Abstract
Purpose
Epidemiological data are conflicting regarding the association between androgenetic alopecia (AA) and prostate cancer (CaP). We examined the relationship between these two conditions.
Materials and Methods
We performed a case-control study at a Veterans Affairs Hospital among 708 men: 312 healthy controls, 167 men with CaP, and 229 men without CaP on prostate biopsy. Participants were asked to self-describe hair patterns at ages 30, 40 and at study enrollment. We tested the association between hair pattern (overall, vertex or frontal) and CaP status using logistic regression analysis adjusting for multiple clinical features. Disease grade was similarly examined as a secondary outcome.
Results
Relative to healthy controls, younger age of AA onset was significantly associated with increased CaP risk (p=0.008). Similar patterns were noted for frontal (p=0.005) and not vertex balding (p=0.22). When compared to biopsy negative men, a similar pattern was seen with younger age of AA onset having higher risk for CaP, though this was not significant (p=0.07). A suggestion for younger age of AA onset for frontal (p=0.07) being associated with CaP vs. biopsy negative men was also observed. Overall balding (yes/no) was associated with > 2-fold increase of high-grade disease (p=0.02).
Conclusions
Men reporting earlier AA onset were at increased CaP risk and suggestively had more aggressive disease. Contrary to other studies, frontal balding was the predominant pattern associated with elevated CaP risk. Further study is required to confirm these findings in a larger sample and to better understand the role of AA, androgens, and CaP biology.
INTRODUCTION
Prostate cancer is considered a hormonally-linked cancer, though to date, most studies have failed to show an association between serum androgen levels and prostate cancer (CaP) risk. (1, 2) As such, the role of androgens in prostate cancer development is unclear. However, serum testosterone levels may not correlate with androgenic activity within the prostate, stressing the importance of understanding intra-prostatic androgen activity. (3, 4) Thus, alternative approaches to studying the association between androgen activity and prostate cancer risk are needed. One such approach is to study androgenetic alopecia (AA). AA is a phenotype which results from the interplay between genetic susceptibility and androgenic activity. (5, 6) AA is associated with increased androgen activity in the skin/hair follicle resulting in increased quantitative and qualitative hair loss. (7) Moreover, AA is a highly prevalent condition which increases with age and indeed the age-specific prevalence of AA is very similar to the age-specific prevalence of CaP. (5, 7) Already shown to be related to benign prostatic hyperplasia, AA also is speculated to be related to androgen activity within prostatic tissue. (8) Indeed, one common treatment for AA is finasteride, a 5 alpha-reductase type-2 inhibitor, which has also been shown to decrease CaP risk. (9, 10) Prior studies are conflicting as to whether AA is associated with increased risk of CaP. (11–18) Several, but not all studies found an increased risk of cancer diagnosis in men with AA, specifically men with vertex balding patterns. (11, 12) Also, only two prior studies have examined AA and grade of disease with conflicting results. (12, 17) Thus, we studied a multi-ethnic cohort of Veterans in an effort to describe the association between hair patterns, CaP risk, and grade of disease.
MATERIALS AND METHODS
Patients
After obtaining institutional review board approval, 1,904 male patients (controls n=992 and men undergoing biopsy n=912) were screened to participate in a prostate cancer case-control study between January 2007 and August 2011 at the Durham Veterans Administration Hospital (DVAMC) in Durham, NC. Controls were male veterans seen at the DVAMC with no prior history of prostate cancer who had a PSA measurement within one year of enrollment and were not recommended to undergo a biopsy. There were no other inclusion or exclusion criteria and thus we did not try to individually match cases and controls for any specific characteristics. Cases were men scheduled for a prostate needle biopsy at the DVAMC due to concerns for prostate cancer: typically an elevated PSA and/or suspicious digital rectal examination (DRE). Of the 1,904 potential participants, a total of 1,195 participants provided written consent and completed the in-person interview (controls n=472; men undergoing biopsy n=723). Participants were measured for heights and weights, and also were given a personal history questionnaire which elicited information on hair balding patterns as well as family history of prostate cancer in any first or second degree relative. They were asked to complete the document either in person at their study visit or at home at their convenience and return it to the study coordinator at the DVAMC in a provided pre-addressed, postage-paid envelope. The goal was to have the biopsy subjects return their questionnaire before they knew the results of the biopsy to minimize recall bias. Of the 1,195 consented participants, 747 (313 healthy controls and 434 biopsied subjects) completed the section of the questionnaire pertaining to hair patterns (response rate of 63%). Of these men, 38 patients were excluded from analysis for missing data points pertaining to BMI, PSA, DRE, and family history of CaP for a study population of 708 patients. For men undergoing biopsy, DRE findings, cancer status, and Gleason grade were abstracted from medical records.
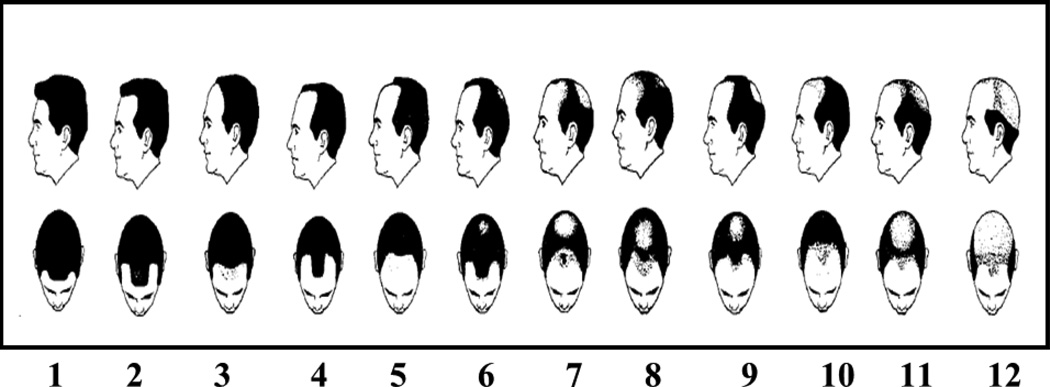
To assess hair baldness patterns, participants were shown a modified version of the Hamilton Scale of Hair Balding Patterns Modified by Norwood (HSB). (18) The HSB is a series of 12 view pairs of balding hair patterns from superior and lateral viewpoints. Each view pair is representative of a different pattern of hair baldness (fig.1). Patterns demonstrated were non-balding (1, 2), frontal (3, 4, 5, and 10), vertex (6), and combination of both vertex and frontal balding (7, 8, 9, 11, and 12). Participants were asked to evaluate their hair patterns at age 30, age 40, and at time of study enrollment (current).
Fig. 1.
Hair Balding Patterns adapted from Demark-Wahnefried et al., 2000
Statistical analysis
Participants were categorized for both frontal and vertex baldness separately as either bald or not bald at each location. HSB scores 1 and 2 were considered not bald for both frontal and vertex. HSB scores 3, 4, 5, 7, 8, 9, 10, 11, and 12 were considered frontal bald. A score of 6, 7, 8, 9, 11, and 12 were considered vertex bald.
The distribution of clinical characteristics and hair balding patterns was compared across the three study populations (i.e. control, biopsy positive, or biopsy negative) using the Kruskal-Wallis test and x2 analysis for continuous and categorical variables, respectively. Our primary outcome was to explore whether onset of AA independently predicted risk of cancer relative to healthy controls or men with a negative biopsy. To accomplish this we used two separate unconditional multivariate logistic regressions: one relative to healthy controls and one relative to biopsy-negative men. In both models we adjusted for age (continuous), race (black vs. non- black), and body mass index (BMI; continuous after logarithmic transformation, kg/m2). In the model examining biopsied subjects, we also controlled for DRE (suspicious vs. not suspicious for cancer), PSA (continuous after logarithmic transformation), and family history of CaP (any first or second degree relative affected). Among biopsy-positive men, we compared the association between onset of AA and high grade disease (biopsy Gleason score ≥7 vs. ≤6) using x2 analysis. Using a multivariate logistic analysis, we investigated if balding patterns independently predicted high-grade disease among men with cancer, mutually adjusting for PSA, BMI, DRE, age, and race. To test for trend, age of AA onset was treated as a continuous variable with men reporting being bald at age 30 assigned a value of 30, men reporting being bald at age 40 but not age 30 assigned a value of 40, and men reporting being bald at study enrollment, but not age 30 or 40 assigned a value equal to their age at study enrollment. Men who reported themselves as non-balding ever were arbitrarily assigned an age of AA onset of 80 years old. Given concerns how this arbitrary selection may influence our analyses, a sensitivity analysis was conducted assigning age of AA onset for non-balding men to 90 and 100 years of age. Our results remained consistent regardless of designated age of AA onset for non-balding men. Similarly, when non-bald men at study enrollment were excluded entirely, the magnitude of the associations between AA and CaP risk were largely unchanged, though due to the removal of over a third of the population, our power was reduced and the associations were no longer statistically significant. Thus, we used age 80 in all analyses for p-trend calculations. Finally, as our population included a large percentage of black men we explored whether race was a modifier in our analyses. Given that we found no significant interactions between race and AA and any of the outcomes examined (data not shown), data from the races were combined for analyses. All statistical analyses were performed using STATA version 11.1 (StataCorp, College Station, Texas) with p<0.05 defined as significant.
RESULTS
Demographics
Clinical and pathological characteristics for all study participants are presented in Table 1. Median age for all men was 62.4 years old, with no significant difference among the three study groups (p=0.08). Approximately 42% of participants reported their race as black. Black men were more likely to have cancer on biopsy than non-black men (p<0.001). The median BMI for all participants was 30.2 kg/m2, with healthy controls having significantly higher BMI than biopsied men (p=0.002). Among men with cancer upon biopsy, 74 (44%) had high-grade disease defined as a Gleason score ≥7.
Table 1.
Clinical and Pathological Characteristics of Study Population (n=708)
| Healthy Controls | Biopsy Negative | Biopsy Positive | P | ||
|---|---|---|---|---|---|
| n=312 | n = 229 | n = 167 | |||
| Median age, years (IQR) | 61.9 (58.2–65.1) | 62.5 (59.5–66.4) | 62.7 (59.6–66.3) | 0.08† | |
| Median PSA, ng/dl (IQR) | 0.8 (0.5–1.5) | 5.5 (4.3–6.5) | 6.1 (4.7–8.5) | <0.001† | |
| Race (%) | <0.001‡ | ||||
| Black | 102 (33) | 98 (43) | 94 (56) | - | |
| Non-Black | 210 (67) | 131 (57) | 73 (44) | ||
| Median BMI Kg/m2, (IQR) | 30.8 (27.0–34.0) | 29.3(26.0–32.8) | 29.0 (25.6–32.3 ) | 0.002† | |
| Family History | 0.21 | ||||
| Negative | 249 (80) | 172 (75) | 122 (73) | ||
| Positive | 63 (20) | 57 (25) | 45 (27) | ||
| Digital Rectal Examination (%) | 0.77† | ||||
| Not suspicious | - | 172 (75) | 123 (74) | ||
| Suspicious | - | 57 (25) | 44 (26) | ||
| Gleason Score (%) | - | ||||
| ≤6 | - | - | 93 (57) | ||
| ≥7 | - | - | 74 (44) | - | |
Generated using Chi squared test
Generated using ANOVA
Across all 3 study populations, 267 men (38%) reported never being bald (table 2). We observed a larger proportion of biopsy positive men reported early onset of hair loss (age 30) relative to both healthy controls and biopsy negative men (28% vs. 22% vs. 24%), though this only approached significance (p=0.07). Furthermore, when examining hair loss patterns separately, there was a suggestion that men with cancer on biopsy were more likely to report early frontal (26% vs. 21% vs. 18%, p=0.054) or vertex (11% vs. 10% vs. 9%, p=0.08) balding versus healthy controls and biopsy negative men.
Table 2.
Incidence of Self-Reported Hair Balding Patterns At Various Ages
| Healthy Controls (%) | Biopsy Negative (%) |
Biopsy Positive (%) | |||
|---|---|---|---|---|---|
| Age of onset | P1 | ||||
| Overall: | 0.07 | ||||
| Age 30 | 68 (22) | 54 (23) | 48 (29) | ||
| Age 40 | 43 (14) | 39 (18) | 35 (21) | ||
| Current | 66 (21) | 54 (23) | 34 (20) | ||
| Never Bald | 135 (43) | 82 (36) | 50 (30) | ||
| Frontal: | 0.054 | ||||
| Age 30 | 55 (18) | 48 (21) | 44 (26) | ||
| Age 40 | 36 (11) | 31 (13) | 28 (17) | ||
| Current | 55 (18) | 49 (21) | 29 (17) | ||
| Never Bald | 166 (53) | 101(44) | 66 (40) | ||
| Vertex: | 0.08 | ||||
| Age 30 | 27 (09) | 24 (10) | 19 (11) | ||
| Age 40 | 40 (13) | 28 (12) | 20 (12) | ||
| Current | 66 (21) | 68 (30) | 53 (32) | ||
| Never Bald | 179 (57) | 109 (48) | 75 (45) | ||
Chi-Squared analyses examining onset of hair balding pattern and subject category
Relative to men who reported hair loss, men who were never bald were younger (p<0.001, table 3) and had lower serum PSA values (p=0.03) at study enrollment.
Table 3.
Clinical and Demographic Features by Age of Balding Onset (n=708)
| Age of Balding Onset |
Never bald | Bald at age 30 |
Bald at age 40 |
Bald at study enrollment |
P | |
|---|---|---|---|---|---|---|
| No. subjects | 267 | 170 | 117 | 154 | ||
| Median age, years (IQR) | 61.6 (58.1–64.7) | 62.5 (59.4–66.6) | 62.7 (59.2–67.0) | 63.5 (61.1–67.2) | <0.001 | |
| Median PSA, ng/dl (IQR) | 3.7 (0.8–5.6) | 4.5 (1.4–6.2) | 4.7 (1.4–6.3) | 4.5 (1.4–6.2) | 0.03 | |
| Race (%) | 0.09 | |||||
| Black | 104 (39) | 67 (39) | 61 (52) | 62 (40) | ||
| Non-Black | 163 (61) | 103 (61) | 56 (48) | 92 (60) | ||
| Median BMI Kg/m2, (IQR) | 29.8 (26.3–34.1) | 30.3 (27.1–34.2) | 28.9 (26.1–32.1 ) | 29.5 (26.1–32.8) | 0.10 | |
| Family History | 0.49 | |||||
| Negative | 204 (76) | 135 (79) | 84 (72) | 120 (78) | ||
| Positive | 63 (24) | 35 (21) | 33 (28) | 34 (22) | ||
| Digital Rectal Examination (%)** | 0.96 | |||||
| Not suspicious | 97 (73) | 75 (74) | 56 (76) | 67 (76) | ||
| Suspicious | 35 (27) | 27 (26) | 18 (24) | 21 (24) | ||
| Gleason Score (%)* | 0.14 | |||||
| ≤6 | 34 (68) | 26 (54) | 15 (43) | 18 (53) | ||
| ≥7 | 16 (32) | 22 (46) | 20 (57) | 16 (47) | ||
Generated using Chi squared test
Generated using Kruskal-Wallis
Data only available for men undergoing prostate biopsy
Data only available for men with cancer
Cases vs. controls
Relative to healthy controls, earlier age of AA onset was significantly associated with increased risk of CaP (p-trend=0.008, table 4). When each age of AA onset was examined separately, men who reported onset of balding at ages 30 and 40 had 88% and 66% increased risk of CaP, though this was significant only for balding at age 30. Onset of balding at study enrollment was not associated with CaP risk (p=0.54). When balding was analyzed according to vertex or frontal balding, early onset of frontal (p-trend: 0.005) and not vertex balding (p-trend: 0.22) was associated with CaP risk.
Table 4.
| vs. Healthy Controls | |||||
|---|---|---|---|---|---|
| Hair Balding Pattern |
Age of Onset3 |
Controls | PCa cases | Odds Ratio | 95% CI |
| General: | 312 | 167 | |||
| Never Bald | 135 | 50 | reference | - | |
| Age 30 | 68 | 48 | 1.88 | 1.12–3.16 | |
| Age 40 | 43 | 35 | 1.66 | 0.92–2.98 | |
| Current | 66 | 34 | 1.19 | 0.68–2.09 | |
| Frontal: | 250 | 138 | |||
| Never Bald | 166 | 66 | reference | - | |
| Age 30 | 55 | 44 | 2.08 | 1.24–3.48 | |
| Age 40 | 36 | 28 | 1.42 | 0.77–2.61 | |
| Current | 55 | 29 | 1.18 | 0.69–2.07 | |
| Vertex: | 224 | 103 | |||
| Never Bald | 179 | 75 | reference | - | |
| Age 30 | 27 | 19 | 1.66 | 0.84–3.27 | |
| Age 40 | 40 | 20 | 1.18 | 0.63–2.21 | |
| Current | 66 | 53 | 1.61 | 0.99–2.59 | |
Multivariate Analyses adjusted for race, age, family history, and BMI.
Reference group were men who were never bald.
Cases vs. biopsy negative men
Relative to biopsy negative men, earlier age of AA onset was associated with increased risk of CaP, though this did not reach statistical significance (p-trend=0.07, table 5). However, when each age of onset was examined separately, no specific age of onset was significantly associated with risk of CaP (p≥0.12). There was a suggestion that earlier age of frontal (p-trend= 0.07) but not vertex (p-trend=0.40) was associated with increased CaP risk relative to biopsy negative men.
Table 5.
| vs. Biopsy Negative | |||||||
|---|---|---|---|---|---|---|---|
| Hair Balding Pattern |
Age of Onset3 |
Biopsy Negative |
PCa cases |
Odds Ratio |
95% CI | P | P-trend |
| General: | 229 | 167 | 0.07 | ||||
| Never Bald | 82 | 50 | reference | - | - | ||
| Age 30 | 54 | 48 | 1.55 | 0.90–2.67 | 0.12 | ||
| Age 40 | 39 | 35 | 1.37 | 0.75–2.52 | 0.31 | ||
| Current | 54 | 34 | 0.96 | 0.54–1.71 | 0.89 | ||
| Frontal: | 229 | 167 | 0.07 | ||||
| Never Bald | 101 | 66 | reference | - | - | ||
| Age 30 | 48 | 44 | 1.63 | 0.93–2.86 | 0.09 | ||
| Age 40 | 31 | 28 | 1.63 | 0.80–3.32 | 0.18 | ||
| Current | 49 | 29 | 0.96 | 0.48–1.91 | 0.91 | ||
| Vertex: | 229 | 167 | 0.57 | ||||
| Never Bald | 109 | 75 | reference | - | - | ||
| Age 30 | 24 | 19 | 1.24 | 0.63–2.48 | 0.53 | ||
| Age 40 | 28 | 20 | 1.04 | 0.53–2.05 | 0.91 | ||
| Current | 68 | 53 | 1.04 | 0.64–1.69 | 0.88 | ||
Multivariate Analyses adjusted for race, age, family history, BMI, DRE, and PSA.
Reference group were men who were never bald.
High-grade disease
Among the 167 men with cancer on their biopsy there were limited men within each age of onset category and thus AA was examined as being bald at study enrollment or not. In general, being bald at study enrollment was associated with a 139% increase risk of high-grade disease (p=0.02, table 6). When frontal and vertex balding patterns were examined separately, we continued to observe a suggestion that being bald at the time of study enrollment was associated with a >1.5-fold increased risk of Gleason 7 or higher on biopsy (p≤0.06) relative to a Gleason 6 or lower, though this only reached statistical significance for frontal baldness (p=0.03).
Table 6.
Onset of Hair Balding Pattern At Various Ages and Risk of High Grade Disease Relative to Low Grade Disease
| Hair Balding Pattern |
Gleason<7 | Gleason≥7 | Odds Ratio |
95% CI | P1,2 | |
|---|---|---|---|---|---|---|
| General: | ||||||
| Bald vs. Not Bald | 93 | 74 | 2.39 | 1.12–5.10 | 0.02 | |
| Frontal: | ||||||
| Bald vs. Not Bald | 93 | 74 | 2.13 | 1.08–4.20 | 0.03 | |
| Vertex: | ||||||
| Bald vs. Not Bald | 93 | 74 | 1.78 | 0.92–3.44 | 0.09 |
Multivariate Analyses adjusted for race, age, family history, BMI, DRE, and PSA.
Reference group were men who were never bald.
DISCUSSION
Despite its categorization as an androgen-linked malignancy, the majority of studies have shown null or weak associations between serum androgen levels and CaP risk. (19–21) However, as serum levels may not reflect intra-prostatic levels nor androgenic activity, and as there is no easy method to assess intra-prostatic androgenic activity, there is a need to study alternative measures of androgen activity and their relationship to CaP risk, such as AA. In a multi-ethnic cohort of veterans, we found that earlier age of AA onset was associated with a significantly increased CaP risk relative to healthy controls. Similar trends were noted for frontal and vertex balding, though frontal balding was more strongly linked with CaP risk. Among men with CaP, we found that balding in general, regardless of age of onset was linked with high-grade disease. If these findings are validated in future studies, this would imply that earlier age of AA onset may be a CaP risk factor. Whether this represents shared pathophysiology, presumably related to androgen activity, or is a non-causal association requires further study.
AA is a common dermatologic condition with an age-specific prevalence similar to that of histological CaP in that 30% of men report hair loss at 30, 60% at 60, and 80% by age 80. (5, 7, 22) The hair loss seen in AA is a result of increased hair follicle sensitivity to androgens, specifically dihydrotestosterone (DHT), which alters the hair growth cycle such that hair density and quality are adversely affected. (22, 23) Polymorphisms in the androgen receptor gene which lead to increased receptor activity as well as higher expression of the 5-alpha-reductase-type 2 enzyme which promotes conversion of testosterone to its more biologically active form DHT, are thought to be important in the development of AA. (21, 24) As such, AA is often viewed as a marker of increased androgenic activity. Given the androgen dependency of CaP, there is great interest in further understanding the relationship between androgen activity and CaP risk. As such, it is not surprising that several studies have examined the association between AA and CaP. (11, 14)
Several prior studies found that AA in general was either associated with a significantly increased CaP risk, or a trend towards increased risk, which was predominantly due to the risk associated with vertex baldness. (11, 12, 14, 17) Our results are in part consistent with these prior reports in that we also found earlier onset of overall balding to be associated with increased CaP risk relative to healthy controls. Thus, AA, especially early onset, does appear to perhaps be a risk factor for CaP. However, contrary to these prior studies, we found that the increased overall risk was more strongly linked to frontal baldness than vertex baldness as reported by prior studies. The pathophysiological differences between frontal and vertex balding are unknown. As such, it is impossible to speculate why in our study frontal balding was more strongly associated with CaP risk compared to vertex baldness in prior studies. (12, 16) Interestingly, similar discrepancies arose when studying the association between AA and cardiovascular disease – a pathology similarly linked to androgens. (25, 26) While there is agreement that AA is associated with myocardial infarction and other adverse cardiovascular pathology, no consensus has been reached as to whether vertex or frontal balding is the dominant risk factor. (25, 27) In the future, as the pathophysiologies of vertex vs. frontal baldness become better understood, these distinguishing characteristics may shed additional light on potential mechanisms linking AA and CaP.
To our knowledge, only two previous studies have examined onset of AA and disease aggressiveness and demonstrate conflicting results. Where Yassa et al. found a null association between AA and high-grade disease, Giles et al. observed trends for increased risk of aggressive disease with earlier age of AA onset, specifically with the vertex hair loss pattern. (12, 17) Similar to Giles et al., we found AA was associated with > 2-fold increase in risk of aggressive disease. However, in this cohort of veterans, risk of aggressive disease did not appear pattern specific. Regardless of whether the associations are due to vertex or frontal – a point which requires further clarification – the finding that AA in general, which is associated with increased androgenicity, is perhaps associated with more aggressive cancers suggests a potential role for prolonged exposure to elevated androgens in the etiology of aggressive CaP. Ultimately, further studies are needed to validate our findings and ultimately better address this important issue.
Our study was limited by the small number of participants. Thus, these findings require validation in external datasets. Unfortunately a sizable percentage of men refused to participate in our study. As we did not keep information on these men, we are unable to compare the men who did and did not participate and as such are unable to comment on any selection bias this may have created. Furthermore, though we adjusted for major known confounders for which we had data available, it remains possible that residual confounding could account for our results. It is possible that some of our controls had CaP which would bias our results toward the null. Thus, our findings may actually underrepresent the true association between baldness and CaP risk. We relied on self-reported baldness to assess balding status from decades prior. However, the agespecific baldness prevalence in this study mirrors the prevalence in the general population. (7) Moreover, men were asked their hair patterns prior to biopsy minimizing bias between biopsy positive and negative men. Also, prior studies have shown this approach is a valid means to assess hair balding patterns. (28, 29) In addition, we studied androgenic alopecia and not other types of alopecia. The link between other types of alopecia and CaP risk remains to be tested. Lastly, we studied baldness. Thus, though prior studies do indeed strongly suggest a link between AA and androgenicity, AA has also been linked with insulin-like growth factor levels, which is also related to CaP risk. (30, 31) Thus, whether the association between AA and CaP is mediated through androgens, some other mechanism, or has no biological basis and is purely coincidental can only be addressed in further studies.
In conclusion, our results add to the body of evidence supporting that earlier onset AA – especially frontal balding – is associated an increased risk of CaP diagnosis. In addition, AA, regardless of pattern was linked with high-grade disease among men with CaP. Our findings require validation as well as further study to assess to what degree age of AA onset and androgenicity may or may not jointly influence CaP development.
Acknowledgments
Funding/Support: Department of Veterans Affairs, Department of Defense, T32 Training and the AUA Foundation/Astellas Rising Star in Urology Award
References
- 1.Kaaks R, Lukanova A, Sommersberg B. Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis. 2000;3:157–172. doi: 10.1038/sj.pcan.4500421. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–253. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Travis RC, Key TJ, Allen NE, Appleby PN, Roddam AW, Rinaldi S, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121:1331–1338. doi: 10.1002/ijc.22814. [DOI] [PubMed] [Google Scholar]
- 4.Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–2361. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- 5.Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4:1–11. doi: 10.1017/S1462399402005112. [DOI] [PubMed] [Google Scholar]
- 6.Otberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36:379–398. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 8.Oh BR, Kim SJ, Moon JD, Kim HN, Kwon DD, Won YH, et al. Association of benign prostatic hyperplasia with male pattern baldness. Urology. 1998;51:744–748. doi: 10.1016/s0090-4295(98)00108-3. [DOI] [PubMed] [Google Scholar]
- 9.Rapaport MJ. Follow-up of 1 mg finasteride treatment of male pattern baldness-difference between clinical trials and private office follow-up: influences on prescribing habits evaluated. Dermatol Surg. 2004;30:761–763. doi: 10.1111/j.1524-4725.2004.30213.x. [DOI] [PubMed] [Google Scholar]
- 10.Thompson IM, Klein EA, Lippman SM, Coltman CA, Djavan B. Prevention of prostate cancer with finasteride: US/European perspective. Eur Urol. 2003;44:650–655. doi: 10.1016/j.eururo.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Denmark-Wahnefried W, Schildkraut JM, Thompson D, Lesko SM, McIntyre L, Schwingl P, et al. Early onset baldness and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:325–328. [PubMed] [Google Scholar]
- 12.Giles GG, Severi G, Sinclair R, English DR, McCredie MR, Johnson W, et al. Androgenetic alopecia and prostate cancer: findings from an Australian case-control study. Cancer Epidemiol Biomarkers Prev. 2002;11:549–553. [PubMed] [Google Scholar]
- 13.Wright JL, Page ST, Lin DW, Stanford JL. Male pattern baldness and prostate cancer risk in a population-based case-control study. Cancer Epidemiol. 2010;34:131–135. doi: 10.1016/j.canep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawk E, Breslow RA, Graubard BI. Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 2000;9:523–527. [PubMed] [Google Scholar]
- 15.Greenwald P, Damon A, Kirmss V, Polan AK. Physical and demographic features of men before developing cancer of the prostate. J Natl Cancer Inst. 1974;53:341–346. doi: 10.1093/jnci/53.2.341. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh CC, Thanos A, Mitropoulos D, Deliveliotis C, Mantzoros CS, Trichopoulos D. Risk factors for prostate cancer: a case-control study in Greece. Int J Cancer. 1999;80:699–703. doi: 10.1002/(sici)1097-0215(19990301)80:5<699::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Yassa M, Saliou M, De Rycke Y, Hemery C, Henni M, Bachaud JM, et al. Male pattern baldness and the risk of prostate cancer. Ann Oncol. 2011;22:1824–1827. doi: 10.1093/annonc/mdq695. [DOI] [PubMed] [Google Scholar]
- 18.Cremers RG, Aben KK, Vermeulen SH, den Heijer M, van Oort IM, Kiemeney LA. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. Eur J Cancer. 2010;46:3294–3299. doi: 10.1016/j.ejca.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Hsing AW, Comstock GW. Serological precursors of cancer: serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol Biomarkers Prev. 1993;2:27–32. [PubMed] [Google Scholar]
- 20.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 21.Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14:1262–1269. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 22.Stough D, Stenn K, Haber R, Parsley WM, Vogel JE, Whiting DA, et al. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin Proc. 2005;80:1316–1322. doi: 10.4065/80.10.1316. [DOI] [PubMed] [Google Scholar]
- 23.Randall VA. Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol. 2007;18:274–285. doi: 10.1016/j.semcdb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116:452–455. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 25.Lesko SM, Rosenberg L, Shapiro S. A case-control study of baldness in relation to myocardial infarction in men. JAMA : the journal of the American Medical Association. 1993;269:998–1003. [PubMed] [Google Scholar]
- 26.Shahar E, Heiss G, Rosamond WD, Szklo M. Baldness and myocardial infarction in men: the atherosclerosis risk in communities study. Am J Epidemiol. 2008;167:676–683. doi: 10.1093/aje/kwm365. [DOI] [PubMed] [Google Scholar]
- 27.Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G. Gray hair, baldness, and wrinkles in relation to myocardial infarction: the Copenhagen City Heart Study. Am Heart J. 1995;130:1003–1010. doi: 10.1016/0002-8703(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 28.Littman AJ, White E. Reliability and validity of self-reported male balding patterns for use in epidemiologic studies. Ann Epidemiol. 2005;15:771–772. doi: 10.1016/j.annepidem.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Taylor R, Matassa J, Leavy JE, Fritschi L. Validity of self reported male balding patterns in epidemiological studies. BMC Public Health. 2004;4:60. doi: 10.1186/1471-2458-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Signorello LB, Wuu J, Hsieh C, Tzonou A, Trichopoulos D, Mantzoros CS. Hormones and hair patterning in men: a role for insulin-like growth factor 1? J Am Acad Dermatol. 1999;40:200–203. doi: 10.1016/s0190-9622(99)70188-x. [DOI] [PubMed] [Google Scholar]
- 31.Platz EA, Pollak MN, Willett WC, Giovannucci E. Vertex balding, plasma insulin-like growth factor 1, and insulin-like growth factor binding protein 3. J Am Acad Dermatol. 2000;42:1003–1007. [PubMed] [Google Scholar]