Abstract
The ultimate goal of prophylactic gene therapy is to confer permanent protection against ischemia. Although gene therapy with inducible nitric oxide synthase (iNOS) is known to protect against myocardial infarction at 3 days and up to 2 months, the long-term effects on myocardial ischemic injury and function are unknown. To address this issue, we created a recombinant adeno-associated viral vector carrying the iNOS gene (rAAV/iNOS), which enables long-lasting transgene expression. The ability of rAAV/iNOS to direct the expression of functional iNOS protein was confirmed in COS-7 cells before in vivo gene transfer. Mice received injections in the anterior LV wall of rAAV/LacZ or rAAV/iNOS; 1 year later, they underwent a 30-min coronary occlusion (O) and 4 h of reperfusion (R). iNOS gene transfer resulted in elevated iNOS protein expression (+3-fold vs. the LacZ group, n = 6; P < 0.05) and iNOS activity (+4.4-fold vs. the LacZ group, n = 6; P < 0.05) 1 year later. Infarct size (% of risk region) was dramatically reduced at 1 year after iNOS gene transfer (13.5 ± 2.2%, n = 12, vs. 41.7 ± 2.9%, n = 10, in the LacZ group; P < 0.05). The infarct-sparing effect of iNOS gene therapy at 1 year was as powerful as that observed 24 h after ischemic preconditioning (six 4-min O/4-min R cycles) (19.3 ± 2.3%, n = 11; P < 0.05). Importantly, compared with the LacZ group (n = 11), iNOS gene transfer (n = 10) had no effect on LV dimensions or function for up to 1 year (at 1 year: FS 34.5 ± 2.0 vs. 34.6 ± 2.6%, EF 57.0 ± 2.0 vs. 59.7 ± 2.9%, LVEDD 4.3 ± 0.1 vs. 4.2 ± 0.2 mm, LVESD 2.8 ± 0.1 vs. 2.9 ± 0.2 mm) (echocardiography). These data demonstrate, for the first time, that rAAV-mediated iNOS gene transfer affords long-term, probably permanent (1 year), cardioprotection without adverse functional consequences, providing a strong rationale for further preclinical testing of prophylactic gene therapy.
Keywords: Nitric oxide synthase, Gene therapy, Myocardial infarction, Cardiac function, Mouse
Introduction
Despite advances in our understanding, treatment, and prevention of acute myocardial infarction (AMI), this disorder remains the leading cause of morbidity and mortality in all industrialized nations and many survivors experience lasting morbidity with progression to heart failure and death [2, 13, 53, 57]. When coronary occlusion is followed by early reperfusion (as is often the case in the clinical arena), the final extent of myocardial injury represents the sum of two components: the injury incurred during ischemia and that incurred after reperfusion. Consequently, therapeutic manipulations that alleviate both components are likely to be more efficacious than manipulations targeted at reperfusion injury alone. In the clinical setting, however, administering cardioprotective drugs prior to the onset of AMI is usually impossible [3, 13].
Therefore, the most effective strategy for limiting infarct size and improving clinical outcomes may be to induce a chronically protected cardiac phenotype, analogous to that elicited by late (sustained) preconditioning (PC). The late phase of ischemic PC is the phenomenon whereby exposure of the heart to brief, sublethal ischemic results in the acquisition of a sustained cardioprotected phenotype. The protection becomes manifest 12–24 h after the PC stimulus and lasts 3–4 days [1-3, 57]. Because of its sustained duration, late PC may have considerable clinical relevance [38, 44, 53].
Conceptually, inducing a late PC-like state would offer significant advantages over treatments given acutely during or after AMI [2, 3]. The protective mechanisms recruited by prophylactic measures are already in place when ischemia starts, thereby enabling limitation of injury from the very beginning of this process. In principle, this should alleviate both the ischemic and the reperfusion components of injury, and thus should be more efficacious than treatments started late into ischemia or after reperfusion. The feasibility of prophylactic cardioprotection is strongly supported by several preclinical studies which suggest that it is indeed possible to induce a chronically protected cardiac phenotype by emulating the phenomenon of late PC with genetic approaches. For example, gene transfer of heat shock protein HSP70, inducible nitric oxide synthase (iNOS), heme oxygenase-1 (HO-1), or extracellular superoxide dismutase (ecSOD) (all of which are upregulated during late PC) has been shown to confer protection against ischemia/reperfusion injury [30, 31, 37, 43, 46].
A similar delayed protective effect can be induced pharmacologically with the administration of NO donors, such as nitroglycerin, S-nitroso-N-acetylpenicillamine, and diethylenetriamine/NO (DETA/NO) [2, 3, 18, 21] highlighting the central role of NO as a trigger, mediator, and potential effector of cardioprotection [19]. However, pharmacologic induction of delayed protection has inherent disadvantages including reliance on the specificity of the drugs, limited duration, and requirement for repeated treatments [3].
Studies by various investigators, including ourselves, have demonstrated that late PC induced by a wide variety of stimuli, including ischemia, physical exercise, and pharmacological agents such as NO donors, is mediated by the upregulation of the cardioprotective protein iNOS [2, 3, 23, 48, 51], pointing to a central role of this enzyme as a common effector of cardioprotection following a multiplicity of different stimuli. Therefore, upregulation of iNOS in myocardium by iNOS gene therapy appears to be a promising strategy for prophylactic cardioprotection resulting in a chronic late PC-like state. This strategy would require only one treatment to achieve sustained protection rather than daily administration of drugs.
Previous studies have shown that gene therapy with iNOS protects against AMI at 3 days and for up to 2 months, due to the short-term gene expression mediated by recombinant adenoviral vector 5 (rAd5) [36, 37, 56]. However, the long-term effects of iNOS gene therapy on myocardial ischemic injury and function (which are of the utmost clinical importance) are unknown. Before iNOS gene therapy can be used clinically to protect the ischemic myocardium, it is necessary to determine whether chronic overexpression of iNOS in the myocardium for 1 year is cardioprotective and is safe in terms of myocardial function. To address this issue, we created a recombinant adeno-associated viral vector (rAAV) carrying the iNOS gene (rAAV/iNOS), which enables long-lasting transgene expression (at least 1 year) because of chromosomal integration of the transgene and lack of inflammation. The gaining popularity of rAAV use in gene therapy can be attributed to its lack of pathogenicity and added safety due to its replication defectiveness and its ability to mediate long-term expression in tissues [12, 17].
Thus, the goal of the present study was to determine whether long-term (up to 1 year) overexpression of iNOS confers protection against myocardial infarction without adverse functional consequences in mice. We used a well-established murine model of AMI [9, 10] and rAAV-mediated iNOS gene transfer. Our results demonstrate that the rAAV vector enables long-lasting iNOS gene expression in the myocardium for up to 1 year and that this results in significant cardioprotection without adverse functional effects. The cardioprotection produced by rAAV-mediated iNOS gene therapy was not only as powerful as the salubrious effects of late PC but also dramatically longer, up to 1 year, thereby overcoming the limitation of the short-term (3 days) protection induced by late PC.
Methods
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. [NIH] 86–23) and with the guidelines of the Animal Care and Use Committee of the University of Louisville, School of Medicine (Louisville, KY, USA).
Construction of the rAAV/iNOS vector
The cDNA for the open-reading frame sequence of mouse iNOS [39] was cloned into the corresponding sites in the AAV2 shuttle plasmid. The recombinant AAV2 containing mouse iNOS under the control of the cytomegalovirus promoter was generated by a triple-transfection technique using a helper virus-free system (Stratagene). rAAV/iNOS was purified from 293 cell extracts by CsCl centrifugation and was routinely concentrated to approximately 2–4 × 1011 vector genomes (vg)/ml as determined by quantitative PCR [42, 60].
Gene transfer in vivo
Male ICR mice (10- to 12-week old; body weight 35.2 ± 0.8 g) were anesthetized with sodium pentobarbital (50 mg/kg i.p.). After opening the chest through a midline sternotomy, mice received two intramyocardial injections in the anterior left ventricular (LV) wall of rAAV/LacZ (1 × 1010 vp, LacZ group) or rAAV/iNOS (1 × 1010 vp, iNOS group). One year later, mice underwent either cardiac tissue collection or the infarction protocol described below (Fig. 1). Each intramyocardial injection was 20 μl in volume and was performed with a 100-μl syringe using a 30-gauge needle; each mouse heart received two injections (total volume, 40 μl) in the soon-to-be-ischemic region of the LV [33, 36, 37]
Fig. 1.

Experimental protocol. Five groups of mice were studied. The sham group underwent a thoracotomy and 1 h of open-chest state (without coronary occlusion) on day 1. The 1 h of open-chest state corresponded to the time interval necessary to perform six occlusion-reperfusion cycles in the late PC group; 24 h later (day 2), mice underwent a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in the late PC group were preconditioned with a sequence of six cycles of 4-min occlusion and 4-min reperfusion on day 1; on day 2, they were subjected to a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in the age-matched control, LacZ, and iNOS groups underwent serial echocardiographic studies at baseline (4 days prior to gene transfer), 3 months and 1 year (4 days prior to coronary occlusion) after gene transfer. The age-matched group did not receive any intervention during the period of the investigation. On day 1, mice in the LacZ and iNOS groups were subjected to intramyocardial injections of rAAV/LacZ or rAAV/iNOS, respectively; at 1 year (4 days after echocardiographic measurements), both the LacZ and iNOS groups underwent a 30-min coronary occlusion followed by 4 h of reperfusion for infarct size determination
Echocardiographic studies
Echocardiographic studies were performed using a HDI 5000 SonoCT ultrasound system (Philips Medical Systems, Bothell, WA, USA) equipped with a 15- to 7-MHz linear transducer. Mice were anesthetized with isoflurane (3% induction and 1.5% maintenance). The chest was shaved, and mice were placed in a supine position. A rectal temperature probe was placed, and the body temperature was carefully maintained between 37.0 and 37.3°C with a heating pad throughout the study. The parasternal short-axis and modified parasternal long-axis views were used to obtain two-dimensional (2-D) and M-mode images. Digital images were analyzed offline in a blinded fashion using ProSolv (version 2.5) image analysis software (Problem Solving Concepts, Indianapolis, IN, USA), according to the American Society of Echocardiography standards [7, 32, 36].
Coronary occlusion/reperfusion protocol
The murine model of myocardial ischemia and reperfusion has been described in detail [9, 10]. Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and ventilated using carefully selected parameters. After administration of antibiotics, the chest was opened through a midline sternotomy, and a nontraumatic balloon occluder was implanted around the mid-left anterior descending coronary artery by using an 8-0 nylon suture. To prevent hypotension, blood from a donor mouse was given at serial times during surgery [10, 33]. Rectal temperature was carefully monitored and maintained between 36.7 and 37.3°C throughout the experiment. In all groups, myocardial infarction was produced by a 30-min coronary occlusion followed by 4 h of reperfusion (Fig. 1). The LacZ and iNOS groups received intramyocardial injections of rAAV/LacZ or rAAV/iNOS, respectively, as described above, 1 year before the 30-min occlusion. Successful performance of coronary occlusion and reperfusion was verified by visual inspection (i.e., by noting the development of a pale color in the distal myocardium after inflation of the balloon and the return of a bright red color due to hyperemia after deflation) and by observing S–T segment elevation and widening of the QRS on the ECG during ischemia and their resolution after reperfusion. After the coronary occlusion/reperfusion protocol was completed, the chest was closed in layers, and a small catheter was left in the thorax for 10–20 min to evacuate air and fluids. The mice were removed from the ventilator, kept warm with heat lamps, given fluids (1.0–1.5 ml of 5% dextrose in water i.p.), and allowed 100% oxygen via nasal cone [9, 10, 33, 36].
Postmortem tissue analysis
At the conclusion of the study, the occluded/reperfused vascular bed and the infarct were identified by postmortem perfusion of the heart with phthalo blue dye and triphenyltetrazolium chloride (TTC), respectively [9, 10]. The corresponding areas were measured by computerized video planimetry (Adobe Photoshop CS3) and from these measurements infarct size was calculated as a percentage of the region at risk [35-37].
Western immunoblotting analysis
Protein samples were isolated from heart tissues as previously described [33, 35-37]. The protein content in the cytosolic and membranous fractions was determined by the Bradford technique (Bio-Rad). The expression of iNOS, eNOS, and GAPDH was assessed by standard SDS/PAGE Western immunoblotting techniques [33, 35-37]. Briefly, 80 μg of protein was separated on an SDS–polyacrylamide gel and transferred to a nitrocellulose membrane. The gel transfer efficiency was recorded carefully by making photocopies of membranes dyed with reversible Ponceau staining; gel retention was determined by Coomassie blue staining [9, 33, 35-37]. Proteins were probed with specific anti-iNOS, anti-eNOS (BD Transduction Laboratories, Lexington, KY, USA), and anti-GAPDH antibodies (Cell Signaling Technology, Inc. Danvers, MA, USA). Immunoreactive bands were visualized with horseradish peroxidase-conjugated anti-rabbit IgG using an enhanced chemiluminescence detection kit (NEN), quantified by densitometry, and normalized to the Ponceau stain density.
Measurement of iNOS activity
Calcium-independent NOS activity (iNOS activity) was determined by measuring the conversion of l-[14C]arginine to l-[14C]citrulline as previously described [9, 37]. Briefly, isolated proteins were incubated at 37°C for 30 min with reaction buffer containing 50 mM Tris–HCl (pH 7.4), 1 mM NADPH, 5 μM flavin adenine dinucleotide, 5 μM flavin mononucleotide, 10 μM tetrahydrobiopterin, 1 mM EGTA, and purified l-[14C]arginine (348 mCi/mmol, 0.1 μCi per reaction, Amersham) without calcium and calmodulin. In all groups, duplicate assays were performed for each sample, and iNOS activity was expressed as picomoles of l-citrulline per minute per milligram protein.
Immunohistochemistry analysis
Immunohistochemical analysis for iNOS protein expression 1 year after gene transfer was performed using the ABC kit (Vector Laboratories) [33]. Frozen 10-μm sections were fixed in 10% formalin for 2 min at room temperature, washed in PBS, and blocked with Mouse-On-Mouse (MOM) IgG blocking reagent. Antigenic epitopes were equilibrated in MOM diluent and then incubated at 37°C with a monoclonal anti-iNOS antibody (BD Transduction Laboratories). Alternate sections were incubated in the absence of the primary antibody (negative control) as previously reported [33, 36].
Statistical analysis
Data are presented as means ± SEM. All data were analyzed with one-way ANOVA for normally distributed data or Kruskal–Wallis one-way ANOVA on ranks for data that are not normally distributed, as appropriate, followed by unpaired Student’s t tests with the Bonferroni correction. A P value <0.05 was considered statistically significant. All statistical analyses were performed using the SigmaStat software system (V 3.5) [32, 33].
Results
Exclusions
A total of 89 mice were used for this study (63 for the infarct size and functional studies and 26 for biochemical analyses). Fourteen mice died during or shortly after the surgical procedure: six after the first surgery (gene transfer) and eight after the second surgery (coronary occlusion). Four mice (5%) were excluded because of technical problems, including body temperature out of normal range (n = 1), balloon occluder malfunction (n = 1), and bleeding during the surgery (n = 2). Three mice (3%) died during the 1-year follow-up. Thus, a total of 68 mice were included in the final analyses. Nineteen mice were used for both this study and the study of rAAV/HO-1 gene therapy reported in the companion manuscript [34], specifically, six mice in the sham group, six mice in the late PC group, and seven mice in the rAAV/LacZ group.
Fundamental physiological parameters
Heart rate, a fundamental-physiological parameter that may impact infarct size [10, 33], was similar in the sham and late PC groups, and in the LacZ and iNOS groups (Table 1). The heart rate of the sham and late PC groups was higher than that of both gene therapy groups, possibly reflecting the effect of surgical trauma 24 h earlier. However, there was no statistically significant difference between the sham and late PC groups or between the LacZ and iNOS groups. Within the same group, heart rate did not differ significantly at any time-point before and during the 30-min occlusion or the ensuing reperfusion (Table 1). By experimental design, rectal temperature remained within a narrow, physiologic range (36.8–37.2°C) in all groups. During the echocardiographic studies, heart rate (which may impact myocardial function) was similar in the age-matched controls, LacZ, and iNOS groups. The average heart rate in the 3 groups before (baseline), and 3 months and 1 year after gene therapy ranged from 487 ± 13 to 466 ± 18 bpm (P > 0.05). The size of the region at risk, expressed as a percentage of left ventricular weight, did not differ among the 4 groups: sham group 35.8 ± 3.0%, late PC group 34.1 ± 4.5%, LacZ group 40.0 ± 3.6%, and iNOS group 36.0 ± 3.3% (P = 0.717).
Table 1.
Heart rate and rectal temperature on the day of the 30-min coronary occlusion
| Groups | Preocclusion | Occlusion
|
Reperfusion
|
||
|---|---|---|---|---|---|
| 5 min | 30 min | 5 min | 15 min | ||
| Heart rate (beats/min) | |||||
| Sham (n = 9) | 590 ± 16 | 617 ± 18 | 616 ± 16 | 632 ± 28 | 633 ± 21 |
| Late PC (n = 11) | 620 ± 23 | 621 ± 24 | 605 ± 35 | 604 ± 32 | 647 ± 30 |
| rAAV/LacZ (n = 10) | 510 ± 12a,b | 520 ± 11a,b | 504 ± 13a,b | 518 ± 17a,b | 518 ± 17a,b |
| rAAV/iNOS (n = 12) | 550 ± 21b | 542 ± 19a,b | 533 ± 21a | 537 ± 26a | 542 ± 23a,b |
| Body temperature (°C) | |||||
| Sham (n = 9) | 36.9 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 | 36.8 ± 0.1 |
| Late PC (n = 11) | 36.9 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 | 36.8 ± 0.1 |
| rAAV/LacZ (n = 10) | 37.1 ± 0.0 | 37.1 ± 0.1 | 37.2 ± 0.0 | 37.1 ± 0.0 | 37.1 ± 0.1 |
| rAAV/iNOS (n = 12) | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
Data are means ± SEM. The heart rate and rectal temperature were recorded before the 30-min coronary occlusion (preocclusion), at 5 min and 30 min during the 30-min occlusion, and at 5 min and 15 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text
P < 0.05 versus sham group
P < 0.05 versus late PC group
iNOS gene expression in vitro
The ability of rAAV/iNOS to induce expression of functionally competent protein was verified in transfected COS-7 cells. Transfection of COS-7 cells with rAAV/iNOS resulted in increased iNOS protein expression 4 days after rAAV/iNOS-mediated gene transfer (Fig. 2). Consistent with the increased level of iNOS protein expression, calcium-independent iNOS activity was also increased in rAAV/iNOS transfected COS-7 cells as compared with control cells (+2.9-fold vs. COS-7 cells transduced with rAAVLacZ, n = 3, P < 0.05), indicating that rAAV/iNOS is able to direct the expression of functional iNOS protein.
Fig. 2.

iNOS gene expression after rAAV-mediated gene transfer in vitro. COS-7 cells were treated with rAAV/LacZ (negative control), rAAV/iNOS, or lipofectamine-mediated pcDNA3.1/iNOS gene transfer (positive control). Four days later, proteins were isolated from cells and subjected to Western blotting with anti-murine iNOS antibody. Each experiment was repeated thrice
iNOS gene expression in vivo
One year after intramyocardial injection of rAAV/LacZ or rAAV/iNOS, the myocardium surrounding the sites of injections (~40 mg) was harvested for the measurement of iNOS protein expression and iNOS activity (each heart received two injections with a total of 40 μl in volume). Western immunoblotting analysis demonstrated that cardiac iNOS gene transfer mediated by the rAAV vector resulted in a significant increase in iNOS protein expression at 1 year (+3-fold vs. the LacZ group, n = 6; P < 0.05; Fig. 3a). In contrast, eNOS protein expression in the transduced myocardium did not change (Fig. 3a). No nNOS immunoreactivity was detected (data not shown). Consistent with the immunoblotting results, iNOS activity was also significantly increased in mice transfected with rAAV/iNOS (4.4-fold greater than in the rAAV/LacZ group, n = 6; P < 0.05; Fig. 3b), indicating that the transgenic protein was functionally competent 1 year after rAAV/iNOS-mediated gene transfer, and that this rAAV vector enables long-term expression of the transgene in myocardium in vivo.
Fig. 3.

iNOS gene expression in myocardium 1 year after rAAV-mediated gene transfer. a Western immunoblots and densitometric analyses of iNOS protein signals in cytosolic and of eNOS protein signals in membranous fractions of the transduced myocardium. b Total calcium-independent NOS (iNOS) activity in the transduced myocardium. Data are mean ± SEM
As illustrated in Fig. 4, immunohistochemical analysis showed robust iNOS immunoreactivity in cardiac myocytes 1 year after transfection with rAAV/iNOS (n = 3). No iNOS immunoreactivity was noted in the rAAV/LacZ group (n = 3) and in cardiac sections incubated with nonimmune serum (not shown). The fact that iNOS expression or activity did not increase in the rAAV/LacZ group (Figs. 3 and 4) demonstrates that the upregulation of iNOS observed in the rAAV/iNOS group 1 year after gene transfer cannot be ascribed to nonspecific factors associated with gene transfer such as trauma and local inflammatory reaction.
Fig. 4.

Expression of the iNOS gene 1 year after rAAV-mediated gene transfer. Hearts transfected with rAAV/LacZ (a 300×) or rAAV/iNOS (b 300×), respectively, are shown. Robust expression of iNOS (brown stain) was observed in the transfected region of myocardium 1 year after gene transfer (n = 3)
Long-term effect of iNOS gene transfer on infarct size
To characterize the long-term (1-year) effects of iNOS gene therapy, the infarct-sparing effect of rAAV/iNOS gene transfer was compared with that of the late phase of ischemic PC. When mice were preconditioned with six cycles of 4-min coronary occlusion/4-min reperfusion 24 h before the 30-min coronary occlusion, infarct size was reduced by an average of 58% versus sham PC mice, indicating a late PC effect (Fig. 5). In mice given rAAV/LacZ, infarct size did not differ from that measured in mice undergoing sham PC (41.7 ± 2.9% of the risk region, n = 10, vs. 46.3 ± 2.3%, n = 9, in sham group; P > 0.05; Fig. 5). However, in mice transduced with rAAV/iNOS, infarct size was smaller than in the control rAAV/LacZ group (13.5 ± 2.2% of the risk region, n = 12, vs. 41.7 ± 2.9%, n = 10, in the LacZ group; P < 0.05; Fig. 5), demonstrating that the expression of iNOS was associated with long-term cardioprotection up to 1 year.
Fig. 5.

Myocardial infarct size expressed as a percentage of the region at risk. Open circles individual mice, solid circles mean values ± SE
Representative examples of the infarctions observed in the rAAV/LacZ and rAAV/iNOS groups 1 year after gene transfer are shown in Fig. 6. Large, confluent areas of infarction spanning most of the thickness of the LV wall, with thin rims of viable subendocardial tissue, were characteristic of the group transduced with rAAV/LacZ control gene. In contrast, in the rAAV/iNOS group only small, sporadic areas of cell death were noted (Fig. 6). The average infarct size observed in the rAAV/iNOS group was similar to that observed in the late PC group (Fig. 5), demonstrating that the infarct-sparing effect of iNOS gene therapy at 1 year is as powerful as that observed 24 h after ischemic PC (six 4-min O/4-min R cycles).
Fig. 6.
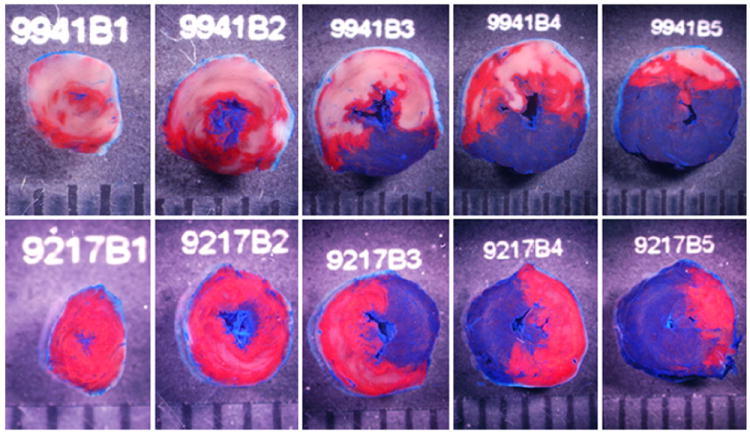
Representative heart slices from rAAV/LacZ and rAAV/iNOS groups 1 year after gene therapy. The slices shown here were obtained from the apex to the base. Top panel rAAV/LacZ. Bottom panel rAAV/iNOS. The region at risk and the infarct were identified by postmortem perfusion with TTC and phthalo blue dye, as described in Methods. As a result of this procedure, the nonischemic portion of the LV was stained dark blue, and viable tissue within the region at risk was stained bright red, whereas infarcted tissue was light yellow/white. The LV endocardial surface was stained dark blue with phthalo blue to facilitate identification of the endocardial border of the slice. rAAV/LacZ-treated heart exhibited large, confluent areas of infarction. In contrast, the heart pretreated with rAAV/iNOS exhibited small patchy areas of infarction, indicating a profound and long-term (1 year) cardioprotective effect of iNOS gene therapy. The scale at bottom is in millimeters
Long-term effect of iNOS gene transfer on cardiac function
Cardiac function was assessed by 2-D echocardiography at baseline, and 3 months and 1 year after gene transfer. To prevent any after-effects of the anesthesia used during the echocardiographic assessments, a 4-day interval was allowed between the baseline echocardiographic measurement and gene transfer, and between the 1-year echocardiographic assessment and the 30-min coronary occlusion followed by 4 h of reperfusion (Fig. 1). As shown in Fig. 7, there were no appreciable changes in LV fractional shortening, LV ejection fraction, or LV end-diastolic or end-systolic diameter at any time point after iNOS gene transfer as compared with the age-matched controls or with the rAAV/LacZ group, indicating that rAAV-mediated iNOS gene transfer in the heart does not induce adverse cardiac functional consequences for at least 1 year.
Fig. 7.

Quantitative analysis of echocardiographic LV functional parameters before (baseline), 3 months and 1 year after rAAV-mediated gene transfer. BSL baseline, FS fractional shortening, EF ejection fraction, LVEDD left ventricular (LV) end-diastolic diameter, LVESD LV end-systolic diameter. Data are mean ± SEM
Discussion
The data presented herein demonstrate, for the first time, that rAAV-mediated transfer of iNOS gene to the heart recapitulates the powerful cardioprotective effect of late PC and extends this effect in time up to 1 year, resulting in the acquisition of chronic tolerance to ischemia/reperfusion injury without cardiac-functional impairment and thereby providing a strong rationale for further preclinical testing of prophylactic gene therapy.
Methodological considerations of vectors
Most investigators, including ourselves, have utilized rAd5 vectors to transfer genes to the heart [11, 22, 33, 35, 37, 56]. While this approach has been very useful to begin to understand the utility and applications of gene therapy, the therapeutic use of rAd is inherently limited by two fundamental (and related) problems: (1) the vigorous inflammatory response to viral proteins, which results in frank myocarditis, and (2) the transient expression of the transgene, which lasts only ~ 2 weeks [25, 40, 45]. Clearly, for gene therapy to become a feasible therapeutic strategy for chronic protection against ischemia, it will be necessary to express the target gene for many months or years. We have also used the second-generation rAd vectors with deletion of the E1, E2a, and E3 regions [8]. These vectors evoke an attenuated inflammatory response, which enables transgene expression to last for up to 2 months [36] but still does not result in permanent transduction. Consequently, in the present study we created recombinant adeno-associated viral vector (rAAV) carrying the therapeutic gene iNOS (rAAV/iNOS).
The rAAV has many properties that make it attractive as a delivery vehicle in gene therapy. AAVs are not associated with any known human disease and are capable of integrating into the host chromosome 19 in a site-specific manner without pathogenic reactions [16, 52]. In particular, the rAAV vector is capable of infecting both mitotic and post-mitotic cells [17]. These unique properties make rAAVs a very promising vector for gene therapy [16, 17]. Indeed, numerous studies in non-cardiac tissue have documented the ability of rAAVs to direct prolonged expression of the gene of interest for 6–12 months and up to 2 years [20, 26, 54]. rAAV vectors have also been successfully employed for long-term gene transfer to the heart. For example, rAAV-mediated transfer of the HO-1 gene protects the heart from ischemia/reperfusion injury for at least 8 weeks [43] and rAAV-mediated LacZ gene transfer is detectable for at least 6 months [29]. The translational potential of rAAV vectors is underscored by the fact that they are increasingly being recognized as gene therapy vectors for clinical disease. Despite theoretical concerns, initial safety results from clinical trials of rAAV therapy are promising. A number of human trials using rAAV vectors for cystic fibrosis and hemophilia B are ongoing, and so far there has been no report of toxicity [6, 41]. This, coupled with the fact that initial studies using rAAV-mediated therapy in patients have given encouraging results with respect to efficacy [16, 24], suggests that rAAV vectors may have important applications for gene delivery in the clinical arena. Besides the obvious advantage of not causing an inflammatory response, the durable transgene expression afforded by rAAV should enable chronic, possibly even permanent, prophylactic cardioprotection in patients with acute AMI.
Efficacy of rAAV/iNOS-mediated gene transfer in vitro and in vivo
Utilizing the rAAV system, we found that the rAAV/iNOS vector not only induced expression of iNOS protein but also increased iNOS activity in the transfected COS-7 cells, demonstrating the ability of rAAV/iNOS vectors to infect mitotic cells (COS-7) and to induce expression of functionally competent protein iNOS in mitotic cells in vitro (Fig. 2). Furthermore, at 1 year, cardiac iNOS gene transfer mediated by the rAAV/iNOS vector resulted in a significant increase in iNOS protein expression (+3-fold vs. the LacZ group, P < 0.05; Fig. 3a). Consistent with the increased level of iNOS protein expression in the myocardium, iNOS activity was also significantly increased in the hearts transfected with rAAV/iNOS (4.4-fold greater than in the rAAV/LacZ group, P < 0.05; Fig. 3b), indicating that the transgenic iNOS protein was functionally competent 1 year after rAAV/iNOS-mediated gene transfer. This rAAV vector enables infection of post-mitotic cells, i.e., cardiac myocytes (Fig. 4), thereby resulting in long-term expression of the transgene in vivo.
Long-term prophylactic cardioprotection with iNOS gene therapy
The late phase of ischemic PC is considered an excellent example of prophylactic cardioprotection because exposure of the heart to brief sublethal ischemia results in the acquisition of a sustained cardioprotected phenotype [2, 3, 51]. PC consistently limits infarct size in every animal model and in every species examined, and there is considerable evidence that it is effective in protecting human myocardium as well [2, 3, 18, 27]. The delayed (or late) phase of PC affords a relatively long-lasting protection (3 days) and can be elicited by pharmacologic or genetic stimuli [3]. However, the pharmacologic stimulation has inherent disadvantages including non-specificity of these drugs in vivo, relatively short duration of protection, necessity of repeated treatments, and requirement for drugs to be administered prior to the onset of AMI. In an effort to overcome these limitations, the present investigation examined a novel non-pharmacologic approach to cardioprotection, i.e., prophylactic cardioprotection induced by gene therapy.
The selection of the gene (iNOS) to be investigated in the present study was motivated by the fact that overexpression of iNOS in the heart affords a protected phenotype and that iNOS is an obligatory mediator of the infarct-sparing effects of the late phase of PC induced by ischemia and several other stimuli [1-3, 9], indicating that upregulation of this enzyme is a common pathway whereby the heart adapts to stress [1, 2]. We created a rAAV vector carrying the iNOS gene and performed rAAV-mediated iNOS gene transfer into the heart in a well-established murine model of AMI. Our results indicate that stable transfer of cardioprotective gene iNOS to the myocardium via rAAV vectors elicited a chronic state of tolerance to ischemia/reperfusion injury up to 1 year after gene transfer (Figs. 5 and 6). As illustrated in Fig. 5, the limitation of infarct size afforded by iNOS gene therapy after 1 year was as powerful as that of late PC at 3 days, demonstrating that iNOS gene therapy-induced protection is long-lasting (up to 1 year). The significance of these results is that rAAV-mediated iNOS gene transfer will alleviate ischemic damage whenever it occurs and from the very onset of the ischemic insult (not just after reperfusion). These data establish a preclinical model of prophylactic cardioprotection mediated by gene therapy.
There are other advantages inherent in the selection of iNOS as the therapeutic gene. When the products of the transgene are not secreted, only cells expressing the transgene will be affected, which might be inefficient, particularly when intracoronary delivery of genes is applied in the clinical setting. In contrast, the highly diffusible nature of NO should enable this gas to access cells that do not express the iNOS transgenes, thereby producing a paracrine effect that amplifies the effects of iNOS gene transfer. This concept is supported by our finding that intramyocardial injection of rAd/iNOS in mice reduced infarct size by approximately 60% despite the fact that only approximately 20% of the risk region exhibited iNOS expression [37], demonstrating that NO released from the transduced cells protected a larger adjacent myocardial region. The ability of NO to diffuse readily in tissues suggests that iNOS gene transfer can overcome, at least in part, the major obstacle to gene therapy, i.e., the limited number of cardiac cells that can be transduced with currently available clinical methods.
Long-term effect of iNOS gene transfer on cardiac function
Experimental and clinical evidence suggests that enhanced production of NO by iNOS may be causally related to the observed contractile dysfunction in heart failure. de Belder et al. [4] were the first to show enhanced iNOS activity in right ventricular tissue from patients with dilated cardiomyopathy. Later, increased iNOS activity has been reported in myocarditis, ischemic heart disease, valvular heart disease, and human cardiac allografts, and iNOS levels have been found to correlate with ventricular contractile dysfunction [14, 28, 50, 59]. Unlike constitutively expressed NOS isoforms, iNOS is more ubiquitously expressed and its expression only increases following induction by events such as hypertrophy or heart failure.
In such cases, iNOS appears to contribute to myocardial dysfunction [5, 58, 59]. To investigate the specific, independent effect of iNOS isoform on cardiac function, Heger et al. [15] used a transgenic mouse model overexpressing iNOS under the cardiac-specific α-myosin heavy chain promoter and found that robust overexpression of iNOS in the heart was not associated with deleterious effects on cardiac hemodynamics and LV oxygen consumption, or with heart failure. Therefore, the effects of chronic upregulation of iNOS on the myocardium remain controversial, and studies performed so far could not answer the question as to whether long-term (1 year) iNOS expression in the heart is associated with the development of cardiac dysfunction.
Before concluding that long-term iNOS gene therapy affords prophylactic cardioprotection and is a clinically promising approach, it is necessary to determine whether chronic overexpression of iNOS in the myocardium for 1 year is safe for myocardial function. To this end, the functional consequences of iNOS overexpression in the heart were studied in mice by means of echocardiography at baseline, 3 months, and 1 year after gene transfer. Since the mice were well over 1-year old at the time of killing, the cellular changes associated with senescence [55] could have contributed to functional deterioration. Significant alterations in myocyte morphology and cardiac function are the most prominent phenotypes associated with senescence [47, 49]. Thus, besides the control group subjected to rAAV/LacZ gene transfer, an age-matched control group was employed to monitor any significant changes in cardiac function associated with aging. As illustrated in Fig. 4, immunohistochemistry analysis demonstrated no significant changes in myocyte morphology 1 year after iNOS gene transfer as compared with that of the control LacZ group. Furthermore, as shown in Fig. 7, among of the age-matched controls, LacZ, and iNOS groups, there were no appreciable differences in LV fractional shortening, LV ejection fraction, and LV end-diastolic or end-systolic diameter at baseline, 3 months, and 1 year after iNOS gene transfer. Our results clearly demonstrate that 1 year of overexpression of iNOS in the heart is not associated with deleterious effects on cardiac function and does not result in myocyte hypertrophy and heart failure (Fig. 7). The fact that the heart can tolerate overexpression of iNOS for 1 year without detrimental functional consequences suggests that the concept that iNOS-derived NO triggers pathophysiological mechanisms leading to heart failure needs to be reevaluated.
Conclusions
The present investigation demonstrates, for the first time, that transfer of the iNOS gene to the myocardium via rAAV vectors affords chronic, possibly permanent (1 year) protection against ischemia/reperfusion injury without adverse cardiac functional consequences. Considerable evidence indicates that the cardioprotective effects observed 24 h after the late phase of PC are mediated by iNOS. Our finding that iNOS gene transfer can reproduce the salubrious effects of the late phase of ischemic PC but dramatically extend their duration (from 3 days to at least 1 year) supports a novel therapeutic strategy for achieving long-term prophylactic cardioprotection in patients with ischemic heart disease based on the transfer to the heart of iNOS or other genes implicated in late preconditioning.
Acknowledgments
This study was supported in part by NIH grants R01 HL55757, HL-70897, HL-76794, and P01HL78825.
Contributor Information
Qianhong Li, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Yiru Guo, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Wen-Jian Wu, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Qinghui Ou, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Xiaoping Zhu, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Wei Tan, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Fangping Yuan, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Ning Chen, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Buddhadeb Dawn, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA; Cardiovascular Research Institute, University of Kansas Medical Center, Kansas City, KS 66160, USA.
Li Luo, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Erin O’Brien, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
Roberto Bolli, Email: rbolli@louisville.edu, Division of Cardiovascular Medicine, Institute of Molecular Cardiology, University of Louisville, 550 S Jackson Street, Louisville, KY 40292, USA.
References
- 1.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Li QH, Tang XL, Guo Y, Xuan YT, Rokosh G, Dawn B. The late phase of preconditioning and its natural clinical application—gene therapy. Heart Fail Rev. 2007;12:189–199. doi: 10. 1007/s10741-007-9031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Belder AJ, Radomski MW, Why HJ, Richardson PJ, Bucknall CA, Salas E, Martin JF, Moncada S. Nitric oxide synthase activities in human myocardium. Lancet. 1993;341:84–85. doi: 10.1016/0140-6736(93)92559-c. [DOI] [PubMed] [Google Scholar]
- 5.de Belder AJ, Radomski MW, Why HJ, Richardson PJ, Martin JF. Myocardial calcium-independent nitric oxide synthase activity is present in dilated cardiomyopathy, myocarditis, and postpartum cardiomyopathy but not in ischaemic or valvar heart disease. Br Heart J. 1995;74:426–430. doi: 10.1136/hrt.74.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flotte TR, Schwiebert EM, Zeitlin PL, Carter BJ, Guggino WB. Correlation between DNA transfer and cystic fibrosis airway epithelial cell correction after recombinant adeno-associated virus serotype 2 gene therapy. Hum Gene Ther. 2005;16:921–928. doi: 10.1089/hum.2005.16.921. [DOI] [PubMed] [Google Scholar]
- 7.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res. 1995;76:907–914. doi: 10.1161/01.res.76.5.907. [DOI] [PubMed] [Google Scholar]
- 8.Gorziglia MI, Kadan MJ, Yei S, Lim J, Lee GM, Luthra R, Trapnell BC. Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, Dec GW, Semigran MJ, Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajjar RJ, Zsebo K. AAV vectors and cardiovascular disease: targeting TNF receptor in the heart: clue to way forward with AAV? Gene Ther. 2007;14:1611–1612. doi: 10.1038/sj.gt.3303047. [DOI] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter workshop recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1094. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 15.Heger J, Godecke A, Flogel U, Merx MW, Molojavyi A, Kuhn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 16.Henckaerts E, Linden RM. Adeno-associated virus: a key to the human genome? Future Virol. 2010;5:555–574. doi: 10.2217/fvl 10.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hester ME, Foust KD, Kaspar RW, Kaspar BK. AAV as a gene transfer vector for the treatment of neurological disorders: novel treatment thoughts for ALS. Curr Gene Ther. 2009;9:428–433. doi: 10.2174/156652309789753383. [DOI] [PubMed] [Google Scholar]
- 18.Heusch G. Nitroglycerin and delayed preconditioning in humans: yet another new mechanism for an old drug? Circulation. 2001;103:2876–2878. doi: 10.1161/01.cir.103.24.2876. [DOI] [PubMed] [Google Scholar]
- 19.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, Powell S, Keller T, McMurray M, Labelle A, Nagy D, Vargas JA, Zhou S, Couto LB, Pierce GF. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 21.Jneid H, Chandra M, Alshaher M, Hornung CA, Tang XL, Leesar M, Bolli R. Delayed preconditioning-mimetic actions of nitroglycerin in patients undergoing exercise tolerance tests. Circulation. 2005;111:2565–2571. doi: 10.1161/CIRCULATIONAHA.104.515445. [DOI] [PubMed] [Google Scholar]
- 22.Jones JM, Wilson KH, Koch WJ, Milano CA. Adenoviral gene transfer to the heart during cardiopulmonary bypass: effect of myocardial protection technique on transgene expression. Eur J Cardiothorac Surg. 2002;21:847–852. doi: 10.1016/s1010-7940(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 23.Kavazis AN. Exercise preconditioning of the myocardium. Sports Med. 2009;39:923–935. doi: 10.2165/11317870-000000000-00 000. [DOI] [PubMed] [Google Scholar]
- 24.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 25.Kyto V, Vuorinen T, Saukko P, Lautenschlager I, Lignitz E, Saraste A, Voipio-Pulkki LM. Cytomegalovirus infection of the heart is common in patients with fatal myocarditis. Clin Infect Dis. 2005;40:683–688. doi: 10.1086/427804. [DOI] [PubMed] [Google Scholar]
- 26.Lai YK, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE. Potential long-term inhibition of ocular neovascularization by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther. 2002;9:804–813. doi: 10.1038/sj.gt.3301695. [DOI] [PubMed] [Google Scholar]
- 27.Leesar MA, Stoddard MF, Dawn B, Jasti VG, Masden R, Bolli R. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 28.Lewis NP, Tsao PS, Rickenbacher PR, Xue C, Johns RA, Haywood GA, von der Leyen H, Trindade PT, Cooke JP, Hunt SA, Billingham ME, Valantine HA, Fowler MB. Induction of nitric oxide synthase in the human cardiac allograft is associated with contractile dysfunction of the left ventricle. Circulation. 1996;93:720–729. doi: 10.1161/01.cir.93.4.720. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Wang D, Qian S, Chen Z, Zhu T, Xiao X. Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Ther. 2003;10:1807–1813. doi: 10.1038/sj.gt.33020783302078. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103:1893–1898. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Bolli R, Qiu Y, Tang XL, Murphree SS, French BA. Gene therapy with extracellular superoxide dismutase attenuates myocardial stunning in conscious rabbits. Circulation. 1998;98:1438–1448. doi: 10.1161/01.cir.98.14.1438. [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O’Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, Shinmura K, Rokosh GD, Wang S, Bolli R. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation. 2009;120:1222–1230. doi: 10.1161/CIRCULATIONAHA.108.778688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Guo Y, Ou Q, Wu WJ, Chen N, Zhu X, Tan W, Yuan F, Dawn B, Luo L, Hunt GN, Bolli R. Gene transfer as a strategy to achieve permanent cardioprotection II: rAAV-mediated gene therapy with heme oxygenase-1 limits infarct size 1 year later without adverse functional consequences. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0208-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, Dawn B, Hunt G, Cui C, Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007;116:1577–1584. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Guo Y, Tan W, Stein AB, Dawn B, Wu WJ, Zhu X, Lu X, Xu X, Siddiqui T, Tiwari S, Bolli R. Gene therapy with iNOS provides long-term protection against myocardial infarction without adverse functional consequences. Am J Physiol Heart Circ Physiol. 2006;290:H584–H589. doi: 10.1152/ajpheart.00855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Guo Y, Xuan YT, Lowenstein CJ, Stevenson SC, Prabhu SD, Wu WJ, Zhu Y, Bolli R. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ Res. 2003;92:741–748. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 39.Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci USA. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowenstein PR, Castro MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.33020483302048. [DOI] [PubMed] [Google Scholar]
- 41.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-32962002-10-3296. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 43.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell’Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 44.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–513. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 45.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 46.Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation. 2001;103:877–881. doi: 10.1161/01.cir.103.6.877. [DOI] [PubMed] [Google Scholar]
- 47.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 48.Patel HH, Hsu A, Gross GJ. Delayed cardioprotection is mediated via a non-peptide delta opioid agonist, SNC-121, independent of opioid receptor stimulation. Basic Res Cardiol. 2004;99:38–45. doi: 10.1007/s00395-003-0438-3. [DOI] [PubMed] [Google Scholar]
- 49.Patel MB, Sonnenblick EH. Age associated alterations in structure and function of the cardiovascular system. Am J Geriatr Cardiol. 1998;7:15–22. [PubMed] [Google Scholar]
- 50.Paulus WJ, Kastner S, Pujadas P, Shah AM, Drexler H, Vanderheyden M. Left ventricular contractile effects of inducible nitric oxide synthase in the human allograft. Circulation. 1997;96:3436–3442. doi: 10.1161/01.cir.96.10.3436. [DOI] [PubMed] [Google Scholar]
- 51.Post H, Heusch G. Ischemic preconditioning Experimental facts and clinical perspective. Minerva Cardioangiol. 2002;50:569–605. [PubMed] [Google Scholar]
- 52.Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, Hunter LA. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulz R, Gres P, Konietzka I, Heusch G. Regional differences of myocardial infarct development and ischemic preconditioning. Basic Res Cardiol. 2005;100:48–56. doi: 10.1007/s00395-004-0497-5. [DOI] [PubMed] [Google Scholar]
- 54.Seppen J, Bakker C, de Jong B, Kunne C, van den Oever K, Vandenberghe K, de Waart R, Twisk J, Bosma P. Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther. 2006;13:1085–1092. doi: 10.1016/j.ymthe.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Szelid Z, Pokreisz P, Liu X, Vermeersch P, Marsboom G, Gillijns H, Pellens M, Verbeken E, Van de Werf F, Collen D, Janssens SP. Cardioselective nitric oxide synthase 3 gene transfer protects against myocardial reperfusion injury. Basic Res Cardiol. 2010;105:169–179. doi: 10.1007/s00395-009-0077-4. [DOI] [PubMed] [Google Scholar]
- 57.Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 58.Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010;333:191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 59.Vejlstrup NG, Bouloumie A, Boesgaard S, Andersen CB, Nielsen-Kudsk JE, Mortensen SA, Kent JD, Harrison DG, Busse R, Aldershvile J. Inducible nitric oxide synthase (iNOS) in the human heart: expression and localization in congestive heart failure. J Mol Cell Cardiol. 1998;30:1215–1223. doi: 10.1006/jmcc.1998.0686. [DOI] [PubMed] [Google Scholar]
- 60.Wright JF, Qu G, Tang C, Sommer JM. Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr Opin Drug Discov Devel. 2003;6:174–178. [PubMed] [Google Scholar]


