Abstract
Objective
Bulimia nervosa (BN) has been characterized as similar to an addiction, though the empirical support for this characterization is limited. This study utilized PET imaging to determine whether abnormalities in brain dopamine (DA) similar to those described in substance use disorders occur in BN.
Method
PET imaging with [11C]raclopride, pre/post methylphenidate administration, to assess dopamine type 2 (D2) receptor binding (BPND) and striatal DA release (ΔBPND).
Results
There was a trend towards lower D2 receptor BPND in two striatal subregions in the patient group compared to the control group. DA release in the putamen in the patient group was significantly reduced and, overall, there was a trend towards a difference in striatal DA release. Striatal DA release was significantly associated with the frequency of binge eating.
Discussion
These data suggest that BN is characterized by abnormalities in brain DA that resemble, in some ways, those described in addictive disorders.
Keywords: Bulimia nervosa, eating disorders, PET imaging, dopamine, addictions, reward
Bulimia nervosa (BN) is a serious psychiatric disorder, characterized by recurrent binge eating and inappropriate compensatory behavior such as self-induced vomiting and laxative abuse. While multiple neurotransmitters may be involved in the initiation and maintenance of BN, dopamine (DA) is of particular interest because of its established role in mediating food reward (1) and because abnormalities in central nervous system DA have been well-described in several substance use disorders (2).
Parallels between the behavioral patterns of individuals addicted to drugs or alcohol and the behavior of individuals with BN have long been noted (3), including difficulty ‘cutting back’ on the substance (drug or food) in question, the ongoing use of the substance despite deleterious health effects, and the use of the substance to temporarily alleviate emotional distress. The development of positron emission tomography (PET) imaging, in the last two decades, has permitted dopaminergic measures, such as the density of type 2 dopamine (D2) receptors and the release of DA in response to pharmacological probes, to be examined in humans. Studies using PET have consistently found that the density of dopamine type 2 (D2) receptors in the striatum is reduced among individuals with a variety of substance use disorders, including cocaine, heroin, methamphetamine, and alcohol use disorders (2). Striatal DA release in individuals who abuse cocaine and alcohol has been evaluated in a smaller number of PET studies (4, 5). These studies, which use a pharmacological probe or ‘challenge’ to provoke DA release, have found striatal DA release to be reduced in patients with substance use disorders. Further, among cocaine abusers, the reduction in DA release is significantly inversely associated with the choice to self-administer cocaine over an alternate reinforcer – i.e., the lower the release of striatal DA, the greater the tendency to choose cocaine (5). These data have thereby established an association, in living humans, between disturbances in striatal DA function and substance abuse.
DA’s role in food reward supports the rationale for studying the DA system in BN. Multiple preclinical studies have demonstrated that DA is released into the striatum of animals receiving food reward, and in response to cues associated with food reward (1). Two human studies using PET have demonstrated striatal DA release in response to food cues and ingestion in healthy control participants (6, 7). Preclinical studies of bulimic-like eating patterns (i.e., models in which periods of food restriction in rodents are alternated with binge-like consumption of sugar solutions) have found alterations in DA release in striatal regions, resembling alterations seen in preclinical models of repeated drug taking (8). Another recent study found that overconsumption of a palatable diet was associated with reduced striatal D2 receptor levels (9).
Only a few studies have attempted to assess brain DA function among individuals with BN, and these have employed indirect measures of DA. Patients with more severe BN were found to low levels of CSF homovanillic acid [a marker of DA turnover] (10), and patients with BN have been found to carry a genetic DA-related polymorphism which has also been implicated in addictive disorders (11). Other neuroimaging studies have shown hypofunctioning in reward-related areas of the brain in BN, though without clear implications on brain DA levels or function (12, 13). We are not aware of previous studies utilizing PET to evaluate striatal D2 receptor density, or striatal DA release in BN.
In summary, given the likely involvement of brain DA circuits in mediating food reward, and the similarity of some symptoms of BN to those of substance abuse, we conducted a study to assess striatal D2 receptor density and striatal DA release in patients with BN. We hypothesized that patients with BN would exhibit abnormalities in DA circuits similar to those found in patients with addictive disorders, relative to controls, Specifically, we hypothesized (1) decreased striatal D2 receptor density in BN, and (2) decreased striatal DA release to a psychostimulant in BN.
METHODS
The study was conducted through the Eating Disorders Research Unit of the New York State Psychiatric Institute/Columbia University Medical Center. The study was reviewed and approved by the New York State Psychiatric Institute/Columbia University IRB.
Recruitment and screening
Based on the effect sizes from PET studies of differences in D2 receptor density and in DA release reported in individuals with substance abuse vs. controls, and in PET studies of obese vs. normal weight individuals, we calculated that a sample size of 15 patients with BN and 15 control participants would provide sufficient (80%) power to detect a statistically significant difference with α = 0.05 (2-tailed).
Treatment-seeking women with BN were recruited via self-referral and referral from clinicians; control participants responded to notices and advertising in local media. During the subject’s initial phone call to the clinic, potential participants were briefly told about the study by a research coordinator, and information was collected from the subject after verbal consent was obtained. Following this telephone assessment, those participants who continued to be interested and eligible underwent an in-person assessment, including (1) full psychiatric and medical assessment, including physical exam, (2) a complete blood count, basic metabolic panel, liver function tests, thyroid stimulating hormone, and serum pregnancy test, (3) urine toxicology, (4) electrocardiogram, (5) Structured Clinical Interview for DSM-IV (SCID) (14), the Beck Depression Inventory (BDI) (15), and the Eating Disorder Examination (EDE-12) (16).
Inclusion/exclusion
Participants were excluded if they met DSM-IV-TR criteria for current or past Axis I disorders, other than BN or past history of anorexia nervosa, for the patient group. Participants were required to weight at least 85% of ideal body weight, thereby excluding individuals with anorexia nervosa. Because of the frequent comorbidity of BN with anxiety and mood disorders, patients with BN were not excluded by the presence of mild or moderate depressive and anxiety symptoms. Of note, PET/SPECT studies of striatal DA measures in unipolar depression have been discrepant and, in many cases, have not strongly demonstrated striatal DA alterations between patients with MDD and control participants (17, 18). Patients who reported a diagnosis of current ADHD during the telephone assessment or in-person interview were excluded from the study. In addition, participants were excluded for the presence of (1) lifetime past histories of abuse or dependency on alcohol or other drugs (assessed by phone interview, in-person MD clinical interview, and urine drug screen on the screening day), (2) active suicidal ideation, (3) use of fluoxetine in the 6 weeks prior to the study, and other psychoactive medications in the 4 weeks prior to the study, (4) ongoing medical or neurological illness, (5) pregnancy, (6) exposure to radiation in the workplace, or nuclear medicine procedures during the previous year, and (7) presence of metallic implants that could be adversely affected by MRI procedures.
Participants who continued to be eligible and interested provided written informed consent. Patients were offered treatment for BN after completion of the study; control participants were given financial compensation. We attempted, when possible, to perform PET studies during the early follicular phase of the menstrual cycle. As this was sometimes not possible, we also attempted to match patients and control participants for menstrual cycle status (follicular vs. luteal phase).
Scanning protocol
PET scanning was conducted using an ECAT EXACT HR+ camera. Scans were acquired on the same day in almost all cases, to maximize convenience and subject retention between scans 1 and 2, and to standardize conditions between the two scans. The radiotracer [11C]raclopride (maximum dose 15 mCi/scan),was synthesized on-site immediately prior to scanning. This radiotracer has been used extensively in several psychiatric populations (19).
Outpatients with BN were admitted to the hospital for 24–48 hours prior to scanning, to control for acute effects of disordered eating patterns on scan results. Both patients and control participants were given a standardized meal of an English muffin, 1 pat of butter, and 8 fl oz apple juice, for whatever meal (breakfast or lunch) immediately preceded scanning procedures.
Two scans with [11C]raclopride were performed on each subject: a baseline scan (for measurement of baseline D2 receptor density) and a second scan which began 60 minutes following administration of methylphenidate 60 mg p.o. (20). Methylphenidate administration results in an increase in extracellular DA and a consequent reduction in [11C]raclopride binding. The magnitude of this decrease is a measure of the change in extracellular DA, and the comparison of the pre- and post-methylphenidate [11C]raclopride scans provides a noninvasive measure of changes in DA concentration in the human brain (21). Vital signs were monitored throughout both scans, and an ECG was obtained 120 minutes after the dose. The subjective response to methylphenidate was evaluated by asking participants to rate four items (euphoria, energy, restlessness, and anxiety) on a scale of 1 (not at all) to 5 (most ever) at baseline (10 min before methylphenidate) and periodically through the scan. An MRI was acquired for co-registration of PET data.
Analyses
PET data were co-registered to each subject’s MRI according to methods previously used by the Division of Functional Brain Mapping, Columbia University Medical Center (19, 22), for anatomical localization of regions of interest. Five ROI were identified on the MRI, including the ventral striatum, the caudate rostral to the anterior commissure (dorsal caudate), the caudate caudal to the anterior commissure (posterior caudate), the putamen rostral to the anterior commissure (anterior putamen), and the putamen caudal to the anterior commissure (posterior putamen). Activities from left and right regions were averaged. The activity of the striatum as a whole was derived as the spatially-weighted average of the ROIs, excluding the posterior caudate, due to the noisiness of this area. The cerebellum was identified as a sixth ROI, and used as the reference region. After regions were drawn on the MRI, they were then pasted on the co-registered PET for activity concentration measurement in the anatomical region.
The simplified reference tissue model (SRTM) (23) was used for derivation of the binding potential (BP) implemented in MATLAB (The Math Works, Inc., South Natick, Massachusetts), using the cerebellum as the reference region. The outcome measure for the PET studies was binding potential, defined as the ratio of specifically bound to nondisplaceable radioligand at equilibrium (BPND) (24). BPND can also be described as:
where Bmax is the concentration of D2/3 receptors, Kd is the inverse of the affinity of the radiotracer for the receptor, and fnd is the free fraction in the nonspecific distribution volume of the brain (25). [11C]raclopride has a similar affinity for D2 and D3 receptors (26), and the signal from these receptors cannot be distinguished. The reduction in D2 receptor availability following MP (referred to as ΔBPND) was calculated as the relative reduction in BPND where ΔBPND = (BPND-Baseline – BPND-MP) / BPND-Baseline).
Primary outcome measures (striatal BPND; striatal ΔBPND) were statistically tested using a two-sided t-test. We then examined between-group differences in each of 5 striatal subregions (ventral striatum, anterior and posterior putamen, dorsal and posterior caudate), using two-sided t-tests. We conducted a post-hoc subgroup analysis within the patient group to assess the impact of a past history of anorexia nervosa. We also examined, using linear regression, potential associations between DA measures and core features of BN: specifically, (1) number of objective binge eating episodes (OBEs) in the last month; (2) number of vomiting episodes in the last month; (3) typical binge size, in kilocalories [based on a representative binge meal as given in the EDE]; and (4) duration of eating disorder. To assess for the potential confound of mild to moderate depression on DA measures, we also examined the association between DA measures and Beck Depression Inventory Scores.
RESULTS
Clinical characteristics
Clinical characteristics of the participants are shown in Table 1. Patients with BN and control participants were similar with respect to age, BMI, smoking status, and phase of menstrual cycle. The patients with BN had a moderate level of illness, as indicated by the number of binge eating and purging episodes per month [objective binge episodes in past month = 35.4; subjective binge episodes in past month = 22.0; vomiting episodes in the past month = 68.3], the average duration of illness [7.8 years], and the fact that the majority were seeking inpatient treatment. The BMI range of subjects with BN was 18.8 – 23.6 kg/m2; the BMI range of control subjects was 19.0 – 23.6 kg/m2.
Table 1.
Characteristics of patients with Bulimia Nervosa (BN) and Controls (CTR)a
| BN | CTR | Stat | |
|---|---|---|---|
| Number enrolled | 16 | 17 | |
| Number completing both scans | 15 | 14 | |
| Mean Age (years) | 24.4 +/− 5.1 | 24.9 +/− 4.2 | p=0.85 |
| Mean weight (pounds) | 132.1 +/− 14.3 | 125.0 +/− 16.1 | p=0.21 |
| Mean BMI (kg/m2) | 21.7 +/− 1.4 | 21.4 +/− 2.0 | p=0.78 |
| Race | 13 Caucasian | 10 Caucasian | χ2=1.96; p=0.26 |
| 3 non-Caucasian (2 mixed Caucasian; 1 Native American) | 7 non-Caucasian (4 mixed Caucasian; 1 Asian; 2 Hispanic) | ||
| Smoking Statusb | 4 current/past smokers (1 current smoker; 2 light smokers; 1 past smoker) | 2 current/past smokers (2 past smokers) | χ2=1.87; p=0.225 |
| 12 non-smokers | 15 non-smokers | ||
| EDE-OBE | 35.4 +/− 29.1 | 0.0 +/− 0.0 | p<0.01 |
| EDE-SBE | 22.0 +/− 25.1 | 0.0 +/− 0.0 | p<0.01 |
| EDE-Purge | 68.3 +/− 74.4 | 0.0 +/− 0.0 | p<0.01 |
| Mean BDI score | 15.6 +/− 9.0 | 0.3 +/− 1.0 | p<0.001 |
| Hormonal Stage at time of PET | 11 Follicular phase | 13 Follicular phase | χ2=0.67; p=0.685 |
| 5 Luteal Phase | 3 Luteal phase | ||
| 1 unknown | |||
| Duration of illness | 7.8 years | ||
| # seeking inpatient treatment | 11 | ||
| # seeking outpatient treatment | 5 |
Abbreviations: BN = Bulimia Nervosa; CTR = Healthy Control participants; N = number of participants; BMI = Body Mass Index (kilograms per square meter); EDE = Eating Disorder Examination; OBE = Objective Binge Episodes (in month prior to study); SBE = Subjective Binge Episodes (in month prior to study); BDI = Beck Depression Inventory; PET = positron emission tomography.
Smoking status was subdivided into current smokers (defined as regularly smoking 1 pack per week or more), light smokers (defined as smoking less than 1 pack per week, or other irregular/”social” smoking); past smokers; and non-smokers.
Scanning procedures
16 patients with BN and 17 control participants completed the baseline scan, and 15 patients and 14 control participants completed both scans. Therefore, D2 receptor BPND measures are reported for 33 participants, and DA release measures are reported for 29 participants.
The mean injected dose of [11C]raclopride did not vary between patients and controls, for either the baseline scan or the post-methylphenidate scans [baseline scan: 11.47 +/− 2.00 mCi (patients) vs. 12.00 +/− 1.82 mCi (control participants), p=0.44; post-methylphenidate scan: 11.18 +/− 1.86 mCi vs. 11.24 +/− 2.02 mCi, p = 0.94]. The mean mass of [11C]raclopride also did not differ between patients and controls, for either the baseline scan or the post-methylphenidate scans [baseline scan: 4.25 +/− 1.86 µg (patients) vs. 4.61 +/− 1.71 µg (control participants), p=0.40; post-methylphenidate scan: 3.07 +/− 1.34 µg (patients) vs. 3.61 +/− 1.35 µg (controls), p = 0.15]. The region of interest sizes (including a between-group comparison of the striatum as a whole, as well as a between-group comparison of the striatal substructures) were not significantly different between patients and control participants (data not shown; all p > 0.16).
Striatal D2 receptor binding potential
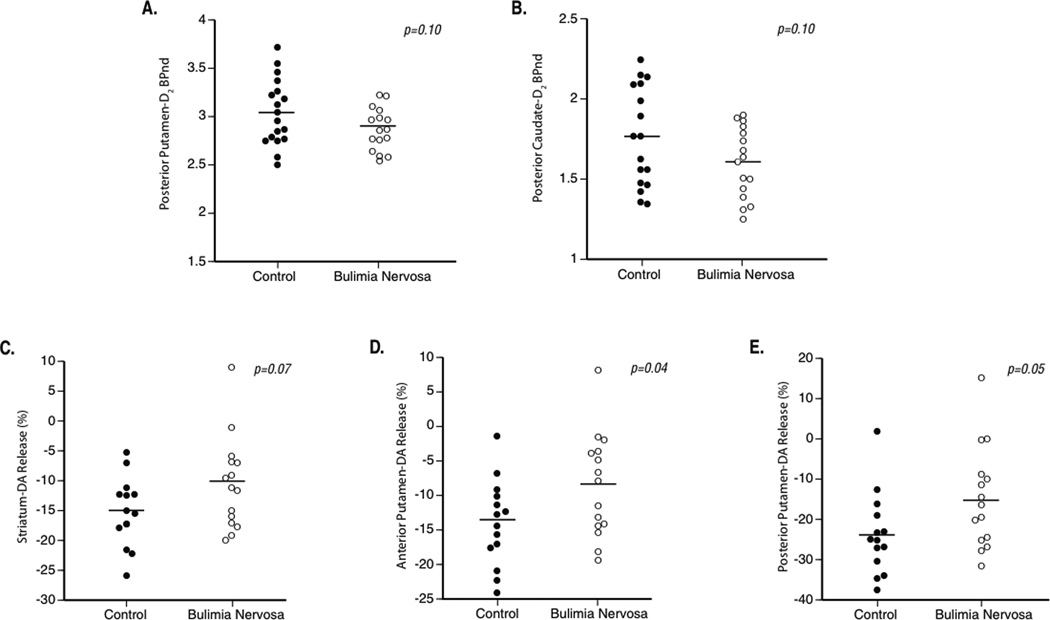
Results are presented in Table 2. For the primary outcome measure (i.e., D2 receptor BPND across the striatum as a whole), the difference in D2 receptor BPND between the patient and control groups was not statistically significant [striatal D2 receptor BPND = 2.53 +/− 0.18 (BN) vs. 2.64 +/− 0.23 (CTR); p=0.14]. By subregion, there was a trend towards a difference in BPND in the posterior putamen and posterior caudate [posterior putamen BPND = 2.88 +/− 0.22 (BN) vs. 3.05 +/− 0.35 (CTR); p=0.10; posterior caudate BPND 1.61 +/− 0.22 (BN) vs. 1.76 +/− 0.31; p=0.10.]
Table 2.
[11C]Raclopride Binding Potential (baseline scan) of patients with Bulimia Nervosa (BN) and controls (CTR) a
| Region | BPND (BN) | BPND (CTR) | p |
|---|---|---|---|
| Striatum, whole | 2.53 ± 0.18 | 2.64 ± 0.23 | 0.14 |
| Ventral striatum | 2.03 ± 0.19 | 2.07 ± 0.19 | 0.57 |
| Putamen, anterior | 2.75 ± 0.27 | 2.86 ± 0.25 | 0.21 |
| Putamen, posterior | 2.88 ± 0.22 | 3.05 ± 0.35 | 0.10* |
| Caudate, dorsal | 2.38 ± 0.20 | 2.49 ± 0.25 | 0.18 |
| Caudate, posterior | 1.61 ± 0.22 | 1.76 ± 0.31 | 0.10* |
Abbreviations: BPND = binding potential; SD = standard deviation; BN = bulimia nervosa; CTR = control participants.
Striatal DA response to psychostimulant
In the striatum as a whole, there was a trend suggesting a difference in DA release between the patient and control groups in response to pharmacological challenge with methylphenidate [striatal BPND = −10.5% +/− 7.7% (BN) vs. −15.1% +/− 5.7% (CTR); two-sided t-test, p=0.07] (Table 3). The difference between patient and control groups was statistically significant in the anterior and posterior putamen [anterior putamen ΔBPND = −8.5% ± 7.5% (BN) vs. −13.9% ± 6.2% (CTR), p=0.04; posterior putamen ΔBPND = − 14.8% ± 12.5% (BN) vs. −23.7% ± 10.1% (CTR), p=0.05]. Although the differences in the other subregions did not reach statistical significance, it is notable that, in all but the ventral striatum, ΔBPND was lower in the patient group than in the control group. BMI did not appear to influence striatal DA response (among patients with BN: r=0.18; p=0.52; among control subjects: r=0.10; p=0.73).
Table 3.
Striatal Dopamine Response to methylphenidate, as measured by the change in striatal D2 receptor Binding Potential after methylphenidate administration, in patients with Bulimia Nervosa (BN) and controls (CTR). a (A more negative displacement indicates a greater release of DA.)
| Region | Δ BPND (BN) | ΔBPND (CTR) | p |
|---|---|---|---|
| Striatum, whole | −10.5% ± 7.7% | −15.1% ± 5.7% | 0.07* |
| Ventral striatum | −11.3% ± 8.5% | −9.7% ± 7.7% | 0.60 |
| Putamen, anterior | −8.5% ± 7.5% | −13.9% ± 6.2% | 0.04* |
| Putamen, posterior | −14.8% ± 12.5% | −23.7% ± 10.1% | 0.05* |
| Caudate, dorsal | −5.8% ± 6.8% | −8.4% ± 6.4% | 0.31 |
| Caudate, posterior | −8.5% ± 14.5% | −10.7% ± 10.6% | 0.65 |
Abbreviations: BPND = binding potential; SD = standard deviation; BN = bulimia nervosa; CTR = control participants
Subgroup analysis – history of anorexia nervosa
Within the BN group, there were no statistically significant differences in measures of D2 receptor density or of DA release between those patients with a history of anorexia nervosa (n=6), and those without a history of anorexia nervosa (n=10) [all p > 0.20].
Association between dopamine measures and symptom severity
There was a statistically significant association between striatal DA release and frequency of objective binge episodes (OBE) as measured by the EDE (r2=0.44, p=0.007; see Figure 1); the lower the striatal DA response to methylphenidate, the greater the frequency of binge eating in the previous 28 days. The association between DA release and vomiting frequency (typically coupled closely with frequency of binge eating) was similar (r2=0.30; p=0.03). Release of DA in the anterior and posterior putamen (ANP and POP, respectively), the subregions with significantly lower DA release, was also inversely related to the frequencies of binge eating and of vomiting [OBE and ANP DA release: r2 = 0.29; p = 0.04; OBE and POP DA release: r2 = 0.46; p = 0.006; vomiting frequency and ANP DA release: r2 = 0.26; p = 0.05; vomiting frequency and POP DA release: r2=0.29; p=0.04].
Figure 1. Scatterplots of between-group differences in dopamine (DA) type 2 (D2) receptor binding, and DA response to methylphenidate.
A. Posterior putamen DA type 2 (D2) receptor binding potential (D2 BPnd; unitless), in control subjects (left) and patients with bulimia nervosa (right). B. A. Posterior caudate D2 receptor BPnd (D2 BPnd; unitless), in control subjects (left) and patients with bulimia nervosa (right). C. Striatal DA response (“DA release”), measured as the percent difference between the baseline PET scan and the post-methylphenidate scan, in control subjects (left) and patients with bulimia nervosa (right). D. Anterior putamen percent DA response in control subjects (left) and patients with bulimia nervosa (right). E. Anterior putamen percent DA response in control subjects (left) and patients with bulimia nervosa (right). For panels C-E, a more negative DA measure indicates greater DA response to methylphenidate.
Data from the individual with the lowest displacement of [11C]raclopride contributed importantly to the statistical significance of these associations [with data point excluded, striatal DA release and OBE: r2=0.11, p=0.24; posterior putamen DA release and OBE: r2=0.16, p=0.15], consistent with a medium effect size.
The kilocalories content of a typical binge described by the patient was inversely associated with DA response in ANP and POP [Binge kilocalorie and ANP DA release: r2 = 0.27, p = 0.05; Binge kilocalorie and POP DA release: r2 = 0.31; p = 0.03]. There were no statistically significant associations between DA measures and Beck Depression Inventory Scores.
Subjective effects of methylphenidate, and association with dopamine
Data on subjective effects of methylphenidate were acquired for 11 patients with BN and 12 control subjects. Peak change from baseline mood rating (scale of 1–5) for ‘happy/euphoric’, ‘restless’, ‘anxious’, and ‘energetic’ were calculated. Most subjects, in both groups, did not report rating changes of more than 2 points, limiting variability detectable in the sample. The average change in ‘happy/euphoric’ was 1.2 (BN) and 0.8 (controls; p=0.21). The average change in ‘restless’ was 1.1 (BN) and 1.9 (controls; p=0.11). The average change in ‘anxious’ was 0.8 (BN) and 1.8 (controls; p=0.07). The average change in ‘energetic’ was 1.3 (BN) and 1.2 (controls; p=0.83).
Associations between striatal DA response and subjective effects were tested, indicating a possible association between striatal DA and change in ‘energetic’ in the patient group (r2=0.55; p=0.009), and a possible association between striatal DA and change in ‘happy/euphoric’ in the control group (r2=0.26; p=0.09). A small number of data points appear to have driven these associations. There were no other significant associations between striatal DA response and change in subjective ratings.
DISCUSSION
The current study found a trend towards decreased mean D2 receptor BPND in the posterior putamen and posterior caudate in the patient group, and a statistically significant difference in DA response to psychostimulant challenge in the putamen. In addition, there was a statistically significant negative association between the frequencies of binge eating and vomiting and the striatal DA response.
These results suggest that patients with BN have disturbances in brain DA function resembling those described as characteristic of individuals with substance abuse, specifically with cocaine and alcohol dependence (4, 5). How such disturbances may relate to the development and perpetuation of the symptoms in BN is unclear. In substance use disorders, blunted DA response has been associated with the choice to self-administer the substance of abuse. Blunted DA response may represent a “downregulation” of the DA system after prolonged responding for reward. It may also be related to a loss of capacity to flexibly shift towards use of alternative reinforcers suggesting that a hypodopaminergic striatal state may contribute to impairments in reward-related learning (5, 27). By extrapolation, low striatal DA in BN may impair the ability of patients to make use of alternative reinforcers to binge eating and/or purging.
In contrast to some studies of substance abuse, neuroanatomically, the findings in this study of low striatal DA response are present at significant levels only in the putamen. The putamen is a heterogeneous region with many projections to associative as well as sensorimotor areas of the brain (28), although the more ventral portions of the putamen are known to form circuits with the gustatory cortex as well as limbic regions such as the amygdala (29). Other neuroimaging studies have implicated the putamen in aberrant eating processes and in deficiencies in reward prediction. In an fMRI study of BOLD response to receipt of a palatable food in obese patients, hypoactivity in the putamen (as well as in the caudate) was associated with high BMI, a finding that was not observed in the ventral striatum (30). An fMRI study in control participants demonstrated greatest BOLD activation in the left putamen during food reward prediction errors (31). The association between putamen DA response and frequency of binge eating episodes found here further supports the notion that a hypofunctioning putamen may confer rigidity in reward behavior in a patient population.
An additional consideration in interpretation of these results is that the posterior putamen DA release reported here in control participants was slightly higher [-23.7% +/− 10.1%] than has been reported in previous studies of psychostimulant-induced DA release in control participants [e.g., −16.6% +/− 9.9%] (32). Previous studies of DA release by striatal subregion have been conducted with amphetamine, as opposed to methylphenidate, which may account for this difference, as may the overall young age (mean = 24.9 years) of this sample. Further, the overall striatal DA release measure of −15.1% in this control sample is quite consistent with the report of Volkow et al (20). Notably, low striatal DA response did not appear to be associated with DA response in the ventral striatum, a striatal subregion classically associated with limbic functions (as it is the region homologous to the nucleus accumbens). Findings from PET studies in addictive disorders, using methodology similar to that implemented here, have revealed low DA response in the caudate, putamen, and ventral striatum in cocaine dependence (5), and low DA response in the putamen and ventral striatum in alcohol dependence (4).
There are several important limitations to the current study. One is sample size. The effect size used to determine the number of participants was based on previous studies of individuals with addictive disorders. The differences described here, while qualitatively consistent with those described among individuals with substance abuse, do not appear as robust.. For example, our results indicating blunted striatal DA release suggest a medium effect size, as does the correlation between striatal DA response and OBE’s, excluding data from the individual with the lowest DA release; these results contrast with differences of large effect size between controls and individuals with substance abuse. It is likely that the sub-regional results would not survive correction for multiple comparisons; nonetheless, these preliminary data suggest the possibility of between-group differences in DA measures, and provide strong support for a larger study.
Other limitations relate to the interpretation of the presence of comorbid illness. As is typical, patients with BN frequently described symptoms of depression. Previous PET/SPECT neuroimaging studies have not consistently found significant striatal DA alternations in unipolar depression (as discussed in the Methods), suggesting that the presence of modest levels of mood disturbances among our patients are unlikely to account for the findings reported here. The absence of a significant association between Beck Depression Inventory scores and DA measures in our sample also argues against depression being a major contributor to the differences between the BN and control groups. A second potential diagnostic confound is that six patients had a history of anorexia nervosa. In almost all cases this history was remote (>1 year prior to study), and anorexia nervosa has been associated with a relative increase in striatal D2 receptor density in the ventral striatum (33), in contrast to the decreases described here. In addition, post-hoc analysis did not detect a significant difference between patients with a history of AN, and those patients without. Third, patients with BN may have had subsyndromal attentional symptoms which did not meet full criteria for current ADHD; however, subsyndromal attentional problems, or past attentional problems not clearly meeting criteria for past ADHD may have been present in some subjects. Such past/present symptoms were not systematically assessed, and might be associated with abnormal DA function. Similarly, while any subject with past/present substance abuse or dependence was excluded, subsyndromal levels of drug use were not systematically assessed with structured instruments, and might be related to the abnormalities in DA function.
Other limitations relate to technical aspects of the PET imaging methods used. For example, interpretation of PET D2 [11C]raclopride data is generally limited by the fact that [11C]raclopride is a competitive antagonist at the D2 receptor, and therefore differences in D2 receptor BPND findings reflect the density of D2 receptors, but may also reflect the quantity of endogenous DA available in the synaptic cleft, receptor affinity, receptor internalization, or other factors (34). Raclopride is also known to have some affinity for the dopamine type 3 (D3) receptor (35), and this affinity may influence interpretation of findings. Another limitation of the data analysis methods used here is that regions of interest (ROI) were drawn bilaterally, not permitting detection of unilateral regional striatal abnormalties. Finally, as in other PET imaging studies utilizing oral psychostimulant challenge (20), the psychostimulant dose used in this study was not adjusted by weight. In this study, the BMI range in both groups was narrow and the mean BMI was very similar, limiting the potential impact of dose adjustment by weight. The association between BMI and DA response was not significant, further suggesting that BMI did not influence pharmacological response to the psychostimulant. Serum methylphenidate concentrations were not obtained, so it is not possible to assess the relationships between those parameters.
Despite these limitations, the current study using PET detected decreased DA neurotransmission in the striatum in patients with BN relative to control participants, a pattern similar to that described among individuals with substance use disorders. Additional studies are needed to confirm these findings, and to elucidate in greater detail similarities and differences between DA abnormalities in BN and in substance use disorders.
ACKNOWLEDGEMENTS
The authors acknowledge those who contributed to this study, including Alessandra Calvo-Friedman, Alexia Spanos, Amanda Brown, Carla Wolper, EdD, RD, Chitra Saxena, Christina Roberto, Dan Richter MD, Daria Orlowska, Diane Klein, MD, Ingrid Carretero, Kaitlin Greene, Joanna Steinglass, MD, Laura Berner, Laurel Mayer, MD, Lilya Deshchenko, Mary Bongiovi, MD/PhD, and Michael Devlin, MD.
FINANCIAL DISCLOSURES
This publication was made possible by NIMH grants R01MH079397, T32MH15144, and K23MH082097; a 2006 NARSAD Junior Investigator Award; and grant number KL2 RR024157 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov.ezproxy.cul.columbia.edu/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.ezproxy.cul.columbia.edu/clinicalresearch/overview-translational.asp.
Dr. Walsh has received research support from AstraZeneca. Dr. Attia has received research support from Eli Lilly. Dr. Abi-Dargham has received support from Bristol-Myers Squibb-Otsuka (as consultant and speaker), Bohringer-Engelheim (as consultant), and GlaxoSmithKline (through research support). Dr. Slifstein has consulted for GlaxoSmithKline and Amgen and has received research support from IntraCellular Therapies and Pierre-Fabre within the past 36 months.
Footnotes
The remaining authors (Broft, Shingleton, Kaufman, Liu, Kumar, Schebendach, Van Heertum, and Martinez) report no conflicts.
REFERENCES
- 1.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006 Jul 29;361(1471):1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009 Aug;53(1):1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005 Nov 15;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 5.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007 Apr;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, et al. "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002 Jun 1;44(3):175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 7.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003 Aug;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 8.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010 May;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992 Feb;49(2):132–138. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, et al. Eating disorders with binge-eating behaviour are associated with the s allele of the 3'-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci. 2004 Mar;29(2):134–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: A functional magnetic resonance imaging study. Int J Eat Disord. 2010 Nov 5; doi: 10.1002/eat.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank GK, Wagner A, Achenbach S, McConaha C, Skovira K, Aizenstein H, et al. Altered brain activity in women recovered from bulimic-type eating disorders after a glucose challenge: a pilot study. Int J Eat Disord. 2006 Jan;39(1):76–79. doi: 10.1002/eat.20210. [DOI] [PubMed] [Google Scholar]
- 14.First MBSR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCIDP), version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 15.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.Fairburn CGCZ. In: The Eating Disorder Examination. 12th edition. Fairburn CGWG, editor. New York: Guilford; 1993. [Google Scholar]
- 17.Hirvonen J, Karlsson H, Kajander J, Markkula J, Rasi-Hakala H, Nagren K, et al. Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology (Berl) 2008 May;197(4):581–590. doi: 10.1007/s00213-008-1088-9. [DOI] [PubMed] [Google Scholar]
- 18.Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, et al. Dopamine D(2) receptor availability and amphetamine-induced dopamine release in unipolar depression. Biol Psychiatry. 2001 Sep 1;50(5):313–322. doi: 10.1016/s0006-3223(01)01089-7. [DOI] [PubMed] [Google Scholar]
- 19.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001 Sep;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002 Mar 1;43(3):181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 21.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993 Jul-Aug;17(4):536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996 Dec;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 24.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007 Sep;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 25.Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001 Jul;28(5):595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- 26.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 27.Schonberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007 Nov 21;27(47):12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000 Mar 15;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005 Sep 19;490(2):101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008 Oct 17;322(5900):449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003 Apr 24;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 32.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003 Mar;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 33.Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biol Psychiatry. 2005 Dec 1;58(11):908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000 Mar;20(3):423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Levant B. The D-3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]