Abstract
Rheumatoid arthritis patients are at heightened risk for infections because of intrinsic disease severity with associated inflammation, comorbid illnesses, and use of glucocorticoids and various immunosuppressives. Although several studies have reported up to a twofold increase in risk of serious infections in RA patients treated with anti–tumor necrosis factor-α agents, results from other studies have been conflicting. Comparing results from different studies is challenging because of differences in patient populations, heterogeneous prevalence of comorbidities, and differing patterns of concomitant medication use. Based on available evidence, an excess risk for infection occurs early after initiation of tumor necrosis factor-α inhibitor therapy. Additionally, special circumstances such as surgical procedures may increase infection risk. The appropriate use of biologics in the perioperative setting remains empiric at best.
Introduction
Animal models have demonstrated an essential role of tumor necrosis factor (TNF) in host defense against infection [1–3]. However, the role played by TNF inhibitors in independently increasing patients’ risk for serious infections has been difficult to define. Patients with rheumatoid arthritis (RA) are at increased risk for infection secondary to the disease itself [4,5], and they have a high rate of comorbidities such as lung disease [6], which also may increase the risk for infection. TNF-inhibitor therapy is often used in combination with corticosteroids, methotrexate (MTX), and other nonbiologic disease-modifying antirheumatic drugs (DMARDs), which contribute to immunosuppression. We review recent data addressing the association between anti-TNF therapy and serious infections, including the special situation of infections in the perioperative setting.
TNF-Inhibitor Therapy and Serious Infections
Results from randomized controlled trials
Randomized clinical studies are inadequately powered to detect most significant adverse events, and meta-analyses seek to overcome this obstacle by pooling data from smaller studies. Bongartz et al. [7•] conducted a meta-analysis using data from nine randomized controlled trials to assess harmful effects of infliximab and adalimumab used for 12 weeks or more compared with MTX or other traditional DMARDs. According to the authors, etanercept was not included due to fundamental differences between the TNF receptor fusion protein and the monoclonal antibodies in molecular structure, binding specificities, and their effect on proinflammatory cytokine release and lymphocyte apoptosis. The pooled odds ratio (OR) for the risk of serious infections associated with anti–TNF-α therapy was 2.0 (95% CI, 1.3–3.1], and the number needed to harm was 59.0 (95% CI, 39–125) over a treatment period of 3 to 12 months. The number of serious infections occurring in the anti-TNF group was 126 among 3493 persons (3.6%), compared with 26 among 1512 persons (1.7%) in the controls. However, this data must be interpreted with caution due to several limitations. There was significant clinical heterogeneity in both the anti-TNF group and the controls in terms of disease duration, disease activity, and previous/concomitant DMARD treatment. The limited number of events resulted in pooled estimates with wide CIs, and because etanercept was omitted, these results are not generalizable to all anti-TNF agents. Moreover, because participants in clinical trials are generally healthier and have fewer comorbidities than those who receive these drugs after US Food and Drug Administration (FDA) approval, the increased risk of serious infections reported in this meta-analysis raises safety concerns for anti-TNF therapy use in the more diverse spectrum of patients in clinical practice.
Results from observational studies
A summary of the largest and most recent observational studies that have investigated the risk of serious infections is shown in Table 1. Similar to infection rates observed in the prebiologic era [5], the rate of serious infections in patients treated with anti–TNF-α ranged from approximately 3 to 6 infections per 100 patient-years. Relative rates varied from 1 (no increased risk with anti–TNF-α therapy) to 2.2-fold greater. Reconciling these seemingly discordant results in the relative rates is challenging, but it is likely that heterogeneity in patient populations (particularly in the control/comparator groups), prevalence of various comorbidities, sites and definitions of the infections under consideration, and timing of the anti–TNF-α use may explain some of these differences. For example, although the rates of lower respiratory tract infections were similar in the anti–TNF-α–treated group in the German Rheumatoid Arthritis Observation of Biologic Therapy (RABBIT) registry [8] and the British Society for Rheumatology Biologics Registry (BSRBR), the rates of infections in the control group varied approximately four-fold, perhaps because the BSRBR control group includes a higher prevalence of participants with chronic obstructive pulmonary disease and who are current smokers. Thus, differences in the control group rather than the group treated with anti–TNF-α may significantly impact estimates of relative risk.
Table 1.
Incidence and relative rate of serious infections associated with anti–TNF-α therapy
| Study | Rate/100 patient-years |
Adjusted relative rate |
|---|---|---|
| Listing et al. [8] | 6.3 | 2.2 |
| Dixon et al. [12] | 5.3 | 1 |
| Askling et al. [13] | 5.4* | 1.4† |
| Curtis et al. [14•] | 2.9‡ | 1.9 |
| Schneeweiss et al. [10] | 2.2 | 1 |
| Carmona et al. [33] | 2.8/6.1 | 1.6 |
In the first year after starting anti-TNF therapy.
In the first 6 months after starting anti-TNF therapy.
Two separate rates: patients < 60 years and patients > 60 years. TNF—tumor necrosis factor.
Reconciling differences: age, comorbidities, and concomitant drug use
Other differences in patient populations may also matter. In one US study by investigators at the University of Alabama at Birmingham (UAB), the mean age of the RA patients was in the early 50s [9]. Concordant with the Bongartz et al. [7•] meta-analysis and the results from the German RAB-BIT registry [8], these investigators showed an adjusted 1.9-fold increase in the risk for infections associated with anti–TNF-α therapy [9]. In contrast, investigators from Brigham and Women’s Hospital (Boston, MA) found no increase in serious bacterial infections among persons receiving anti–TNF-α therapy compared with MTX [10]. The patients in this latter study were Medicare beneficiaries ages 65 years and older (mean age, 77 years). Although no increased relative rate of serious bacterial infections was found for those who initiated anti–TNF-α agents (rate ratio, 1; 95% CI, 0.6–1.7), the researchers observed a strong dose-dependent relationship with glucocorticoids. These data are consistent with a report by Wolfe et al. [11] that did not find increased risk for pneumonia in RA patients receiving anti–TNF-α therapy. However, prednisone use resulted in an increased risk for pneumonia hospitalization (HR, 1.7; 95% CI, 1.5–2), including a dose-related increase in risk (< 5 mg/day: HR, 1.4; 95% CI 1.1–1.6; > 5–10 mg/day: HR, 2.1; 95% CI 1.7–2.7; > 10 mg/day: HR, 2.3; 95% CI, 1.6–3.2). The site and definition of infections may also matter. For example, Dixon et al. [12] found significantly different risks in the incidence of skin infections (incidence rate ratio [IRR], 4.3; 95% CI, 1.1–17.2) compared with all sites of infection (IRR, 1; 95% CI, 0.7–1.6) [12].
Some concern has been raised about a detection or workup bias in which rheumatologists might be more likely to hospitalize patients receiving anti–TNF-α therapy, which could increase hospitalization rates for infections. To address this concern, the study by investigators at UAB collected hospitalized medical records for all suspected infections and centrally adjudicated them by independent infectious disease consultants blinded to exposure status [9]. Using this process and varying the case definitions for infections across a range of more sensitive to more specific definitions, they showed that as the specificity of the infection case definition increased, so did the risk estimates associated with anti–TNF-α therapy. In other words, imprecision in the classification of infections appeared to partially mask the infection risk associated with anti–TNF-α therapy. This observation has implications for classifying infections in future studies that evaluate infection risk.
Although somewhat speculative, differences in the mean age of the cohorts, RA-specific and general comorbidity profiles, duration of disease, patterns of glucocorticoid use, and case identification methodology for infections are factors that may contribute to the discordant findings between these studies.
Risk of Serious Infections over Time
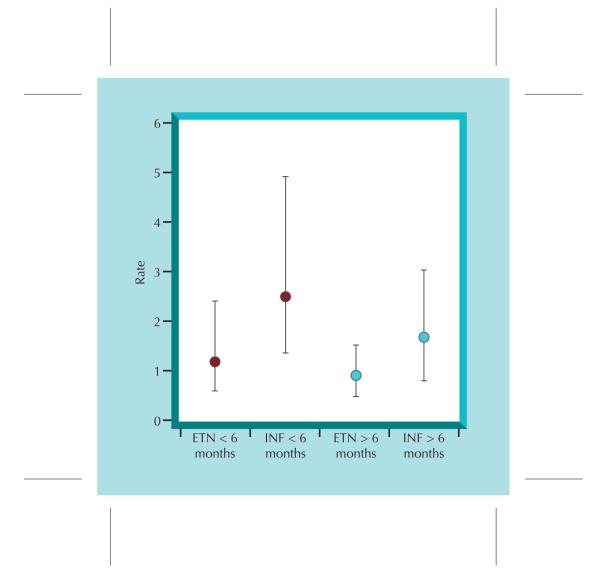
Several studies have now suggested that the risk for infection varies with the duration of exposure to the anti–TNF-α therapy. Data from RA patients in the Swedish Biologics Register (ARTIS) and other national Swedish registers assessed first hospitalization for serious infection [13]. With data on 4167 patients (7776 person-years of follow-up), the risk for infection associated with anti–TNF-α agents decreased over time; a rate ratio of 1.4 (95% CI, 1.2–1.7) was found during the first year of treatment, 1.2 (95% CI, 0.9–1.5) during the second year, and 0.8 (95% CI, 0.6–1.1) in patients who remained on their first anti–TNF-α agents after 2 years. In this study as in most, infections of the respiratory tract and pneumonia were the most common. A follow-up report from the UAB investigators Author: Curtis Editor: Theresa Artist: Wieslawa demonstrated a similar finding, in which infection risk was greatest in the first 6 months after initiating anti-TNF therapy, particularly for infliximab users [14•] (Fig. 1). A re-analysis of the BSRBR also demonstrated that in contrast to these authors’ previous report of no significant association between anti–TNF-α therapy and infection overall, a significantly increased risk for infection was observed within the first 90 days after starting treatment (IRR, 4.6; 95% CI, 1.8–11.9) [15••]. This was partly explained by the low early rates of infection in the control group. An early increased risk for infection has also been observed for nonbiologic agents as well [10], which may support a link to uncontrolled inflammation contributing to infection rates.
Figure 1.
Time-dependent relationship in the risk for serious infections associated with etanercept (ETN) and infliximab (INF).
The length of time that persons are exposed to drug therapies may also impact the rate of infection. In the BSRBR, rates of infection were increased in the 90 days immediately following drug discontinuation and even beyond this time. This finding may be explained by selection factors for drug discontinuation whereby patients may have their anti–TNF-α therapy discontinued due to an outpatient infection that ultimately evolves into a more serious infection requiring hospitalization. Exposure windows that are too short and fail to consider this possibility may bias infection rates. Defining appropriate exposure windows in future studies for long-acting agents like rituximab may prove particularly challenging.
Although the exact reasons for an early risk for infection shortly after starting anti-TNF therapy are yet unresolved, several factors may be important. For infliximab, large induction doses routinely given in the first 6 weeks of therapy may be important. The absence of a significantly increased risk after 6 months also may be a result of a “healthier” cohort later in time, because of patients who experienced an early infection that caused them to discontinue therapy. Finally, it is possible that those who remain on anti–TNF-α therapy attain better control of inflammation and thereby decrease subsequent infection risk.
Perioperative Anti–TNF-α Therapy and Infections
RA is an independent risk factor for postoperative orthopedic infection, with infection rates two to four times higher than those reported in patients without RA [16]. However, the relationship between perioperative use of anti-TNF agents in RA patients and an increased risk for postoperative infection has received only limited attention. A particular focus on TNF-inhibitor therapy and risk for Staphylococcus aureus infection following orthopedic surgery may also be warranted. S. aureus is a common cause of surgical site infection (SSI), and animal models have demonstrated that TNF-α plays a critical role in host defense to S. aureus. [17,18]
In a retrospective study of 91 RA patients who attended the Johns Hopkins Arthritis Clinic (Baltimore, MD) at least twice between January 1, 1999 and March 15, 2004 and underwent at least one orthopedic surgical procedure during the study period, Giles et al. [16] assessed the role of TNF-inhibitor therapy in the incidence of serious postoperative infection. A serious postoperative infection was defined as septic arthritis, osteomyelitis, or deep-wound infection in an instrumented bone or joint occurring within 30 postoperative days and requiring a prolonged course of intravenous antibiotics. Cellulitis and superficial wound infections were not included. Ten (11%) of the 91 patients who underwent an orthopedic surgical procedure developed a serious postoperative orthopedic infection. Seven of these 10 patients were receiving TNF-inhibitor therapy with or without traditional DMARDs, and S. aureus was identified in four of them. In univariate analysis, TNF-inhibitor therapy was significantly associated with the development of a serious postoperative infection (OR, 4.4; 95% CI, 1.1–18.41) and remained statistically significant after adjustment for age, sex, and disease duration, prednisone use, diabetes, and rheumatoid factor seropositivity (OR, 5.3; 95% CI, 1.1–24.9).
The Nederlandse Vereniging voor Reumatologie (NVR, the Dutch Society for Rheumatology) advises cessation of anti-TNF treatment based on the half-life of each drug. Cessation prior to an operation is recommended for infliximab, 39 days; etanercept, 12 days; and adalimumab, 56 days [19]. To assess the effect of these guidelines on the incidence of surgical site infections, a Dutch group conducted a retrospective parallel cohort study of 768 RA patients who underwent elective orthopedic surgery [20]. Cohort 1 did not use anti-TNF agents, and cohort 2 used anti-TNF agents but had stopped therapy preoperatively according to the NVR guidelines (cohort 2A) or continued preoperatively (cohort 2B). Infection rates were compared among cohorts, and logistic regression analysis was performed to examine risk factors for infection. In total, 1219 procedures were included, and crude infection risks were 4% (41/1023), 5.8% (6/104), and 8.7% (8/92) in cohorts 1, 2A, and 2B, respectively. Positive cultures occurred in 22 of 55 infections, of which 14 were S. aureus, four mixed culture (three of which included S. aureus), one Klebsiella pneumoniae, and one Enterobacter cloacae. Perioperative use of anti-TNF was not significantly associated with increased surgical site infection rates (OR, 1.5; 95% CI, 0.43–5.2), and the most important risk factor for these infections was prior history of surgical or skin infection (OR, 13.8; 95% CI, 5.2–36.7). An interesting secondary end point observation was that wound dehiscence and bleeding occurred more frequently in patients who had continued anti-TNF therapy.
An important limitation of the study was its lack of power to detect small differences in infection rates; the incidence of infection was numerically higher in the anti-TNF treated group and higher yet in those who continued anti-TNF therapy. However, this trend was not statistically significant. Another limitation of the study is the nonrandomized comparison between stopping and continuing anti-TNF, which may be subject to uncontrolled confounding. In particular, the authors note that the rate of prior surgical and skin infections was higher in patients who continued anti-TNF treatment, which may have accounted for the numerically greater incidence of infection in this group.
Results of these studies demonstrate the need for further investigation of the relationship between perioperative TNF inhibitors and infection risk following orthopedic surgery. At present, it may be most prudent to hold anti-TNF therapy around the time of surgery, although the optimal duration of interruption that balances the risk for infection against the risk for disease flare has yet to be determined.
New data on tuberculosis
Reactivation of latent tuberculosis infection (LTBI), particularly in the early phase of treatment, is a well-documented risk in patients receiving TNF-antagonist therapy [21–23]. Data from the BSRBR and the FDA’s Adverse Event Reporting System reported a high proportion of extrapulmonary tuberculosis among anti-TNF inhibitor users. Hamdi et al. [24•] has demonstrated that TNF antagonists have a dual action on antimycobacterial CD4+ T lymphocytes. When administered in vivo, they decrease the frequency of the subpopulation of memory CD4+ T lymphocytes that rapidly release interferon (IFN)-γ upon challenge with mycobacterial antigens, and when added in vitro, they inhibit the activation of CD4+ T lymphocytes by mycobacterial antigens.
In 2005, the US Centers for Disease Control and Prevention (CDC) published guidelines to prevent tuberculosis in patients initiating TNF-inhibitor therapy [25]. Recommendations include screening these patients for tuberculosis risk factors and for LTBI with a tuberculin skin test (TST). Skin indurations of 5 mm or greater should be interpreted as a positive result for LTBI in any patient considered for TNF blockade, and treatment for LTBI should begin before initiating TNF-blocking agents, preferably with 9 months of daily isoniazid. Although no data exist on the optimal time interval for which patients must be on isoniazid before beginning anti-TNF therapy, limited evidence suggests that concurrent initiation is safe.
Although the purified protein derivative (PPD) skin test is well established and standardized, it has several disadvantages. It is inconvenient in an outpatient setting, because the patient must return after 48 to 72 hours for the determination of the test result. In the presence of chronic immunosuppression (eg, glucocorticoid use), the test result may be falsely negative. Moreover, previous bacille Calmette-Guérin (BCG) vaccination and nontuberculous mycobacterial exposure can result in false-positive results.
In 2005, QuantiFERON-TB Gold (QFT-G; Cellestis Ltd., Carnegie, Victoria, Australia), received final approval from the FDA as an aid for diagnosing Mycobacterium tuberculosis infection. This enzyme-linked immunosorbent assay test detects the release of IFN-γ by peripheral blood CD4+ T lymphocytes from sensitized persons when it is incubated with mixtures of synthetic peptides, simulating two proteins present in M. tuberculosis: early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10).
A recently published study compared QFT-G with a classic TST for detection of LTBI in patients with chronic inflammatory conditions who were receiving immunosuppressive drugs [26]. Because a gold standard does not exist for diagnosing LTBI, investigators were unable to determine directly the sensitivity and specificity of the IFN-γ assay. Therefore, they compared the performance of QFT-G to the TST in relation to the presence of risk factors for latent tuberculosis and BCG vaccination status. The study population included 142 patients with chronic inflammatory rheumatic conditions, 126 of whom were receiving immunosuppressive therapy. All the TSTs (but not all the QFT-G tests) were performed before the patients had undergone TNF-α–inhibitor treatment. The investigators found that a positive result from the IFN-γ assay was more strongly associated with the presence of LTBI risk factors than the association between TST and these risk factors (OR, 31; 95% CI, 6–162 for QFT-G vs OR, 5; 95% CI, 2–14 for TST). The authors noted that the high BCG vaccination rate of 83% in the study patients may have led to increased false-positive TST results. Additionally, the odds for a positive IFN-γ assay were lower in patients treated with TNF-α inhibitors (adjusted OR, 0.19; 95% CI, 0.05–0.76). This negative result may reflect true negatives that resulted from appropriate evaluation and treatment prior to beginning TNF-α inhibitors. The authors suggest that it more likely resulted from false-negative testing, due to decreased mitogen-induced IFN-γ response in patients treated with TNF-α inhibitors [26].
Further investigation is needed before the QFT-G assay should be recommended in addition to, or instead of, the TST for screening of LTBI in patients being evaluated for or under treatment with TNF-α inhibitors. Repeating either of these tests (eg, every 1 to 2 years) is potentially useful in certain high-risk populations, but further evaluation is needed. The regional prevalence of tuberculosis, travel history, and prior BCG vaccinations are also important considerations in determining which test(s) to use and how often. Right now, these issues are mostly matters of clinical judgment and guided by little data.
Preventing Infections: the Role of Vaccination
Whenever possible, clinicians should provide indicated vaccines to all persons before initiating anti–TNF-α agents. Caution should be exercised regarding administration of live vaccines such as influenza (nasal administration), oral polio, measles/mumps/rubella, yellow fever, and smallpox. Until additional information becomes available, current guidelines from the CDC Advisory Committee on Immunization Practices generally recommend avoiding live attenuated vaccines during immunosuppressive therapy unless the benefit of vaccination outweighs the hypothetical increased risk for an adverse reaction after vaccination [27••], although a revised recommendation for the zoster virus was recently made. Live attenuated vaccines should not be administered for at least 3 months after discontinuation of immunosuppressive therapy. Inactivated vaccines can be administered safely during anti–TNF-α therapy.
Recommended vaccinations in patients receiving anti–TNF-α agents
Vaccination with trivalent inactivated influenza vaccine is indicated for persons with altered immunocompetence and should be administered annually to patients receiving anti–TNF-α agents. Live-attenuated influenza vaccine administered nasally is generally contraindicated. The pneumococcal polysaccharide vaccine should be administered to patients receiving anti–TNF-α agents, and patients should also receive a one-time revaccination at least 5 years after the first dose. Other inactivated vaccines such as meningococcal and hepatitis B vaccines can be administered as clinically indicated.
Although some uncertainty exists about the immunogenicity of vaccines in immunocompromised patients, anti–TNF-α agents have not been demonstrated to affect the humoral response to influenza or pneumococcal vaccinations. Fomin et al. [28] found that infliximab did not affect humoral response to influenza vaccination in RA patients, although the response was lower in patients with RA compared to healthy controls for one of the antigens (67% in patients with RA vs 87% in controls; P = 0.05). Kaine et al. [29] investigated the immune responses following influenza vaccination in RA patients receiving adalimumab and found that in patients without protective antibody titers at baseline, response rates were similar in the two groups (adalimumab 73.3% vs placebo 73.9%; 95% CI for the difference, −19.3 to 17.8) [29]. They also reported that following pneumococcal vaccination, the proportion of patients achieving a vaccine response was similar in the adalimumab and placebo groups (37.4% and 40.4%, respectively; 95% CI for the difference −16.2% to 10.3%) [29]. Likewise, Visvanathan et al. [30] reported no significant difference in RA patients’ responses to pneumococcal vaccine polyvalent (Pneumovax 23; Merck and Co. Inc., Whitehouse Station, NJ) between the infliximab plus MTX versus placebo plus MTX treatment groups [30]. These observations seemed consistent in non-RA patients; Mease et al. [31] investigated responses to pneumococcal vaccine polyvalent in patients with psoriatic arthritis receiving etanercept. They found that the responses to the vaccine were similar in patients treated with etanercept and those who received placebo [31].
Zoster vaccine
Recently, the CDC Advisory Committee on Immunization Practices recommended routine vaccination of all persons older than 60 years with one dose of zoster vaccine, a live vaccine (Zostavax; Merck and Co. Inc., Whitehouse Station, NJ) [32•]. This recommendation allows for zoster vaccination administration to RA patients receiving MTX and prednisone at doses up to 20 mg/day. However, the safety and efficacy of zoster vaccine administered concurrently with adalimumab, infliximab, and etanercept is unknown, and caution with immunization in patients receiving these agents was advised. Ideally, zoster vaccine should be administered at least 14 days before initiation of anti-TNF therapy; otherwise, it should be deferred for at least 1 month after discontinuation of such therapy. If it is not possible to administer zoster vaccine to patients before initiating anti-TNF therapy, physicians should evaluate patients on a case-by-case basis to determine risks and benefits.
Conclusions
Although several studies have reported up to a twofold increase in risk of serious infections in RA patients treated with anti–TNF-α agents, particularly during the initial treatment period, other studies have not demonstrated this relationship. Additionally, even a twofold increased risk for infection is likely to be of similar magnitude to doses of glucocorticoids commonly used for RA patients. Thus, any infection risk associated with anti–TNF-α therapy may be partially or fully offset if glucocorticoids can be tapered. The ability of anti–TNF-α therapy to achieve better long-term control of inflammation and thereby reduce infection risk is an attractive theoretical possibility, but it has yet to be demonstrated.
Differences between studies in patient populations, comorbidities, use of DMARDs by patients who were not exposed to TNF-α antagonists, patterns of glucocorticoid use, and different analytical approaches may account for seemingly discordant results between studies. Methodology standardization may help harmonize results from different investigations, and greater transparency is needed in assessing and reporting adverse events. Further investigation is required to assess relationships between infections and anti–TNF-α therapy and newer biologics to develop appropriate clinical practice guidelines, and to effectively communicate this information to patients.
Acknowledgments
This work was supported by the Doris Duke Charitable Foundation, the National Institutes of Health (AR053351), and the Arthritis Foundation.
The authors thank Dr. Jack Cush for his review of this manuscript.
Footnotes
Disclosures Dr. Curtis consulted for Roche, UCB, and Proctor and Gamble. He has served on the speaker’s bureaus of Merck and Co., Proctor and Gamble, Eli Lilly, Roche, and Novartis, and he has received research grants from Merck, Proctor and Gamble, Eli Lily, Amgen, and Novartis. Dr. Crawford has reported no potential conflicts of interest relevant to this article.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988;56:2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato K, Minagawa T, Kato K, et al. Human tumor necrosis factor increases the resistance against Listeria infection in mice. Med Microbiol Immunol. 1989;178:337–346. doi: 10.1007/BF00197452. [DOI] [PubMed] [Google Scholar]
- 3.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Effect of late administration of anti-TNF alpha antibodies on a Salmonella infection in the mouse model. Microb Pathog. 1993;14:473–480. doi: 10.1006/mpat.1993.1046. [DOI] [PubMed] [Google Scholar]
- 4.Doran MF, Crowson CS, Pond GR, et al. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–2300. doi: 10.1002/art.10529. [DOI] [PubMed] [Google Scholar]
- 5.Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 6.Hyrich K, Symmons D, Watson K, et al. Baseline comorbidity levels in biologic and standard DMARD treated patients with rheumatoid arthritis: results from a national patient register. Ann Rheum Dis. 2006;65:895–898. doi: 10.1136/ard.2005.043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.•.Bongartz T, Sutton AJ, Buchan I, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. This meta-analysis applied a validated technique for pooling sparse event data from randomized controlled trials to assess the extent to which anti-TNF antibody therapies may increase the risk of serious infections and malignancies in patients with RA. Based on the reviewed studies, evidence exists of an increased risk of serious infections and a dose-dependent increased risk of malignancies associated with anti-TNF therapy in patients with RA.
- 8.Listing J, Strangfield A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 9.Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125–1133. doi: 10.1002/art.22504. [DOI] [PubMed] [Google Scholar]
- 10.Schneeweiss S, Setoguchi S, Weinblatt ME, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:1754–1764. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–634. doi: 10.1002/art.21568. [DOI] [PubMed] [Google Scholar]
- 12.Dixon WG, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 13.Askling J, Fored CM, Brandt L, et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis. 2007;66:1339–1344. doi: 10.1136/ard.2006.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Curtis JR, Xi J, Patkar N, et al. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:4226–4227. doi: 10.1002/art.23050. A particular strength of this study was that the outcome of bacterial infection was confirmed through a rigorous validation process with a review of hospital medical records, including physician-assessed “definite” diagnoses of infection, which intentionally excluded those cases with less certain diagnoses.
- 15••.Dixon WG, Symmons DP, Lunt M, et al. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56:2896–2904. doi: 10.1002/art.22808. In a previously published observational study, these authors found that overall risk of severe infection was not increased following anti–TNF-α therapy. In this extended analysis, however, when the at-risk period was limited to the first 90 days, there was an adjusted incidence rate ratio of 4.6 (95% CI, 1.8–11.9) in patients receiving anti–TNF-α therapy compared with patients receiving DMARD therapy. This underscores the point that varied methodologies can seriously affect data interpretation.
- 16.Giles JT, Bartlett SJ, Gelber AC, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55:333–337. doi: 10.1002/art.21841. [DOI] [PubMed] [Google Scholar]
- 17.Hultgren O, Eugster HP, Sedgwick JD, et al. TNF/lymphotoxin-alpha double-mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J Immunol. 1998;161:5937–5942. [PubMed] [Google Scholar]
- 18.Stenzel W, Soltek S, Miletic H, et al. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Dutch Society for Rheumatology . Medicijnen: het toepassen van TNF blokkade in de behandeling van reumatoïde artritis. Dutch Society for Rheumatology; Utrecht: 2004. [Google Scholar]
- 20.den Broeder AA, Creemers MC, Fransen J, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34:689–695. [PubMed] [Google Scholar]
- 21.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(Suppl 3):S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41(Suppl 3):S199–S203. doi: 10.1086/429998. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Tuberculosis associated with blocking agents against tumor necrosis factor-alpha--California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2004;53:683–686. [PubMed] [Google Scholar]
- 24•.Hamdi H, Mariette X, Godot V, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006;8:R114. doi: 10.1186/ar1994. This interesting study proposes two effects of TNF antagonists on antimycobacterial CD4+ T lymphocytes, which may explain the increased incidence of tuberculosis in patients treated with TNF antagonists. Administered in vivo, TNF antagonists decrease the frequency of the subpopulation of memory CD4+ T lymphocytes rapidly releasing IFN-γ upon challenge with mycobacterial antigens. Added in vitro, they inhibit the activation of CD4+ T lymphocytes by mycobacterial antigens.
- 25.Winthrop KL, Siegel JN, Jereb J, et al. Tuberculosis associated with therapy against tumor necrosis factor alpha. Arthritis Rheum. 2005;52:2968–2974. doi: 10.1002/art.21382. [DOI] [PubMed] [Google Scholar]
- 26.Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 27••.Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-15):1–48. This comprehensive report addresses common vaccination concerns for clinicians and guidelines for special situations including altered immunocompetence.
- 28.Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006;65:191–194. doi: 10.1136/ard.2005.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272–279. [PubMed] [Google Scholar]
- 30.Visvanathan S, Keenan GF, Baker DG, et al. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol. 2007;34:952–957. [PubMed] [Google Scholar]
- 31.Mease PJ, Ritchlin CT, Martin RW, et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J Rheumatol. 2004;31:356–361. [PubMed] [Google Scholar]
- 32•.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. quiz CE2–4. Updated recommendations on the use of the live herpes zoster vaccine.
- 33.Carmona L, Descalzo MA, Perez-Pampin E, et al. BIOBADASER and EMECAR Groups: All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann Rheum Dis. 2007;66:880–851. doi: 10.1136/ard.2006.067660. [DOI] [PMC free article] [PubMed] [Google Scholar]