). In contrast, the Fos response to hypoxia was less robust than that seen following restraint (p < 0.05), and increased substantially after BMI treatment (B). Cell counts revealed that the number of Fos immunopositive cells decreased in the dorsal parvocellular region of the PVN of BMI treated animals following restraint, whereas decrements in the medial parvocellular PVN were not significant (p = 0.10)(C,E). In contrast, BMI increased the number of Fos responsive medial parvocellular neurons following hypoxia, but did not affect staining in the dorsal parvocellular zone (D,F). (n = 6 – 8/group) * = p < 0.05, Fisher’s LSD test.
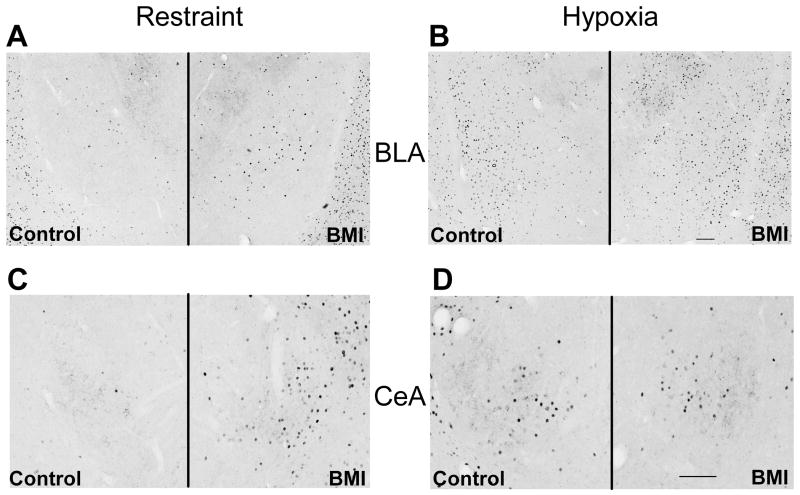
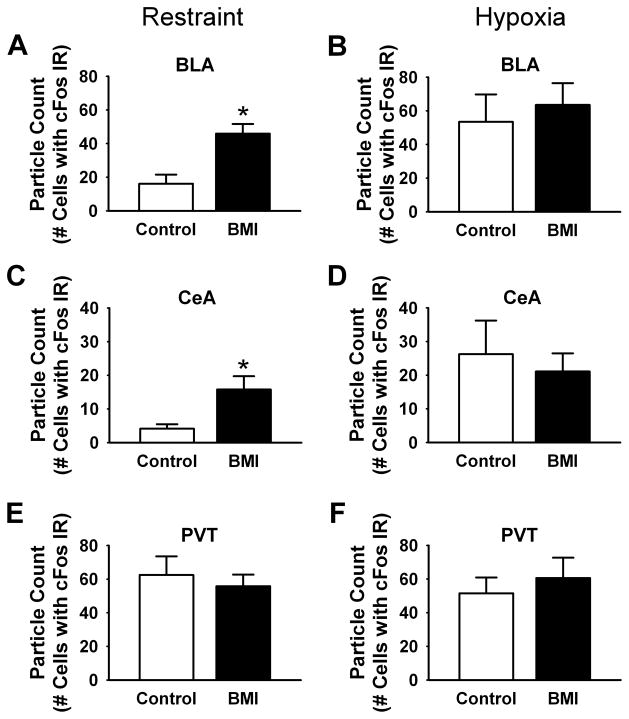
BMI injections in the PL increased the number of c-Fos immunoreactive neurons in the BLA and central nucleus of the amygdala (CeA) of animals exposed to restraint (Fig. 5). Quantification of particle counts in response to restraint stress revealed a significant effect of treatment in the BLA [t(11) = −4.1, p < 0.01] and CeA [t(10) = −2.4, p < 0.05] (Fig. 6A,C), as there were significantly more Fos-positive neurons in BMI-injected rats (n = 7) than saline controls (n = 6; Fig. 4). Injections of BMI did not further increase hypoxia-induced Fos expression in the amygdala (Fig. 6B, D). Additionally, BMI did not affect Fos expression in the PVT, also a principle efferent target of the PL (Fig. 6E, F) [13].
Figure 5.
Fos expression in extrahypothalamic regions following BMI injection into the PL prior to restraint (A,C) or hypoxia (B,D). BMI injection increased the extent of Fos labeling in the BLA (A) and CeA (C) after restraint. Fos staining was not affected in the BLA (B) or CeA (D) of BMI treated rats subjected to hypoxia.
Figure 6.
Analysis of Fos expression in extrahypothalamic regions following BMI injection into the PL prior to restraint (A,C) or hypoxia (B,D). BMI injection increased the number of Fos-positive neurons in the BLA (A) and CeA (C) after restraint. Greater numbers of Fos-positive neurons were observed in the BLA (B) and CeA (D) following hypoxia in the saline group (p <0.05). However, BMI did not affect the number of Fos-immunoreactive cells in the BLA or CeA of hypoxia-treated rats. No changes in the number of Fos-positive neurons were observed in the PVT following either restraint (E) or hypoxia (F). (n = 5 – 8/group) * = p < 0.05, Fisher’s LSD test.
Discussion
Our study supports a role for the PL in inhibition of HPA axis responses to acute psychogenic stress. Stimulation of the PL region by BMI is sufficient to reduce both ACTH and corticosterone secretion following restraint stress. However, regional PL stimulation had an opposing action on HPA responses to hypoxia, causing increased corticosterone release, enhanced adrenal sensitivity and medial parvocellular PVN Fos activation. The data suggest differential roles of this PFC region in responses to psychogenic and systemic stimuli.
The observation that PL-targeted stimulation inhibits HPA axis stress responses complements the existing literature on medial PFC lesions [4, 5, 7], and suggests that this region is necessary and sufficient to cause inhibition of HPA axis responses to restraint stress. Effects of PL stimulation were evident throughout the HPA axis, including pituitary ACTH secretion and adrenal corticosterone release, suggesting that effects of PL stimulation were mediated via inhibition at the level of the CNS. However, we did not observe significant decrements in hypophysiotrophic parvocellular PVN Fos immunoreactivity (p = 0.1). The failure to observe significant decrements in the complement of Fos neurons may be due to any of several factors. First, PL stimulation reduces, but does not block psychogenic stress responses. Thus, the PL may reduce duration of the PVN cellular response rather than its initiation, which may not be easily detectable when using counts of Fos-immunostained neurons as a marker. Second, PL inputs may selectively target specific complements of PVN neurons, which may not be a high enough number to impact the total pool of stress-activated cells. Recent studies indicate significant rostro-caudal differences in innervation of the PVN [18], suggesting the possibility that PL-PVN relays may only affect a portion of the CRH neuron pool. Finally, PL stimulation may affect feedback circuits that only become active after corticosterone levels reach a critical value (see [19]), and therefore cannot completely prevent the initial Fos induction engendered by the stressor.
The ability of PL stimulation to enhance hypoxia-mediated corticosterone responses and PVN Fos activation was surprising, given previous findings that lesions of the PL region do not affect responses to stimuli such as ether inhalation [4, 5]. Overall, our data suggest that PL activation is sufficient to drive responses to hypoxia, but may not be required for these responses to occur. Interestingly, enhanced corticosterone responses to hypoxia occur without concomitant increases in ACTH, and the adrenal appears to be more sensitive to ACTH. Together, the data suggest that stimulation of the PL affects HPA axis drive by altering sensitivity of the adrenal. Enhanced stress reactivity may be mediated by enhancement of sympathetic nervous system activation, which is known to increase adrenal ACTH responsiveness [20].
Previous studies report that retrodialysis of dopaminergic antagonists into the PL/IL region inhibits ACTH responses to systemic (interleukin 1-beta) but not psychogenic (air puff) stress [21]. These data suggest that PL and/or IL may be differential modulated by different classes of afferent input, which may be sufficient to alter the net valence of prefrontal regulation of the stress response.
Activation of the PL by BMI differentially affected extrahypothalamic Fos production by restraint vs. hypoxia. In the case of restraint, Fos induction is increased in the BLA, a known target of the PL [12 ], as well as in the CeA. Efferents from the PFC are known to project to inhibitory interneurons in the other amygdalar regions (e.g., intercalated nuclei) [22]. If this arrangement is maintained in the BLA, PL stimulation may contribute to inhibition of the HPA axis by dampening excitatory BLA output. Activation of the CeA in BMI treated rats following restraint is somewhat surprising, given that very few CeA neurons are stimulated by restraint in vehicle-treated rats (see also [23, 24]). These data indicate that PL activation recruits this cellular population. In contrast, PL stimulation does not augment activation of BLA and CeA by hypoxia. Hypoxia activates larger numbers of BLA [t(12) = −2.4, p < 0.05] and CeA [t(11) = −2.3, p < 0.05] neurons than restraint, and raises the possibility that PL stimulation is insufficient to recruit additional neurons in these regions.
No differences in Fos activation of the PVT were observed following PL stimulation in conjunction with hypoxia or restraint. The PVT is a prominent target of the PL [12, 13, 25], and is thought to be involved in facilitation as well as habituation of responses to repeated stress [26, 27]. Thus, single stimulation of PL may not be sufficient to alter activation of this region by acute stressors.
The ability of GABA-A inhibitors to cause local excitation in vivo is well documented [22, 28, 29]. Previous studies indicate that picrotoxin causes activation of Fos in known PFC targets in the amygdala (intercalated nuclei) [22]. In addition, local injections of BMI increase PVN activation [30], verifying the efficacy of this method for producing activation of defined neurocircuits. However, we need to acknowledge that some neurons may not be responsive to BMI, and that BMI may also result in stimulation of inhibitory neurons, which can then dampen local excitation. Nonetheless, our data clearly indicate that local BMI induces widespread neuronal activation in output layers of the PL, and alters activation of monosynaptic (e.g., BLA) as well as trans-synaptic (e.g., CeA, PVN) targets of the PL.
Studies performed in naïve animals prove that we can target BMI activation to primarily the PL. However, we acknowledge that we cannot preclude the possibility that IL activation also contributes to our results. For example, the IL projects to the CeA and may be responsible for recruitment of this structure by PL-targeted stimulation in the restraint test [11, 13, 31]. In addition, the role of the IL in stress regulation appears to oppose that of the PL [7, 10, 14], and may be more important in control of autonomic responses to stress (see [14]). Thus, it is possible that the potentiating effects on responses to hypoxia may be the result of a specific contribution of dorsally-situated IL neurons activated by the BMI injections.
Conclusions
The prefrontal cortex is critical for regulation of brain stress processing, and is implicated in numerous stress-related disease states. Our data indicate that the prelimbic region is sufficient (as well as necessary (see [14])) for inhibition of central HPA axis responses to psychogenic stress, and is positioned to play a major role in linking glucocorticoid dyshomeostasis with stress-related disease states such as depression.
Acknowledgments
This work was supported by MH049698 and MH069860. We would like to thank Ben Packard for his excellent technical assistance with these studies.
References
- 1.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–8. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. J Clin Endocrinol Metab. 2006;91:1591–4. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- 4.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamo-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 6.Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–76. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- 7.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–76. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 9.Tavares RF, Correa FM, Resstel LB. Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J Neurosci Res. 2009;87:2601–7. doi: 10.1002/jnr.22070. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–40. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–76. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 12.McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–38. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- 13.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 14.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–9. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 17.Engeland WC, Byrnes GJ, Presnell K, Gann DS. Adrenocortical sensitivity to adrenocorticotropin (ACTH) in awake dogs changes as a function of the time of observation and after hemorrhage independently of changes in ACTH. Endocrinology. 1981;108:2149–53. doi: 10.1210/endo-108-6-2149. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich-Lai YM, Jones KR, Zielger DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. doi: 10.1002/cne.22571. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology. 151:4811–9. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76:79–92. doi: 10.1159/000064426. [DOI] [PubMed] [Google Scholar]
- 21.Spencer SJ, Ebner K, Day TA. Differential involvement of rat medial prefrontal cortex dopamine receptors in modulation of hypothalamic-pituitary-adrenal axis responses to different stressors. Eur J Neurosci. 2004;20:1008–16. doi: 10.1111/j.1460-9568.2004.03569.x. [DOI] [PubMed] [Google Scholar]
- 22.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–53. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 24.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 25.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 27.Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–10. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 28.Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–98. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- 29.Oishi T, Mikami A, Kubota K. Local injection of bicuculline into area 8 and area 6 of the rhesus monkey induces deficits in performance of a visual discrimination GO/NO-GO task. Neurosci Res. 1995;22:163–77. doi: 10.1016/0168-0102(96)80436-6. [DOI] [PubMed] [Google Scholar]
- 30.Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–69. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 32.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]