Abstract
Purpose
To assess the relationships of walking distance, frequency, and intensity to the prevalence of antidiabetic, antihypertensive, and LDL cholesterol–lowering medications use.
Methods
Cross-sectional analyses of 32,683 female and 8112 male participants of the National Walkers’ Health Study, of whom 2.8% and 7.4% reported antidiabetic, 14.3% and 29.0% reported antihypertensive, and 7.3% and 21.5% reported LDL cholesterol–lowering medication use, respectively.
Results
Weekly walking distance, longest walk, and walking intensity were inversely related to the prevalence of antidiabetic (males: P < 0.001, females: P < 0.0001), antihypertensive (males: P < 0.01, females: P < 0.0001), and LDL cholesterol–lowering medications (males: P < 0.01, females: P < 0.0001). Each medication remained significantly related to both walking intensity and longest weekly walk when adjusted for total weekly distance. Compared with men and women who walked at a speed of < 1.2 m/s, those who walked > 2.1 m/s had 48% and 52% lower odds for antihypertensive, 68% and 59% lower odds for antidiabetic, and 53% and 40% lower odds for LDL cholesterol–lowering medications, respectively, when adjusted for age, smoking, and diet. The longest usual weekly walk was a better discriminator of medication status than the total cumulative distance per week, particularly in men.
Conclusion
These results are consistent with the hypothesis that antidiabetic, antihypertensive, and LDL cholesterol–lowering medication use may be reduced substantially by walking more intensely and farther each week, and by including longer walks.
INTRODUCTION
Diabetes, hypertension, and high cholesterol are common chronic conditions of modern society. These conditions increase risks for cardiovascular disease morbidity and mortality and require lifetime treatments, with no expectation of cure. The annual economic costs for medical treatments and of lost productivity from diabetes and cardiovascular disease are estimated at $130 billion and $368 billion, respectively [10]. Although exercise is recommended for the prevention of all three maladies, 41% percent of women and 35% of men engaged in no leisure-time physical activity, and 73% of women and 66% of men are inadequately active [23].
Recent physical activity recommendations by governmental and nongovernmental organizations emphasize the accumulation of 30 min of moderately intense physical activity on most days of the week, which may be obtained from multiple activity bouts of at least 8–10 min each [22,25]. In addition to being specifically recommended for meeting recent guideline levels, walking is also the most common exercise reported by Americans [25]. There is substantial evidence from clinical trials and prospective studies relating walking and other physical activities to lower risks for diabetes, hypertension, and dyslipoproteinemia [2,4,7,8,11,13,16,17, 19,22,24,25]; however, the optimal dose, intensity, and frequency for reducing these risks remain less certain.
The National Walkers’ Health Study was selected and surveyed specifically to assess the dose–response relationships of walking distance, intensity, and frequency to health. Previous analyses of this cohort have demonstrated that walking longer and faster are inversely related to body weight and regional body circumferences [27,28]. The purposes of the current report are to 1) assess the dose–response relationships between the prevalence of antidiabetic, antihypertensive, and LDL-lowering medication use and walking distance, intensity, and frequency, and 2) assess whether these walking parameters are associated with disease prevalence independently of each other and independently of the lower body weight of faster, longer-distance walkers.
MATERIALS AND METHODS
A two-page questionnaire was mailed to walkers identified through a walking magazine subscriber list [27,28]. Approximately 8% of the 575,000 subscribers solicited elected to join the National Walkers’ Health Study. Our goal was to obtain a sufficiently large cohort for a prospective epidemiologic study of health in walkers rather than a comprehensive survey of these magazine subscribers; thus, recruitment among subscribers ceased once more than 50,000 questionnaires had been received (including multiple surveys from the same individuals). Subscribers were primarily women who chose Walking Magazine through stamp-sheet sweepstakes, and thus they were a socially diverse readership. The study protocol was approved by the University of California Berkeley committee for the protection of human subjects, and each subject provided a signed statement of informed consent.
Walking quantity was taken as the participant’s usual weekly walking distance for the year in which the survey was completed. Walking intensity was the participant’s reply to the survey question, “During your usual walk, how many minutes does it take for you to walk one mile?” We also asked, “During your average week, how many miles is your longest walk?” and “During an average week, how many walks do you take that are over 10 min long?” Height and weight were determined by asking the participant, “What is your current height (in inches, without shoes)?” and “What is your current weight (prepregnancy weight if pregnant)?” Walking distances were reported in miles walked per week and body weights in pounds, which were then converted to kilometers and kilograms for this report. Body mass index (BMI) was calculated as the weight in kilograms divided by height in meters squared. The survey also solicited information on demographics (age, race, education), age at which each respondent commenced walking at least 12 miles per week, weight history (weight and hip, waist, and chest body circumferences when each respondent commenced walking 12 or more miles per week, at greatest weight, and at 18 yr old), diet (vegetarianism and the current weekly intakes of alcohol, red meat, fish, fruit, vitamin C, and vitamin E), aspirin use, current and past cigarette use, history of heart attacks and cancer, and medication use for blood pressure, thyroid condition, cholesterol level, and diabetes. The survey questions were designed to be identical to those used in our study of runners, except for the type of activity [29,30]. Among runners, the reproducibility of the distance run between duplicate questionnaires was r = 0.89.
Logistic regression analyses were used to assess the significance of the inverse relationships of medication use with walking distance, frequency, and intensity, adjustment for age (age and age squared), and intakes of meat, fish, fruit, and alcohol. Additional adjustments for BMI (BMI and BMI squared) and walking distance (km/wk) were included where indicated. The logistic regression coefficients are presented with their standard errors, from which probabilities or odds ratios may be calculated. We also computed the logistic regression coefficients for indicator variables representing distance and intensity categories, and we have used these to calculate the odds ratios of each category relative to the least-active and slowest walkers for the figures. To test whether walking farther or walking faster were associated with additional reductions in the prevalence of medication use relative to each distance or speed category, we also tested whether the odds ratios between each category and all higher values were significantly less than one.
RESULTS
There were 32,683 female and 8112 male walkers who provided complete data on height, weight, age, and weekly walking distance. Respondents were 91.7% white, 2.5% Hispanic, 3.7% black, 0.9% Native American, and 1.2% Asian. The women were on average younger than the men (females mean ± SD: 50.4 ± 13.0, males: 61.1 ± 13.1 yr), had lower BMI (females: 25.8 ± 5.6, males: 27.2 ± 4.7 kg/m2), drank less alcohol (females: 38.5 ± 73.1, males: 72.9 ± 117.5 mL/wk), consumed fewer weekly servings of meat (females: 2.6 ± 2.6, males: 3.3 ± 3.1) and fish (females: 1.5 ± 1.5, males: 1.7 ± 1.7), but slightly more fruit (females: 11.0 ± 7.8, males: 10.6 ± 8.4 pieces per week). Women and men walked similar amounts (females: 20.1 ± 14.2, males: 20.7 ± 15.0 km/wk) at similar speeds (females: 1.7 ± 0.5, males: 1.8 ± 0.5 m/s); however, women took slightly fewer walks per week (females: 5.5 ± 3.5, males: 5.8 ± 4.0), and their usual longest walk was slightly shorter (females: 6.4 ± 4.1, males: 6.6 ± 4.9 km). The women had walked 12 or more miles per week for 8.1 ± 9.2 yr, and the men for 12.4 ± 13.2 yr (excluding those never attaining 12 miles per week).
Nine hundred nineteen women (2.8%) and 599 men (7.4%) reported taking medications for diabetes, 4668 women (14.3%) and 2349 men (29.0%) reported taking medications for high blood pressure, and 2388 women (7.3%) and 1741 men (21.5%) reported taking medications for high cholesterol. As expected, use of these medications was strongly affected by age and BMI (Table 1). Compared with those under 40 yr old, women and men over 70 were six times more likely to take antidiabetic medications; 16 times and 9 times more likely to take antihypertensive medications, respectively, and 17 times and 11 times more likely to take LDL cholesterol–lowering medications, respectively. Compared with lean women and men whose BMI was less than 22.5 kg/m2, those whose BMI exceeded 32.5 kg/m2 were 7.2 times and 3.7 times more likely to take antidiabetic medications, 4.2 times and 2.6 times more likely to take antihypertensive medications, and 2.9 times and 2 times more likely to take LDL cholesterol–lowering medications, respectively.
TABLE 1.
Adjusted prevalence of medication use (%) by age and BMI from logistic regression analyses of 32,683 female and 8112 male walkers.
| N | Antidiabetic Medication Use (%) | Antihypertensive Medication Use (%) | LDL Cholesterol- Lowering Medication Use (%) | |
|---|---|---|---|---|
| Females | ||||
| Age (yr) | ||||
| < 40 | 6918 | 0.8 | 2.4 | 1.2 |
| 40–54 | 14,868 | 1.4§ | 9.3§ | 4.1§ |
| 55–69 | 8217 | 3.0§ | 23.5§ | 13.8§ |
| ≥ 70 | 2680 | 5.0§ | 39.2§ | 21.1§ |
| BMI (kg/m2) | ||||
| < 22.5 | 5850 | 0.9 | 6.2 | 3.0 |
| 22.5–24.9 | 3195 | 1.1* | 9.3§ | 4.4§ |
| 25–27.4 | 5914 | 1.6§ | 11.8§ | 6.4§ |
| 27.5–29.9 | 7878 | 2.3§ | 15.3§ | 7.7§ |
| 30–32.4 | 2227 | 3.8§ | 17.9§ | 8.9§ |
| > 32.5 | 3623 | 6.5§ | 26.3§ | 8.9§ |
| Males | ||||
| Age (yr) | ||||
| < 40 | 473 | 1.4 | 4.7 | 2.5 |
| 40–54 | 2199 | 3.8† | 16.6§ | 13.6§ |
| 55–69 | 3256 | 7.4§ | 32.7§ | 27.1§ |
| ≥ 70 | 2184 | 8.5§ | 43.3§ | 28.7§ |
| BMI (kg/m2) | ||||
| < 22.5 | 907 | 5.0 | 19.9 | 17.3 |
| 22.5–24.9 | 1812 | 4.5 | 22.5 | 21.1* |
| 25–27.4 | 2313 | 5.3 | 28.2§ | 26.2§ |
| 27.5–29.9 | 1366 | 7.3* | 36.2§ | 28.1§ |
| 30–32.4 | 801 | 13.2§ | 42.5§ | 33.3§ |
| > 32.5 | 913 | 18.4§ | 52.3§ | 33.7§ |
All analyses were adjusted to nonsmokers who consumed the average intakes of alcohol, meat, fish, and fruit. In addition, the analyses of age were adjusted to the their mean BMI, and the analyses of BMI were adjusted to their mean age. Significance levels for the odds ratios from logistic regression analyses are coded:
P < 0.05,
P < 0.01,
P < 0.001, and
P < 0.0001.
Table 2 examines the association of smoking and diet with medication use. The percentages were adjusted for age, smoking, and intakes of other foods as required, and with and without adjustments for BMI. Women who took antidiabetic or LDL cholesterol–lowering medications consumed significantly less alcohol and were significantly less likely to smoke. Diabetic-medication use in men was also inversely related to alcohol intake. In both sexes, those who consumed more than four meat servings per week were significantly more likely to take antidiabetic medications than those who ate no meat. Antihypertensive medications were more prevalent among women who ate diets containing more meat and fish and less fruit, even when adjusted for BMI, but these trends were not significant in the smaller sample of men. The relationship of meat intake to LDL cholesterol–lowering medications was inconsistent in men (inversely related) and women (concordantly related).
TABLE 2.
Adjusted prevalence of medication use (%) by current smoking status and intakes of alcohol, meat, fish, and fruit from logistic regression analyses of 32,683 female and 8112 male walkers.
| Antidiabetic Med Use (%) | Antihypertensive Med Use (%) | LDL Cholesterol Lowering Med Use (%) | |||||
|---|---|---|---|---|---|---|---|
| Independent Variables | N | BMI Unadjusted | BMI Adjusted | BMI Unadjusted | BMI Adjusted | BMI Unadjusted | BMI Adjusted |
| Women | |||||||
| Smoking | |||||||
| Smokers | 1805 | 2.1 | 1.7 | 12.1 | 11.3 | 5.6 | 5.6 |
| Nonsmokers | 30,878 | 4.2§ | 3.4§ | 12.9 | 11.8 | 7.9‡ | 7.9‡ |
| Alcohol (mL/wk) | |||||||
| None | 16,830 | 3.4 | 2.5 | 13.9 | 12.1 | 6.5 | 6.1 |
| 1–49 | 7868 | 1.4§ | 1.2§ | 10.3§ | 10.0§ | 5.1§ | 5.2† |
| 50–99 | 4242 | 1.0§ | 1.0§ | 9.7§ | 10.0‡ | 4.8§ | 5.2† |
| ≥ 100 | 3743 | 0.9§ | 0.9§ | 11.3§ | 12.2 | 4.1§ | 4.6§ |
| Meat (servings/wk) | |||||||
| None | 5762 | 1.6 | 1.6 | 10.2 | 10.7 | 5.0 | 5.5 |
| 1 or 2 | 12,517 | 1.7 | 1.5 | 11.2* | 10.8 | 5.4 | 5.6 |
| 3 or 4 | 9169 | 2.3† | 1.7 | 13.8§ | 12.2† | 6.1‡ | 5.8 |
| > 4 | 5235 | 3.2§ | 2.3† | 13.7§ | 11.5 | 5.7* | 5.3 |
| Fish (servings/wk) | |||||||
| None | 7469 | 2.0 | 1.7 | 10.7 | 10.1 | 5.1 | 5.2 |
| 1 | 12,394 | 1.9 | 1.6 | 11.5 | 11.0 | 5.5 | 5.6 |
| 2 | 7398 | 2.2 | 1.8 | 13.1§ | 11.9‡ | 5.9* | 5.8 |
| 3 or more | 5422 | 2.4* | 1.9 | 14.2§ | 12.7§ | 6.0* | 5.8 |
| Fruit (servings/wk) | |||||||
| < 5 | 6373 | 2.2 | 1.7 | 13.1 | 11.7 | 6.1 | 6.0 |
| 5–9 | 9065 | 2.1 | 1.7 | 13.0 | 12.0 | 5.8 | 5.7 |
| 10–14 | 9168 | 1.9 | 1.6 | 11.3‡ | 10.6* | 5.7 | 5.7 |
| ≥ 15 | 8077 | 2.0 | 1.8 | 11.4† | 11.0 | 4.9‡ | 5.1† |
| Men | |||||||
| Smoking | |||||||
| Smokers | 385 | 7.9 | 7.0 | 32.5 | 31.4 | 26.6 | 26.7 |
| Nonsmokers | 7727 | 7.9 | 7.4 | 26.5* | 26.7 | 21.1* | 21.9 |
| Alcohol (mL/wk) | |||||||
| None | 3451 | 12.3 | 10.5 | 33.5 | 31.6 | 26.7 | 26.4 |
| 1–49 | 1475 | 8.8‡ | 7.8† | 31.9 | 30.9 | 26.5 | 26.6 |
| 50–99 | 1191 | 5.3§ | 4.9§ | 30.0* | 29.9 | 27.7 | 28.3 |
| ≥ 100 | 1995 | 4.2§ | 3.8§ | 33.0 | 32.7 | 25.7 | 26.0 |
| Meat (servings/wk) | |||||||
| None | 1055 | 6.4 | 6.5 | 30.5 | 32.2 | 31.8 | 33.9 |
| 1 or 2 | 2793 | 6.7 | 6.2 | 32.5 | 32.0 | 27.2† | 27.6‡ |
| 3 or 4 | 2335 | 8.1 | 7.1 | 32.1 | 30.6 | 24.9‡ | 24.7§ |
| > 4 | 1929 | 10.6‡ | 8.6* | 34.0 | 31.1 | 25.1‡ | 24.5§ |
| Fish (servings/wk) | |||||||
| None | 1215 | 7.1 | 6.0 | 31.0 | 29.6 | 24.2 | 24.3 |
| 1 | 3094 | 7.7 | 6.9 | 32.1 | 31.3 | 25.7 | 25.8 |
| 2 | 2106 | 8.6 | 7.7 | 34.1 | 33.1 | 28.7† | 28.8† |
| 3 or more | 1697 | 8.1 | 6.9 | 32.2 | 30.6 | 27.1 | 27.1 |
| Fruit (servings/wk) | |||||||
| < 5 | 1796 | 7.6 | 6.5 | 34.0 | 32.2 | 26.4 | 26.1 |
| 5–9 | 2328 | 7.6 | 6.7 | 33.2 | 31.9 | 26.9 | 26.8 |
| 10–14 | 2079 | 8.5 | 7.5 | 31.5 | 30.5 | 27.8 | 28.0 |
| ≥ 15 | 1909 | 7.8 | 7.3 | 31.4 | 31.3 | 25.0 | 25.6 |
All analyses were adjusted to nonsmokers of average age who consumed the average intakes of alcohol, meat, fish, and fruit (not including the categorical variable displayed; e.g., the smoking was adjusted for age, alcohol, meat, fish, and fruit intake, but not for smoking). Adjusted to the average BMI for males and females as indicated. Significance levels for the odds ratio from logistic regression analyses are coded:
P < 0.05,
P < 0.01,
P < 0.001, and
P < 0.0001.
Quantity
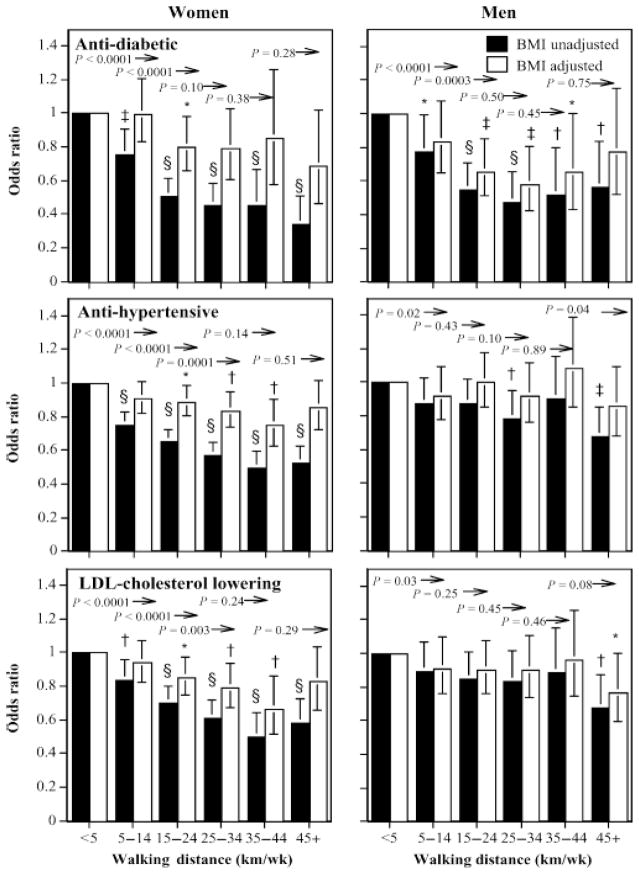
Weekly walking distance was associated with declines in the log odds for antidiabetic, antihypertensive, and LDL cholesterol–lowering medications (Table 3). Men and women who walked as little as 5–14 km/wk had 24% and 23% lower odds, respectively, for using antidiabetic medications than those who walked < 5 km/wk, and those who walked more than 15 km/wk had significantly lower odds for using these medications than those averaging 5–15 km/wk (Fig. 1). The odds for antidiabetic medications were 64% lower in women and 53% lower in men who walked 45 km/wk vis-a-vis < 5 km/wk. The inverse associations of antidiabetic medication use with distance were attributable to the lower BMI values of the longer-distanced male walkers, but the inverse relationships in women remained statistically significant when adjusted for BMI (Table 3).
TABLE 3.
Logistic regression analyses (coefficient ± SE) for the log odds of prevalence of antidiabetic, antihypertensive, and LDL cholesterol–lowering medication use by weekly walking distance, usual walking speed, and longest usual weekly walk per week.
| BMI Adjusted | Intercept | Distance (km/wk) | Speed (m/s) | Longest Walk (km) | |
|---|---|---|---|---|---|
| Antidiabetic | |||||
| Females | |||||
| Distance | Unadjusted | −3.4411 | −0.0230 ± 0.0029§ | ||
| Adjusted | −3.9579 | −0.0069 ± 0.0028† | |||
| Speed | Unadjusted | −2.5088 | −0.8191 ± 0.0850§ | ||
| Adjusted | −3.4722 | −0.3749 ± 0.0853§ | |||
| Longest | Unadjusted | −3.5943 | −0.0592 ± 0.0127§ | ||
| Adjusted | −4.0612 | −0.0177 ± 0.0011 | |||
| Distance and speed | Unadjusted | −2.4023 | −0.0153 ± 0.0031§ | −0.7062 ± 0.0871§ | |
| Adjusted | −3.4235 | −0.0040 ± 0.0030 | −0.3555 ± 0.0862§ | ||
| Distance and longest | Unadjusted | −3.4579 | −0.0147 ± 0.0036§ | −0.0321 ± 0.0133* | |
| Adjusted | −4.0166 | −0.0035 ± 0.0035 | −0.0127 ± 0.0116 | ||
| Males | |||||
| Distance | Unadjusted | −2.2357 | −0.0115 ± 0.0032‡ | ||
| Adjusted | −2.4669 | −0.0052 ± 0.0032 | |||
| Speed | Unadjusted | −1.4242 | −0.6141 ± 0.1002§ | ||
| Adjusted | −1.8426 | −0.4428 ± 0.1000§ | |||
| Longest | Unadjusted | −2.2157 | −0.0488 ± 0.0128§ | ||
| Adjusted | −2.4441 | −0.0323 ± 0.0123† | |||
| Distance and speed | Unadjusted | −1.3327 | −0.0070 ± 0.0034* | −0.5803 ± 0.1009§ | |
| Adjusted | −1.8146 | −0.0018 ± 0.0034 | −0.4357 ± 0.1007§ | ||
| Distance and longest | Unadjusted | −2.1851 | −0.0027 ± 0.0039 | −0.0441 ± 0.0143† | |
| Adjusted | −2.4633 | 0.0015 ± 0.0039 | −0.0347 ± 0.0138† | ||
| Antihypertensive | |||||
| Females | |||||
| Distance | Unadjusted | −1.7438 | −0.0134 ± 0.0013§ | ||
| Adjusted | −2.0145 | −0.0036 ± 0.0013† | |||
| Speed | Unadjusted | −1.2694 | −0.4371 ± 0.0419§ | ||
| Adjusted | −1.8618 | −0.1421 ± 0.0424‡ | |||
| Longest | Unadjusted | −1.8611 | −0.0291 ± 0.0050§ | ||
| Adjusted | −2.0794 | −0.0078 ± 0.0046 | |||
| Distance and speed | Unadjusted | −1.1891 | −0.0099 ± 0.0014§ | −0.3672 ± 0.0427§ | |
| Adjusted | −1.8368 | −0.0021 ± 0.0014 | −0.1312 ± 0.0430† | ||
| Distance and longest | Unadjusted | −1.8368 | −0.0103 ± 0.0016§ | −0.0133 ± 0.0053† | |
| Adjusted | −2.0409 | −0.0029 ± 0.0016 | −0.0040 ± 0.0051 | ||
| Males | |||||
| Distance | Unadjusted | −0.6226 | −0.0054 ± 0.0018† | ||
| Adjusted | −0.7330 | −0.0010 ± 0.0018 | |||
| Speed | Unadjusted | −0.0362 | −0.4009 ± 0.0564§ | ||
| Adjusted | −0.2714 | −0.2915 ± 0.0571§ | |||
| Longest | Unadjusted | −0.5975 | −0.0238 ± 0.0061§ | ||
| Adjusted | −0.7021 | −0.0146 ± 0.0060* | |||
| Distance and speed | Unadjusted | 0.0132 | −0.0034 ± 0.0019 | −0.3863 ± 0.0568§ | |
| Adjusted | −0.2798 | 0.0005 ± 0.0019 | −0.2934 ± 0.0576§ | ||
| Distance and longest | Unadjusted | −0.5505 | −0.0035 ± 0.0022 | −0.0189 ± 0.0067† | |
| Adjusted | −0.6971 | −0.0004 ± 0.0022 | −0.0142 ± 0.0067* | ||
| LDL Lowering | |||||
| Females | |||||
| Distance | Unadjusted | −2.5949 | −0.0133 ± 0.0017§ | ||
| Adjusted | −2.7347 | −0.0060 ± 0.0018‡ | |||
| Speed | Unadjusted | −2.0729 | −0.4613 ± 0.0550§ | ||
| Adjusted | −2.4240 | −0.2545 ± 0.0555§ | |||
| Longest | Unadjusted | −2.6631 | −0.0349 ± 0.0069§ | ||
| Adjusted | −2.7766 | −0.0174 ± 0.0064† | |||
| Distance and speed | Unadjusted | −1.9901 | −0.0102 ± 0.0019§ | −0.3894 ± 0.0560§ | |
| Adjusted | −2.3715 | −0.0044 ± 0.0019* | −0.2317 ± 0.0561§ | ||
| Distance and longest | Unadjusted | −2.5431 | −0.0107 ± 0.0021§ | −0.0179 ± 0.0072† | |
| Adjusted | −2.7141 | −0.0049 ± 0.0021* | −0.0107 ± 0.0069 | ||
| Males | |||||
| Distance | Unadjusted | −0.9028 | −0.0056 ± 0.0019† | ||
| Adjusted | −0.9267 | −0.0032 ± 0.0019 | |||
| Speed | Unadjusted | −0.6307 | −0.2244 ± 0.0610‡ | ||
| Adjusted | −0.7292 | −0.1631 ± 0.0615† | |||
| Longest | Unadjusted | −0.8262 | −0.0313 ± 0.0071§ | ||
| Adjusted | −0.8537 | −0.0256 ± 0.0071‡ | |||
| Distance and speed | Unadjusted | 0.5534 | −0.0053 ± 0.0021† | −0.2022 ± 0.0615‡ | |
| Adjusted | −0.6791 | −0.0032 ± 0.0021 | −0.1516 ± 0.0618† | ||
| Distance and longest | Unadjusted | −0.8002 | −0.0020 ± 0.0024 | −0.0282 ± 0.0079‡ | |
| Adjusted | −0.8496 | −0.0003 ± 0.0024 | −0.0252 ± 0.0078‡ | ||
All analyses were adjusted to nonsmokers of average age who consumed the average intakes of alcohol, meat, fish, and fruit. Additional adjustment for BMI as specified. Significance levels for the log odds from logistic regression analyses are coded:
P < 0.05,
P < 0.01,
P < 0.001, and
P < 0.0001. The proportion of medication users can be calculated from the formula 1/(1 + exp (−intercept - coefficientkilometers per week × kilometer-per-week - coefficientspeed × meter-per-second - coefficientlongest × kilometers)).
FIGURE 1.
Odds ratios for medication use by walking distance relative to < 5 km/wk, adjusted for age, smoking, and intakes of meat, fish, fruits, and BMI where indicated. Brackets designate 95% confidence intervals. Significance levels for the odds relative to < 5 km/wk are coded: * P < 0.05, † P < 0.01, ‡ P < 0.001, and § P < 0.0001. Significance levels relative to all men of women who walked greater distances are presented above the bars and to the left of the arrows.
Antihypertensive and LDL cholesterol–lowering medications use were also inversely related with walking distance in both sexes, and in women even when adjusted for BMI. Compared with women who walked < 5 km/wk, those who walked > 5 km/wk were significantly less likely to use antihypertensive or LDL cholesterol-lowering medications, with significant incremental reductions in odds for both medications with each 10 km/wk increment in distance up to 25 km/wk. Adjustment for BMI reduced the coefficient for the women’s log odds for antihypertensive medications by nearly three fourths and for LDL cholesterol-lowering medications by more than one half; however, both associations remained significant when adjusted for BMI (Table 3). Figure 1 shows that compared with women who walked < 5 km/wk, when adjusted for BMI those who walked > 15 km/wk generally had significantly lower odds for antihypertensive and LDL cholesterol–lowering medications.
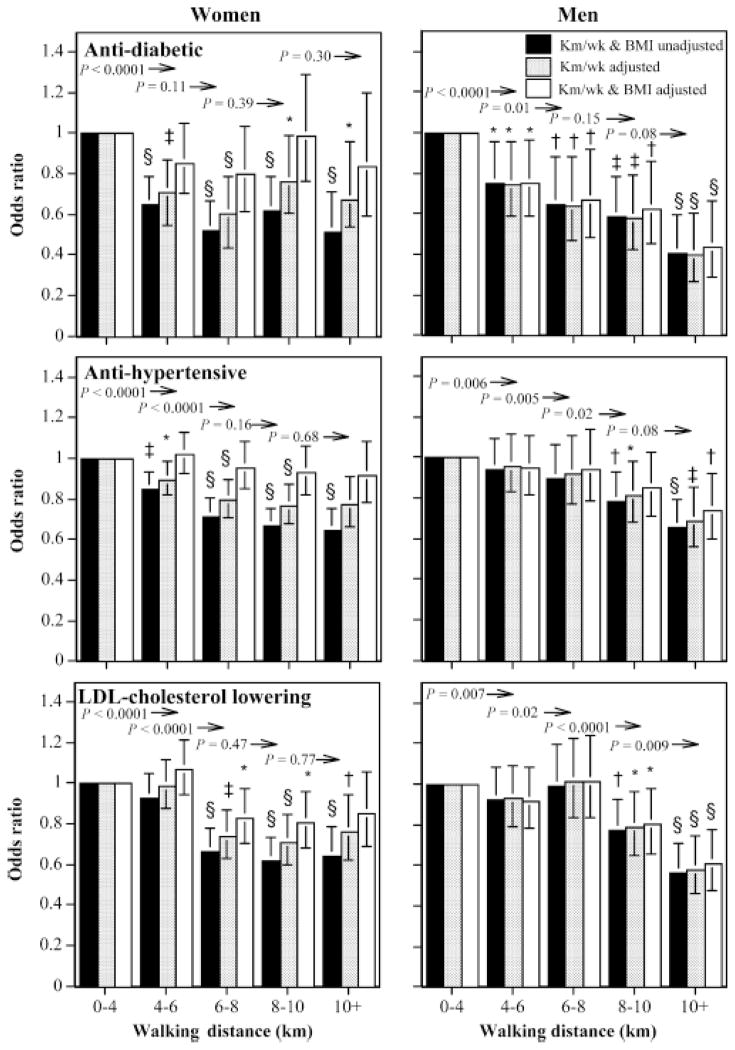
Longest walk
Table 3 shows that the length of the longest weekly walk was significantly associated with reduced log odds for the use of all three medications in both sexes, even when adjusted for total distance per week. Adjustment for BMI eliminated the lower use of antidiabetics and antihypertensives with longest distance for women, but not for men. Moreover, the inverse relationship between men’s log-odds for antidiabetic, antihypertensive, and LDL cholesterol-lowering medication use versus longest walking distance remained significant when adjusted simultaneously for both BMI and average weekly distance.
Figure 2 shows that including a walk of at least 4–6 km each week was associated with significantly lower odds for antidiabetic medication use in men and women and antihypertensive use in women, regardless of total weekly distance. The odds for antihypertensive and LDL cholesterol–lowering medications were 28% and 33% lower for women whose longest walk was 6–8 km than for those who never exceeded 4 km, respectively. Among women, longer walks were not associated with further reductions in these medications, whereas in men lower use was most pronounced when walks of eight or more kilometers were included. BMI accounted for the lower medication use with longer walks by women but not by men.
FIGURE 2.
Odds ratios for medication use by longest usual weekly walk relative to < 4 km, adjusted for age, smoking, and intakes of meat, fish, fruits, and kilometers per week and BMI where indicated. Brackets designate 95% confidence intervals. Significance levels for the odds relative to < 4 km are coded: * P < 0.05, † P < 0.01, ‡ P < 0.001, and § P < 0.0001. Significance levels relative to all men of women who walked greater distances are presented above the bars and to the left of the arrows.
The lower odds for men’s diabetic medication use in association with the length of the longest walk were particularly robust to adjustments for total weekly distances and BMI values. Men who included at least one walk > 10 km had 56% lower odds for antidiabetic medication use, 39% lower odds for antihypertensive medication use, and 26% lower odds for LDL cholesterol–lowering medication use when adjusted for both BMI and total distance walked per week.
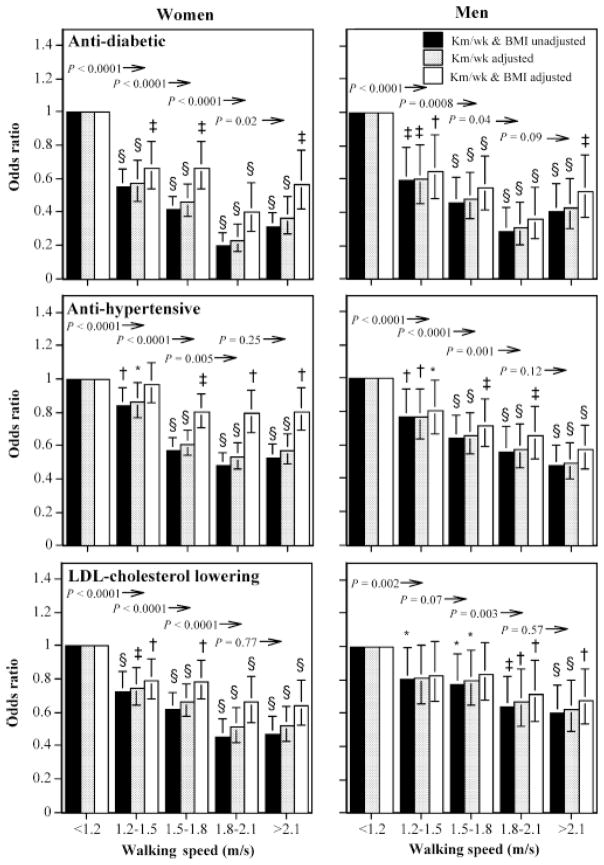
Intensity
The analyses of Table 3 suggest that the strongest predictor of medication use was self-reported walking speed. Speed was significantly associated with reduced antidiabetic, antihypertensive, and LDL cholesterol–lowering medication use, independently of both BMI and total distance. Adjustment for weekly walking distance generally reduced the logistic regression coefficient for speed by about 15% in women and by 10% or less in men. In contrast, adjusting the coefficient for weekly walking distance for walking speed reduced the logistic regression coefficients for diabetic medication use by 33% in women and 39% in men, and it reduced those for antihypertensive medication use by 26% and 37% in women and men, respectively. Although the inverse association between medication use and walking speed remained statistically significant when adjusted for BMI, the effects were considerably weakened.
Figure 3 shows that the odds ratios for greater speed were only minimally affected by adjustments for walking distance. The odds for medication use showed similar trends in men and women for each 0.3-m/s increment in walking speed from 1.2 through 1.8 m/s. The graphs illustrate the greater impact of BMI adjustment on the odds in women than in men. However, with few exceptions, the lower odds ratio for faster men and women remained significant.
FIGURE 3.
Odds ratios for medication use by usual walk speed relative to < 1.2 m/s, adjusted for age, smoking, and intakes of meat, fish, fruits, and kilometers per week and BMI where indicated. Brackets designate 95% confidence intervals. Significance levels for the odds relative to < 1.2 m/s are coded: * P < 0.05, † P < 0.01, ‡ P < 0.001, and § P < 0.0001. Significance levels relative to all men of women who walked greater distances are presented above the bars and to the left of the arrows.
Frequency
The frequency of walks taken per week was largely unrelated to medication use (analyses not displayed).
DISCUSSION
These data demonstrate pronounced dose–response relationships of walking distance and intensity with the prevalence of antidiabetic, antihypertensive, and LDL cholesterol–lowering medications. High cholesterol, hypertension, and diabetes are all strong predictors of cardiovascular disease, and their inverse relationship with physical activity may partly mediate the reduction in cardiovascular disease risk associated with being more physically active.
Quantity
Our analyses suggest that the odds for diabetes were inversely related to walking distance through at least 15 km/wk in both men and women, and that the odds for hypertension and high cholesterol in women were inversely related to distance through at least 25 km/wk. The BMI-adjusted associations for diabetes shown in Figure 1 are consistent with reductions in adjusted incidence reported from cohort studies in women and men and controlled clinical trials [2,4,8,11,17,19]. Our observations that the inverse relationships for women’s diabetes are greater than in men, and that the proportions of these relationships attributable to BMI is also greater in women than in men, has been suggested by cohorts followed prospectively [8,17,19] and by comparisons between the Nurses’ and Physicians’ Health Studies [4]. The lower prevalence of LDL cholesterol–lowering medication use with walking distance reported here agrees with meta-analyses of 948 subjects in 22 randomized controlled trials that showed that walking decreased non-HDL cholesterol by about 4% (−0.15 mM) [13]. Meta-analyses by Leon and Sanchez [16] also concluded that exercise training, including both moderate and vigorous prescriptions, decreased LDL cholesterol by about 5%.
In his meta-analysis of 35 randomized controlled trials, Fagard [7] estimates that training decreases systolic and diastolic blood pressure by 3.4 and 2.4 mm Hg, respectively. Moreover, he notes that the reductions were greater in hypertensive (7.4 and 5.8 mm Hg, respectively) than normotensive subjects (2.6 and 1.8 mm Hg, respectively), suggesting that exercise might have a greater affect on reducing the prevalence of hypertension than on lowering mean blood pressure for the population [7]. Whereas Fagard’s meta-analyses does not suggest a clear dose–response relationship between reductions in blood pressure and the frequency, duration, or intensity of the intervention [7], Figures 1–3 show that the prevalence of antihypertensive medication use declined proportionately with volume, length of longest walk, and intensity. The trials examined by Fagard, which ranged from 4 to 52 wk, suggest that longer exercise trials were less effective in lowering systolic blood pressure than were shorter trials [7]. It is noteworthy, therefore, that the lower prevalence in antihypertensive medication use shown here pertain to men and women who had walked for exercise an average of 8 and 12 yr, respectively.
Excess body weight, particularly intraabdominal adiposity, increases the risks for diabetes, hypertension, and elevated cholesterol. Elsewhere, we have shown that BMI and body circumferences decline in association with walking quantities and intensities [27,28]. Declines in women’s BMI and regional adiposity with walking distance were shown to be convex [27]. Correspondingly, in this paper we show that incremental decreases in the prevalence of women’s use of antidiabetic and LDL cholesterol-lowering medications also diminished above 35 km/wk (Fig. 1).
Our analyses were repeated with and without BMI as a covariate to assess quantitatively the extent to which BMI explained the association of medication use with walking (Table 3). Adjustment for BMI accounted for one half to two thirds of the inverse association with antidiabetic medication use, three-fourths to four-fifths of the inverse association with antihypertensive medication use, and about one-half of the inverse association with LDL cholesterol–lowering medication use with walking distance (Table 3). Although substantial, these proportions are expected to underestimate the true percentages attributable to the inherent errors in assessing adiposity, including both the approximate nature of BMI for estimating the physiologically relevant fat and the imprecision in obtaining heights and weights by self-report.
The significant contributions of BMI to the dose–response relationships reported here do not necessarily negate the importance of exercise. The change in the coefficient for walking distance when BMI is added to the model may reflect in part the mediating effects of BMI; that is, exercise attenuates age-related weight gain, thereby reducing the risks for diabetes, hypertension, and high cholesterol. In Western societies, men and women usually gain weight with age [29]. We have shown that long-term runners experience less weight gain over time in proportion to their weekly distance run [29], but they are subject to accelerated weight gain when they quit running [30]. Although we have not shown that walking also attenuates age-related weight gain, walking has been shown to help maintain diet-induced weight loss [12], and weight maintenance is the basis for the Institute of Medicine’s recommendation of walking briskly for 60 instead of 30 min/day [12].
There are also the confounding effects of BMI attributable to self-selection. Specifically, the distance relationships shown in Figures 1 and Table 3 would become irrelevant if lean men and women (i.e., those at low risk for diabetes, hypertension, and high cholesterol) simply choose to walk farther. Walking distance and medication use would then be secondarily related through preexercise adiposity, but not through cause and effect. We have reported that 40% of the decline in BMI with walking distance in women and 17% of the decline in men are attributable to self-selection—that is, these declines are attributable to initially leaner sedentary men and women choosing to walk farther each week when they begin exercising [28]. Thus, particularly in women, some of the association between walking distance and medication use may be attributable to leaner women (i.e., those who are at lower risk for disease) choosing to walk farther.
Intensity
We also have shown that the odds for antidiabetic, antihypertensive, and LDL cholesterol–lowering medications were inversely related to walking intensity, independently of weekly distance (Table 3, Fig. 3). Walking may be performed at light (2–3 METs) or moderate absolute intensities (3–4.5 METs) [1]. Incidence rates for diabetes, hypertension, and metabolic syndrome have been demonstrated to relate prospectively to cardiorespiratory fitness [3,15,26], and high-intensity walking produces significantly greater increases in fitness than does low-intensity walking [14].
Adjustment for adiposity accounted for only one-quarter of the men’s decrease in the prevalence of antidiabetic and antihypertensive medication use, and one-quarter of the decrease in the prevalence of LDL cholesterol–lowering medication use with walking intensity. The corresponding proportions in women were substantially greater than in men, between one-half and two-thirds. These proportions are less likely to be attributable to the mediating effect of BMI than to the effects of self-selection. Specifically, we have reported that self-selection accounts for more than 70% of the decline in BMI with walking intensity [28]. Yet, in both women and men, Figure 3 shows that when adjusted for BMI, the odds ratios for medication use continued to be significantly inversely related to walking speed. Thus, there seems to be a residual association of walking intensity on diabetes, hypertension, and high cholesterol (or the converse), independent of adiposity.
The Nurses’ Health Study showed that walking intensity predicted lower incident diabetes during follow-up [11]. The inverse association in diabetes with walking intensity reported here (Table 3, Fig. 3) and by the Nurses’ Health Study may be unexpected, given reports that equivalent energy expenditures by moderately intense (3–6 METs) and vigorously intense (>6 METs) physical activities produce comparable reductions in incident diabetes [11], and non-vigorous and vigorous activity correspond to equivalent improvements in insulin sensitivity [18]. Although the Nurses’ Health Study also reports that the risk reduction was significantly independent of the time spent walking each week [11], this was not exactly a test of whether walking volume and intensity were independently predictive of diabetes risk. Walking volume in their study is reported as MET-hours, which is the product of time and pace. They show that diabetes risk declined with MET-hours of activity, and if this were true, irrespective of walking intensity, then both time and pace effects would be expected to be significant.
In our study, walking volume is reported as distance, and the analyses of Table 3 and Figure 3 show that walking intensity is associated with lower prevalence of diabetes, hypertension, and high cholesterol, independently of walking volume (i.e., distance that is comparable to MET-hours or kilocalories). Individual differences in walking speed and fitness that are not attributable to exercise dose might reflect inherent genetic dissimilarities. Walking speed in older female twins has been attributed to significant genetic (20%), common environment (26%), and individual environmental effects (54%) [21]. Genetic factors are estimated to account for approximately 40% of the variance in VO2max in family sets [20]. Fifty percent heritability was reported for VO2max in the sedentary state [5] and ΔVO2max in response to training [6] in the HERTIAGE family study.
Longest walk
The longest usual weekly walk was a better discriminator of medication status than the total cumulative distance per week, particularly in men. The usual length of the longest regular walk remained significant when adjusted for total weekly distance. This suggests a possible disadvantage of accumulating only short bouts of exercise compared with including some bouts of longer duration. Prior studies of fractionization of exercise have primarily used fitness and body weight as outcomes, and although most report no significant differences between fractionized versus continuous exercise [9], they may have limited statistical power to detect differences.
Caveats
The recruitment of walkers through Walking Magazine subscription lists and walking events is likely to have generated a more health conscious and active sample then the population at large. This strategy was pursued in order to target higher doses of walking than represented in other population studies. This may limit the generalizability of these results. However, we believe that the biological processes relating walking to hypertension, hypercholesterolemia, and diabetes would not dramatically differ between this sample and a less selected population.
Our use of self-reported medications for diabetes, hypertension, and high cholesterol may provide greater specificity for these maladies than self-reported physician diagnoses; however, medication use may also provide less sensitivity than physician diagnosis, because not all patients will initiate and remain compliant with pharmacological treatment. We also recognize that the dose–response relationships described herein are cross-sectional rather than prospective. An inherent limitation of cross-sectional analyses is the uncertainty of whether walking preceded the disease, or whether the converse occurred. For example, diabetes may cause peripheral neuropathy or vascular disease, which could diminish walking duration or intensity attributable to muscular discomfort, weakness, or cramping. Walking intensity or duration would be the consequence rather than the antecedent of diabetes, and the decline in prevalence with intensity or duration would overestimate the effect of walking on diabetes risk. Alternatively, walking is prescribed for type 2 diabetes mellitus, peripheral neuropathy, peripheral vascular disease, hypertension, and high cholesterol, and this fact may cause the decline in diabetes prevalence to be underestimated. Despite these possible biases, the decline in the prevalence of diabetes in intensity and distance as shown in Figures 1–3 are entirely consistent with prospective data reported by others, leading us to surmise that at least some of the declines in antidiabetic, antihypertension, and LDL cholesterol-lowering medications are attributable to walking distance and intensity.
Acknowledgments
Supported in part by grants HL-45652, HL-072110, and DK066738 from the National Heart Lung and Blood Institute, and conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women: a systematic review of randomised controlled trials. Sports Med. 2004;34:753–78. doi: 10.2165/00007256-200434110-00004. [DOI] [PubMed] [Google Scholar]
- 3.Barlow CE, LaMonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–50. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 4.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99:1193–204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for V· O2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30:252–8. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, An P, Rice T, et al. Familial aggregation of V· O2max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol. 1999;87:1003–8. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 7.Fagard AH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33(6 suppl):S484–92. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 8.Haapanen N, Miilunpalo S, Vuori I, Oja P, Pasanen M. Association of leisure time physical activity with the risk of coronary heart disease, hypertension and diabetes in middle-aged men and women. Int J Epidemiol. 1997;26:739–47. doi: 10.1093/ije/26.4.739. [DOI] [PubMed] [Google Scholar]
- 9.Hardman AE. Issues of fractionization of exercise (short vs long bouts) Med Sci Sports Exerc. 2001;33(6 suppl):S421–7. doi: 10.1097/00005768-200106001-00010. [DOI] [PubMed] [Google Scholar]
- 10.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2203;26:917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington (DC): The National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 13.Kelley GA, Kelley KS, Tran ZV. Walking and non-HDL-C in adults: a meta-analysis of randomized controlled trials. Prev Cardiol. 2005;8:102–7. doi: 10.1111/j.1520-037x.2005.3474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness and lipoproteins in men and women age 50 to 65 years. Circulation. 1995;91:2596–604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- 15.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112:505–12. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 16.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33(6 Suppl):S502–15. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- 17.Lipton RB, Liao Y, Cao G, Cooper RS, McGee D. Determinants of incident non-insulin-dependent diabetes mellitus among blacks and whites in a national sample. The NHANES I Epidemiologic follow-up study. Am J Epidemiol. 1993;138:826–39. doi: 10.1093/oxfordjournals.aje.a116786. [DOI] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ, D’Agostino R, Jr, Karter AJ, et al. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–74. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 19.Meisinger C, Thorand B, Schneider A, Stieber J, Doring A, Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162:82–9. doi: 10.1001/archinte.162.1.82. [DOI] [PubMed] [Google Scholar]
- 20.Montoye HJ, Gayle R. Familial relationships in maximal oxygen uptake. Hum Biol. 1978;50:241–9. [PubMed] [Google Scholar]
- 21.Pajala S, Era P, Koskenvuo M, et al. Contribution of genetic and environmental factors to individual differences in maximal walking speed with and without second task in older women. J Gerontol A Biol Sci Med Sci. 2005;60:1299–303. doi: 10.1093/gerona/60.10.1299. [DOI] [PubMed] [Google Scholar]
- 22.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 23.Schoenborn CA, Barnes PM. Advance Data From Vital and Health Statistics. 325. Hyattsville (MD): National Center for Health Statistics; 2002. Leisure-Time Physical Activity Among Adults: United States, 1997–98. [Google Scholar]
- 24.Tully MA, Cupples ME, Hart ND, et al. Randomised controlled trial of home-based walking programmes at and below current recommended levels of exercise in sedentary adults. J Epidemiol Community Health. 2007;61:778–83. doi: 10.1136/jech.2006.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 26.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130:89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- 27.Williams PT. Nonlinear relationships between weekly walking distance and adiposity in 27,596 women. Med Sci Sports Exerc. 2005;37:1893–901. doi: 10.1249/01.mss.0000175860.51204.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams PT. Self-selection contributes significantly to the lower adiposity of faster, longer-distanced, male and female walkers. Int J Obes. 2007;31:652–62. doi: 10.1038/sj.ijo.0803457. [DOI] [PubMed] [Google Scholar]
- 29.Williams PT, Thompson PD. Dose-dependent effects of training and detraining on weight in 6406 runners during 7.4 years. Obesity (Silver Spring) 2006;14:1975–84. doi: 10.1038/oby.2006.231. [DOI] [PubMed] [Google Scholar]
- 30.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int J Obes. 2006;30:543–51. doi: 10.1038/sj.ijo.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]