Abstract
Context
In February 2002, the allocation system for liver transplantation became based on the Model for End-Stage Liver Disease (MELD) score. Before MELD, black patients were more likely to die or become too sick to undergo liver transplantation compared with white patients. Little information exists regarding sex and access to liver transplantation.
Objective
To determine the association between race, sex, and liver transplantation following introduction of the MELD system.
Design, Setting, and Patients
A retrospective cohort of black and white patients (≥ 18 years) registered on the United Network for Organ Sharing liver transplantation waiting list between January 1, 1996, and December 31, 2000 (pre-MELD cohort, n=21 895) and between February 28, 2002, and March 31, 2006 (post-MELD cohort, n=23 793).
Main Outcome Measures
Association between race, sex, and receipt of a liver transplant. Separate multivariable analyses evaluated cohorts within each period to identify predictors of time to death and the odds of dying or receiving liver transplantation within 3 years of listing. Patients with hepatocellular carcinoma were analyzed separately.
Results
Black patients were younger (mean [SD], 49.2 [10.7] vs 52.4 [9.2] years; P < .001) and sicker (MELD score at listing: median [interquartile range], 16 [12–22] vs 14 [11–19]; P < .001) than white patients on the waiting list for both periods. In the pre-MELD cohort, black patients were more likely to die or become too sick for liver transplantation than white patients (27.0% vs 21.7%) within 3 years of registering on the waiting list (odds ratio [OR], 1.51; 95% confidence interval (CI), 1.15–1.98; P = .003). In the post-MELD cohort, black race was no longer associated with increased likelihood of death or becoming too sick for liver transplantation (26.5% vs 22.0%, respectively; OR, 0.96; 95% CI, 0.74–1.26; P = .76). Black patients were also less likely to receive a liver transplant than white patients within 3 years of registering on the waiting list pre-MELD (61.6% vs 66.9%; OR, 0.75; 95% CI, 0.59–0.97; P = .03), whereas post-MELD, race was no longer significantly associated with receipt of a liver transplant (47.5% vs 45.5%, respectively; OR, 1.04;95%CI, 0.84–1.28; P = .75).Women were more likely than men to die or become too sick for liver transplantation post-MELD (23.7% vs 21.4%; OR, 1.30; 95%CI, 1.08–1.47; P = .003) vs pre-MELD (22.4% vs 21.9%; OR, 1.08; 95% CI, 0.91–1.26; P = .37). Similarly, women were less likely than men to receive a liver transplant within 3 years both pre-MELD (64.8% vs 67.6%; OR, 0.80; 95% CI, 0.70–0.92; P = .002) and post-MELD (39.9% vs 48.7%; OR, 0.70; 95% CI, 0.62–0.79; P < .001).
Conclusion
Following introduction of the MELD score to the liver transplantation allocation system, race was no longer associated with receipt of a liver transplant or death on the waiting list, but disparities based on sex remain.
The Model for End-Stage Liver Disease (MELD) score has been used by the Organ Procurement and Transplantation Network (OPTN) since February 2002 as the basis for allocation of deceased donor livers for transplantation among adults in the United States.1,2 Its use reflects the Institute of Medicine’s recommendations for a system based on objective criteria with less emphasis on waiting time.3
The previous allocation system included subjective assessments of disease, such as the severity of ascites and encephalopathy, and placed great emphasis on waiting time. Under that system, a patient referred late in the course of cirrhosis was disadvantaged and could die waiting for an organ, while another patient with more accrued time on the waiting list but less severe disease received a transplant.
The MELD score is based on objective laboratory variables (bilirubin, creatinine, and the international normalized ratio for the prothrombin time) and predicts the risk of mortality within 3 months. In the current system, patients with higher MELD scores receive greater priority for organ allocation regardless of the amount of time spent on the waiting list. The current system also prioritizes patients with hepatocellular carcinoma (HCC) to increase their likelihood of undergoing liver transplantation before disease progression renders them ineligible. Studies of the first year under the MELD system reported improved transplantation rates and decreased mortality on the waiting list.4,5
An investigation of the previous allocation system by Reid et al6 assessed access to liver transplantation by race using OPTN data. They demonstrated that black patients were underrepresented on the waiting list, had more advanced disease at listing, and were more likely to die while awaiting liver transplantation.6 These data confirmed previous studies and highlighted decreased access to liver transplantation among black patients despite greater burden of liver disease.7,8 Relatively little is known about access to liver transplantation based on sex and whether the use of the MELD score has disadvantaged one sex over another. One study,9 reported only in abstract form, showed that women in the MELD-based allocation system had higher mortality and were less likely to undergo liver transplantation than men. Our study uses a national database to determine whether race- or sex-based disparities exist in access to liver transplantation in the MELD era.
METHODS
Study Population and Data Collection
The OPTN database of the United Network for Organ Sharing (UNOS) was used to identify patients registered on the waiting list on or before April 24, 2006, during 2 periods, pre-MELD (January 1, 1996–December 31, 2000) and post-MELD (February 28, 2002–March 31, 2006). The study population comprised all non-Hispanic black and non-Hispanic white patients aged 18 years or older who were liver transplant waiting list registrants during those periods. Race is identified by patients when registering on the UNOS waiting list. Hispanics were not included as the UNOS database has multiple variables that identify race and ethnicity with often discordant results.
Patients listed for retransplantation or multiorgan transplantation were excluded. Given the different criteria for organ allocation, patients were excluded if they were listed as status 1, defined as fulminant liver failure with a life expectancy without liver transplantation of less than 7 days. We also excluded patients listed as temporarily inactive because they could not be properly assessed for receipt of liver transplantation. The UNOS variables collected included listing date, age, sex, blood type, listing diagnoses, race, education level, insurance payer, UNOS region, calculated MELD score at listing and removal, waiting time, reason for removal from the waiting list, and comorbid illnesses. Waiting time was determined using the first date each patient was placed on the waiting list and the date of removal from the list.
Education level was grouped into 3 categories comprising no education or grade school education, high school and attended college without degree, and college degree or higher. The UNOS regions were grouped into 4 categories according to region of the country (northeast = UNOS regions 1, 2, and 9; southeast = UNOS regions 3, 4, and 11; midwest = UNOS regions 7, 8, and 10; and west = UNOS regions 5 and 6). Listing diagnoses were grouped into 11 common diagnostic categories including cryptogenic cirrhosis, hepatitis C virus, hepatitis B virus, Laennec cirrhosis, nonalcoholic steatohepatitis, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, alpha-1 antitrypsin deficiency, hemochromatosis, and HCC. All other diagnoses were combined into a category designated “other.”
Patients with reasons for removal from the waiting list, such as emergency transplant, died during transplant, and transplanted at another center, were combined into a category called “transplanted.” Similarly, patients removed from the waiting list with reasons such as medically unsuitable or too sick for liver transplantation were combined into a category called “too sick for liver transplantation,” and patients whose reasons for removal from the waiting list were identified as refused transplant, transferred to another center, other, condition improved, living donor, and removed in error were combined into a category called “other.” Patients who died before liver transplantation were kept as 1 category and called “died.”
Statistical Analyses
The primary outcome measure was the association between black or white race and liver transplantation in the MELD era compared with the previous allocation system. Secondary outcomes of interest included the identification of other sociodemographic characteristics associated with liver transplantation, including sex.
Patients listed for liver transplantation with and without HCC were analyzed independently. Patients with HCC were systematically given priority in both the pre-MELD and post-MELD cohorts, because neither the MELD score nor the previous allocation system accurately reflects the prognosis for HCC. Our initial analyses confirmed that patients with HCC compared with patients without HCC waited significantly less time for liver transplantation (median [range], 53 [19–143] vs 155.5 [37–478] days; P < .001). These observations confirmed that patients with and without HCC should be analyzed separately.
To assess the association between MELD score and race in receipt of liver transplantation, we conducted separate sets of analyses for patients listed between 1996 and 2000 (pre-MELD cohort) and patients listed between 2002 and 2006 (post-MELD cohort). First, logistic regression analyses were performed to determine whether race was associated with the likelihood of death or becoming too sick for liver transplantation or the likelihood of liver transplantation within 3 years of listing. These analyses were restricted to patients listed between January 1, 1996, and January 1, 1997 (pre-MELD analysis), and February 28, 2002, and March 31, 2003 (post-MELD analysis), to allow at least 3 years of follow-up.
Second, Cox proportional hazard regression models were used to study the association between prognostic factors such as race and sex on death or becoming too sick for liver transplantation while controlling for confounders.10 The Cox proportional hazard regression models covered the entirety of the 2 periods and were used to account for differences in follow-up times after listing among patients. They were also used to account for time-dependent covariates such as age and calculated MELD score, because MELD is not a static characteristic. Patients who did not die were censored. The assumption of proportionality was tested via Kolmogorov-type supremum tests for each variable in the models.11 No violations to this assumption were found for any of the models. Patients listed with and without HCC were analyzed separately.
To determine which variables to include in the multivariable models, we first performed univariate comparisons by the outcome of interest (liver transplantation, death, or becoming too sick for liver transplantation) by each covariate including age, sex, blood type, listing diagnoses, education level, insurance payer, region, calculated MELD score at listing and removal, reason for removal from the waiting list, and comorbid illnesses, retaining those variables with P < .05. Race, sex, and age were considered for inclusion regardless of the univariate analysis. The models were built using forward selection of covariates retaining covariates that improved the likelihood ratio or goodness of fit of the model. We also compared each covariate by race for each group of patients.
Differences were tested by using t tests or Wilcoxon rank sum tests for continuous predictors and Pearson χ2 or Fisher exact tests for categorical variables depending on their distribution. Covariates were selected a priori to any comparisons. Multicollinearity was tested for predictor variables and linearity of the log assumption was verified for all continuous predictors.12 Interactions between clinically relevant covariates were examined including between race and calculated MELD score, race and blood type, race and region, race and insurance payer, and sex and calculated MELD score. We determined that calculated MELD score at listing and removal were highly correlated; therefore, only 1 was included in the multivariable models. For all multivariable models, we excluded patients with missing data.
All tests of significance were 2-sided, with P ≤ .05 considered significant. Hazard ratios (HRs) are reported for the Cox proportional hazard regression models and adjusted odds ratios (ORs) are reported for the logistic regression analyses. All statistical analyses were performed by using SAS statistical software version 9.1 (SAS Institute Inc, Cary, North Carolina).
This study was approved by the institutional review board at Duke University Medical Center, Durham, North Carolina.
RESULTS
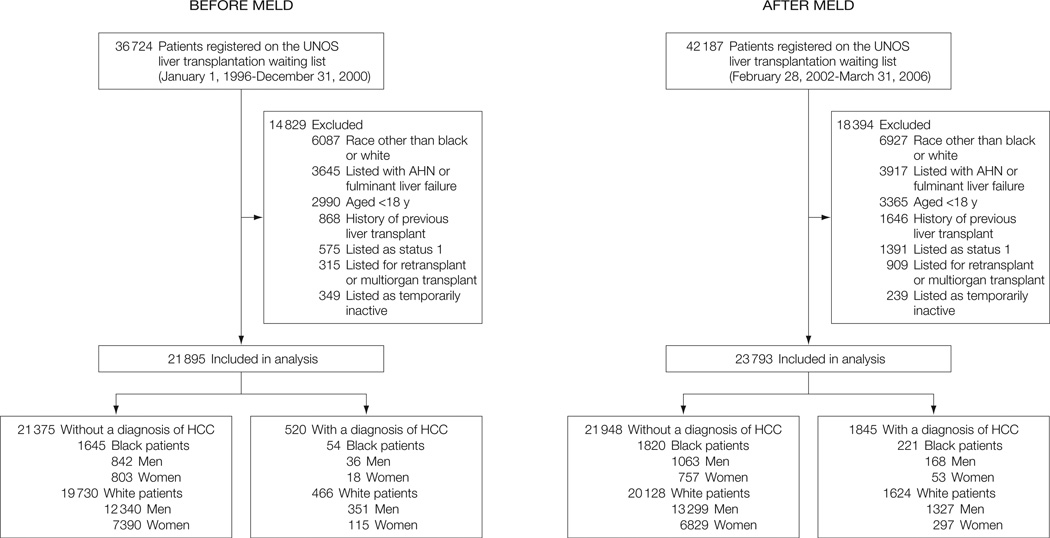
A total of 36 724 patients were registered on the UNOS liver transplantation waiting list in the pre-MELD cohort, with 21 895 of these patients being included in the analyses. A total of 42 187 patients were on the waiting list in the post-MELD cohort, with 23 793 of these patients being included in the analyses (Figure).
Figure.
Flow of Patients Registered on the UNOS Liver Transplantation Waiting List Before and After MELD
Characteristics of the pre- and post-MELD cohorts of black and white patients listed for liver transplantation with and without HCC are shown in Table 1. Black patients comprised between 7.7% and 12.0% of listed patients, respectively. For both periods, differences between black and white patients were more apparent among patients without HCC. Black patients were significantly younger, more likely to be women, and had a lower education level than white patients. Significantly more black patients had diabetes mellitus, drug-treated systemic hypertension, and renal insufficiency in the post-MELD cohort.
Table 1.
Characteristics of Black and White Patients on the UNOS Liver Transplantation Waiting List Before and After MELDa
| No. (%) of Patients Listed Without a Diagnosis of HCC | No. (%) of Patients Listed With a Diagnosis of HCC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-MELD Cohort (n = 21 375) |
Post-MELD Cohort (n = 21 948) |
Pre-MELD Cohort (n = 520) |
Post-MELD Cohort (n = 1845) |
|||||||||||||
| Characteristics | White (n = 19 730) |
Black (n = 1645) |
P Value |
White (n = 20 128) |
Black (n = 1820) |
P Value |
White (n = 466) |
Black (n = 54) |
P Value |
White (n = 1624) |
Black (n = 221) |
P Value |
||||
| Age, mean (SD), y | 50.4 (9.7) | 46.4 (10.9) | <.001 | 52.4 (9.2) | 49.2 (10.7) | <.001 | 54.2 (10.1) | 52.1 (9.6) | .15 | 56.1 (8.0) | 53.7 (8.3) | <.001 | ||||
| Male sex | 12 340 (62.5) | 842 (51.2) | <.001 | 13 299 (66.1) | 1063 (58.4) | <.001 | 351 (75.3) | 36 (66.7) | .17 | 1327 (81.7) | 168 (76) | .04 | ||||
| Blood type | ||||||||||||||||
| A | 7931 (40.2) | 432 (26.26) | <.001 | 8213 (40.8) | 493 (27.09) | <.001 | 165 (35.41) | 11 (20.37) | .05 | 645 (39.72) | 58 (26.24) | .002 | ||||
| AB | 721 (3.65) | 74 (4.5) | 763 (3.79) | 67 (3.68) | 11 (2.36) | 0 | 56 (3.45) | 9 (4.07) | ||||||||
| B | 2069 (10.49) | 311 (18.91) | 2176 (10.81) | 390 (21.43) | 55 (11.8) | 13 (24.07) | 183 (11.27) | 44 (19.91) | ||||||||
| O | 9009 (45.66) | 828 (50.33) | 8976 (44.59) | 870 (47.8) | 235 (50.43) | 30 (55.56) | 740 (45.57) | 110 (49.77) | ||||||||
| Education level | (n = 13 255) | (n = 1118) | (n = 14 653) | (n = 1299) | (n = 305) | (n = 34) | (n = 1234) | (n = 171) | ||||||||
| No education or grade school | 454 (3.43) | 42 (3.76) | <.001 | 415 (2.83) | 41 (3.16) | <.001 | 11 (3.61) | 1 (2.94) | .15 | 37 (3) | 3 (1.75) | .02 | ||||
| High school or attended some college | 9720 (73.33) | 899 (80.41) | 10 813 (73.79) | 1039 (79.98) | 197 (64.59) | 27 (79.41) | 867 (70.26) | 140 (81.87) | ||||||||
| College degree or graduate degree | 3081 (23.24) | 177 (15.83) | 3425 (23.37) | 219 (16.86) | 97 (31.8) | 6 (17.65) | 330 (26.74) | 28 (16.37) | ||||||||
| Regionb | ||||||||||||||||
| Northeast | 5614 (28.45) | 598 (36.35) | <.001 | 5097 (25.32) | 574 (31.54) | <.001 | 158 (33.91) | 31 (57.41) | .01 | 401 (24.69) | 67 (30.32) | .002 | ||||
| Southeast | 5271 (26.72) | 511 (31.06) | 6377 (31.68) | 641 (35.22) | 86 (18.45) | 3 (5.56) | 449 (27.65) | 67 (30.32) | ||||||||
| Midwest | 5062 (25.66) | 375 (22.8) | 5008 (24.88) | 415 (22.8) | 111 (23.82) | 12 (22.22) | 378 (23.28) | 56 (25.34) | ||||||||
| West | 3783 (19.17) | 161 (9.79) | 3646 (18.11) | 190 (10.44) | 111 (23.82) | 8 (14.81) | 396 (24.38) | 31 (14.03) | ||||||||
| Removal codesc | ||||||||||||||||
| Still waiting | 1192 (6.04) | 103 (6.26) | .38 | 6900 (34.28) | 539 (29.62) | .11 | 8 (1.72) | 0 | .56 | 160 (9.85) | 24 (10.86) | .46 | ||||
| Transplanted | 10 202 (51.71) | 810 (49.24) | 8492 (42.19) | 849 (46.65) | 251 (53.86) | 27 (50) | 1177 (72.48) | 162 (73.3) | ||||||||
| Too sick for liver transplantation | 1044 (5.29) | 103 (6.26) | 818 (4.06) | 87 (4.78) | 75 (16.09) | 9 (16.67) | 92 (5.67) | 11 (4.98) | ||||||||
| Died | 4008 (20.31) | 356 (21.64) | 2385 (11.85) | 249 (13.68) | 69 (14.81) | 12 (22.22) | 102 (6.28) | 14 (6.33) | ||||||||
| Other | 3284 (16.64) | 273 (16.6) | 1533 (7.62) | 96 (5.27) | 63 (13.52) | 6 (11.11) | 93 (5.73) | 10 (4.52) | ||||||||
Abbreviations: HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; UNOS, United Network for Organ Sharing.
Numbers of patients are listed for education level because some patients were not included due to missing data. Pre-MELD cohort was between January 1, 1996, and December 31, 2000, and post-MELD cohort was between February 28, 2002, and March 31, 2006.
Northeast includes UNOS regions 1, 2, and 9; southeast includes UNOS regions 3, 4, and 11; midwest includes UNOS regions 7, 8, and 10; and west includes UNOS regions 5 and 6.
Removal refers to patients who are removed from the waiting list for reasons such as liver transplantation, death, too sick for transplant, or other reason.
Black patients without HCC were listed and removed from the liver transplantation waiting list with significantly higher median (interquartile range) calculated MELD scores compared with white patients (at listing: 16 [12–22] vs 14 [11–19], P < .001; and at removal: 18 [13–26] vs 16 [11–22], P < .001). In the pre-MELD cohort, 810 black patients (49.2%) and 10 202 white patients (51.7%) listed without HCC received liver transplantation. The frequency of liver transplantation was somewhat lower in the post-MELD cohort for both races, with 849 black patients (46.6%) and 8492 white patients (42.2%) receiving liver transplantation.
Women comprised between 19.0% and 38.3% of patients depending on the period. Women were older than men at listing in both the pre-MELD (mean [SD]: 51.0 [10.7] vs 49.9 [9.4] years; P < .001) and post-MELD (mean [SD]: 53.8 [9.9] vs 52.2 [8.9] years; P < .001) cohorts. The rates of drug-treated systemic hypertension were lower for women vs men in both the pre-MELD (4.75% vs 7.1%, P = .03) and post-MELD (17.9% vs 19.9%, P = .04) cohorts, whereas the rates of diabetes mellitus were not significantly different by sex in either period. Women who did not have HCC were listed and removed with lower median (interquartile range) calculated MELD scores (at listing: 14 (10–18) for women vs 15 (11–19) for men, P < .001; at removal: 15 (11–22) for women vs 16 (12–23) for men, P < .001). A greater percentage of men received liver transplantation compared with women in the pre-MELD cohort if listed without HCC (53.5% vs 48.3%) but not if listed with HCC (52.4% vs 56.4%). In the post-MELD cohort, fewer patients received liver transplantation overall and men still had a greater frequency of liver transplantation than women if listed without HCC (45.1% vs 37.7%). If listed with HCC in the post-MELD cohort, both men and women received liver transplantation at high rates (72.2% vs 74.0%).
To assess the primary outcome of the association of race with receipt of liver transplantation while controlling for factors known to be associated with liver transplantation or death on the waiting list, several adjusted analyses were performed. The first of these analyses revealed the odds of death or becoming too sick for liver transplantation within 3 years of listing to be significantly higher in black vs white patients in the pre-MELD cohort (27.0% vs 21.7%; OR, 1.51; 95% confidence interval [CI], 1.15–1.98; P = .003) but not in the post-MELD cohort (26.5% vs 22.0%; OR, 0.96; 95% CI, 0.74–1.26; P = .76). This was also the case for patients listed with a diagnosis of HCC (Table 2).
Table 2.
Multivariable Analyses of Likelihood of Death or Becoming Too Sick for Liver Transplantation and Likelihood of Liver Transplantation Within 3 Years of Registering on the Waiting List by Race
| Frequency (%) | ||||
|---|---|---|---|---|
| Black Patients |
White Patients |
Adjusted OR (95% CI)a |
P Value |
|
| Death or becoming too sick for liver transplantation | ||||
| Without hepatocellular carcinomab | ||||
| Pre-MELD cohort (n = 4066) | 85 (27.0) | 816 (21.7) | 1.51 (1.15–1.98) | .003 |
| Post-MELD cohort (n = 5163) | 113 (26.5) | 1063 (22.0) | 0.96 (0.74–1.26) | .76 |
| With hepatocellular carcinoma | ||||
| Pre-MELD cohort (n = 91) | 4 (50.0) | 23 (27.7) | 7.80 (1.15–71.00) | .04 |
| Post-MELD cohort (n = 387) | 5 (11.6) | 45 (13.1) | 1.03 (0.39–3.27) | .95 |
| Liver transplantation | ||||
| Without hepatocellular carcinoma | ||||
| Pre-MELD cohort (n = 4067) | 194 (61.6) | 2511 (66.9) | 0.75 (0.59–0.97) | .03 |
| Post-MELD cohort (n = 5289) | 200 (47.5) | 2215 (45.5) | 1.04 (0.84–1.28) | .75 |
| With hepatocellular carcinoma | ||||
| Pre-MELD cohort (n = 91) | 3 (33.3) | 47 (55.9) | 3.42 (0.67–21.8) | .15 |
| Post-MELD cohort (n = 387) | 34 (79.1) | 266 (77.3) | 0.97 (0.41–2.09) | .94 |
Abbreviations: CI, confidence interval; MELD, Model for End-Stage Liver Disease; OR, odds ratio.
Adjusted for core set of covariates: sex, age, blood type, region, listing diagnoses, calculated MELD score (for post-MELD models).
Pre- and post-MELD analyses also adjusted for insurance payer and diabetes mellitus.
After adjustment for all relevant covariates, we found that black patients were significantly less likely than white patients to receive liver transplantation within 3 years of listing in the pre-MELD cohort (61.6% vs 66.9%; OR, 0.75; 95% CI, 0.59–0.97; P = .03) but not in the post-MELD cohort (47.5% vs 45.5%; OR, 1.04; 95% CI, 0.84–1.28; P = .75). However, race was not a significant predictor of receipt of liver transplantation within 3 years of listing if listed with a diagnosis of HCC in the pre- or post-MELD cohorts (Table 2). Interaction between race and calculated MELD score was not predictive of results as it was nonsignificant in our multivariable analyses.
Sex was significantly associated with death and liver transplantation after adjusting for race despite the use of the MELD score (Table 3). Women were more likely to die or become too sick for liver transplantation within 3 years of listing in the post-MELD cohort (23.7% of women vs 21.4% of men; OR, 1.30; 95%CI, 1.08–1.47; P = .003) but not in the pre-MELD cohort (22.4% of women vs 21.9% of men; OR, 1.08; 95% CI, 0.91–1.26; P = .37). Women were also less likely to receive a liver transplant within 3 years of listing in both the pre-MELD (64.8% of women vs 67.6% of men; OR, 0.80; 95% CI, 0.70–0.92; P = .002) and post-MELD (39.9% of women vs 48.7% of men; OR, 0.70; 95% CI, 0.62–0.79; P < .001) cohorts. Such differences were not observed if patients were listed with HCC (pre-MELD: 70.4% of women vs 48.4% of men; OR, 2.48; 95% CI, 0.91–7.30; P = .08; vs post-MELD: 74.4% of women vs 78.4% of men; OR, 0.72; 95% CI, 0.41–1.31; P = .27). Interaction between sex and MELD was also assessed and found to be nonsignificant.
Table 3.
Multivariable Analyses of Likelihood of Death or Becoming Too Sick for Liver Transplantation and Likelihood of Liver Transplantation Within 3 Years of Registering on the Waiting List by Sexa
| Frequency (%) | Adjusted OR (95% CI)a |
P Value |
||
|---|---|---|---|---|
| Women | Men | |||
| Death or becoming too sick for liver transplantation | ||||
| Without hepatocellular carcinomab | ||||
| Pre-MELD cohort (n = 4066) | 353 (22.4) | 548 (21.9) | 1.08 (0.91–1.26) | .37 |
| Post-MELD cohort (n = 5163) | 437 (23.7) | 739 (21.4) | 1.30 (1.08–1.47) | .003 |
| With hepatocellular carcinoma | ||||
| Pre-MELD cohort (n = 91) | 4 (14.8) | 23 (35.9) | 0.28 (0.07–0.99) | .05 |
| Post-MELD cohort (n = 387) | 9 (10.0) | 41 (13.8) | NA | |
| Liver transplantation | ||||
| Without hepatocellular carcinoma | ||||
| Pre-MELD cohort (n = 4067) | 1109 (64.8) | 1686 (67.6) | 0.80 (0.70–0.92) | .002 |
| Post-MELD cohort (n = 5289) | 736 (39.9) | 1679 (48.7) | 0.70 (0.62–0.79) | <.001 |
| With hepatocellular carcinoma | ||||
| Pre-MELD cohort (n = 91) | 19 (70.4) | 31 (48.4) | 2.48 (0.91–7.30) | .08 |
| Post-MELD cohort (n = 387) | 67 (74.4) | 233 (78.4) | 0.72 (0.41–1.31) | .27 |
Abbreviations: CI, confidence interval; MELD, Model for End-Stage Liver Disease; NA, not applicable (sex was not included in this final multivariable model); OR, odds ratio.
Adjusted for core set of covariates: race, age, blood type, region, listing diagnoses, calculated MELD score (for post-MELD models).
Pre- and post-MELD analyses also adjusted for insurance payer and diabetes mellitus.
Lastly, we assessed the risk of death or becoming too sick for liver transplantation by race over the course of the entire 2 periods by using Cox proportional hazard regression models. These models allowed us to assess the association between race and the risk of death at any given point while controlling for other predictors known to affect the risk of death, such as age and calculated MELD score. Black patients listed in the pre-MELD cohort without HCC had a significantly higher risk of death compared with white patients (HR, 1.21; 95% CI, 1.09–1.34; P < .001).
In the post-MELD cohort, the risk of death was no longer associated with race in these patients (HR, 1.03; 95% CI, 0.92–1.16; P = .61). Race also was not associated with the risk of death in the post-MELD (HR, 0.86; 95% CI, 0.56–1.30; P = .48) or pre-MELD (HR, 1.24; 95% CI, 0.78–1.97; P = .36) cohorts if listed with a diagnosis of HCC. After controlling for race, sex was also associated with the risk of death. In the post-MELD cohort, women listed without a diagnosis of HCC had a significantly higher risk of death than men did while on the waiting list (HR, 1.09; 95% CI, 1.02–1.17; P = .01), whereas in the pre-MELD cohort, this was not the case (HR, 0.95; 95% CI, 0.89–1.00; P = .06). Like race, sex was not associated with risk of death if patients were listed with a diagnosis of HCC in both the pre-MELD (HR, 0.70; 95% CI, 0.49–1.04; P = .08) or post-MELD (HR, 0.80; 95% CI, 0.56–1.14; P = .21) cohorts.
Calculated MELD score was associated with all of the outcomes assessed, including the risk of death, death or becoming too sick for liver transplantation within 3 years of listing, and likelihood of liver transplantation within 3 years of listing in the post-MELD cohort. For example, patients with more severe liver disease (ie, those with a higher calculated MELD score) had significantly increased risk of death for each 1-point increase in calculated MELD score (HR, 1.16; 95% CI, 1.14–1.18; P < .001) if listed without HCC.
COMMENT
A major strength of the current MELD score allocation system is its emphasis on objective measures of disease severity rather than on subjective assessments and waiting times. When OPTN adopted the MELD score as the basis for liver transplantation allocation in 2002, one of its goals was to eliminate possible biases presented by the previous allocation system.13 Since its introduction, refinements to the MELD system continue to be made and a recent analysis demonstrated that the addition of serum sodium to the MELD formula may improve its ability to predict survival on the waiting list.14 To our knowledge, this is the first comprehensive investigation of the new allocation system to assess improvements in its ability to address race- or sex-based disparities in access to liver transplantation.
Before the MELD era, we found that black race was associated with an increased likelihood of death or becoming too sick for liver transplantation and a decreased likelihood of liver transplantation within 3 years of listing. Under the new MELD allocation system, these differences between black and white race resolved. Similarly, black patients had a 20% increased risk of death in the pre-MELD cohort if listed without HCC and an equal risk in the post-MELD cohort. These findings suggest that the present allocation system is achieving a critical goal of ensuring more equitable organ allocation by race. Our pre-MELD results concur with those found by Reid et al6 in a similar analysis performed between 1994 and 1998. Their investigation found race to be associated with a shorter time to death or becoming too sick for liver transplantation when black patients were compared with white patients (HR, 1.36; 95% CI, 1.23–1.49; P < .001). Black patients were also less likely to undergo liver transplantation (OR, 0.67; 95% CI, 0.52–0.87; P = .003) and more likely to die or become too sick for liver transplantation within 4 years of listing (OR, 1.52; 95% CI, 1.15–1.99; P = .003) when compared with white patients.6 The authors also reported that black patients were underrepresented on the liver transplantation waiting list (8.4%) and among liver transplantation recipients (7.9%) compared with their percentage of the general population (13.6%).
This elimination in racial disparity in the post-MELD cohort likely reflects the fact that the MELD score now accounts for the severity of a patient’s disease when listed. A critical issue to understand is that although black and white patients now have equal time to death and equal odds of death or receipt of liver transplantation once listed, there remains an important difference that persists between the races. Black patients continue to be listed with more severe liver disease (as reflected in their higher MELD scores) than white patients. Once MELD is added to the adjusted post-MELD model, it accounts for this difference in severity of liver disease and puts black patients at equal position at listing. Race likely accounted for this difference in the pre-MELD cohort.
It remains to be determined why black patients get listed at a more advanced stage of disease. The reasons may reflect barriers faced by black patients in referral for liver transplantation. Although our study evaluated the use of liver transplantation among patients already listed for liver transplantation, disparities between race in getting listed for a liver transplantation remain and other studies have shown this to be a persistent issue. Julapalli et al15 reported a decreased likelihood of referral for liver transplantation among black patients in a Veterans Affairs hospital. Eckhoff et al7 performed a retrospective analysis at 1 institution and found black patients made up 14.1% of referrals despite making up 25.6% of the population. Most of these referrals for black patients were for children.7 It is somewhat encouraging that despite getting listed with more severe disease, black patients no longer die or become too sick for liver transplantation while waiting compared with white patients. This mortality benefit is likely explained by the addition of the MELD score, which prioritizes disease severity.
After controlling for differences based on race, there remain persistent differences in access to liver transplantation based on sex and region. Women experienced an approximately 30% increased odds of death or becoming too sick for liver transplantation compared with men after the introduction of the MELD allocation system. Women also had decreased odds of liver transplantation within 3 years of listing and a shorter time to death than men did in the post-MELD cohort. Recent studies have questioned if women are disadvantaged with the MELD score due to lower creatinine values.16,17 Our study did not address this important issue in detail given that organ allocation for women may also be influenced by other factors such as organ size matching not evaluated in this study. The methods and database were limited in the ability to study this question adequately. But to our knowledge, these are the first published data addressing access to liver transplantation by sex before and after MELD.
Similarly, we found that regional variation in access to liver transplantation continues to occur in the MELD era. Our findings concur with previous investigations demonstrating geographic and center disparity despite the utilization of the MELD score for organ allocation.18–22 Patients listed in regions other than the southeast region (UNOS regions 3, 4, and 11) in our analyses waited longer for liver transplantation in both the pre-MELD and post-MELD cohorts.
Hepatocellular carcinoma remains a controversial issue in the MELD era through the use of exception points aimed to improve the likelihood of liver transplantation for patients with HCC. The number of cadaveric transplants for this indication has increased nearly 6-fold since 2002.23 The incidence of HCC has more than doubled in the United States during the past 2 decades largely due to an increase among minority populations.24–26 To date, there is only limited information regarding racial disparity and utilization of liver transplantation for HCC. Our analyses of patients listed from the pre- and post-MELD cohorts demonstrate that most patients listed with a diagnosis of HCC, both black and white, were transplanted. Black patients with HCC, however, had a significantly increased risk of death or becoming too sick for liver transplantation within 3 years of listing before MELD. These findings are consistent with a recent investigation of the Surveillance, Epidemiology, and End Results database in which black patients were approximately half as likely as white patients to receive liver transplantation for HCC between 1998 and 2002.27 Although the allocation plan for HCC has been refined repeatedly in the MELD era, we found that this system appears to be functioning equitably in terms of race and sex.
The strengths of our study include the use of a large national database, the ability to assess numerous factors influencing liver transplantation, and the large number of patients involved. Our study also has several limitations. First, the results rely on the accuracy of recorded data through the UNOS database. Variables have been increasingly collected throughout the life of the database resulting in missing data for the pre-MELD era for potentially relevant predictors such as MELD score and body mass index. For most predictors, including race, sex, calculated MELD scores (in the MELD era), removal codes, and region, there was no missing data. Missing data regarding comorbidities of renal insufficiency and coronary artery disease as well as education level were apparent both before and after MELD. We chose to exclude missing data rather than to perform imputation techniques given the changing qualities of the database over time and fear of bias. Given the overall high numbers of patients included in the analyses and the fact that these covariates were not included in most of the multivariable models, we do not think that the frequency of missing values significantly impacted the results, particularly with regards to race or sex. However, this missing data may limit our ability to fully assess the effect of predictors such as education level on the outcomes.
Second, the database and the allocation system have been dynamic during the period under study and while the general concept of MELD has been in practice since the beginning of the MELD era, the specific effect of these refinements was not assessed. This is particularly relevant for the analyses of HCC, because the methods of diagnosis and requirements for listing have changed during these periods. The MELD score is analyzed as a static variable in our analyses. Much of the liver transplant literature treats MELD as a time-dependent covariate that factors in the change in MELD score over time. We found that the initial MELD score and removal MELD score were highly correlated; therefore, we chose to use only 1 MELD score in the multivariable models.
Third, a potential limitation with respect to retrospective cohort studies is that failure to identify differences in the post-MELD era may result from chance alone based on sample sizes (type 2 error). We performed a post hoc power calculation and found that given the samples size and α = .05, we had a 90% power to detect at least a 4% difference in liver transplantation between black and white patients.
Finally, this study assessed only patients listed for liver transplantation by UNOS. It did not assess whether black patients continue to be underrepresented on the waiting list when compared with the racial distribution of the general population. There remains an unknown denominator of patients with end-stage liver disease needing liver transplantation not listed. Variables that influence which patients get listed include patient and physician preferences, referral patterns, insurance providers, and regional and physician differences on appropriateness for listing and removal.
Although our study identified significant differences between the clinical and sociodemographic characteristics of black and white patients listed for liver transplantation, race was not significantly associated with receipt of liver transplantation or death on the waiting list during the MELD era. These findings differ significantly from those findings before the use of the MELD score in which black race was associated with a decreased likelihood of liver transplantation as well as an increased risk of death or becoming too sick for liver transplantation. Sex differences persist despite the use of MELD. Whether these differences result from true anatomic differences or represent a problem not addressed by the use of the MELD score mandates further investigation. The use of the MELD score allocation system appears to have reduced at least racial disparity in liver transplantation. We hope that ongoing investigations and refinements of MELD can provide even greater equity in the allocation of this precious resource.
Acknowledgments
Funding/Support: This work was supported by grant 5T32-DK07568-17 from the National Institutes of Health.
Role of the Sponsor: The sponsor provided financial support for the study only and had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the study, or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Moylan and Muir had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Moylan, Brady, Muir.
Acquisition of data: Moylan, Tuttle-Newhall.
Analysis and interpretation of data: Moylan, Johnson, Smith, Muir.
Drafting of the manuscript: Moylan, Muir.
Critical revision of the manuscript for important intellectual content: Moylan, Brady, Johnson, Smith, Tuttle-Newhall, Muir.
Statistical analysis: Moylan, Johnson.
Obtained funding: Moylan, Muir.
Administrative, technical, or material support: Moylan, Tuttle-Newhall, Muir.
Study supervision: Moylan, Brady, Smith, Muir.
Financial Disclosures: None reported.
Disclaimer: The Transplant and Waiting List Patient Information data reported herein have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
Previous Presentation: The data from this study were presented in part at Digestive Disease Week; May 20, 2007; Washington, DC.
REFERENCES
- 1.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, terBorg PL. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner R, Edwards E, Freeman R, et al. Model for end stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine Committee on Organ Procurement and Transplantation Policy. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: National Academy Press; 1999. pp. 1–29. [PubMed] [Google Scholar]
- 4.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10(1):7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 5.Kanwal F, Dulai GS, Spiegel BMR, Yee HF, Gralnek IM. A comparison of liver transplantation outcomes in the pre- vs. post-MELD eras. Aliment Pharmacol Ther. 2005;21(2):169–177. doi: 10.1111/j.1365-2036.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- 6.Reid AE, Resnick M, Chang Y, Buerstatte N, Weissman JS. Disparity in use of orthotopic liver transplantation among black and whites. Liver Transpl. 2004;10(7):834–841. doi: 10.1002/lt.20174. [DOI] [PubMed] [Google Scholar]
- 7.Eckhoff DE, McGuire BM, Young CJ, et al. Race: a critical factor in organ donation, patient referral and selection, and orthotopic liver transplantation? Liver Transpl Surg. 1998;4(6):499–505. doi: 10.1002/lt.500040606. [DOI] [PubMed] [Google Scholar]
- 8.Wei YL, Detre KM, Everhart JE. The NIDDK liver transplantation database. Liver Transpl Surg. 1997;3(1):10–22. doi: 10.1002/lt.500030102. [DOI] [PubMed] [Google Scholar]
- 9.Kanwal F, Dulai GS, Gralnek IM, Han SB, Spiegel BM. The impact of gender on access to liver transplantation in the MELD era. Gastroenterology. 2005;128:A701. [abstract]. [Google Scholar]
- 10.Lin DY, Wej LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 11.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute Inc.; 1995. [Google Scholar]
- 13.Institute of Medicine Committee on Organ Procurement and Transplantation Policy. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 14.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julapalli VR, Kramer JR, El Serag HB. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005;11(11):1370–1378. doi: 10.1002/lt.20434. [DOI] [PubMed] [Google Scholar]
- 16.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores: a systematic bias. Am J Transplant. 2007;7(3):685–692. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 17.Flodén A, Castedal M, Friman S, Olausson M, Backman L. MELD score in patients accepted for liver transplantation 1994 to 2004. Transplant Proc. 2006;38(8):2673–2674. doi: 10.1016/j.transproceed.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RB, Harper A, Edwards EB. There is minimal variation among UNOS regions in prospectively collected MELD scores for patients receiving liver transplants. Am J Transplant. 2002;2(suppl):S251. [abstract]. [Google Scholar]
- 19.Barshes NR, Becker NS, Washburn WK, Halff GA, Aloia TA, Goss JA. Geographic disparities in deceased donor liver transplantation within a single UNOS region. Liver Transpl. 2007;13(5):747–751. doi: 10.1002/lt.21158. [DOI] [PubMed] [Google Scholar]
- 20.Schaffer RL, Kulkarni S, Harper A, Millis JM, Cronin DC. The sickest first? disparities in the model for end-stage liver disease-based organ allocation: one region’s experience. Liver Transpl. 2003;9(11):1211–1215. doi: 10.1053/jlts.2003.50192. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad J, Bryce CL, Cacciarelli T, Roberts MS. Differences in access to liver transplantation: disease severity, waiting time, and transplantation center volume. Ann Intern Med. 2007;146(10):707–713. doi: 10.7326/0003-4819-146-10-200705150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299(2):202–207. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 23.Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9(1):12–18. doi: 10.1053/jlts.2003.50009. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 25.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(suppl 1)(5):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 26.el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5(1):87–107. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 27.Siegel AB, Russell RB, El-Serag HB, et al. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998–2002. Am J Gastroenterol. 2008;103(1):120–127. doi: 10.1111/j.1572-0241.2007.01634.x. [DOI] [PubMed] [Google Scholar]