Abstract
Cortisol responses are typically more pronounced under low controllability conditions, yet little is known about the role of individual differences. This study examined whether cortisol response to a situation with low controllability differs as a function of preexisting control beliefs and age. We manipulated level of controllability using a driving simulator. Control beliefs were assessed prior to the lab session. Salivary cortisol was measured before and after the driving simulation. Participants were 152 adults aged 22-84 from a Boston area sample. In comparison to the normal controllability condition, those in the low controllability condition reported less perceived control over driving, supporting the effectiveness of the manipulation. In the low controllability condition those with higher control beliefs showed a greater cortisol response than those with low control beliefs. Older adults showed a greater cortisol response than younger adults during the challenge. Implications of acute cortisol responses for performance outcomes are discussed.
Keywords: Control beliefs, Situational controllability, Cortisol response, Age differences
Introduction
Stress-related neuroendocrine responses, such as in the hypothalamic-pituitary-adrenal (HPA) axis, under challenging situations are especially pronounced when the amount of controllability over the situation is limited (Dess et al., 1983; Dickerson and Kemeny, 2004; Hanson et al., 1976). There is also some evidence that the cortisol response to challenge varies as a function of individual differences in factors such as general beliefs about control over desired outcomes (Pruessner et al., 2005; Pruessner et al., 1999). Higher control beliefs are generally associated with lower cortisol response. It may be that the greatest reactivity occurs when situational expectations for control conflict with actual experience – as, for example, when someone with high expectations of control (high control beliefs) is confronted with a situation where despite their best efforts, situational controllability is low. Previous studies (Evans et al., 1993) have indicated that a lack of correspondence, or incongruence, between situational controllability (i.e., environmental opportunities to exercise control) and personal control (e.g., control beliefs, desire for control) leads to negative psychological, physiological, and behavioral outcomes. We are aware of two studies that have focused on cortisol reactivity as a function of both general control beliefs and situational controllability over the stressor (Bollini et al., 2004; Peters et al., 2003). In one study control beliefs did not moderate the effects of controllability on cortisol response (Peters et al., 2003). The second study (Bollini et al., 2004) showed that participants with high control beliefs had a smaller cortisol response when given the opportunity for high controllability. In the present study we examined the effects of low controllability. We hypothesized a greater cortisol response when those with higher control beliefs are placed in a situation with low controllability.
Age is another potential moderator of cortisol response (Kudielka et al., 2009). Older adults typically show greater cortisol response than younger adults in challenging situations, yet the domains associated with challenge may vary by age. For instance older adults react more when exposed to circumstances susceptible to threats of age declines such as performance in cognitive tasks or driving challenges (Neupert et al., 2006; Seeman and Robbins, 1994).
The goal of the current study was to experimentally create a low controllability situation and to analyze the level of cortisol response as a function of general control beliefs and age. Other studies have demonstrated that it is possible to experimentally manipulate situational controllability, such as by varying the opportunity to regulate noise intensity (Bollini et al., 2004). In the current study we adapted a paradigm for inducing a low controllability situation using a driving simulation scenario. In the low controllability situation we expected a greater cortisol response, especially for those with preexisting higher control beliefs. Older adults were expected to show greater cortisol response than younger adults, particularly when in the low controllability driving scenario.
Method
Participants
Participants were 152 adults recruited from a list of names randomly sampled by Survey Sampling International from all the zip codes located within a ten mile radius of the test site in west suburban Boston. The sample was obtained after applying several exclusion criteria including: not a current driver, poor self-rated health compared to other people the same age, low level of educational attainment (no high school degree or General Equivalency Diploma), and a history of stroke in the last five years, serious head injury, Parkinson’s disease, or other neurological disorders. Also, non-English speakers and those who learned English after age 10, as well as those with more than two errors on the Pfeiffer Short Portable Mental Status Questionnaire (Pfeiffer, 1975) were excluded. The 145 respondents who completed the study ranged in age from 22 to 84 years (M = 56.84, SD = 15.80) and included 44.8 % women. We created two age groups: younger (N = 72, M = 43.39) and older (60 years old or more, N = 73, M = 70.11). The level of education ranged from 12 to 20 years (M = 16.92, SD = 2.13), with 82.7 % having a Bachelor’s degree or higher.
Procedure
Participants were informed that the study involved problem solving, memory tasks, a driving simulation scenario, as well as mailed questionnaires. During the lab session, we manipulated the level of actual control over the situation using a driving simulator. The participants were randomly assigned to one of two experimental driving conditions: normal (N = 72) and low situational controllability (N = 73). In the low controllability condition, control over steering and braking was reduced using a lowered coefficient of friction on the road surface (.4) and 18 wind gusts (10 to 25 seconds each) to simulate slippery and windy conditions (Funke et al., 2007). For the normal driving condition the coefficient of friction was .8 and there was no wind.
For both conditions, the scenario began with 8,800 feet of easy driving (straight roads, no obstacles) followed by more complex driving, which included curvature of the roadway, stop signs, traffic lights, obstacles (e.g., construction sites) and concurrent cognitive tasks (i.e., digit monitoring task, visual attention task, and the logical memory task from the Wechsler Memory Scale Third Edition (Wechsler, 1997). The entire scenario lasted 19.13 minutes on average, with some variation due to differences in driving speed (SD = 2.22; range = 15.96 to 33.40). The driving apparatus used was the STISIM Driving Simulator - M100 (www.stisimdrive.com). Participants seated themselves a comfortable distance from a 24 inch widescreen flat monitor with a sixty degree field of view as if from the driver seat of a car. Steering, acceleration, and braking were accomplished with the Logitech G25 Racing Wheel and pedals. The simulator was set for automatic transmission.
Participants were asked ahead of time not to eat or brush their teeth within thirty minutes of the appointment. Four saliva samples were taken during the lab session, which was scheduled for times ranging from 8 AM to 7:30 PM, at the participants’ convenience. The first saliva sample was taken right after the participants arrived and had read and signed the consent form. The second saliva sample was taken 30 minutes later, right after participants completed questionnaires and a brief period of driving instruction and adaptation, and just before the driving challenge. A third sample was taken immediately after the driving challenge. The fourth sample was taken about 30 minutes after the end of the driving session. During this 30-minute post-driving period, participants were engaged in cognitive tasks. The second and fourth samples were used to obtain the cortisol response.
Saliva samples were frozen immediately after each laboratory session and stored at -20°C until delivery to the Laboratory for Biological Health Psychology at Brandeis University for assay. After thawing, salivettes were centrifuged at 2,000 × g for 10 minutes, which resulted in a clear supernatant of low viscosity. Salivary cortisol concentrations were measured in duplicate using a commercially available chemiluminescence immunoassay (CLIA; RE62019) with a sensitivity of .16 ng/ml (IBL International, Toronto, Canada). Inter-assay variability was 3.73% and intra-assay variability was 5.94%.
Measures
General Control Beliefs
This measure, completed before the lab session via a mailed questionnaire, examines participants’ beliefs about the degree they can influence what happens in their lives. Participants completed the Midlife in United States (MIDUS) Control Beliefs scale (Lachman and Weaver, 1998) which includes twelve statements (Cronbach’s α = .81) regarding personal mastery (e.g., “I can do just about anything I really set my mind to”) and perceived constraints (e.g., “Other people determine most of what I can and cannot do”). Participants indicated the degree to which they agree or disagree with each statement as applied to themselves on a scale from 1 (strongly agree) to 7 (strongly disagree). The personal mastery items were reverse coded so that higher scale scores indicate higher control beliefs. Based on a median split (median = 6) we created two groups: lower control beliefs (N = 70, M = 5.14) and higher control beliefs (N = 73, M = 6.51).
Situational Controllability
As presented in the procedure section, we experimentally created a low controllability situation and a normal controllability situation, by modifying the parameters of the driving simulator (i.e., coefficient of friction on the road surface and instances of wind).
Perceived Control over the Driving Situation
For a manipulation check, after the driving period was completed, the participants reported their level of perceived control: “On a scale of 1 to 5 where 1 is no control and 5 is a lot of control, how much control did you feel you had during the driving segment?”
Cortisol Response
Cortisol reactivity response was operationalized as the difference between the fourth (30 minutes after driving) and the second cortisol samples (just before the driving session).
Covariates
Driving Performance
The driving simulator recorded the position of the vehicle relative to the roadway dividing line and calculated the percentage of distance traveled out of lane (both over the center line and off the road edge). This value was multiplied by -1 and was used as an indicator of driving performance. Higher scores indicated higher performance.
Cognitive Performance during Driving
Three concurrent cognitive tasks were included during the driving segment: digit monitoring, visual attention, and logical memory. During the first task, participants listen to a three-minute recording of a series of single-digit numbers and had to indicate when they hear three odd digits in a row. The final score was the total number of correct responses provided. For the visual attention task, two gray boxes each containing a red diamond shape were located in the upper left and right hand corners of the screen. Participants were instructed to respond by pressing a button on the steering wheel if they notice that the diamond in one of the boxes has changed to a triangle. The task was performed for a distance of 13,600 feet. The average reaction time for all the correct responses was computed in seconds and then multiplied by -1, so that higher values indicate better performance. Logical memory was measured at the very end of the driving simulation. Participants listened to a recording of a short, three sentences long story and then they had to immediately repeat the short story as close to verbatim as possible. Participants were given a score of 0 to 25 for recall of individual story units.
Time Since Awakening
The number of hours since awakening at the start of the lab session was computed for each participant.
Medication Use
Participants also listed all medications they took within two days of the interview. Using information about medications that could affect cortisol from http://www.epocrates.com, we created a new variable indicating the absence (49%) or presence (51%) of at least one of the following types of medications (Granger et al., 2009): corticosteroids, glucocorticoids, beta blockers, beta agonists, birth control, estrogen, testosterone, cholesterol medication, and nonsteroidal anti-inflammatory drugs.
Data Analysis
The data analysis included all available data from the participants who completed the experimental driving session (N = 145).
Data Preparation for Cortisol
Cortisol scores greater than 60 nmol/l were assigned as missing data in order to minimize the influence of outliers or measurement inaccuracies. This was the case for two participants, for the fourth saliva sample. Nevertheless, when their data were included in the analysis, the pattern of results did not change. The cortisol scores were log-transformed to correct for nonnormality (Nicolson, 2008).
Manipulation Check
The effectiveness of the experimental manipulation was tested in an ANCOVA model with age, sex, and general control beliefs as covariates. The outcome variable was the level of perceived control during the experimental manipulation.
The Role of Age, Control Beliefs, and Situational Controllability in Cortisol Response
The hypotheses were tested using a 2 (situational controllability: low vs. normal) × 2 (general control beliefs: low vs. high levels) × 2 (age: younger vs. older) ANCOVA model. The dependent variable was the cortisol response operationalized as the difference between the two log-transformed cortisol values (i.e., saliva sample #4 - saliva sample #2). Given that the difference of two logarithms is equivalent to the logarithm of their ratio, our outcome represents the proportion of change. Several covariates were identified based on the simple correlations with cortisol response, cortisol levels, general control beliefs, and experimental condition (see Table 1): education, time since awakening, driving performance, and visual attention task (the only measure of cognitive performance during driving associated with cortisol). When the total duration of the driving segment or medication use were included as covariates, the pattern of results did not change.
Table 1.
Means, Standard Deviations, and Intercorrelations of Study Variables
| M | SD | Age | Sex | Education | Situational Controllability | Perceived Control over the Driving Situation | General Control Beliefs | |
|---|---|---|---|---|---|---|---|---|
| Age | 56.84 | 15.80 | ||||||
| Sex (1 = male, 2 = female) | --- | --- | -.13 | |||||
| Education (years) | 16.92 | 2.13 | -.13 | -.01 | ||||
| Situational Controllability (1 = low control, 2 = normal control) | --- | --- | -.02 | .05 | -.06 | |||
| Perceived Control over the Driving Situation | 3.57 | .91 | -.12 | -.14 | -.01 | .32*** | ||
| General Control Beliefs | 5.84 | .86 | -.03 | .11 | .27** | .08 | -.04 | |
| Cortisol Sample #1 | .89 | .26 | .19* | -.12 | -.13 | -.01 | -.06 | .01 |
| Cortisol Sample #2 | .85 | .25 | .24** | -.10 | -.12 | .01 | .02 | -.01 |
| Cortisol sample #4 | .87 | .34 | .46*** | .01 | -.03 | -.01 | -.07 | .04 |
| Cortisol Response | .02 | .30 | .32*** | .10 | .07 | -.01 | -.10 | .05 |
| Time since Awakening | 6.36 | 3.27 | -.18* | -.02 | .09 | -.01 | .02 | -.07 |
| Driving Performance | -.34 | .56 | -.16 | .13 | .19* | .22* | .23** | .19* |
| Digit Monitoring | 12.52 | 3.13 | -.36*** | .04 | .23** | -.02 | .10 | .02 |
| Visual Attention | -1.48 | .52 | -.40*** | -.09 | .11 | -.10 | .16 | .03 |
| Logical Memory | 13.83 | 3.91 | -.40*** | .21* | .14 | .03 | -.01 | .03 |
| Driving Duration (minutes) | 19.13 | 2.22 | .34*** | .17* | .04 | -.16 | -.24** | .16 |
| Medication (0 = absence, 1 = presence) | --- | --- | .34*** | -.05 | -.15 | -.12 | -.05 | -.03 |
| Cortisol sample #1 | Cortisol sample #2 | Cortisol sample #4 | Cortisol Response | Time since Awakening | Driving Performance | Digit Monitoring | Visual Attention | |
| Cortisol sample #2 | .83*** | |||||||
| Cortisol sample #4 | .44*** | .52*** | ||||||
| Cortisol Response | -.21* | -.26** | .69*** | |||||
| Time since Awakening | -.38*** | -.42*** | -.35*** | -.04 | ||||
| Driving Performance | -.12 | -.06 | -.09 | -.05 | .05 | |||
| Digit Monitoring | -.14 | -.04 | -.14 | -.13 | .02 | .29** | ||
| Visual Attention | -.11 | -.12 | -.22* | -.14 | .04 | .22* | .37*** | |
| Logical Memory | -.08 | -.11 | -.16 | -.10 | .11 | .24** | .37*** | .15 |
| Cortisol sample #1 | Cortisol sample #2 | Cortisol sample #4 | Cortisol Response | Time since Awakening | Driving Performance | Digit Monitoring | Visual Attention | Logical Memory | Driving Duration | |
| Driving Duration | .05 | .09 | .17 | .11 | -.07 | .05 | -.17* | -.32*** | -.02 | |
| Medication | .09 | .09 | .16 | .10 | -.17* | -.22** | -.17* | -.08 | -.10 | .07 |
Notes.
p < .05;
p < .01;
p < .001; cortisol values are log-transformed
The correlations between the variables included in the analytic model are presented in bold.
Results
Descriptives
The means, standard deviations, and intercorrelations of all variables are presented in Table 1. The correlations between the variables included in the analytic model are presented in bold.
There was a positive significant association between age and cortisol, both in terms of levels and change. Overall, older participants had higher levels of cortisol at all time points in the study and had a greater cortisol response to the experimental manipulation. The descriptive results were in line with previous studies showing negative associations between age and cognitive functioning, in our case cognitive performance during driving. In addition, higher performance on the visual attention task was associated with better driving performance. The associations between situational controllability (i.e., experimental conditions) and age, sex, and education were not significant.
Manipulation Check
Participants in the low controllability condition reported significantly less perceived control over the driving situation [M = 3.29 vs. M = 3.86; F(1,137) =17.09, p <.001] than those in the normal controllability condition, suggesting that the experimental manipulation was successful in inducing low levels of situational controllability.
The Role of Control Beliefs and Situational Controllability in Cortisol Response
The main effects of control beliefs and situational controllability on cortisol response were not significant. Analyses (see Figure 1) revealed the expected significant interaction between the level of control beliefs and situational controllability [F(1, 113) = 5.50, p =.021]. In the low controllability condition, those with higher prior control beliefs reacted more than those with lower control beliefs [F(1, 113) = 4.90, p =.029]. In the normal controllability condition, cortisol response did not vary significantly as a function of control beliefs.
Figure 1.
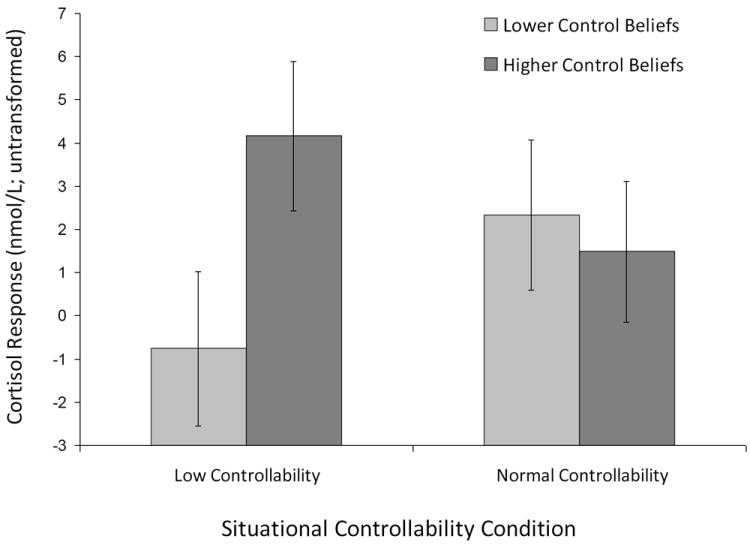
Cortisol Response as a Function of Level of Situational Controllability and Control Beliefs; Error bars are one standard error of the mean
Age Differences in Cortisol Response
As predicted, compared to younger adults, older adults showed a greater cortisol response during the driving challenge [Figure 2; F(1, 113) = 4.49, p = .036]. Age did not interact with control beliefs or situational controllability and the three-way interaction was not significant.
Figure 2.
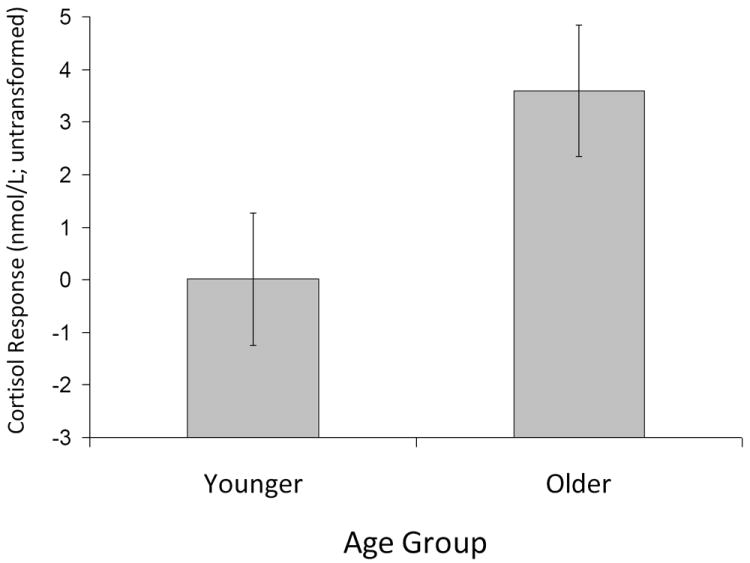
Age Differences in Cortisol Response; Error bars are one standard error of the mean
Discussion
The aim of this study was to examine the effects of low situational controllability on cortisol response and the role of individual differences in general control beliefs and age. First, our driving simulation procedure was found to be effective for manipulating levels of situational controllability. We examined the level of perceived control over the driving situation as a manipulation check, and on average, the level of perceived control was higher for the participants in the normal controllability condition than for those in the low controllability condition.
In addition, as predicted, the results showed that under low controllability, individual differences in control beliefs make a difference for cortisol response. Previously, Bollini and her colleagues found that in a highly controllable situation those with high control beliefs had a lower cortisol response (Bollini et al., 2004). In contrast, we examined the effects of a low controllability situation. In this condition we found that those with higher control beliefs had a greater cortisol response.
Our results provide additional evidence regarding the role of individual differences in control in relation to cortisol response to situations with limited opportunities for control. In the low controllability situation, those with high control beliefs had a greater cortisol response than those with low control beliefs. Irrespective of age, the low controllability situation led to greater cortisol response for those who generally have high expectations of control.
Consistent with previous studies (Neupert et al., 2006; Seeman and Robbins, 1994), older participants showed, on average, greater levels of cortisol response. This was found regardless of general control beliefs or controllability condition, perhaps because the driving domain coupled with divided attention involving cognitive tests is particularly stressful for older adults (Matthews et al., 1999). Interestingly, younger participants did not show a cortisol response to the driving challenge, on average, as shown in Figure 2, perhaps because they have more experience with video/driving games or find driving and divided attention to cognitive tasks less stressful than older adults.
In summary, the results support the importance of considering individual differences in the cortisol response to challenge. General control beliefs were found to be a source of individual differences in cortisol response. In a situation with low controllability, it was those who typically expect to have high control who showed the greater cortisol response.
Future studies are needed to understand the consequences of higher cortisol response, especially for those with high general control beliefs. In the context of our study, a higher cortisol response could be indicative of either heightened stress and anxiety or the mobilization of resources to regain control over the situation. The latter possibility is compatible with past work suggesting that, when placed in a low control situation, the participants with a high-level of control make attributions that allow them to regain control (Taylor and Sherman, 2008). In order to disentangle and isolate these processes, in future work we will examine the implications of the heightened cortisol response for cognitive performance and the mechanisms involved (Domes et al., 2002; Kirschbaum et al., 1996; Lupien et al., 1997; Wright et al., 2005) especially for older adults and those with high control beliefs. We can examine whether those who have high control beliefs, who typically show better performance on behavioral and cognitive tasks (Lachman et al., 2011), will show greater resilience, recover more quickly from the low controllability situation, and thereby maintain good performance.
Highlights.
-
▪
We examined cortisol response to a low controllability situation as a function of age and prior general control beliefs.
-
▪
The low controllability situation was experimentally induced using a driving simulation.
-
▪
In the low controllability situation those with higher general control beliefs showed a greater cortisol response.
-
▪
Older adults showed a greater cortisol response than younger adults across conditions.
Acknowledgments
This research was supported by a grant from the NIA (# RO1 AG 17920).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bollini AM, Walker EF, Hamann S, Kestler L. The influence of perceived control and locus of control on the cortisol and subjective responses to stress. Biological Psychology. 2004;67:245–260. doi: 10.1016/j.biopsycho.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dess NK, Linwick D, Patterson J, Overmier JB, Levine S. Immediate and proactive effects of controllability and predictability on plasma cortisol responses to shocks in dogs. Behavioral Neuroscience. 1983;97:1005–1016. doi: 10.1037//0735-7044.97.6.1005. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middleaged women: high responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 2002;27:843–853. doi: 10.1016/s0306-4530(01)00085-3. [DOI] [PubMed] [Google Scholar]
- Evans GW, Shapiro DH, Lewis MA. Specifying dysfunctional mismatches between different control dimensions. British Journal of Psychology. 1993;84:255–273. [Google Scholar]
- Funke G, Matthews G, Warm JS, Emo AK. Vehicle automation: a remedy for driver stress? Ergonomics. 2007;50:1302–1323. doi: 10.1080/00140130701318830. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Hanson JD, Larson ME, Snowdon CT. The effects of control over high intensity noise on plasma cortisol levels in rhesus monkeys. Behavioral Biology. 1976;16:333–340. doi: 10.1016/s0091-6773(76)91460-7. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Science. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Neupert SD, Agrigoroaei S. The relevance of control beliefs for health and aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. Academic Press; Burlington, MA: 2011. pp. 175–190. [Google Scholar]
- Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. Journal of Personality and Social Psychology. 1998;74:763–773. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NPV, Hauger RL, McEwen BS, Meaney MJ. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. The Journal of Clinical Endocrinology & Metabolism. 1997;82:2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- Matthews G, Joyner LA, Newman R. Age and gender differences in stress responses during simulated driving. Proceedings of the Human Factors and Ergonomics Society Annual Meeting; 1999. pp. 1007–1011. [Google Scholar]
- Neupert SD, Miller LMS, Lachman ME. Physiological reactivity to cognitive stressors: variations by age and socioeconomic status. International Journal of Aging and Human Development. 2006;62:221–235. doi: 10.2190/17DU-21AA-5HUK-7UFG. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Sage Publications; Thousand Oaks, CA: 2008. pp. 37–74. [Google Scholar]
- Peters ML, Godaert GLR, Ballieux RE, Heijnen CJ. Moderation of physiological stress responses by personality traits and daily hassles: Less flexibility of immune system responses. Biological Psychology. 2003;65:21–48. doi: 10.1016/s0301-0511(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatric Society. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord K, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality and Individual Differences. 1999;27:477–489. [Google Scholar]
- Seeman TE, Robbins RJ. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocrine Reviews. 1994;15:233–260. doi: 10.1210/edrv-15-2-233. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Sherman DK. Self-enhancement and self-affirmation: The consequences of positive self-thoughts for motivation and health. In: Shah JY, Gardner WL, editors. Handbook of Motivation Science. Guilford Press; New York, NY: 2008. pp. 57–70. [Google Scholar]
- Thompson SC, Sobolew-Shubin A, Galbraith ME, Schwankovsky L, Cruzen D. Maintaining perceptions of control: Finding perceived control in low-control circumstances. Journal of Personality and Social Psychology. 1993;64:293–304. doi: 10.1037//0022-3514.64.2.293. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) manual. The Psychological Corporation; New York: 1997. [Google Scholar]
- Wright CE, Kunz-Ebrecht SR, Iliffe S, Foese O, Steptoe A. Physiological correlates of cognitive functioning in an elderly population. Psychoneuroendocrinology. 2005;30:826–838. doi: 10.1016/j.psyneuen.2005.04.001. [DOI] [PubMed] [Google Scholar]


