Abstract
Zebrafish provide a highly versatile model in which to study vertebrate development. Many recent studies have elucidated early events in the organogenesis of the zebrafish pancreas; however, several aspects of early endocrine pancreas formation in the zebrafish are not homologous to the mammalian system. To better identify mechanisms of islet formation in the zebrafish, with true homology to those observed in mammals, we have temporally and spatially characterized zebrafish secondary islet formation. As is the case in the mouse, we show that Notch inhibition leads to precocious differentiation of endocrine tissues. Furthermore, we have used transgenic fish expressing fluorescent markers under the control of a Notch-responsive element to observe the precursors of these induced endocrine cells. These pancreatic Notch-responsive cells represent a novel population of putative progenitors that are associated with larval pancreatic ductal epithelium, suggesting functional homology between secondary islet formation in zebrafish and the secondary transition in mammals. We also show that Notch-responsive cells persist in the adult pancreas and possess the classical characteristics of centroacinar cells, a cell type believed to be a multipotent progenitor cell in adult mammalian pancreas.
Introduction
Eukaryotic organisms have evolved multiple strategies for achieving reliable glucose homeostasis, manifested by a variety of cell types capable of producing insulin- and/or other glucoregulatory peptide hormones. In vertebrates, these include: a) the dispersed enteroendocrine system, present in all bilaterian organisms (Drucker, 2007), b) the Brockmann body, a foregut-associated collection of endocrine cells observed in larval lamprey and other jawless fish (Youson and Al-Mahrouki, 1999), c) immature endocrine cells observed in the early pancreatic bud of mammals (Herrera, 2000; Teitelman et al., 1993), and, d) mature α-, β-, δ-, PP and ghrelin-producing cells located within the pancreatic islets of cartilaginous and bony fish, as well as in all tetrapods (Burlison et al., 2008; Chiang and Melton, 2003; Kawaguchi et al., 2002; Offield et al., 1996; Prado et al., 2004). In mammals, the stages of pancreas development corresponding to active morphogenesis and formation of early endocrine cells (E9.5-E12.5) are often referred to as the “primary transition”, while the later appearance of mature α-, β-, ε-, δ-, and PP-producing cells is often referred to as the “secondary transition” (Kemp et al., 1972; Wang et al., 2005). During the secondary transition, endocrine cells are formed by delamination of dedicated endocrine progenitor cells from the nascent branching ductal epithelium, followed by their differentiation, migration and consolidation into islet clusters (Jensen, 2004; Murtaugh and Melton, 2003). Several observations have indicated that initiation of islet neogenesis occurs in association with ductal epithelium in a variety of settings (Bonner-Weir et al., 2008; Gu and Sarvetnick, 1993; Hayashi et al., 2003; Rosenberg et al., 1983; Song et al., 1999; Xu et al., 2008; Zhang et al., 2002). Furthermore, recent work using lineage tracing in the mouse has demonstrated that endocrine progenitors, responsible for compensatory regeneration following tissue injury, are also ductal in nature (Inada et al., 2008).
Type 1 diabetes mellitus is characterized by the autoimmune destruction of pancreatic β-cells, resulting in insulin deficiency. The path to a cure for diabetes will rely on achieving a cessation of β-cell destruction, followed by restoration of β-cell mass, either by replacement with exogenous cells or by regeneration from endogenous progenitors. For these reasons, there continues to be considerable interest in understanding how β-cell specification and differentiation are regulated in both adult and embryonic pancreas.
Over the past decade, the zebrafish (Danio rerio) has been increasingly utilized as complementary system in which to study β-cell differentiation, both during normal development (reviewed in (Gnugge et al., 2004; Kinkel and Prince, 2009) and in the context of regeneration following injury (Curado et al., 2007; Moss et al., 2009; Pisharath et al., 2007). Zebrafish pancreas development shares many cellular and molecular events in common with pancreas development in mammals (reviewed in (Gnugge et al., 2004; Kinkel and Prince, 2009). As in the mouse, the zebrafish pancreas is formed from both dorsal and ventral anlagen (Field et al., 2003). Cells of the dorsal pancreas appear to exclusively form endocrine cells, which by 24 hours post fertilization (hpf) have coalesced to form the ‘principal islet’. In contrast, cells in the ventral anlage serve as progenitors for all acinar and ductal cells of the future pancreas, as well as an additional population of early endocrine cells (Dong et al., 2007; Dong et al., 2008; Field et al., 2003; Lin et al., 2004). While the embryonic and larval zebrafish pancreas consist of a single principal islet, the adult pancreas contains many secondary islets, similar to the situation observed in mammals (Chen et al., 2007). Because these cells do not commonly appear during the first 6 days of development, it seems likely that a population of endocrine progenitors must exist in larval zebrafish pancreas (Biemar et al., 2001). However, the location and identity of these cells has not yet been determined.
Among the different cell populations in zebrafish endocrine pancreas, prior studies of β-cell differentiation have been largely limited to cells in the principal islet, as the early differentiation of these cells facilitates transient genetic manipulations including gene knockdown using antisense morpholinos. However, the mechanism and timing of principal islet development differs considerably from islet formation in mammals, as the zebrafish principal islet forms prior to formation of an actual gut tube and in absence of exocrine elements. This discrepancy leads to uncertainty regarding the relationship between principal islet formation in zebrafish and formation of endocrine cells during either the primary or secondary transition in mammals. In order to better identify mechanisms of islet formation in zebrafish with true homology to those observed in mammals, we have characterized secondary islet formation in zebrafish pancreas. In so doing, we have defined a novel population of pancreatic Notch-responsive cells that reside within the larval pancreatic ductal epithelium. These cells differentiate concomitantly with the appearance of new endocrine cells of the secondary islets, suggesting functional homology between secondary islet formation in zebrafish and the secondary transition in mammals.
Materials and Methods
Transgenic lines used
The Tg(ptf1a:Gal4-VP16)jh16 transgenic line was made using a recombineered BAC that drives expression of Gal4-VP16 under the control of the endogenous ptf1a promoter/enhancer (Pisharath and Parsons, 2009). The recombineering and line generation was carried out in an analogous way as previously reported (Davison et al., 2008; Park et al., 2008). When this line is crossed to Tg(T2KUAS-E1b:nfsB-mCherry)jh17 fish (available from ZIRC, http://zebrafish.org/zirc/home/guide.php (Davison et al., 2007)), the resulting progeny express a fusion protein of NTR and mCherry in a mosaic fashion, which is restricted to the expression domain of ptf1a.
To generate nuclear tagged fluorescent proteins, we cloned the zebrafish high-mobility group box 1 (hmgb1) gene, which encodes a nuclear factor associated with chromatin. Primers were designed to amplify from cDNA, the sequence encoding the full 205aa zebrafish Hmgb1. Using standard fusion PCR protocols, this sequence (minus the termination codon) was fused to either the genes for eGFP or mCherry. The backbones of the constructs used to generate Tg(T2KIns:hmgb1-eGFP)jh10 and Tg(T2KTp1bglob:hmgb1-mCherry)jh11 fish, were obtained from the vector T2KXIGΔIN (Kawakami, 2004). The ins promoter refers to the promoter region of the preproinsulin gene (insa (Irwin, 2004)), the details previously described (Huang et al., 2001; Pisharath et al., 2007). As a Notch responsive element we utilized the promoter from the Epstein Barr Virus terminal protein 1 (TP1) gene, which contains two Rbp-Jκ binding sites (Grossman et al., 1994; Henkel et al., 1994). A tp1blgob element consisting of 6 copies of the TP1 promoter (12 Rbp-Jκ binding sites), upstream of the rabbit β-globin minimal promoter (Minoguchi et al., 1997) was cloned upstream of a Gateway cassette in the pTol-Dest vector (Villefranc et al., 2007). Gateway cloning was used to generate the pTol-tp1bglob:eGFP plasmid. The final constructs were injected along with Tol2 transposase RNA to generate mosaic F0 fish (Kawakami, 2004). F1 lines were screened for expression in the expected tissues and raised.
Tg(P0-pax6b:GFP)ulg515 line was obtained from Bernard Peers (University of Liege, Belgium (Delporte et al., 2008)), Tg(-3.5kbnkx2.2:GFP)ia3 and Tg(gcga:GFP)ia1 lines from Francesco Argenton (University of Padova, Italy (Pauls et al., 2007)). The Tg(kdrl:GRCFP)zn1 transgenic line was originally made in the Rubinstein lab (Cross et al., 2003). Over expression of the Notch intra-cellular domain (NICD), from Notch 1a, was carried out using fish transgenic for both Tg(hsp70l:Gal4)1.5kca4 and Tg(UAS:myc-Notch1a-intra)kca3 (Scheer and Campos-Ortega, 1999; Scheer et al., 2001). These embryos, at 24hpf, were incubated at 39°C for 30 minutes in groups of 30 in 1.5ml Eppendorfs tubes containing a total volume of 500μl E3. Following incubation embryos were returned to 10cm petri dishes containing E3 at 28°C. Tg(hsp70l:Gal4)1.5kca4 and Tg(UAS:myc-Notch1a-intra)kca3 fish were genotyped using primers and protocol provided by the Zebrafish International Resource Center (http://zebrafish.org/zirc/home/guide.php).
A complete list of transgenic lines used in this report is included in a Supplemental Table (ST1).
Transmission electron microscopy
Tp1bglob:eGFP larvae at 10 days post fertilization (dpf) and the viscera from juvenile 35 dpf transgenic fish were fixed in 1% paraformaldehyde and 2.5% glutaraldehyde mixture made in 0.06M phosphate buffer containing 3mM of magnesium chloride and 3% sucrose at pH 7.2 -7.4 and processed for transmission electron microscopy as per (Schmitt and Dowling, 1999). This fix was reformulated to contain 4% paraformaldehyde and 0.1% glutaraldehyde mixture in 0.1M phosphate buffer containing 3mM of magnesium chloride and 1% sucrose at pH 7.2-7.4 and processed for immunoelectron microscopy as per (Luby-Phelps et al., 2003). Anti-eGFP immunolabeling was performed using rabbit polyclonal anti-eGFP IgG 1:25 dilution (Invitrogen) overnight at 4°C, followed by incubation for 1 h at room temperature in 1:25 dilution of 12nm gold conjugated donkey anti-rabbit IgG (Jackson Immunoresearch). Sections were contrasted with 3% uranyl acetate, dried and viewed with a Hitachi 7600 TEM (Hitachi High Technologies America, Pleasanton, CA).
Immunofluorescence
For anti E-cadherin immunofluorescent staining in whole mounts, 5dpf embryos were fixed in 4% PFA for 24 h at 4°C followed by three 5 min washes in PBS containing 0.1% Triton-X100(PBST) at room temperature. This was followed by an overnight permeabilization in ice-cold methanol at -20°C. Rehydrated embryos were labeled with 1/100 rabbit anti E-cadherin primary antibody (Cell Signaling Systems): 1/100 anti-rabbit cy3 (Jackson Immunoresearch).
For immunofluorescence on juvenile/adult fish sections, dissected viscera were fixed in 10% formalin, embedded in paraffin and 5μm sections cut and processed as per standard procedures (Cell signaling technology) followed by double labeling using rabbit polyclonal anti-eGFP (Invitrogen; 1:250): goat anti rabbit Cy2 (Jackson ImmunoResearch; 1:300) and guinea pig polyclonal anti-insulin (Biomeda, 1:250): goat anti guinea pig Cy3 (Jackson ImmunoResearch; 1:300).
Hematoxylin & Eosin staining of paraffin sections
Adult zebrafish were fixed in 10% neutral buffered formalin for 4 days. The mid section containing the pancreas was cut longitudinally, placed cut face down on cassettes (62500 series, Tissue Tek) and processed for paraffin embedding. 6μm sections were cut and stained with hematoxylin and eosin (H & E) in a routine manner.
Images were taken using a Eclipse E800 (Nikon Inc, Tokyo) camera attached to a compound microscope.
Drug inhibition of Notch-signaling in larvae
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT, D 5942, Sigma), a presenilin γ-secretase inhibitor was used to block Notch signaling. A 100x stock solution of 10mM DAPT in DMSO was made and stored at -20°C until use. Depending on the experiment, transgenic embryos were incubated from 80% epiboly to 24hpf in 100μM of DAPT in E3 (Geling et al., 2002; Westerfield, 1993) or dechorionated and incubated in DAPT in E3 from 3 dpf until 5 dpf. Embryos incubated in 1% DMSO in E3 were used as control. All the embryos were incubated at 28°C in dark.
Embryo dissection
To image by confocal microscopy, transgenic embryos were fixed overnight in 4% PFA and then depending on their age imaged directly (1-2dpf) or micro-dissected (2-5dpf). To micro-dissect, fixed embryos were placed in PBS on an agarose lined plate. Then using pulled capillaries as tools, first the yolk and then the whole foregut were pried away from the embryo. At later stages (2-5dpf), the pancreas can clearly be seen and separated from the rest of the foregut. The pancreas was placed islet down on a cover slip and dried by removing all excess water using a drawn capillary with a filament. This cover slip was then mounted onto a microscope slide using columns of vacuum grease to prevent the pancreas from being crushed. Water was introduced under the cover slip to rehydrate the sample.
Confocal microscopy and β-cell quantification
Widefield and confocal sections were acquired using an Axiovert 200M microscope coupled to the Zeiss LSM 5 Pascal system. A Plan-Apochromat 10X/0.45 lens for widefield or Plan-NeoFluar 40X/1.3 Oil DIC objective for confocal sections were used. Blue, green and red channels were excited using a UV, Argon and He/Ne laser, and emissions were detected using BP 420-480, BP 505-530 and LP 560 filters, respectively, and controlled through the Multi-track mode on Zeiss AIM software. All rendering and quantification used the AIM software.
In order to count β-cells in the principal islet, we used a transgenic line that expresses eGFP in the nuclei only. This allows the visualization of individual nuclei surrounded by a negative cytoplasm. The whole principal islet is between 45-50μM thick and we imaged 2μM Z-sections through the islet. To assist in counting individual cells a camera lucida approach was taken. An example of the Z-sections through one islet is shown in supplemental figure S2.
Statistical analysis
To answer the question if Notch inhibition has a significant effect on β-cell number, the β-cells were counted from Tg(T2Kins:Hmgb1-eGFP)jh10 fish that were either mib (-/-) or their wildtype sibs (-/+, +/+). Two separate time points were used (48 and 72 hpf). Analysis of variance (ANOVA) was performed, between the time points for each treatment group. As this was not significant, the data from each time point was compiled to give one set of results for wt and one for mib samples. ANOVA was used again to compare these two samples. Note, if data from different time points were kept separate and comparisons made between mib (-/-) and wildtype sibs (-/+, +/+), the outcome was not affected and there was still a significant difference. Statistical analyses were performed with the use of SAS software, version 9.1 (SAS Institute Inc., Cary, NC). The length of the larvae was measured by placing the fish on their side on a microscope slide, imaging by a Zeiss AxioCam on a Zeiss Stemi SV II microscope and measured using AxioVision 4.6.3 SP1 software.
Results
Occurrence and localization of secondary islets
In order to characterize a secondary transition in islet formation in the zebrafish, we analyzed both the timing and localization of forming secondary islets during larval development. Secondary islets form within the tail of the pancreas, posterior and distinct from the principal islet. We examined, using a dissecting stereoscope, larvae of both ins:mCherry (Pisharath et al., 2007) and pax6b:GFP (Delporte et al., 2008) through larval development. Larvae were raised in petri dishes as normal until 5dpf. After 5dpf, these larvae were placed in tanks and fed in our fish facility in the usual manner. A dissecting stereoscope was used to observe the presence of fluorescent islets in the pancreatic tail of living larvae. By counting the numbers of larvae displaying fluorescence in the tail of the pancreas over time, we established the temporal pattern of secondary islet formation. The two lines used mark either early pan-endocrine (pax6b:GFP) or a mature endocrine cell-type type; namely, β-cells (ins:mCherry). At the level of detection afforded by a dissecting scope on living larvae, only a minority of larvae possessed detectable secondary islets at 5dpf, in either line (Fig. 1A); pax6b:GFP (average 17.7%, n=36) or ins:mCherry (average 9.2%, n=86). Conversely, by 20dpf the majority have developed secondary islets as seen in both lines; pax6b:GFP (average 96%, n=54) or ins:mCherry (average 89%, n=101). At all time points more larvae display pax6b driven expression in a secondary islet position than express ins driven mCherry, consistent with pax6b marking both nascent and mature endocrine tissue.
Figure 1.
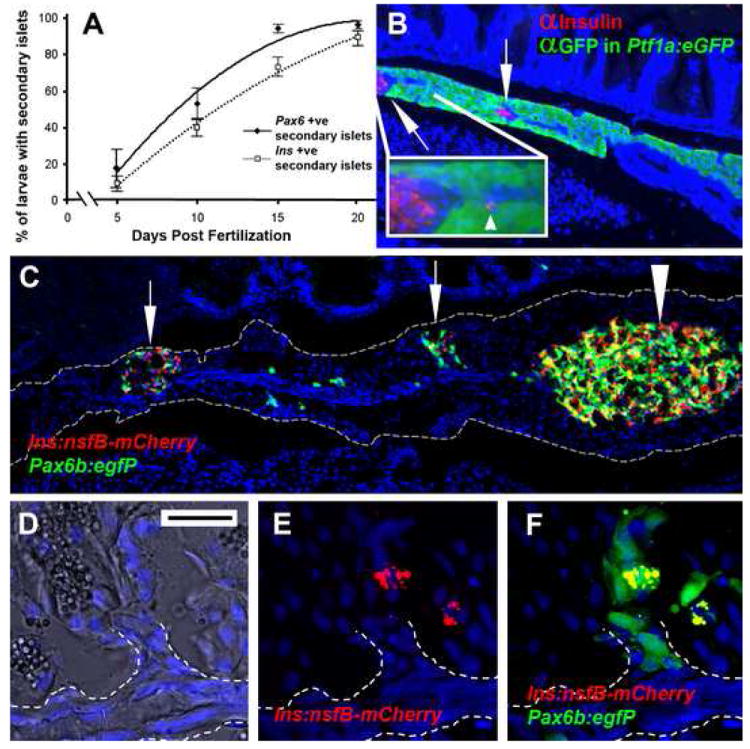
Secondary islets are established during the first 3 weeks of larval life and are associated with the main pancreatic duct. (A) The results of counting, over time, the occurrence of secondary islets in either Tg(P0-pax6:GFP)ulg515 or Tg(ins:mCherry)jh2 larvae. Starting at 5 days, few larvae display a secondary islet as detected by either transgene. By 20 days almost all larvae have at least one secondary islet.
(B) Paraffin section through the pancreas of a 28dpf, Tg(ptf1a:eGFP)jh1 juvenile following immunofluorescent detection for GFP (green) and insulin (red). The main pancreatic duct can be distinguished as a GFP negative strip of cells running through the middle of the pancreatic tail. Two secondary islets can be seen in the pancreatic tail (↑) closely associated with the duct. Inset is an enlargement of the secondary islet on the left. An isolated β-cell can be seen located right on the duct (◄). (C) Cryosection of adult pancreas lobe from a Tg(ins:nfsB-mCherry)jh5;Tg(P0-pax6b:GFP)ulg515, which includes the principal islet (large arrowhead) and two significant secondary islets (↑). (D,E,F) Close up of secondary islet (second from the left); (D) bright field image merged with nuclei staining reveals a morphological epithelial duct (outlined by white dotted line). (E) β-cells can be discerned by red fluorescence and are located in close association of an offshoot of the duct. (F) Pax6b expressing cells are marked green and in a merged image demonstrate endocrine cells adjacent to the duct. (D,E,F scale bar = 20μm)
Next we used a combination of transgenic analysis and immunofluorescent detection to characterize secondary islets in juvenile fish. Our results show that in juvenile fish (28dpf) secondary islets are located near the center of the pancreas trunk/tail, in close proximity to a morphologically distinct and ptf1a negative, main pancreatic duct (Fig. 1B). The secondary islets maintain this position in adults (Fig. 1C), an observation also made in other teleosts (Morrison et al., 2004). At high magnification it can be seen that these secondary islets are in direct contact with branch points along the main duct (Fig. 1D-F). This observation is consistent with islets forming from progenitors located within or immediately adjacent to the ductal epithelium, as demonstrated for mammalian perinatal islet formation and neogenesis following pancreas injury (Bonner-Weir and Weir, 2005; Inada et al., 2008).
Generation of Notch-responsive reporter fish
Given that the Notch-signaling pathway plays a critical role in secondary transition in mammals (Apelqvist et al., 1999; Esni et al., 2004; Hald et al., 2003; Jensen et al., 2000a; Jensen et al., 2000b; Murtaugh et al., 2003), we next investigated the role of Notch-signaling in endocrine formation in the zebrafish; both early in development during formation of the principal islet and later during the secondary transition. To observe the temporal and spatial pattern of Notch – signaling during this process, we generated transgenic lines that expressed either GFP [Tg(Tp1bglob:eGFP)um13] or a nuclear mCherry fluorescent marker [Tg(T2KTp1bglob:hmgb1-mCherry)jh11] under the control of an element containing 12 RBP-Jκ binding sites (Minoguchi et al., 1997). These lines will henceforth be abbreviated to Tp1bglob:eGFP and Tp1bglob:hmgb1-mCherry respectively. The regulatory sequence in these transgenes directs expression of eGFP or nuclear mCherry when the Notch intra-cellular domain (NICD) and its cofactor RBP-Jκ bind to the sites present in the TP1 element (Fig. 2C and reviewed in (Bray, 2006)).
Figure 2.
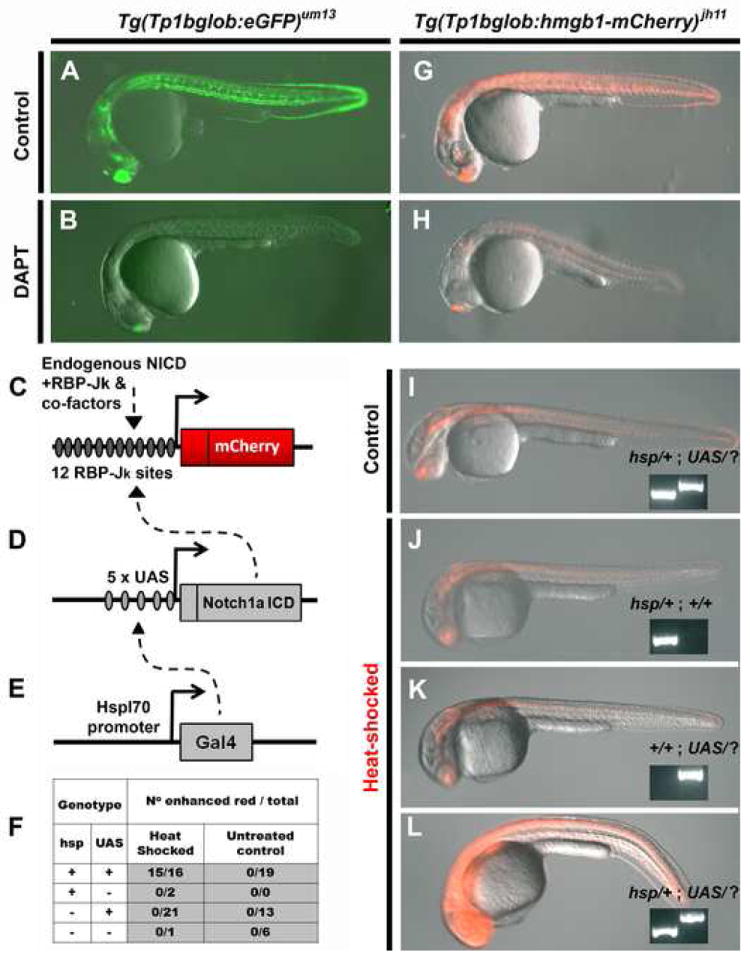
Transgenic zebrafish can report on Notch-signaling activity. The Notch-responsive element was used to generate two transgenic lines Tg(Tp1bglob:eGFP)um13 and Tg(Tp1bglob:hmgb1-mCherry)jh11. In-crossing homozygous animals for both lines gave clutches expressing fluorescent marker in all individuals. Representatives (at 24hpf) for each lines are shown in (A) and (G). Incubation of clutch mates with the Notch-signaling inhibitor, DAPT (100μM) from 80% epiboly to 24hpf, results in reduction in fluorescent protein (B and H). This demonstrates that inhibiting Notch-signaling concomitantly reduces Tp1 transgene activity.
Overexpression of NICD in our red Notch-responsive line was carried out using a Gal4/UAS bipartite system (Scheer and Campos-Ortega, 1999). (C-E) Schematics of the following constructs: (C) Tp1bglob:hmgb1-mCherry, (D) UAS:notch1a-intra and (E) hsp70l:Gal4. In the presence of all three constructs and following heat-shock, the transcriptional activator Gal4 is expressed and transactivates through its upstream-activating site (UAS) (dashed arrow, E), and this directs expression of NICD. This exogenous NICD augments the endogenous NICD (dashed arrow, D), and in concert with RBP-Jκ and co-factors increases activity of the Tp1 element. (F) Progeny from a Tg(Tp1bglob:hmgb1-mCherry ; UAS:notch1a-intra ; hsp70l:Gal4) × Tg(UAS:notch1a-intra) cross were selected for red fluorescence and then either heat-shocked or kept as a control. 6 hours later embryos were scored for red fluorescence that was enhanced above that seen for controls. All embryos were then genotype for presence of hsp70l:Gal4 (hsp) and UAS:notch1a-intra (UAS) transgenes. As can been seen in (F) and the examples shown in (I-L), enhanced red fluorescence is dependent on heat-shock and possession of both the Gal4 and UAS transgenes (inset panel shows genotyping, UAS/? Refers to either UAS/UAS or UAS/+). Together these results indicate that over-expression of NICD augments expression from the Tp1 element.
Exposure time used to record fluorescence images was constant in A and B, G and H and for I-L.
The TP1 element has shown to be Notch-responsive in both mammalian cell culture (Kato et al., 1997) and in transgenic mice (Kohyama et al., 2005; Souilhol et al., 2006). To verify that our transgenic lines using the same TP1 enhancer element were similarly Notch-responsive, we performed the following experiments. 1) Marker expression in both lines Tp1bglob:eGFP and Tp1bglob:hmgb1-mCherry was observed in tissues known to be Notch-responsive such as the developing CNS, vasculature, liver, intestine and pancreas (Fig. 2A, S1, 3). 2) Antagonizing Notch-signaling with a commonly used antagonist, DAPT (Geling et al., 2002), drastically reduces expression from either transgene (Fig. 2B and 2H). 3) Over expression of NICD (from Notch 1a) in Tp1bglob:hmgb1-mCherry fish leads to significant increase in expression of the mCherry marker (Fig. 2F and 2L). NICD overexpression was performed by use of the bipartite and heat-shock inducible system created by Scheer and colleagues (Scheer and Campos-Ortega, 1999; Scheer et al., 2001). As shown in Fig. 2C-E, the augmentation of the mCherry expression is dependent on both transgenic components of the NICD over-expression system being present and only occurs following induction by heat-shock.
Figure 3.
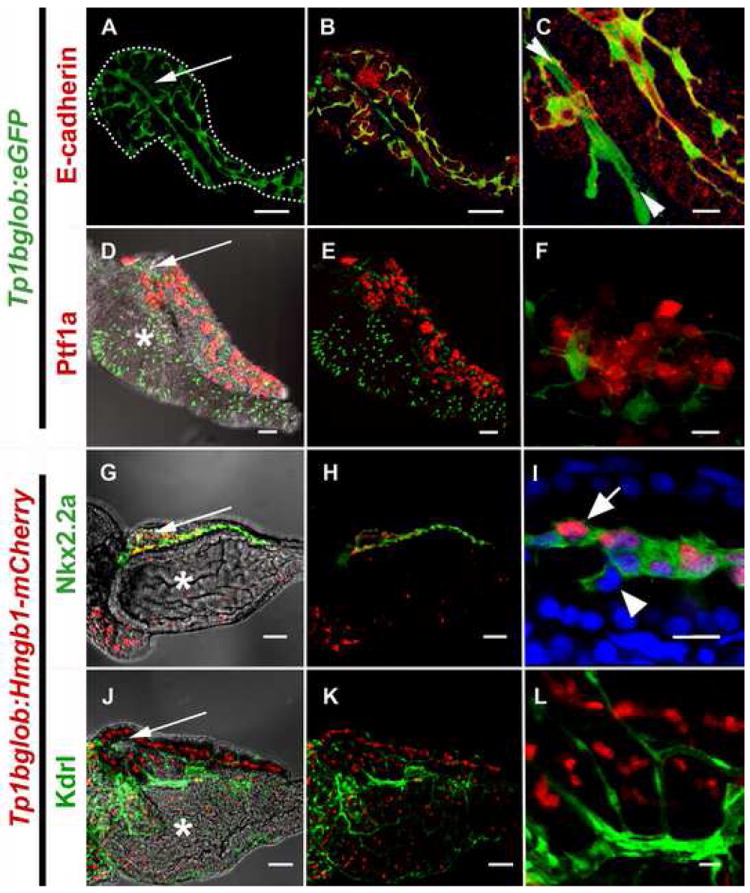
The localization of pancreatic Notch-responsive cells in the 5dpf zebrafish pancreas as imaged by confocal microscopy. (A,B,D,E,G,H,J,K) low power images to see the whole developing organ, outlined (white dots) in (A) (scale bars = 50μM). (D,G,J) brightfield merged images allows visualization of pancreas oriented so that the pancreas lies on top of the intestinal bulb (white *) with the principal islet (white →) and head of the pancreas on the left, and the tail of the pancreas on right. (C,F,I,L) high magnification shows detailed cellular structure (scale bars = 10μM).
(A-F) images of micro-dissected pancreata from Tg(Tp1bglob:eGFP)um13 larvae; PNCs can be visualized as green due to cytoplasmic GFP. GFP positive PNCs can be detected through out the pancreas (A) and immunofluorescent staining to detect E-cadherin (red) indicates vast majority of PNCs are epithelial in nature (B,C). At higher mag. (C), Notch responsive cells that are E-cad negative can also be seen (► ◄). These cells represent endothelium of the early pancreatic, arterial blood supply. (D-F) Tg(ptf1a:Gal4VP16)jh16; Tg(T2KUAS:nfsB-mCherry)jh17 larvae express red fluorescence in a mosaic fashion in the developing exocrine pancreas. The domain of ptf1a driven red fluorescence is exclusive of PNCs. (G-L) images of micro-dissected pancreata from Tg(T2KTp1bglob:hmgb1-mCherry)jh11, larvae; nuclei in PNCs can be visualized in red. (G-I) Tg(-3.5kbnkx2.2:GFP)ia3 larva expressing green fluorescence in a cell-type previously reported to be pancreatic ducts. (I) some but not all GFP expressing cells are also responding to Notch activity. (J-L) Tg(kdrlG-RCFP)zn1 larva expressing green fluorescence in the vasculature. With the exception of the same artery imaged in (C), all endothelium is Notch non-responsive.
Notch responsive cells are located in the developing pancreas surrounding the principal islet
To ascertain if pancreatic Notch-responsive cells (PNCs) are present in larval zebrafish we imaged, by confocal microscopy, micro-dissected pancreata from Tp1bglob:hmgb1-mCherry ; ins:hmgb1-eGFP, compound transgenic larvae. In these fish the nuclei of Notch-responding cells are marked fluorescent red and the nuclei of β-cells fluorescent green. PNCs are clearly located around the principal islet from 2dpf (Fig. S1A). At the time points tested (2, 3, 5dpf) we never detected co-localization of mCherry with eGFP protein. This confirms that differentiated β-cells are not actively responding to Notch signaling. We did detect a few PNCs closely associated with β-cells of the principal islet (Fig. S1B, S1C); however, the majority of PNCs adjacent to principal islet are equatorial.
Quantifying β-cells in the principal islet
Prior to determining whether Notch inhibition could enhance or accelerate the differentiation of β-cells, we first carefully quantified the number of β-cells in the principal islet during early development (2-5dpf). To do this we utilized the transgenic line ins:hmgb1-eGFP, where the nuclei of β-cells can be clearly discerned and easily counted by micro-dissection and confocal microscopy. The result of plotting length against β-cell number is shown in Supplemental figure S1. As expected, β-cell number in the principal islet increases over time. This is consistent with two previous observations; namely, that the β-cells of the principal islet are mitotically active during this stage of larval development (Pisharath et al., 2007) and that β-cells of the principal islet are joined by new cells differentiating from the extra-pancreatic duct (Dong et al., 2007). It is clear that there is a considerable degree of variance in β-cell occurrence between larvae suggesting that β-cell formation is an idiosyncratic process.
Pancreatic Notch-responsive cells are localized throughout the pancreatic parenchyma
The pancreata of 5dpf larvae contain many of the cell types found in an adult pancreas. By 5 days of development (5dpf), the pancreas lies to the right of the intestinal bulb and consists of the head of the pancreas where both the principal islet and the extra-pancreatic duct are located. Posterior to the head, an elongation, called the tail of the pancreas, is situated. Using Tp1bglob:eGFP larvae, the shape and structure of PNCs can be imaged throughout the pancreas, both surrounding the islet and continuing through the tail of the pancreas (Fig. 3A). PNCs appear to be in direct contact with each other through long cellular projections. To better characterize these cells, we simultaneously marked other cell types in the pancreas. E-cadherin localization is a marker of epithelial tissues and immunofluorescent staining demonstrated that nearly all green cells in Tp1bglob:eGFP pancreata are also positive for this marker (Fig. 3B-C). The only Notch-responsive cells that are negative for E-cadherin belong to a linear structure that runs through the islet (Fig. 3C). As it is known that the formation of arterial tissue requires Notch-signaling (Lawson et al., 2001), these E-cadherin-negative PNCs likely represent vascular endothelial cells. To completely rule out an endothelial origin for all PNCs, we utilized Tg(kdrl:GRCFP)zn1 transgenic larvae that express green fluorescent protein in all developing endothelial cells (Cross et al., 2003). We crossed these fish to our Tp1bglob:hmgb1-mCherry line and analyzed localization of red and green fluorescence. These studies confirmed that the only non-epithelial Notch-responsive cells observed were endothelial and located in the islet artery. Close inspection did reveal many developing blood vessels on the surface of the pancreas that are Notch non-responsive and presumably part of the developing venous system (Fig. 3L).
Ptf1a:Gal4-VP16 ; UAS-E1b:nfsB-mCherry fish mark exocrine tissue with red fluorescence. We crossed these fish to the Tp1bglob:eGFP line and looked for co-localization in subsequent progeny. Due to the mosaic nature of the Gal4/UAS system, the degree of exocrine labeling with red fluorescence was mosaic, which facilitated detection of co-labeling by confocal microscopy. Using this approach, in all pancreata analyzed (n=5), no co-localization of mCherry and eGFP was seen (Fig. 3f). This suggests that PNCs are separate from the exocrine pancreatic progenitor population.
Work from Pauls and colleagues characterized Tg(-3.5kbnkx2.2:GFP)ia3 transgenic fish, and demonstrated the utility of this line for marking larval pancreatic ductal epithelium (Pauls et al., 2007). By crossing our red Notch responder line to Tg(-3.5kbnkx2.2:GFP)ia3 fish, we generated transgenic larvae where we can simultaneously observe PNCs and ductal cells. Co-expression of the Notch-responsive reporter and the ductal marker and was restricted to the center of the pancreatic tail and circumferential to the islet (Fig. 3G-H); however, this population is mixed. Some of the green fluorescent cells are also bright red, some weakly red and some not red at all (Fig. 3I). Based on these results, it is clear that there is a heterogeneous population of non-acinar cells forming a linear network through the pancreas. Our observation of heterogeneity in red fluorescence is consistent with two hypotheses: 1/ this cell population is characterized by varying degrees of Notch-activity; and 2/ some cells within this population are differentiating, no longer subject to Notch-signaling and extinguishing expression of mCherry. We see a decline in the number of double positive cells over time as larvae mature, which strongly supports the second hypothesis.
Notch inhibition has profound effects on secondary islet appearance
The mib mutation abrogates Notch-signaling by preventing normal function of the ligand Delta (Koo et al., 2005). This mutant can be used to ascertain the role of Notch in early developmental events including the formation of the principal islet and a host of other events during embryogenesis (Dutta et al., 2008; Itoh and Chitnis, 2001; Leslie et al., 2007). By generating homozygous mib fish that also carried the ins:hmgb1-eGFP transgene, we have shown that impaired Notch signaling, associated with the mutation mibta52b, does cause a moderate increase in β-cell number in the principal islet (Supplemental Figure S1).
However, as the pleiotropic nature of the mib mutation hinders the study of Notch function in the later events of pancreatic organogenesis, such as secondary islet formation, we took advantage of the commonly used Notch-signaling antagonist, DAPT (Geling et al., 2002). We incubated 3 dpf larvae for 48 hs from a variety of transgenic lines, namely: pax6:GFP, gcga:GFP and ins:mCherry (Fig. 4A-E). 3dpf represents a time point where secondary islet formation has not yet occurred. When compared to untreated controls at 5dpf (Fig. 4A, 4D), Notch inhibition with DAPT causes a concomitant loss of PNCs and an increase in occurrence of endocrine cells (Fig. 4B, 4C, 4E).
Figure 4.
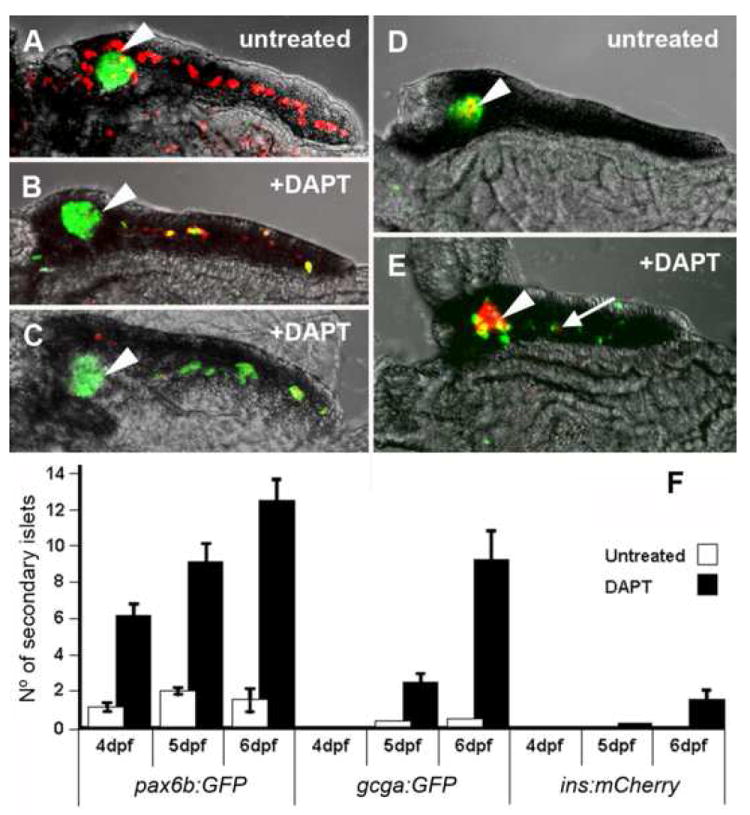
Inhibition of Notch-signaling dramatically induces precocious secondary islet formation. (A-E) Confocal images of 5dpf micro-dissected pancreata. (A) Tg(Tp1bglob:hmgb1-mCherry)jh11; Tg(P0-pax6b:GFP)ulg515 untreated larva display pax6b expressing cells (green) localized only to the principal islet (◄), and PNCs (red) around the islet and through the middle of the pancreatic tail. (B,C) The same compound transgenic larvae, when treated with 100μM DAPT from 3dpf to 5dpf, show a dramatic loss of PNCs concurrent with the appearance of Pax6b endocrine cells along the pancreatic tail. (B) Represents a typical result and (C) an extreme example.
(D) Tg(T2Kins:mCherry)jh2; Tg(gcga:GFP)ia1 larva show a principal islet containing β-cells (red) surrounded by α-cells (green). (E) DAPT treatment causes appearance of numerous α- and a few β-cells (→) along the tail of the pancreas. (F) Larvae of transgenic lines that mark early pan-endocrine cells [Tg(P0-pax6b:eGFP)ulg515], α-cells [Tg(gcga:GFP)ia1] and β-cells [Tg(T2Kins:mCherry)jh2] were examined at three time points for the number of secondary islets. By treating with 100μM of DAPT, significantly more secondary islets were observed. Within either the treated or untreated groups, more pax6b expressing islets were observed, and at an early development time, than either other marker. The marker for β-cells was the most infrequently observed.
In order to quantify DAPT induced secondary islet formation, we micro-dissected pancreata from the treated and untreated transgenic fish, counter stained with DAPI to visualize nuclei and imaged by confocal microscopy. This technique afforded not only the ability to see substantial secondary islets discernible in living larvae, but also smaller islets including isolated single cells, dispersed throughout the pancreatic parenchyma. For the purposes of quantification, endocrine clusters of one or more cells were classed as secondary islets (Fig. 4F). As shown in Fig. 4F, one day after DAPT addition, an effect can already be detected in the number of larvae with pax6b:GFP-positive secondary islets. Following 2 days of treatment with DAPT, the induction of pax6b:GFP-positive secondary islets is further augmented, and an effect can also be detected in the number of secondary islets as determined by use of a glucagon reporter line (gcga:GFP). It takes longer to see a significant increase in insa positive secondary islets, which becomes evident 3 days following initiation of treatment with DAPT. This sequential effect on the precocious expression of pax6b followed by glucagon followed by insa is reminiscent of the normal progression of endocrine differentiation seen in mammalian pancreogenesis (Herrera et al., 1991).
Work from others has shown that inhibiting Notch-signaling, via knockdown of Notch-ligands, prevents formation of zebrafish intra-hepatic and intra-pancreatic ducts (Lorent et al., 2004; Yee et al., 2005). Together these observations are consistent with Notch-inhibition causing precocious endocrine tissue at the expense of ductal epithelium. It is also clear from performing a detailed study by micro-dissection and confocal microscopy, that there are many more single endocrine cells in a secondary islet position than can be discerned by imaging live larvae.
Ultra structural analysis of pancreatic Notch-responsive cells
From confocal images of PNCs in Tp1bglob:eGFP pancreata, it was clear that these cells possess long projections and a very different morphology than those of classical endocrine or acinar tissue. To further characterize these cells we performed ultrastructural analysis using transmission electron microscopy. Transverse sectioning through a 10dpf pancreas indicates a small central lumen (Fig. 5A) consistent with there being one main pancreatic duct at this time point. Higher magnifications indicate that this lumen is enclosed by cells with a distinct morphology, with the remainder of the lumen being comprised of the apical surface of acinar cells. These luminal cells are easily distinguished from acinar cells for several reasons: firstly, these cells are much smaller with smaller nuclei than the surrounding acinar cells; and secondly, these cells display irregular and contorted cell boundaries (Fig. 5B). Finally, where these cells are in contact with each other, clear tight junctions are apparent (Fig. 5C). This last observation is consistent with these cells being an epithelial cell type, as would be predicted for a ductal cell. To ascertain if these ductal cells are the PNCs described previously, we processed pancreata from Tp1bglob:eGFP for electron microscopy and detected GFP by immuno-gold labeling. Cells in the same luminal position and displaying the same morphology were indeed labeled with the immuno-gold particles (Fig. 5D). Altogether, this data demonstrates that the PNCs are epithelial and partially surround the lumen of the central duct.
Figure 5.
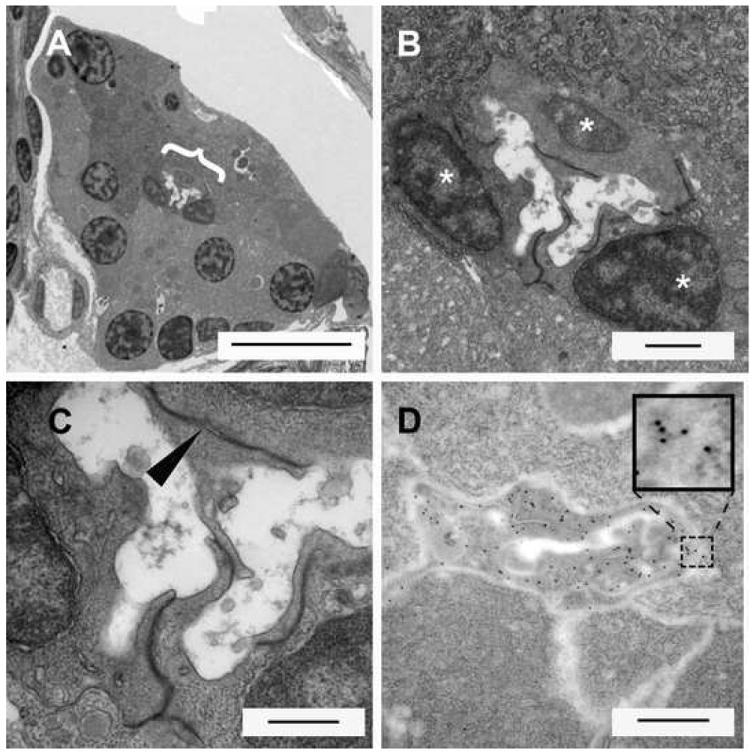
Electromicrographs of transverse sections through the 10dpf larval pancreas. (A) At low power the whole transverse section of the pancreas appears triangular. Large nuclei and zymogen granules characterize acinar cells of the exocrine pancreas. Right in the middle of the pancreas the lumen of the pancreatic duct can be seen ({, scale bar = 10μms). (B) Close up of lumen region in (A). Three cells can be seen around the circumference of the lumen (scale bar = 1μm). (C) These duct localized cells have projection into the lumen. Wherever the cell membranes of these cells are in direct contact with each other, distinctive tight junctions are observed (►, scale bar = 500nm). (D) Immunoelectron microscopy using a gold conjugated secondary to detect GFP protein in a pancreas of a Tg(Tp1bglob:eGFP)um13 larva (scale bar = 500nm). The ultra-structure appears slightly different due to changes in fixation to permit immunolabelling. Gold particles appear very dark and circular (see inset enlargement) and are detected throughout the cells lining the duct. These cells are morphologically similar and in the same position as the cells previously observed by EM. This demonstrates that the previously described PNCs are located in the lining of the pancreatic duct.
Notch-signaling activity persists in adult centroacinar cells
Analysis of Notch responsivity in the adult pancreata of Tp1bglob:hmgb1-mCherry ; ptf1a:eGFP, indicate adult PNCs are found throughout the pancreas parenchyma (Fig. 6 B). At high magnification, adult PNCs can be seen to reside as nodes of 2-3 cells, in the center of the acini, surrounded by polarized mature acinar cells (Fig. 6 C). Not only are PNCs found in both larval to adult stages, but at both stages theses cells display a very similar morphology. Adult PNCs continue to display long extensions (Fig. 6D-E). The localization of adult PNCs within acini and the presence of cellular extensions are characteristics of specialized terminal duct cells, known as centroacinar cells (CACs). Mammalian CACs have been shown to send out projections that contact both endocrine and exocrine cells (Leeson and Leeson, 1986). Further evidence that adult PNCs in adult zebrafish are equivalent to CACs comes from our EM studies. Using immuno-gold labeling of PNCs in Tp1bglob:eGFP fish, demonstrates that GFP positive cells in the adult zebrafish pancreas have irregular shaped nuclei and cytoplasm of low electron density (Fig. 6F), both characteristics of mammalian CACs (Ekholm et al., 1962).
Figure 6.
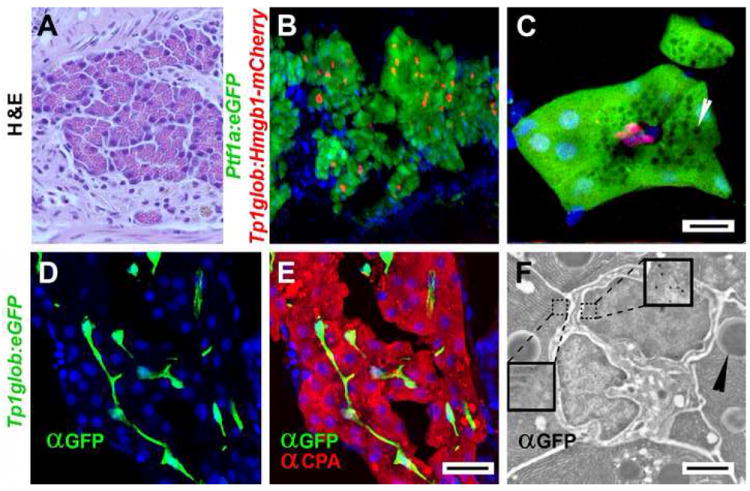
Notch-responsivity marks centroacinar cells in adult zebrafish pancreas. (A) H & E stained sectioned from an adult zebrafish pancreas, demonstrating very similar histology to mammalian pancreas. (B-C) Confocal images of adult pancreata from Tg(ptf1a:eGFP)jh1 ; Tg(Tp1bglob:hmgb1-mCherry)jh11 fish. Acinar cells are easily identified by ptf1a transgene expression (green). The nuclei of PNCs are marked by red fluorescence. (C) High mag image of a single acinus. Apically localized zymogen granules (white arrowheads) appear as dark circles due to exclusion of GFP. The apical surface of the acinar cells surround three red nuclei of PNCs. Due to their location in the adult pancreas and their Notch responsivity, these cells are definitive centroacinar cells (scale bars = 10μm). (D-F) Images from adult Tg(Tp1bglob:eGFP)um13 pancreata. (D,E) Confocal images of immunofluorescent detection for GFP (green) and Carboxypeptidase A (CPA, red) performed on longitudinal cryo-sectioned pancreas (scale bars = 20μm). (F) Immunogold detection of GFP on section through an acinus of Tg(Tp1bglob:eGFP)um13 adult pancreas. EM clearly shows two centroacinar cells with irregular nuclei, surrounded by acinar cells (characterized by the presence of zymogen granules ►). Gold particles are localized to the centroacinar cells (see right inset enlargement to see gold particles) but not acinar cells (see left inset). Scale bar = 2μm)
(B,C,D,E) Nuclei are stained blue with Hoechst.
Discussion
Previous studies in mouse (Apelqvist et al., 1999; Esni et al., 2004; Hald et al., 2003; Jensen et al., 2000a; Jensen et al., 2000b; Murtaugh et al., 2003) and in zebrafish (Esni et al., 2004; Zecchin et al., 2007) suggest that both endocrine and exocrine progenitor cells are tightly regulated by Notch pathway activity. However, the location and identity of such progenitors in zebrafish pancreas have remained unknown. In this current study, we have identified and characterized a novel population of zebrafish Pancreatic Notch-responsive cells (PNCs). There are 3 observations we have made that together suggest PNCs can act as progenitors of endocrine cells: 1) the PNCs are located in ductal epithelium, similar to the location of mammalian endocrine progenitors. 2) PNCs are the only cells undergoing active Notch transduction in the duct. 3) Inhibition of Notch signaling via DAPT leads to a concomitant loss of PNCs and the precocious appearance of endocrine cells along the duct. Whether PNCs go onto form endocrine cells during normal development and whether they form the CACs of the adult or maintain progenitor capacity will require further investigation and the development of genetic-inducible lineage tracing using the Notch responsive element. Our findings also suggest homology between secondary islet formation in zebrafish and the secondary transition in mammals, establishing the study of secondary islet formation as a means to identify conserved mechanisms of β-cell differentiation within vertebrate pancreas.
Prior assessment of Notch pathway activation in adult and embryonic mouse pancreas has been limited to analyzing expression of Hes1, the product of a Notch-target gene. During the secondary transition, Hes1 expression in developing mouse pancreas is confined to the trunks of the branching tubular epithelium (Esni et al., 2004; Jensen et al., 2000b). These observations suggest, but do not rigorously document a domain of activated Notch-signaling, as Notch-independent Hes1 expression has been observed in variety of settings (Curry et al., 2006; Katoh and Katoh, 2007). Later in development and in adult mouse pancreas, Hes1 becomes progressively restricted to centroacinar cells (Miyamoto et al., 2003; Stanger et al., 2005). This poorly characterized cell type lies at the junction between the acinar cells and adjacent terminal ductal epithelium. These cells send out projections that contact both endocrine and exocrine cells (Leeson and Leeson, 1986), and have been shown to rapidly proliferate following either partial pancreatectomy (Hayashi et al., 1999; Hayashi et al., 2003; Nagasao et al., 2005; Seymour et al., 2007), streptozotocin administration, or administration of caerulein (Gasslander et al., 1992).
Our analysis of Notch reporter activity in zebrafish pancreas now rigorously confirms a spatial and temporal pattern of progressively restricted Notch-pathway activation, and also suggest that Notch-responsive cells in larval zebrafish pancreas act as a source of secondary islets. Using this more direct readout of Notch-pathway activation, our studies extend and validate prior mouse studies limited to analysis of Hes1 expression, and also suggest that in this setting Hes1 does indeed represent a valid surrogate marker of Notch pathway activation. During larval life, PNCs share ultrastructual features in common with centroacinar cells, and may represent their larval functional equivalent. These cells exist as nodes comprised of 2-3 cell bodies positioned on the apical surface of acinar cells, and send out long cytoplasmic projections forming tight junctions with other PNCs. In adult zebrafish pancreas, Notch-responsive centroacinar cells form a surprisingly dense network of projections coursing along apical acinar cell membranes and also extending between acinar cells to contact projections from centroacinar cells in neighboring acini. Although not formally quantified, it is our impression that Notch-responsive centroacinar cells are more abundant in zebrafish pancreas than in mouse. However, our ability to appreciate the richness of the centroacinar network in zebrafish pancreas is a direct result of our being able to visualize both cell bodies and projections using a genetic label (Tp1bglob:eGFP) allowing visualization of the entire cytoplasm; the development of similar tools in the mouse may reveal the presence of a similarly rich centroacinar network in this species.
In addition to providing novel insights regarding the presence, location and identity of PNCs in zebrafish pancreas, these studies strongly suggest that these cells carry an endocrine progenitor capacity. While pancreatic expression of a Ngn3 orthologue has not yet been observed in zebrafish pancreas, previous studies have demonstrated expression of both pax6b and neuroD in endocrine progenitor cells giving rise to the principal islet (Biemar et al., 2001; Delporte et al., 2008). Our studies similarly suggest a role for pax6b-positive precursors in zebrafish secondary islet formation. We observe insulin-positive, pax6b-positive cells appearing immediately peripheral to insulin-negative, pax6b-positive cells located within the branching ductal epithelium. As in developing mouse pancreas, our studies also suggest a temporal chain of events in secondary islet formation where Pax6 is expressed first followed by markers of α-cells (i.e. glucagon) and β-cells (i.e. insulin) (Jensen et al., 2000a). Furthermore, it seems very likely that both mammalian and zebrafish secondary islets originate from Notch-responsive progenitor cells located within the nascent ductal epithelium.
Together, these observations allow further comment on the analogy between formation of endocrine cells in zebrafish pancreas and the primary and secondary transitions observed during mammalian pancreas development. In this regard, the substantial increase in endocrine cell mass associated with formation of secondary islets in zebrafish pancreas appears to most closely resemble events occurring during the secondary transition in mammals. Specifically, these corresponding phases of pancreatic development share multiple similarities between mouse and zebrafish, including the spatial association of Notch-responsive progenitors with developing ductal epithelium. Extending this analogy, we would propose that earlier appearing endocrine cells arising from the ventral bud (Dong et al., 2008; Field et al., 2003; Lin et al., 2004) may be analogous to early endocrine cells appearing during the primary transition in mammals (Herrera, 2000; Teitelman et al., 1993). Under this model, the zebrafish principal islet would be considered analogous to Brockmann body-associated endocrine tissue observed in larval lamprey and other jawless fish (Youson and Al-Mahrouki, 1999), and without a mammalian correlate. From an evolutionary point of view, the developmental program(s) required for Brockmann body formation appear to predate the consolidation of endocrine and exocrine tissue into a single tissue, first apparent in cartilaginous fishes. The precise relationship between early appearing endocrine cells in mouse and zebrafish pancreas obviously remains speculative, and better delineation of these evolutionary relationships will require more specific markers for endocrine cell types arising during the different stages of vertebrate pancreas development.
Supplementary Material
Quantification of β-cells in the principal islet, and the effects of Notch inhibition. (A-C) islet confocal images from Tg(T2Kins:hmgb1-eGFP ; T2KTp1bglob:hmgb1-mCherry) larvae. Pictures generated from multiple optical sections rendered to a single image. Very few PNCs (red nuclei) are associated with the β-cells of the principal islet (green nuclei). No cells were detected that were double positive. (D) Results of measuring larvae and then imaging the head of the pancreas to count β-cell number. Following confocal Z- section analysis through the islet, the number of GFP positive nuclei was plotted against larval length. The graph demonstrates that β-cell number increases with larval size.
The same β-cell counting approach was taken with ins:hmgb1-eGFP larvae that were homozygous for the mibta52b (-/-) mutation or phenotypically wildtype (wt) clutch mates (-/+ and +/+). Pancreata from mib and wt larvae were collected at 48 and 72hpf, dissected and imaged by confocal microscopy. β-cell number for the 2 time points were combined for each group (wt and mib) as there has no significant change in number within each group over this time period (48-72hpf). Comparison of β-cell number in mib (mean=26.1, n=10) and wt (20.0, n=10) is shown in a box plot (E). Boxes represent 50% of the data, with the median marked as a dotted line. The vertical lines represent the whole data range. With mib dependent Notch-inhibition, there is a statistically significant, but less than dramatic, effect on β-cell number (ANOVA, p=0.0123). [Note, when the data from the 2 time points used was not combined, there was still significant increase in β-cells at either 48 hpf (p=0.048) and 72 hpf (p=0.033)].
This confirms that Notch inhibition, via the mib mutation, leads to increased number of β-cells in the principal islet.
In order to count β-cells in the principal islet, we used the tg(ins:hmgb1-eGFP) transgenic line that expresses eGFP in the nuclei only. This allows the visualization of individual nuclei surrounded by a negative cytoplasm. The whole principal islet is between 45-50μM thick and we imaged 2μM Z-sections through the islet. In this way each nuclei is captured on multiple sections. Each section was magnified and projected onto paper. By outlining each nucleus, a detailed record of the number of GFP positive nuclei could be recorded.
Acknowledgments
Authors would like to thank the help and expertise of Karishma Fatabhoy and the staff of the Finzcenter, Mike Delannoy (Microscopy Core Facility, Department of Cell Biology, JHU) for EM work, and Marta Gilson (Department of Surgery, JHU) for assistance with statistical analysis. We are also appreciative of Marnie Halpern, Bernard Peers, Michael Pack and Francesco Argenton for generously supplying transgenic lines. This work was supported by grants from the Juvenile Diabetes Research Foundation (MJP, HRP, SY), and NIH (DK61215, DK56211 -SDL, DK080730 - MJP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–6. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–61. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li C, Yuan G, Xie F. Anatomical and histological observation on the pancreas in adult zebrafish. Pancreas. 2007;34:120–5. doi: 10.1097/01.mpa.0000246661.23128.8c. [DOI] [PubMed] [Google Scholar]
- Chiang MK, Melton DA. Single-cell transcript analysis of pancreas development. Dev Cell. 2003;4:383–93. doi: 10.1016/s1534-5807(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Cross LM, Cook MA, Lin S, Chen JN, Rubinstein AL. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscler Thromb Vasc Biol. 2003;23:911–2. doi: 10.1161/01.ATV.0000068685.72914.7E. [DOI] [PubMed] [Google Scholar]
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–35. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 2006;86:842–52. doi: 10.1038/labinvest.3700442. [DOI] [PubMed] [Google Scholar]
- Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol. 2007;304:811–24. doi: 10.1016/j.ydbio.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison JM, Woo Park S, Rhee JM, Leach SD. Characterization of Kras-mediated pancreatic tumorigenesis in zebrafish. Methods Enzymol. 2008;438:391–417. doi: 10.1016/S0076-6879(07)38027-0. [DOI] [PubMed] [Google Scholar]
- Delporte FM, Pasque V, Devos N, Manfroid I, Voz ML, Motte P, Biemar F, Martial JA, Peers B. Expression of zebrafish pax6b in pancreas is regulated by two enhancers containing highly conserved cis-elements bound by PDX1, PBX and PREP factors. BMC Dev Biol. 2008;8:53. doi: 10.1186/1471-213X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- Dong PD, Provost E, Leach SD, Stainier DY. Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas. Genes Dev. 2008;22:1445–50. doi: 10.1101/gad.1663208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Westerfield M, Varga ZM. Notch signaling regulates endocrine cell specification in the zebrafish anterior pituitary. Dev Biol. 2008;319:248–57. doi: 10.1016/j.ydbio.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm R, Zelander T, Edlund Y. The ultrastructural organization of the rat exocrine pancreas. II. Centroacinar Cells, Intercalary and Intralobular Ducts. J Ultrastruct Res. 1962;7:73–83. doi: 10.1016/s0022-5320(62)80029-x. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–24. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Gasslander T, Ihse I, Smeds S. The importance of the centroacinar region in cerulein-induced mouse pancreatic growth. Scand J Gastroenterol. 1992;27:564–70. doi: 10.3109/00365529209000120. [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–94. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnugge L, Meyer D, Driever W. Pancreas development in zebrafish. Methods Cell Biol. 2004;76:531–51. doi: 10.1016/s0091-679x(04)76024-0. [DOI] [PubMed] [Google Scholar]
- Grossman SR, Johannsen E, Tong X, Yalamanchili R, Kieff E. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the J kappa recombination signal binding protein. Proc Natl Acad Sci U S A. 1994;91:7568–72. doi: 10.1073/pnas.91.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–37. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Takahashi T, Kakita A, Yamashina S. Regional differences in the cellular proliferation activity of the regenerating rat pancreas after partial pancreatectomy. Arch Histol Cytol. 1999;62:337–46. doi: 10.1679/aohc.62.337. [DOI] [PubMed] [Google Scholar]
- Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 2003;66:163–74. doi: 10.1679/aohc.66.163. [DOI] [PubMed] [Google Scholar]
- Henkel T, Ling PD, Hayward SD, Peterson MG. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science. 1994;265:92–5. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–22. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257–65. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- Huang H, Vogel SS, Liu N, Melton DA, Lin S. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol Cell Endocrinol. 2001;177:117–24. doi: 10.1016/s0303-7207(01)00408-7. [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–9. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DM. A second insulin gene in fish genomes. Gen Comp Endocrinol. 2004;135:150–8. doi: 10.1016/j.ygcen.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Itoh M, Chitnis AB. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech Dev. 2001;102:263–6. doi: 10.1016/s0925-4773(01)00308-2. [DOI] [PubMed] [Google Scholar]
- Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000a;49:163–76. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000b;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kato H, Taniguchi Y, Kurooka H, Minoguchi S, Sakai T, Nomura-Okazaki S, Tamura K, Honjo T. Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development. 1997;124:4133–41. doi: 10.1242/dev.124.20.4133. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Integrative genomic analyses on HES/HEY family: Notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. Int J Oncol. 2007;31:461–6. [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transgenesis and Gene Trap Methods in Zebrafish by Using the Tol2 Transposable Element. Methods in Cell Biology. 2004;77:201–224. doi: 10.1016/s0091-679x(04)77011-9. [DOI] [PubMed] [Google Scholar]
- Kemp JD, Walther BT, Rutter WJ. Protein synthesis during the secondary developmental transition of the embryonic rat pancreas. J Biol Chem. 1972;247:3941–52. [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays. 2009;31:139–52. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama J, Tokunaga A, Fujita Y, Miyoshi H, Nagai T, Miyawaki A, Nakao K, Matsuzaki Y, Okano H. Visualization of spatiotemporal activation of Notch signaling: live monitoring and significance in neural development. Dev Biol. 2005;286:311–25. doi: 10.1016/j.ydbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, Lee J, Chitnis AB, Kim CH, Kong YY. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132:3459–70. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Leeson TS, Leeson R. Close association of centroacinar/ductular and insular cells in the rat pancreas. Histol Histopathol. 1986;1:33–42. [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–44. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev Biol. 2004;274:491–503. doi: 10.1016/j.ydbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–66. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K, Ning G, Fogerty J, Besharse JC. Visualization of identified GFP-expressing cells by light and electron microscopy. J Histochem Cytochem. 2003;51:271–4. doi: 10.1177/002215540305100301. [DOI] [PubMed] [Google Scholar]
- Minoguchi S, Taniguchi Y, Kato H, Okazaki T, Strobl LJ, Zimber-Strobl U, Bornkamm GW, Honjo T. RBP-L, a transcription factor related to RBP-Jkappa. Mol Cell Biol. 1997;17:2679–87. doi: 10.1128/mcb.17.5.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–76. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Morrison CM, Pohajdak B, Tam J, Wright JR., Jr Development of the islets, exocrine pancreas, and related ducts in the Nile tilapia, Oreochromis niloticus (Pisces: Cichlidae) J Morphol. 2004;261:377–89. doi: 10.1002/jmor.10256. [DOI] [PubMed] [Google Scholar]
- Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes. 2009 doi: 10.2337/db08-0628. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasao J, Yoshioka K, Amasaki H, Tsujio M, Ogawa M, Taniguchi K, Mutoh K. Morphological changes in the rat endocrine pancreas within 12 h of intravenous streptozotocin administration. Anat Histol Embryol. 2005;34:42–7. doi: 10.1111/j.1439-0264.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Park SW, Davison JM, Rhee J, Hruban RH, Maitra A, Leach SD. Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology. 2008;134:2080–90. doi: 10.1053/j.gastro.2008.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls S, Zecchin E, Tiso N, Bortolussi M, Argenton F. Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev Biol. 2007;304:875–90. doi: 10.1016/j.ydbio.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Parsons MJ. Nitroreductase mediated cell ablation in transgenic zebrafish embryos. In: Lieschke GJ, O AC, K K, editors. Zebrafish - Methods and ProtocolsMethods in Molecular Biology. Vol. 546. 2009. in press. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–29. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983;35:63–72. doi: 10.1016/0022-4804(83)90127-0. [DOI] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–8. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol. 1999;404:515–36. [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–70. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ, Jr, Wright CV, Leach SD. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–26. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- Souilhol C, Cormier S, Monet M, Vandormael-Pournin S, Joutel A, Babinet C, Cohen-Tannoudji M. Nas transgenic mouse line allows visualization of Notch pathway activity in vivo. Genesis. 2006;44:277–86. doi: 10.1002/dvg.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, Depinho RA, Wu H, Melton DA, Dor Y. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–95. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–9. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- Villefranc JA, Amigo J, Lawson ND. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn. 2007;236:3077–87. doi: 10.1002/dvdy.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev Biol. 2005;286:182–94. doi: 10.1016/j.ydbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon; 1993. [Google Scholar]
- Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Yee NS, Lorent K, Pack M. Exocrine pancreas development in zebrafish. Dev Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Youson JH, Al-Mahrouki AA. Ontogenetic and phylogenetic development of the endocrine pancreas (islet organ) in fish. Gen Comp Endocrinol. 1999;116:303–35. doi: 10.1006/gcen.1999.7376. [DOI] [PubMed] [Google Scholar]
- Zecchin E, Filippi A, Biemar F, Tiso N, Pauls S, Ellertsdottir E, Gnugge L, Bortolussi M, Driever W, Argenton F. Distinct delta and jagged genes control sequential segregation of pancreatic cell types from precursor pools in zebrafish. Dev Biol. 2007;301:192–204. doi: 10.1016/j.ydbio.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Zhang H, Maeshima A, Kurihara H, Miyagawa J, Takeuchi T, Kojima I. Up-regulation of the expression of activins in the pancreatic duct by reduction of the beta-cell mass. Endocrinology. 2002;143:3540–7. doi: 10.1210/en.2002-220089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantification of β-cells in the principal islet, and the effects of Notch inhibition. (A-C) islet confocal images from Tg(T2Kins:hmgb1-eGFP ; T2KTp1bglob:hmgb1-mCherry) larvae. Pictures generated from multiple optical sections rendered to a single image. Very few PNCs (red nuclei) are associated with the β-cells of the principal islet (green nuclei). No cells were detected that were double positive. (D) Results of measuring larvae and then imaging the head of the pancreas to count β-cell number. Following confocal Z- section analysis through the islet, the number of GFP positive nuclei was plotted against larval length. The graph demonstrates that β-cell number increases with larval size.
The same β-cell counting approach was taken with ins:hmgb1-eGFP larvae that were homozygous for the mibta52b (-/-) mutation or phenotypically wildtype (wt) clutch mates (-/+ and +/+). Pancreata from mib and wt larvae were collected at 48 and 72hpf, dissected and imaged by confocal microscopy. β-cell number for the 2 time points were combined for each group (wt and mib) as there has no significant change in number within each group over this time period (48-72hpf). Comparison of β-cell number in mib (mean=26.1, n=10) and wt (20.0, n=10) is shown in a box plot (E). Boxes represent 50% of the data, with the median marked as a dotted line. The vertical lines represent the whole data range. With mib dependent Notch-inhibition, there is a statistically significant, but less than dramatic, effect on β-cell number (ANOVA, p=0.0123). [Note, when the data from the 2 time points used was not combined, there was still significant increase in β-cells at either 48 hpf (p=0.048) and 72 hpf (p=0.033)].
This confirms that Notch inhibition, via the mib mutation, leads to increased number of β-cells in the principal islet.
In order to count β-cells in the principal islet, we used the tg(ins:hmgb1-eGFP) transgenic line that expresses eGFP in the nuclei only. This allows the visualization of individual nuclei surrounded by a negative cytoplasm. The whole principal islet is between 45-50μM thick and we imaged 2μM Z-sections through the islet. In this way each nuclei is captured on multiple sections. Each section was magnified and projected onto paper. By outlining each nucleus, a detailed record of the number of GFP positive nuclei could be recorded.


