Abstract
Background
Many epidemiologic studies have considered the association between blood pressure (BP) and Alzheimer disease, yet the relationship remains poorly understood.
Methods
In parallel with work on the AlzRisk online database (www.alzrisk.org), we conducted a systematic review to identify all epidemiologic studies meeting pre-specified criteria reporting on the association between hypertension, systolic BP, or diastolic BP and incident Alzheimer disease. When possible, we computed summary measures using random-effects models and explored potential heterogeneity related to age at BP assessment.
Results
Eighteen studies reporting on 19 populations met the eligibility criteria. We computed summary relative risks (RRΣ) for three measures of BP: hypertension (RRΣ=0.97 [95% confidence interval= 0.80–1.16]); a 10 mm Hg-increase in systolic BP (RRΣ=0.95 [0.91–1.00]); and a 10 mm Hg-increase in diastolic BP (RRΣ=0.94 [0.85–1.04]). We were unable to compute summary estimates for the association between categories of systolic or diastolic BP and Alzheimer disease; however, there did not appear to be a consistent pattern across studies. After stratifying on age at BP assessment, we found a suggestion of an inverse association between late-life hypertension and Alzheimer disease and a suggestion of an adverse association between midlife diastolic hypertension and Alzheimer disease.
Conclusions
Based on existing epidemiologic research, we cannot determine whether there is a causal association between BP and Alzheimer disease. Selection bias and reverse causation may account for the suggested inverse association between late-life hypertension on Alzheimer disease, but, given the expected direction of these biases, they are less likely to account for the suggestion that midlife hypertension increases risk. We advocate continuing systematic review; the Alzrisk database entry on this topic (www.alzrisk.org), which was completed in parallel with this work, will be updated as new studies are published.
There is growing acceptance of a relationship between cardiovascular disease and Alzheimer disease,1,2 yet much of this hypothesis is based largely on studies of cognition and dementia, which encompasses Alzheimer disease, vascular dementia, frontotemporal dementia and other conditions. Although Alzheimer disease is the most common form of dementia, studies of the relationship between vascular risk factors and cognition or dementia do not provide sufficient evidence to support a causal relationship between these risk factors and the incidence of Alzheimer disease. Cerebrovascular pathology is common in older adults, is associated with cognitive impairment, and often co-exists with Alzheimer disease pathology.3–6 Moreover, studies have shown that, for a given level of Alzheimer disease pathology, the probability of manifesting Alzheimer disease dementia is related to the degree of vascular pathology.7–10 Thus, an association between vascular risk factors and cognition or dementia may reflect an independent link between cerebrovascular pathology and cognition or dementia rather than a causal relationship between vascular risk factors and Alzheimer disease incidence or progression.
Despite almost 15 years of study11 and the presence of a substantial body of epidemiologic literature on the relationship between blood pressure and dementia, there has been relatively little discussion of whether there is a relationship specifically between BP and particular subtypes of dementia.12–14 In particular, no comprehensive review has focused on the prospective relationship between blood pressure and Alzheimer disease, the most common form of dementia. The aim of this systematic review and meta-analysis is to assess whether there is sufficient evidence to characterize the relationship between blood pressure and Alzheimer disease. In addition, we consider whether the reported associations could be attributable to bias or alternative explanations, a discussion currently lacking in the literature.
To this end, the current review is limited to prospective cohort and nested case-control studies, which avoid the introduction of bias due to issues of control selection15 and can provide evidence of a temporal relationship between blood pressure and Alzheimer disease onset. As recent reviews have suggested an age-dependent association between blood pressure and impaired cognition or dementia,14,16 we also examined whether any relationship between blood pressure and incident Alzheimer disease is related to age. This work was conducted in parallel with a similar review for the AlzRisk online database of Alzheimer disease epidemiology findings (available at www.alzrisk.org), hosted by the Alzheimer Research Forum (www.alzforum.org), but does not exclude non-English language articles, which are excluded from the AlzRisk database. In addition, the AlzRisk review has slightly different inclusion and exclusion criteria and will be updated as additional studies are published. Thus, there are slight differences across reviews, although the overall conclusions do not differ.
METHODS
Systematic Review and Data Extraction
We used the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines in the reporting of this systematic review and meta-analysis.17 Eligible studies met the following criteria: (1) were prospective cohort studies or nested case-control studies conducted in well-defined cohorts; (2) presented original epidemiologic data on the association between incident Alzheimer disease and measures of blood pressure (BP), i.e., hypertension, systolic blood pressure, or diastolic blood pressure; (3) were not based on a cohort consisting exclusively of individuals with a specific disease; (4) considered blood pressure to be an exposure variable of interest, even if it was one of many exposures considered, rather than exclusively as a potential confounder of another exposure-Alzheimer disease relationship; (5) diagnosed Alzheimer disease through clinical examination, using defined diagnostic criteria; (6) defined the comparison of interest for the reported association (e.g. defined the relative risk as comparing “hypertension” vs. “no hypertension” rather than reporting a relative risk for “blood pressure”); (7) provided confidence intervals or other information that allowed estimation of standard errors; (8) adjusted for, at minimum, age and sex; and (9) were judged to be of sufficient quality by meeting at least 5 of the criteria on a modified version of the Newcastle-Ottawa Scale18 (eAppendix 1, http://links.lww.com). Non-English language articles were eligible for inclusion if they met these criteria, but conference abstracts and unpublished manuscripts were excluded. If more than one published paper reported on the association between blood pressure and Alzheimer disease within a single study population, we included the study with the longest follow-up period or (in the case of identical follow-up periods) the study for which the primary focus was the association between blood pressure and Alzheimer disease.
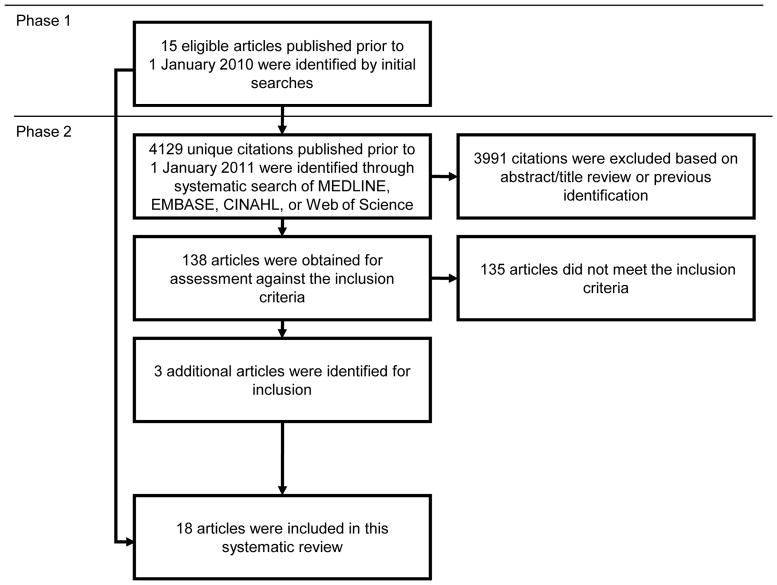
A two-phase approach was used to identify all eligible studies published prior to 1 January 2011 (Figure 1). First, two of the authors of the present paper (MP and JG) independently searched the literature through 1 January 2010 to identify eligible epidemiologic studies using non-systematic searches of electronic databases (MEDLINE, EMBASE, Web of Science, Google Scholar), review of citations from relevant articles, consultation with experts in the field, and hand searches of publication archives from well-known cohorts. The two lists of eligible studies were compared and disagreements were resolved by discussion. Second, to ensure all eligible studies were identified, one of the authors (MP) created separate search filters for the EMBASE and MEDLINE databases such that the combined results generated by searches using these filters included all previously identified studies, and created similar search filters for CINAHL and Web of Science. Unique citations from papers published prior to 1 January 2011 in the combined results from these four database searches were reviewed to identify any additional eligible studies not previously captured (eAppendix 2, http://links.lww.com).
Figure 1.
Flowchart describing the two-phase approach used to identify all eligible studies.
For each eligible study, two of the authors for the present paper (MP and JG) independently extracted data (or, in the case of the one non-English language article that met the eligibility criteria, a colleague of the authors extracted data). The following data items were obtained: year of publication, study location, cohort, number of participants, number of Alzheimer disease cases, follow-up time, percent of respondents lost to follow-up, baseline age, Alzheimer disease screening process, diagnostic criteria, and diagnostic process, effect measure, effect estimates, standard errors or information to compute standard errors, model covariates, and additional information pertaining to the modified Newcastle-Ottawa Scale criteria. A subset of this information is provided in tabular form (Table). When more than one model was reported, we chose the maximally adjusted model that did not include vascular outcomes as a covariate (e.g., cerebrovascular disease), as these may mediate any relationship between blood pressure and Alzheimer disease. When a study reported associations from follow-up periods of varying lengths, we chose the association corresponding to the longest follow-up period.
Data Analysis
We grouped study findings according to blood pressure measure (e.g., diastolic BP, systolic BP). We chose not to further stratify based on study design and reported effect measure (odds ratio or hazard ratio) given the small number of studies reporting on the association between Alzheimer disease and any specific blood pressure measure; all effect measures are referred to as relative risks (RRs) throughout the text. We recognize that the apparent strength of an association is in part a function of the effect measure used; the Table lists the reported effect measure for each study for the reader’s benefit.
If the only findings were from stratified analyses, we combined estimates from individual strata into a single measure using fixed-effects methods with Mantel-Haenszel weights.19 If a study evaluated blood pressure as a continuous variable and reported effect estimates per blood pressure interval other than a 10 mm Hg-increase, we transformed the effect estimates and confidence intervals to reflect a 10 mm Hg-increase. Specifically, we computed a relative risk for a 10-unit increase by dividing the ln(RR) by the original interval, multiplying this quantity by 10 and exponentiating the result. (For example, if ln[RR5] corresponded to a 5 mm Hg-increase, then ln[RR10] per 10 mm Hg increase was (ln[RR5]/5)*10.) Similarly, to compute the standard error of the ln(RR) for a 10 mm Hg-increase, we divided the standard error of the ln(RR) by the original interval and multiplied this quantity by 10. We used the resulting quantity to calculate a 95% confidence interval (CI) for the RR for a 10 mm Hg-increase in blood pressure.
When data from three or more studies were available, we summarized the reported associations in a forest plot. When these studies were similar enough to be combined (typically binary or continuous exposure data, or categorical data with sufficiently similar categories), we computed summary measures using random-effects models20 and included these summary measures in the forest plot. We chose to use random-effects models because these models incorporate between-study heterogeneity into the overall summary measures; we expect between-study heterogeneity given (1) reports of modification of the BP-dementia association by age at baseline,14,16 (2) reports suggesting modification of the effect of BP on Alzheimer disease by antihypertensive drug use,21,22 and (3) variability across studies in study design and covariate adjustment. If there is no between-study heterogeneity, a random-effects model equals a fixed-effects model. We evaluated the presence of heterogeneity across associations using the I2 test, which quantifies the degree of heterogeneity due to between-study characteristics.23 We assessed publication bias using Egger’s regression asymmetry test24 and by visually inspecting the Begg’s funnel plot. These tests may have limited power to detect heterogeneity in our data. We also considered whether age at exposure assessment (midlife versus late-life) could account for heterogeneity in associations. When possible, we computed separate summary measures for midlife and late-life measures of blood pressure.
We chose not to pursue a meta-regression approach for several reasons. We have few studies, a limited range of mean ages at baseline in any single analysis, and large variability in age within studies compared with the variability across studies all of which would make it difficult to detect a true effect. In addition, the effect of mean age at baseline on the association of interest is difficult to interpret when the various populations considered have different age structures (e.g. some require a minimum age for entry while others do not).
All analyses were performed using STATA, version 11 (StataCorp, USA). Sample code is available in eAppendix 3 (http://links.lww.com).
RESULTS
The literature search (Figure 1) yielded 18 eligible publications,21,22,25–40 reporting on 19 unique study populations (one publication32 reported on two separate populations). Study populations from two publications29,36 overlap, but both were included separately as the overlap was modest (~25%). All publications either explicitly excluded persons with prevalent dementia at baseline22,26,28–40 or were reasonably expected to have begun with a dementia-free cohort due to the age of the participants at baseline (mean age at baseline < 60).21,25,27 Blood pressure measures differed by study population, with 10 considering “history of hypertension,”22, 25, 26, 29, 30, 32, 35, 36,39 3 considering “hypertension at enrollment,”31,33,40 4 considering systolic and diastolic BP as a continuous variable,30,34,35,37 and 6 considering systolic and diastolic BP in categories.21,27,28,30,37,38 Four studies assessed blood pressure in midlife,21,25,27,30 defined here as prior to age 65.
“History of hypertension” was assessed via self-report for all but one study reporting on this measure. The one exception26 relied on medicine-chest inventories of antihypertensive medication use, in addition to self-report of previous diagnosis or antihypertensive medication use. The three studies considering “hypertension at enrollment”31,33,40 defined this condition as a baseline blood pressure measurement above 130 mm Hg systolic/85 mm Hg diastolic or self-report of antihypertensive medication use. All studies considering blood pressure as a continuous or categorical variable relied on measurements of blood pressure at baseline or, in one case,21 repeated measures of blood pressure across several baseline interviews.
BP and Alzheimer disease
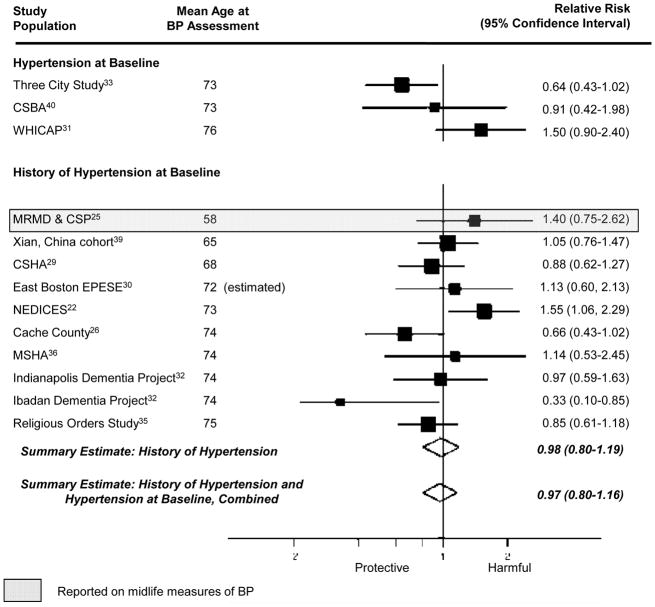
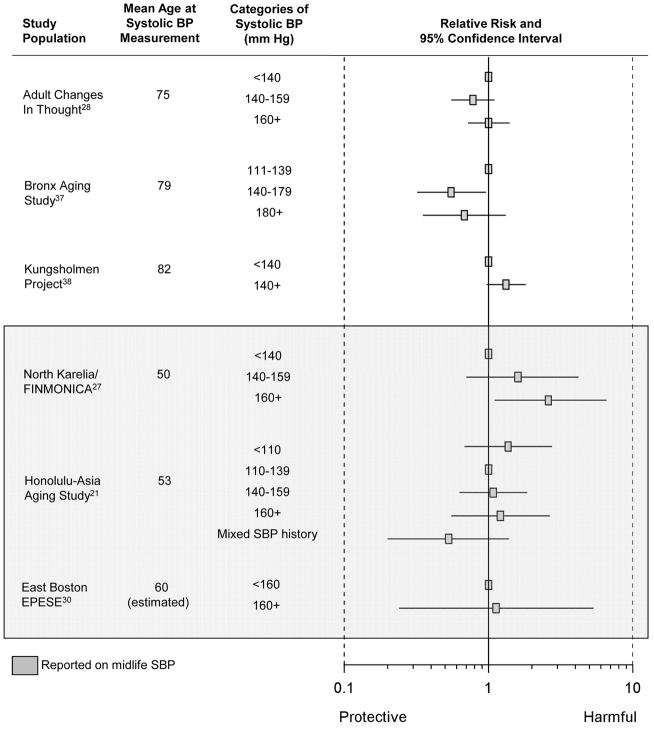
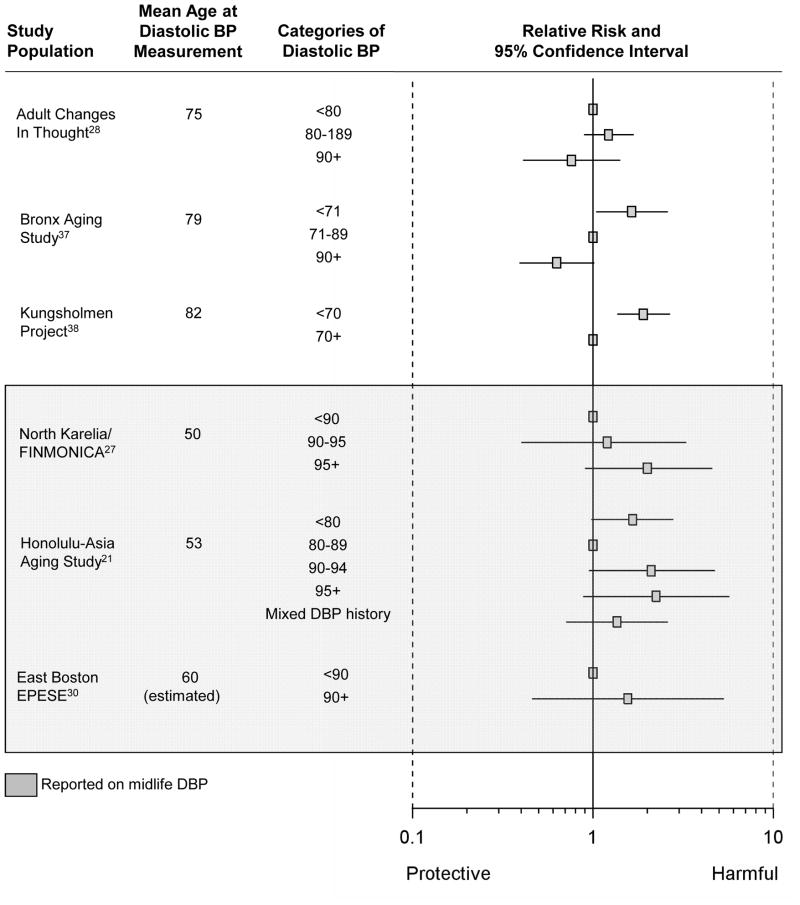
The pooled estimate from the 10 studies reporting on association between a “history of hypertension” and Alzheimer disease was 0.98 (95% CI= 0.80–1.19; Figure 2). Results were similar when we combined data from studies reporting on “history of hypertension” and “hypertension at enrollment” (summary relative risk [RRΣ]= 0.97 [95% CI= 0.80–1.16]; Figure 2). The pooled estimate from the 4 studies considering the association between a 10 mm Hg-increment in systolic BP and Alzheimer disease was 0.95 (95% CI= 0.91–1.00; Figure 3) while the pooled estimate from the 4 studies considering the association between a 10 mm Hg increment in diastolic BP and Alzheimer disease was 0.94 (95% CI= 0.85–1.04; Figure 4). We did not observe a consistent pattern of association for either BP measure (Figures 5 and 6), and categories were not sufficiently similar to compute pooled estimates.
Figure 2.
Individual and pooled estimates of the association between measures of hypertension and Alzheimer disease. The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate. Reference category is no hypertension.
Figure 3.
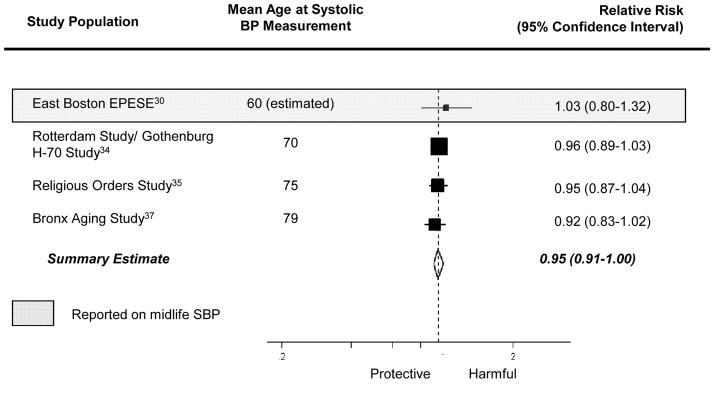
Individual and pooled effect estimates of the association between a 10 mm Hg-increase in systolic BP (SBP) and Alzheimer disease. The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate.
Figure 4.
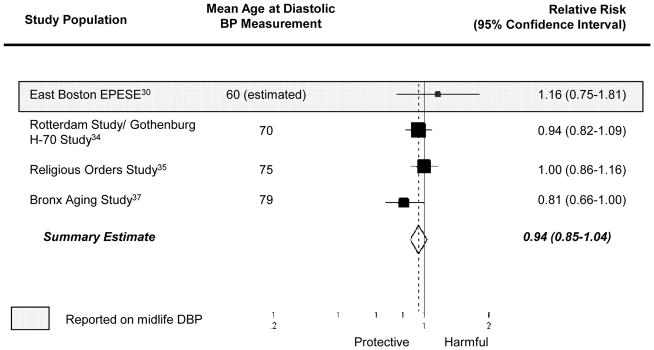
Individual and pooled effect estimates of the association between a 10 mm Hg-increase in diastolic BP (DBP) and Alzheimer disease. The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate.
Figure 5.
Individual estimates of the association between categories of systolic BP and Alzheimer disease.
Figure 6.
Individual estimates of the association between categories of diastolic BP and Alzheimer disease.
Statistical evaluation of heterogeneity found, for “history of hypertension”, I2= 42% (95% CI= 0–71%), for combined results from “history of hypertension” and “hypertension at enrollment” I2= 47% (95% CI= 0–71%), for 10 mm Hg-increment in systolic BP, I2= 0% (95% CI= 0–68%), and for 10 mm Hg-increment in diastolic BP, I2= 14% (95% CI= 0–72%). These findings suggest that moderate heterogeneity of effect may be present in some analyses, but these statistics are greatly underpowered, making it difficult to draw strong inferences. Begg’s funnel plots and Egger’s test of asymmetry failed to indicate the presence of publication bias for any summary estimate (eAppendix 4, http://links.lww.com). However, these statistics are also likely underpowered, given the small number of studies included in each summary estimate, and inference about publication bias should be made with caution.
Midlife BP and Alzheimer disease
Four studies assessed blood pressure in midlife21,25,27,30—defined here as prior to age 65 years. Given that these four studies used different blood pressure classification schemes, we could not compute summary estimates. The single study that considered the association between midlife history of hypertension and incident Alzheimer disease suggested an adverse relationship (RR= 1.40 [95% CI= 0.75–2.62]).25 Three studies21,27,30 evaluated the association between midlife systolic BP and incident Alzheimer disease, but their findings were inconsistent (Figures 3 and 5). Little evidence of an association was apparent in two of the three studies,21,30 while the third reported increased risk of incident Alzheimer disease with highly elevated systolic BP (RR=2.6, [95% CI=1.1–6.6], comparing midlife systolic BP ≥160 mm Hg to <140 mm Hg).27 However, in another study where there was no association in the cohort overall, there appeared to be a nonlinear association among participants who had never been treated with antihypertensive medications.21 The data suggest that those with low (<110 mm Hg) or high midlife systolic BP (>160 mm Hg) had an increased risk of Alzheimer disease, compared with those with intermediate systolic BP (110–139 mm Hg). Conversely, the three studies21,27,30 reporting on the association between midlife diastolic BP and incident Alzheimer disease consistently suggested an adverse association with midlife diastolic hypertension (Figures 4 and 6).
Late-life BP and Alzheimer disease
Fifteen studies, covering 16 populations, reported associations with late-life measures of blood pressure,22,26,28–40 with 9 reporting on an association with “history of hypertension,”22,26,29,30,32,35,36,39 3 with “hypertension at enrollment,”31,33,40 3 with continuous measures of systolic or diastolic BP,28,37,38 and 3 with categories of systolic or diastolic BP. 28,37,38 The summary relative risk among the 9 studies reporting on the association between “history of hypertension” and Alzheimer disease was 0.95 (95% CI= 0.78–1.17). Results were similar when we combined studies reporting on late-life “history of hypertension” and on late-life “hypertension at enrollment” to compute a single summary estimate (RRΣ=0.95 [95%CI= 0.78–1.14]). The pooled estimate from those studies reporting on the association per 10 mm Hg-increment in late-life systolic BP and incident Alzheimer disease was 0.95 (95%CI= 0.90–1.00), but there was no clear pattern in the associations corresponding to categories of late-life systolic BP (Figure 5). The pooled estimate from those studies reporting on the association per 10 mm Hg-increment in diastolic BP and incident Alzheimer disease was 0.93 (95%CI= 0.84–1.04), and studies reporting on the association between incident Alzheimer disease and categories of late-life diastolic BP also suggested decreased risk of Alzheimer disease with higher diastolic BP (Figure 6).
DISCUSSION
This systematic review and meta-analysis of prospective epidemiologic research does not provide clear evidence for a relationship between blood pressure and Alzheimer disease. There was some suggestion of an age-dependent effect of blood pressure on Alzheimer disease, although the evidence is relatively weak: there is a suggestion that midlife diastolic, but not midlife systolic, hypertension in midlife may have an adverse effect on risk of incident Alzheimer disease and a suggestion that elevated late-life BP may actually be beneficial.
Potential Mechanisms of Action
A number of autopsy studies9,10 have shown that the probability of manifesting dementia for a given level of Alzheimer disease pathology is increased by the presence of cerebrovascular pathology, which is strongly linked to hypertension.41–48 Thus, an increased incidence of dementia in those with hypertension could be due solely to its impact on cerebrovascular pathology. However, uncontrolled hypertension appears to predict the level of neurofibrillary tangles and neuritic plaques (pathologic indicators of Alzheimer disease) in the brain,49–51 which could be a direct effect of hypertension on Alzheimer disease pathology. While there is little mechanistic evidence linking blood pressure to the formation or toxicity of tau proteins or neurofibrillary tangles in the brain,7 blood pressure may be related to Alzheimer disease initiation or progression through mechanisms involving beta-amyloid peptides,52 which aggregate to form neuritic plaques. Animal studies suggest cerebral ischemia may be involved in the initiation of Alzheimer disease through upregulation of amyloid precursor protein gene expression,53–55 promotion of amyloid precursor protein cleavage into beta-amyloid peptides,56 or reduction in beta-amyloid peptide clearance.57
Alternately (or additionally), blood pressure may be related to Alzheimer disease progression, rather than initiation, through mechanisms involving beta-amyloid peptides. In mouse models of Alzheimer disease with mutations in the amyloid precursor protein or presenilin genes (which in humans lead to Alzheimer disease in an autosomal dominant fashion and are associated with increased production of beta-amyloid peptides), regulation of cerebral blood flow is impaired, and episodes of hypotension or hypertension result in undesired fluctuations in cerebral blood flow that may contribute to neuronal dysfunction.58,59 Several studies have also observed greater brain atrophy (specifically hippocampal and cortical atrophy) with increased blood pressure in both non-demented and demented persons.60–64 The mechanisms underlying this association are unclear, but some evidence suggests that it could result from an effect of subcortical vascular pathology on cortical neuronal apoptosis (the process of programmed cell death) which, in exaggerated form, is thought to lead to brain atrophy.65
Causal Inference
Systematic review and meta-analysis do not protect from bias and other challenges common to epidemiologic research, even when, as in this review, we only consider prospective epidemiologic studies. As such, we must consider whether observed results are indicative of the true causal relationship between BP and Alzheimer disease or whether they can be attributed to bias.
Selection bias, which can arise when study participation is incomplete and is related to both the exposure and the outcome of interest, can have a substantial impact in studies of older adults. Such studies enroll those who have survived into older adulthood and remain healthy enough to participate in a research study; over time, loss to follow-up related to declining health and death is common. For example, the majority of studies looking at the association between late-life BP and Alzheimer disease enrolled participants after age 65. If initial enrollment or continued participation once enrolled is related to blood pressure and to Alzheimer disease (or their strong correlates), estimates of the association between blood pressure and Alzheimer disease will be biased,66–70 and this bias will be reflected in our summary estimates. Reported loss to follow-up in the studies included in this review ranges from approximately 20% to 80%, indicating that there is ample potential for selection bias.
The potential for selection bias may be large because hypertension is an established risk factor for cardiovascular morbidity and mortality.71–73 Risk of cardiovascular disease appears to double with each 20 mm Hg-increase in systolic BP or 10 mm Hg-increase in diastolic BP in adults ages 40–69 years. The risk ratio associated with these increases in blood pressure is somewhat smaller in adults aged 70 and older. However, the blood-pressure-related risk in this older group remains considerable, especially viewed in terms of the risk difference, which may in fact be greater for the older group, among whom baseline risk is considerably higher.74 In addition, clinical trials demonstrate a robust benefit of antihypertensive medication use on coronary heart disease, congestive heart failure, stroke, other cardiovascular events, cardiovascular-related mortality, and all-cause mortality.75 Those with higher blood pressure would therefore be expected to have decreased participation due to increased cardiovascular mortality and morbidity, which would limit initial participation or continued follow-up.
Likewise, cognitive impairment itself (for which the most common cause is Alzheimer disease, whether full-blown dementia or milder syndromes) is an established risk factor for non-participation76 and mortality.77,78 Often defined as having a low Mini-Mental State Examination score or increased difficulty with activities of daily living, cognitive impairment appears to be associated specifically with loss to follow-up and drop-out due to death in longitudinal cohorts of older adults.79,80 In summary, it is reasonable to expect that persons with hypertension-associated morbidity or mortality and cognitive impairment would be the least likely to be enrolled in or successfully continue participation in a longitudinal cohort study. This pattern of selection would lead to an underestimation of the blood pressure-Alzheimer disease relationship, which may account, at least in part, for the suggestion of an inverse association between late-life hypertension and Alzheimer disease. It also suggests that the true adverse association between midlife measures of hypertension and Alzheimer disease may be stronger than that observed in the literature.
In addition, other evidence supports the existence of bias due to selection effects. The age-dependent pattern observed in the current review, where the mean or minimum age at baseline predicts the direction of the association, has been observed for several other proposed Alzheimer disease risk factors that are in themselves significant predictors of mortality, including smoking70 and total cholesterol.81 While we cannot exclude a true age-dependent association for each of these risk factors, the consistency of this pattern, in which the risk factor appears to have an adverse effect at younger ages and an inverse effect at older ages, suggests that there may be a common underlying source of bias.
Reverse causation may also account for the observed pattern of association. Given that the follow-up time for many of the studies of late-life hypertension and Alzheimer disease was less than 5 years and the time between onset of the disease process and diagnosis of Alzheimer disease is likely much longer,4 the suggestion of an inverse association between late-life blood pressure and Alzheimer disease probably does not reflect a true effect of blood pressure on Alzheimer disease initiation. Instead, the pathologic process of Alzheimer disease may influence blood pressure regulation, leading to a decline in blood pressure during disease development and progression.12 However, reverse causation is less likely to account for the suggestion of an adverse effect of midlife blood pressure on Alzheimer disease risk given that the dementia typically developed ten to twenty years after blood pressure assessment. This suggestion of an adverse effect of midlife blood pressure on Alzheimer disease risk may instead be due to a real effect of blood pressure on cognition. In late life, it is thought that persons with less compliant blood vessels may require higher blood pressure (particularly systolic blood pressure) to maintain adequate brain perfusion.82–87
Confounding may partially account for the current findings. Our eligibility criteria required all studies to report results adjusted for, at minimum, age and sex. Most also adjusted for education. Additional adjustment for covariates such as alcohol consumption, presence of apolipoprotein E4, and smoking status did not materially affect results of individual studies,21,27 and few established risk factors for Alzheimer disease are likely to be strong confounders. However, we cannot preclude the possibility of residual confounding or confounding by unknown factors, particularly other aspects of the metabolic syndrome or physical activity.88–91
Misclassification of blood pressure is likely, given that most studies relied on self-report or measured blood pressure at a single study visit, but this is probably not a significant source of bias. There are several factors that can introduce variability into blood pressure measurements. Factors related to the measurement process, including body size and cuff position, can introduce error.92 In addition, blood pressure exhibits natural variation, both within a day and from day to day, and in some people may be elevated primarily in medical settings (“white coat hypertension”).93 Despite these factors, James at al. 94 found that reproducibility of blood pressure measures in a clinical setting is high, both within a visit and across visits separated by two weeks, and clinical measures are highly correlated with measures taken outside of the clinical setting. In addition, the validity of blood pressure measurements is supported by their clear association with vascular mortality74 and other health endpoints.71 As such, the degree of misclassification of blood pressure is unlikely to render study effect estimates unreliable. Moreover, given that all studies included in this review were prospective, it is unlikely that misclassification of blood pressure was differential relative to ultimate Alzheimer disease status. Therefore, the direction of bias resulting from misclassification would be, most likely, toward the null, and might contribute to the overall null findings, although it is unlikely to account for the suggestion of either an adverse association between midlife blood pressure and Alzheimer disease or an inverse association between late life blood pressure and Alzheimer disease.
Misclassification of Alzheimer disease status may be differential based on history of hypertension or measured blood pressure because diagnosis of Alzheimer disease often uses cardiovascular risk factors such as hypertension—either explicitly or implicitly—to exclude people who are likely to have underlying vascular dementia or vascular cognitive impairment.95–98 If the presence of hypertension leads to a greater likelihood of diagnosing vascular dementia or a mixed dementia rather than Alzheimer disease, the effect of blood pressure on Alzheimer disease risk would be underestimated. This source of bias could contribute to the suggested inverse associations between measures of late-life blood pressure and Alzheimer disease, but cannot account for the suggestion of an adverse association between measures of midlife hypertension and Alzheimer disease. The magnitude of such a bias is difficult to quantify, and depends on the diagnostic criteria used and how they were implemented, which varied across studies. However, in one study,21 exclusion of Alzheimer disease cases with contributing cerebrovascular disease identified through CT scan did not appreciably change effect estimates, suggesting that the effect may not be large.
In summary, selection bias, reverse causation, and differential misclassification of Alzheimer disease status may contribute to the suggestion of an inverse association between late-life blood pressure and Alzheimer disease, but cannot account for the suggestion of an adverse relationship between midlife hypertension and Alzheimer disease. However, the possibility of a true relationship between late-life blood pressure and Alzheimer disease, mediated by an effect of blood pressure on Alzheimer disease progression, cannot be dismissed.
Additional Complicating Issues
Even if the suggested adverse relationship between midlife hypertension and Alzheimer disease is confirmed, the overlap between vascular and Alzheimer disease pathology will make it difficult to infer a causal relationship between midlife hypertension and Alzheimer disease pathology. As noted earlier, increasing evidence shows that Alzheimer disease and vascular dementia often coexist,3,4 and midlife hypertension also appears to be a risk factor for vascular dementia21,25,27 and vascular cognitive impairment.99 In addition, autopsy studies have suggested that, for a given level of Alzheimer disease pathology, concurrent cerebrovascular pathology is associated with earlier onset of clinically significant symptoms of dementia,8–10 which would lead to earlier diagnosis. However, it is currently unknown whether these concurrent conditions act additively or synergistically. As such, it is difficult to distinguish whether blood pressure is an independent risk factor for both Alzheimer disease and vascular dementia or whether the association between blood pressure and Alzheimer disease is driven partly or primarily by the impact of blood pressure on vascular pathology.
The role of medication use must also be considered. If blood pressure is causally related to Alzheimer disease risk, treatment of hypertension may make it difficult to detect such an effect in the population, particularly in later birth cohorts where treatment of blood pressure has been more aggressive.100–102 In one study that considered the association between midlife blood pressure and Alzheimer disease, no association was apparent in the study population as a whole, but there was a suggestion of an association between midlife hypertension and Alzheimer disease in untreated persons.21 In a second study that considered the impact of late-life blood pressure on Alzheimer disease, risk of Alzheimer disease was much stronger in participants with drug-untreated hypertension compared with those with drug-treated hypertension.22 The potential modification of the association between blood pressure and Alzheimer disease by antihypertensive medication use has major public health importance and deserves further study.103
Limitations
Our review and meta-analysis has several limitations. Although we have summarized the prospective epidemiologic literature, the reported associations may not reflect the true causal effect of blood pressure on Alzheimer disease risk due to the presence of selection bias, reverse causation, or misclassification, as discussed above. As the current review is based on published work, we cannot exclude the possibility that publication bias influences our findings. However, if publication bias were important, we would expect to see much stronger findings than those we observed. Furthermore, the cohorts reporting on the BP-Alzheimer disease association include a majority of the current major Alzheimer disease cohort studies. Finally, our consideration of an age-dependent association between blood pressure and Alzheimer disease risk is limited due to the very small number of studies reporting on the association between midlife measures of blood pressure and Alzheimer disease and the disparate treatment of information about hypertension, systolic BP, and diastolic BP across studies.
Recommendations for future research
To promote comparability across studies, we suggest that future studies report estimates of risk per 10 mm Hg-increment in systolic BP and in diastolic BP, as well as estimates of risk across standard categories of blood pressure, such as those recommended by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.71 Future epidemiologic research should focus on the influence of midlife measures of blood pressure on Alzheimer disease risk, as further work in older cohorts is unlikely to provide additional information given the intractable issues of selection and reverse causation. Furthermore, future epidemiologic research on the association between blood pressure and Alzheimer disease should report on the pattern of anti-hypertension medication use and achieved control; it should also consider the potential modifying effects of antihypertensive drug use, including aspects of duration, intensity, and achieved level of blood pressure control.
In addition, we advocate continued systematic review of epidemiologic findings in a centralized database. This analysis was conducted in parallel with a similar review undertaken for the AlzRisk centralized database (www.alzrisk.org), hosted by the Alzheimer Research Forum (www.alzforum.org), which attempts to systematically catalogue all prospective cohort data on non-genetic risk factors for Alzheimer disease. Data on the relationship between Alzheimer disease and several risk factors, including blood pressure, are currently available, with additional risk factors being added regularly. AlzRisk will be updated as new and updated studies become available over time; we anticipate a substantial volume of new data given the aging of existing, long-established cohort studies and increasing interest in cognitive outcomes.
Finally, there may be value in collaboration to combine the data from these cohorts to conduct patient data-level analysis of this relationship. This would allow for a systematic exploration of the role of age of BP measurement, antihypertensive medication use, and other factors in the association between BP and Alzheimer disease.
CONCLUSIONS
The current epidemiologic literature is insufficient for a definitive conclusion about a causal relationship between blood pressure and Alzheimer disease. However, the benefits of midlife blood pressure control on cardiovascular outcomes are well established and justify current clinical recommendations.
Supplementary Material
Table.
Characteristics of studies evaluating the association between blood pressure and incident Alzheimer disease
| Study Population | Country | Mean Age in Years (Range) at Baseline | Mean (Max) Follow-Up in Years | No. Persons (No. AD cases) | Effect Measure | Alzheimer disease diagnostic criteria | Covariates |
|---|---|---|---|---|---|---|---|
| North Karelia/FINMONICA27 | Finland | 50 (NR) | 21 (26) | 1287 (48) | OR | NINCDS-ADRDA | Age, sex, education, alcohol, presence of APOE4, smoking status |
| Honolulu-Asia Aging Study21 | USA | 53 (45–68) | 27 (NR) | 3703 (118) | OR | NINCDS-ADRDA | Age, (restricted to males), education, alcohol intake, APOE genotype, smoking status |
| Multiple Risk Factors for Major Diseases and Cancer Screening Program (MRMD &CSP)25 | Taiwan | 58 (30+) | NR (10–20) | 365 (73) | OR | Chinese version of the DSM-IV | Age, sex, duration of enrollment, residential township |
| East Boston Established Populations for Epidemiologic Study of the Elderly (East Boston EPESE)30 | USA | 60 a (54+) or 72a (65+) | 13.6 (NR) or 4.5 (NR) | 378 (41) or 634 (99) | OR | Modified NINCDS-ADRDA | Age, sex, education, interval to follow-up, stratified sampling |
| Xian, China cohort39 | China | 65 (55+) | 3.2 (NR) | 2197 (37) | OR | NINCDS-ADRDA | Age, sex, education, occupation, rural/urban residence, stroke |
| Canadian Study of Health and Aging (CHSA)29 | Canada | 68 (65–100) | 5 (NR) | 4088 (157) | OR | NINCDS-ADRDA | Age, sex, education |
| Rotterdam Study/Gothenburg H-70 Study 34 | Sweden and the Netherlands | 70 (55+) | 2 (3) | 6985 (124) | HR | NINCDS-ADRDA | Age, sex, study population |
| Neurological Disorders in Central Spain (NEDICES)22 | Spain | 73 (65+) | 3.2 (NR) | 3824 (113) | HR | NINCDS-ADRDA | Age, gender, education, daily sleep duration, depressive symptoms or antidepressant use, diabetes, geographical area, heart disease, hypercholesterolemia, stroke |
| Conselice Study of Brain Ageing (CSBA)40 | Italy | 73 (65+) | 3.9 (0.8) | 749 (53) | HR | NINCDS-ADRDA | Age, gender, education, abdominal obesity, cerebrovascular disease, hyperhomocysteinemia, hyperglycemia, hypertriglyceridemia, history of stroke, inflammation status, low HDL cholesterol, presence of APOE4, sedentary lifestyle |
| Three City Study33 | France | 73 (65+) | 3.5 (4) | 7087 (134) | HR | NINCDS-ADRDA | Age, sex, education, glycemia, HDL cholesterol, study population, triglycerides, waist circumference |
| Cache County Study of Memory, Health and Aging (Cache County)26 | USA | 74 (65+) | 3.2 (5) | 3264 (104) | HR | NINCDS-ADRDA | Age, sex, education, coronary artery bypass graft, diabetes, high cholesterol, history of myocardial infarction, number of APOE4 alleles, overweight/obesity, stroke history |
| Indianapolis-Ibadan Dementia Project, Ibadan (Ibadan)32 | Nigeria | 74 (65+) | NR (5) | 523 (62) | OR | NINCDS-ADRDA | Age, sex |
| Indianapolis-Ibadan Dementia Project, Indianapolis (Indianapolis)32 | USA | 74 (65+) | NR (5) | 470 (89) | OR | NINCDS-ADRDA | Age, sex |
| Manitoba Study of Health and Aging (MSHA)36 | Canada | 74 (65–93) | 5 (NR) | 694 (36) | OR | NINCDS-ADRDA | Age, sex, education |
| Religious Orders Study35 | USA | 75 (NR) | 6.5 (10) | 824 (151) | HR | NINCDS-ADRDA | Age, sex, education |
| Adult Changes in Thought28 | USA | 75.1 (65+) | 6.3 (NR) | 2356 (204) | HR | NINCDS-ADRDA | Age, sex, education, presence of APOE4, race |
| Washington Heights-Inwood Columbia Aging Project (WHICAP)31 | USA | 76 (65+) | 4.4 (NR) | 1833 (147) | HR | NINCDS-ADRDA | Age, sex, education, presence of APOE4, race/ethnicity, smoking, study population |
| Bronx Aging Study37 | USA | 79 (75–85) | NR (21) | 406 (65) | HR | NINCDS-ADRDA | Age, sex, education |
| Kungsholmen Project38 | Sweden | 82 (75–100) | 5.0 (10.5) | 1301 (333) | HR | Study-specific criteria similar to NINCDS-ADRDA | Age, sex, education, antihypertensive drugs, diabetes, baseline MMSE, BMI, follow-up survival status, heart failure, pulse rate, stroke, systolic BP or diastolic BP |
Mean age at blood pressure assessment was estimated based on reported information.
Abbreviations: AD: Alzheimer disease; APOE: Apolipoprotein E; BMI: Body mass index; DSM-IV: Diagnostic and Statistical Manual, Version IV; HDL: high density lipoprotein; HR: hazard ratio; MMSE: Mini-Mental State Examination; NINCDS-ADRDA: National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; NR: Not reported; OR: odds ratio
Acknowledgments
Sources of financial support: Work supported by a grant from an anonymous foundation. Melinda C. Power is also supported by a National Institute of Environmental Health Sciences training grant (T32 ES007069).
Portions of this work also appear on the online database of Alzheimer disease epidemiology findings AlzRisk (www.alzrisk.org), hosted by the Alzheimer Research Forum (www.alzforum.org), and are reprinted with permission. We also thank Xiang Gao, Fumiaki Imamura, Sanae Kishimoto, Martin Lajous, and Peter Liang for their help in determining eligibility for non-English language articles, and Shanshan Li for extracting data from a non-English language article included in this review.
Footnotes
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006;260(3):211–23. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 2.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging. 2000;21(2):153–60. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 3.Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50(8):1431–8. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
- 4.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 5.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531–4. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 6.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128(Pt 9):2034–41. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Ruiz C, Wang J, Ksiezak-Reding H, Ho L, Qian X, Humala N, Thomas S, Martinez-Martin P, Pasinetti GM. Role of Hypertension in Aggravating Abeta Neuropathology of AD Type and Tau-Mediated Motor Impairment. Cardiovasc Psychiatry Neurol. 2009;2009:107286. doi: 10.1155/2009/107286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354(9182):919–20. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 9.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 10.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277(10):813–7. [PubMed] [Google Scholar]
- 11.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Oden A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):141–145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 12.Qiu C, von Strauss E, Winblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia: a longitudinal study from the Kungsholmen project. Stroke. 2004;35(8):1810–5. doi: 10.1161/01.STR.0000133128.42462.ef. [DOI] [PubMed] [Google Scholar]
- 13.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23(1):1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009;8(2):61–70. doi: 10.1016/j.arr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case-control studies. I. Principles. Am J Epidemiol. 1992;135(9):1019–28. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 16.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute; 2009. [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 22.Bermejo-Pareja F, Benito-Leon J, Louis ED, Trincado R, Carro E, Villarejo A, de la Camara AG. Risk of Incident Dementia in Drug-Untreated Arterial Hypertension: A Population-Based Study. Journal of Alzheimers Disease. 22(3):949–958. doi: 10.3233/JAD-2010-101110. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang CJ, Yip PK, Wu SC, Lu CS, Liou CW, Liu HC, Liu CK, Chu CH, Hwang CS, Sung SF, Hsu YD, Chen CC, Liu SI, Yan SH, Fong CS, Chang SF, You SL, Chen CJ. Midlife risk factors for subtypes of dementia: a nested case-control study in Taiwan. Am J Geriatr Psychiatry. 2007;15(9):762–71. doi: 10.1097/JGP.0b013e318050c98f. [DOI] [PubMed] [Google Scholar]
- 26.Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JC, Welsh-Bohmer KA. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20(2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 27.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Rhew IC, Shofer JB, Kukull WA, Breitner JC, Peskind E, Bowen JD, McCormick W, Teri L, Crane PK, Larson EB. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc. 2007;55(8):1161–7. doi: 10.1111/j.1532-5415.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 30.Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. 2001;58(10):1640–6. doi: 10.1001/archneur.58.10.1640. [DOI] [PubMed] [Google Scholar]
- 31.Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24(3):185–92. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogunniyi A, Hall KS, Gureje O, Baiyewu O, Gao S, Unverzagt FW, Smith-Gamble V, Evans RE, Dickens J, Musick BS, Hendrie HC. Risk factors for incident Alzheimer’s disease in African Americans and Yoruba. Metab Brain Dis. 2006;21(2–3):235–40. doi: 10.1007/s11011-006-9017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, Portet F, Dartigues JF, Alperovitch A, Barberger-Gateau P. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes Care. 2009;32(1):169–74. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JC, Lernfelt B, van Harskamp F, Hofman A, Breteler MM. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. 2001;12(1):33–9. doi: 10.1159/000051233. [DOI] [PubMed] [Google Scholar]
- 35.Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Relation of blood pressure to risk of incident Alzheimer’s disease and change in global cognitive function in older persons. Neuroepidemiology. 2006;26(1):30–6. doi: 10.1159/000089235. [DOI] [PubMed] [Google Scholar]
- 36.Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer’s disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30(3):590–7. doi: 10.1093/ije/30.3.590. [DOI] [PubMed] [Google Scholar]
- 37.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61(12):1667–72. doi: 10.1212/01.wnl.0000098934.18300.be. [DOI] [PubMed] [Google Scholar]
- 38.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166(9):1003–8. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 39.Qu QM, Qiao J, Han JF, Yang JB, Guo F, Luo GG, Yang H, Cao HM, Ju XC, Wu CB. The incidence of dementia among elderly people in Xi’ an, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(7):529–32. [PubMed] [Google Scholar]
- 40.Forti P, Pisacane N, Rietti E, Lucicesare A, Olivelli V, Mariani E, Mecocci P, Ravaglia G. Metabolic syndrome and risk of dementia in older adults. J Am Geriatr Soc. 58(3):487–92. doi: 10.1111/j.1532-5415.2010.02731.x. [DOI] [PubMed] [Google Scholar]
- 41.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765–72. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 42.Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37(2):550–5. doi: 10.1161/01.STR.0000199847.96188.12. [DOI] [PubMed] [Google Scholar]
- 43.Kazui S, Levi CR, Jones EF, Quang L, Calafiore P, Donnan GA. Risk factors for lacunar stroke: a case-control transesophageal echocardiographic study. Neurology. 2000;54(6):1385–7. doi: 10.1212/wnl.54.6.1385. [DOI] [PubMed] [Google Scholar]
- 44.Veldink JH, Scheltens P, Jonker C, Launer LJ. Progression of cerebral white matter hyperintensities on MRI is related to diastolic blood pressure. Neurology. 1998;51(1):319–20. doi: 10.1212/wnl.51.1.319. [DOI] [PubMed] [Google Scholar]
- 45.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55(9):1217–25. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 46.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70(6):425–30. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33(1):21–5. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 48.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70(14):1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 49.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging. 2000;21(1):57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, Haroutunian V. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–6. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC., 3rd Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131(2):162–9. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- 52.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34(2):335–7. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 53.Shi J, Yang SH, Stubley L, Day AL, Simpkins JW. Hypoperfusion induces overexpression of beta-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. 2000;853(1):1–4. doi: 10.1016/s0006-8993(99)02113-7. [DOI] [PubMed] [Google Scholar]
- 54.Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, Greenberg DA. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol. 2001;50(1):93–103. doi: 10.1002/ana.1073. [DOI] [PubMed] [Google Scholar]
- 55.Nihashi T, Inao S, Kajita Y, Kawai T, Sugimoto T, Niwa M, Kabeya R, Hata N, Hayashi S, Yoshida J. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir (Wien) 2001;143(3):287–95. doi: 10.1007/s007010170109. [DOI] [PubMed] [Google Scholar]
- 56.Saido TC, Yokota M, Maruyama K, Yamao-Harigaya W, Tani E, Ihara Y, Kawashima S. Spatial resolution of the primary beta-amyloidogenic process induced in postischemic hippocampus. J Biol Chem. 1994;269(21):15253–7. [PubMed] [Google Scholar]
- 57.Weller RO, Yow HY, Preston SD, Mazanti I, Nicoll JA. Cerebrovascular disease is a major factor in the failure of elimination of Abeta from the aging human brain: implications for therapy of Alzheimer’s disease. Ann N Y Acad Sci. 2002;977:162–8. doi: 10.1111/j.1749-6632.2002.tb04812.x. [DOI] [PubMed] [Google Scholar]
- 58.Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283(1):H315–23. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 59.Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97(17):9735–40. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MM. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64(2):263–7. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- 61.Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol. 2007;254(6):713–21. doi: 10.1007/s00415-006-0238-4. [DOI] [PubMed] [Google Scholar]
- 62.Skoog I, Andreasson LA, Landahl S, Lernfelt B. A population-based study on blood pressure and brain atrophy in 85-year-olds. Hypertension. 1998;32(3):404–9. doi: 10.1161/01.hyp.32.3.404. [DOI] [PubMed] [Google Scholar]
- 63.Jouvent E, Viswanathan A, Chabriat H. Cerebral atrophy in cerebrovascular disorders. J Neuroimaging. 2010;20(3):213–8. doi: 10.1111/j.1552-6569.2009.00370.x. [DOI] [PubMed] [Google Scholar]
- 64.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44(1):29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 65.Viswanathan A, Gray F, Bousser MG, Baudrimont M, Chabriat H. Cortical neuronal apoptosis in CADASIL. Stroke. 2006;37(11):2690–5. doi: 10.1161/01.STR.0000245091.28429.6a. [DOI] [PubMed] [Google Scholar]
- 66.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol. 2002;31(1):163–5. doi: 10.1093/ije/31.1.163. [DOI] [PubMed] [Google Scholar]
- 67.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3(2):143–55. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–6. [PubMed] [Google Scholar]
- 69.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 70.Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19(3):448–50. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 71.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 72.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161(12):1501–8. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 73.Psaty BM, Furberg CD, Kuller LH, Cushman M, Savage PJ, Levine D, O’Leary DH, Bryan RN, Anderson M, Lumley T. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med. 2001;161(9):1183–92. doi: 10.1001/archinte.161.9.1183. [DOI] [PubMed] [Google Scholar]
- 74.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 75.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289(19):2534–44. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 76.Euser SM, Schram MT, Hofman A, Westendorp RG, Breteler MM. Measuring cognitive function with age: the influence of selection by health and survival. Epidemiology. 2008;19(3):440–7. doi: 10.1097/EDE.0b013e31816a1d31. [DOI] [PubMed] [Google Scholar]
- 77.Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151(7):676–88. doi: 10.1093/oxfordjournals.aje.a010262. [DOI] [PubMed] [Google Scholar]
- 78.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62(5):779–84. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 79.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58(1):13–9. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Matthews FE, Chatfield M, Freeman C, McCracken C, Brayne C. Attrition and bias in the MRC cognitive function and ageing study: an epidemiological investigation. BMC Public Health. 2004;4:12. doi: 10.1186/1471-2458-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16(5):343–54. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 82.Chabriat H, Pappata S, Ostergaard L, Clark CA, Pachot-Clouard M, Vahedi K, Jobert A, Le Bihan D, Bousser MG. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. 2000;31(8):1904–12. doi: 10.1161/01.str.31.8.1904. [DOI] [PubMed] [Google Scholar]
- 83.Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord. 2010;24(1):19–27. doi: 10.1097/WAD.0b013e3181b4f736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fayed N, Davila J, Oliveros A, Jr, Medrano J, Castillo J. Correlation of findings in advanced MR techniques with global severity scales in patients with some grade of cognitive impairment. Neurol Res. 2010;32(2):157–65. doi: 10.1179/174313209X405164. [DOI] [PubMed] [Google Scholar]
- 85.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122(7):690–7. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luckhaus C, Cohnen M, Fluss MO, Janner M, Grass-Kapanke B, Teipel SJ, Grothe M, Hampel H, Peters O, Kornhuber J, Maier W, Supprian T, Gaebel W, Modder U, Wittsack HJ. The relation of regional cerebral perfusion and atrophy in mild cognitive impairment (MCI) and early Alzheimer’s dementia. Psychiatry Res. 2010;183(1):44–51. doi: 10.1016/j.pscychresns.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Pappata S, Varrone A, Vicidomini C, Milan G, De Falco C, Sansone V, Iavarone A, Comerci M, Lore E, Panico MR, Quarantelli M, Postiglione A, Salvatore M. SPECT imaging of GABA(A)/benzodiazepine receptors and cerebral perfusion in mild cognitive impairment. Eur J Nucl Med Mol Imaging. 2010;37(6):1156–63. doi: 10.1007/s00259-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 88.Harrington M, Weuve J, Blacker D. The AlzRisk Database Alzheimer Research Forum. Physical Activity. [Google Scholar]
- 89.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–12. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4(2):363–81. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weuve J, McQueen M, Blacker D. The AlzRisk Database Alzheimer Research Forum. Diabetes. [Google Scholar]
- 92.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcomittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 93.Pickering TG. Blood pressure variability and ambulatory monitoring. Curr Opin Nephrol Hypertens. 1993;2(3):380–5. doi: 10.1097/00041552-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 94.James GD, Pickering TG, Yee LS, Harshfield GA, Riva S, Laragh JH. The reproducibility of average ambulatory, home, and clinic pressures. Hypertension. 1988;11:545–549. doi: 10.1161/01.hyp.11.6.545. [DOI] [PubMed] [Google Scholar]
- 95.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 96.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV. 1998;xxvii:886. [Google Scholar]
- 97.Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN) Stroke. 1996;27(1):30–6. doi: 10.1161/01.str.27.1.30. [DOI] [PubMed] [Google Scholar]
- 98.Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, Russell RW, Symon L. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–7. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 99.Barone FC, Rosenbaum DM, Zhou J, Crystal H. Vascular cognitive impairment: dementia biology and translational animal models. Curr Opin Investig Drugs. 2009;10(7):624–37. [PubMed] [Google Scholar]
- 100.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 101.Antikainen RL, Moltchanov VA, Chukwuma C, Sr, Kuulasmaa KA, Marques-Vidal PM, Sans S, Wilhelmsen L, Tuomilehto JO. Trends in the prevalence, awareness, treatment and control of hypertension: the WHO MONICA Project. Eur J Cardiovasc Prev Rehabil. 2006;13(1):13–29. doi: 10.1097/00149831-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 102.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26(1):60–9. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 103.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72(4):368–74. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.