Abstract
Certain particulate hexavalent chromium [Cr(VI)] compounds are human respiratory carcinogens that release genotoxic soluble chromate, and are associated with fibrosis, fibrosarcomas, adenocarcinomas and squamous cell carcinomas of the lung. We postulate that inflammatory processes and mediators may contribute to the etiology of Cr(VI) carcinogenesis, however the immediate (0–24 hours) pathologic injury and immune responses after exposure to particulate chromates have not been adequately investigated. Our aim was to determine the nature of the lung injury, inflammatory response, and survival signaling responses following intranasal exposure of BALB/c mice to particulate basic zinc chromate. Factors associated with lung injury, inflammation and survival signaling were measured in airway lavage fluid and in lung tissue. A single chromate exposure induced an acute immune response in the lung, characterized by a rapid and significant increase in IL-6 and GRO-α levels, an influx of neutrophils, and a decline in macrophages in lung airways. Histological examination of lung tissue in animals challenged with a single chromate exposure revealed an increase in bronchiolar cell apoptosis and mucosal injury. Furthermore, chromate exposure induced injury and inflammation that progressed to alveolar and interstitial pneumonitis. Finally, a single Cr(VI) challenge resulted in a rapid and persistent increase in the number of airways immunoreactive for phosphorylation of the survival signaling protein Akt, on serine 473. These data illustrate that chromate induces both survival signaling and an inflammatory response in the lung, which we postulate may contribute to early oncogenesis.
Keywords: chromium, hexavalent, particulate, carcinogenesis, lung, inflammation, injury, Akt, intranasal, mouse model
Introduction
Chronic tissue inflammation is coupled to increased risk of cancer in a wide variety of organs including those of the stomach, colon, bladder, and liver (Coussens et al., 2001;Hold et al., 2008). A similar pathogenesis has also been suggested in the lung, where the incidence of cancer is strongly coincident with pulmonary inflammation, particularly in individuals with chronic bronchitis and interstitial lung diseases (Brown et al., 2004;Daniels et al., 2005;Malkinson, 2005). Inflammatory cells and their chemical mediators are key participants in the generation of a tumor microenvironment that promotes angiogenesis and participates in metastases (Albini et al., 2007). Further evidence of the importance of inflammation in neoplastic progression is illustrated by the reduced risk of colon, stomach, esophagus and lung cancer among individuals that are long-term users of aspirin and nonsteroidal anti-inflammatory drugs (Khuder et al., 2005;Van Dyke et al., 2008;Garcia-Rodriguez et al., 2001;Baron et al., 2000). Given the link between inflammation and cancer, we hypothesize that inhalation of toxic agents that are associated with increased lung cancer risk will induce tissue injury and an inflammatory environment, accompanied with increase survival signaling.
Akt, also known as protein kinase B, is a serine-threonine protein kinase that was originally identified as a retroviral oncogene (Bellacosa et al., 1991;Staal et al., 1977). Akt activation is generally triggered by the interaction of receptor tyrosine kinases with growth factors and cytokines. This activates the phosphoinositide-3-kinase (PI3K) pathway and results in activation of Akt at the plasma membrane through phosphorylation of thr-308 and ser-473 residues (Altomare et al., 2005;Bellacosa et al., 1998). Akt activation initiates a cascade of downstream signaling through a variety of targets that participate in glucose metabolism, neovascularization, proliferation, and inhibition of apoptosis (Altomare and Testa, 2005). Increased levels of phosphorylated Akt have been associated with non-small cell lung cancer and bronchial dysplasia. Increased phospho-Akt staining intensity correlates with poor response to therapy and poor patient prognosis (Altomare and Testa, 2005). In the present study, we postulated that Akt is activated in lung airway epithelium in vivo as an early response to genotoxic exposure.
Particulate hexavalent chromium [Cr(VI)] compounds are well established human respiratory toxins and carcinogens that are used commercially in welding, chrome plating, chrome pigmenting, leather tanning, and in the ferrochrome industry (Fishbein, 1981;PHS US Department of Health and Human Services, 2000). Cr(VI) is of additional concern since it is a component of industrial waste and atmospheric pollution (1991;Fishbein, 1981). Upon inhalation, chromium particles accumulate at the bifurcations of the bronchi and the concentration of Cr in these regions of the lung can reach up to 15.8 mg/g tissue (dry weight) (Ishikawa et al., 1994b;Ishikawa et al., 1994a). As observed in the lungs of chromate workers at autopsy, higher lung-Cr burdens have been shown to correlate with increased lung tumor incidence (Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994b;Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994a). Epidemiological studies in Europe, Japan and the United States have consistently shown that workers in the chromate production industry have an elevated risk of respiratory disease including: fibrosis, perforation of the nasal septum, development of nasal polyps, hyperplasia of the bronchial epithelium, lung fibrosarcomas, adenocarcinomas and squamous cell carcinomas (World Health Organization International Agency for Research on Cancer, 1990;Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994b;Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994a;Dalager et al., 1980). Animal studies of chromate exposure by inhalation or intratracheal/intrabronchial instillations illustrate that the slightly soluble and highly insoluble Cr(VI) particulates such as zinc, lead, strontium and sintered calcium chromate consistently induced lung tumors (Levy et al., 1986;HUEPER et al., 1959;Steffee C.H. and Baettjer A.M., 1965). Furthermore, Cr(VI) is a potent genotoxin and initiates a variety of cellular and molecular damage that includes Cr-DNA adducts, DNA single and double strand breaks, chromosomal aberrations and apoptosis (O'Brien et al., 2003).
While certain particulate chromates are well documented carcinogens, little is known about the mechanisms by which chromate exposure initially injures the lung and subsequently induces inflammation in vivo. Thus, development of a mouse model of particulate Cr(VI) inhalation would provide valuable new insights into the disease process and provide a means to evaluate the contribution of the inflammatory environment in the initiation and promotion of neoplastic cells. We hypothesize that particulate Cr(VI) inhalation will induce lung injury and an acute inflammatory response with the potential to promote carcinogenesis through selection of growth-altered cells and induction of a supportive micro-environment predisposed towards neoplasia. Here we conducted a systematic characterization of the immediate lung injury and inflammatory response induced by a single exposure to a defined particulate chromate compound (basic zinc chromate), using a new mouse model of exposure.
Methods
Chromium Preparation, Animal Care and Intranasal Administration
Basic zinc chromate (ZnCrO44Zn(OH)2), an insoluble particulate form of hexavalent chromium, was obtained from Rockwood Pigments, and had a purity of 99–100% (Beltsville, MD). As a hazardous agent particulate hexavalent chromium was handled by trained staff in chemical or laminar flow hoods. The chromate was suspended in sterile 0.9% sodium chloride solution (Sigma-Aldrich, St. Louis, MO) at a concentration of 1.2 mg/mL, sonicated for 10 pulses × 10 sec at 50% amplitude, and stirred for at least 1 hour (but no more than 24 hours) to assure even distribution of particles in suspension. The chromate and saline suspensions were shown to have less than 0.005 units/mL of endotoxin as determined by a standard microplate assay (Clonogen, Germantown, MD). Female BALB/cJ mice were obtained from the National Cancer Institute (Frederick, MD), and used at 6–8 weeks of age. Animals under a light anesthesia (Isoflurane) were intranasally exposed to a 50 µL dose of basic zinc chromate, and sacrificed by exposure to carbon dioxide at indicated time points up to 48 hours after Cr(VI) exposure. Bronchoalveolar lavage (BAL) was performed with 3×1 mL of PBS (Mediatech, Inc, Herndon VA). All experiments were done in accordance with and under the approval of the George Washington University Institutional Animal Care and Use Committee (IACUC).
Characterization of Basic Zinc Chromate
A basic zinc chromate suspension, prepared with our standard protocol, was analyzed on a Malvern Multisizer 2000 laser diffractor with a Hydro 2000S attachment at Particle Technology Labs, Ltd (Downers Grove, IL). The sample was ultrasonicated for 1 minute prior to analysis and a refractive index of 1.870 was used for zinc chromate. To determine chromium content in the lungs, samples were collected 15–30 minutes after Cr(VI) exposure and the trachea and the lobes in the left lung were freeze dried and digested in 1 mL of trace metal grade HCl (Fisher Scientific, Pittsburgh, PA) for 2 h. 200 µL of 30% hydrogen peroxide (Mallinckrodt Baker, Phillipsburg, NJ) was added and samples remained at room temperature for two days. The top 1 mL volume of the solution was collected, mixed with 6 mL of nanopure water, and analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) on an IRIS Thermo Jarrell Ash where the detection limit for Cr was 0.01 ppm (ThermoElectron, Waltham, MA). Quantification of basic zinc chromate particles was completed on a standard hemocytometer using a 10 µL volume of chromate that was prepared at a concentration of 1.2 mg/mL of saline. Any group of greater than 4 particles was considered an aggregate.
Histology
Following BAL, whole lungs from each mouse were perfused with PBS to remove red blood cells, and fixed in Periodate-Lysine-Paraformaldehyde (PLP) fixative (0.01 M Sodium m-periodate, 0.075 M Lysine, 1% paraformaldehyde). Fixed lung tissue was paraffin embedded, sectioned (4-µm), and stained by Hematoxylin and Eosin (H&E) (HistoServ, Germantown, MD). Giemsa staining of paraffin embedded sections (4-µm) was completed using Giemsa (Sigma-Aldrich) diluted 1:20 with deionized water, rinsed, and air dried. Lung sections were stained for immunohistochemistry with phosphospecific serine-473 Akt antibody (Cell Signaling, Danvers, MA) using a 1:50 dilution at HistoServ (Germantown, MD) (Tsurutani et al., 2006). For phosporylated Akt staining intensity was scored for every airway in an entire lung section as absent (0), mild (1), moderate (2), or strong (3). Brightfield microscopy pictures were obtained using an Olympus DP70 microscope digital camera (Center Valley, PA) with supporting DPController and DPManager software on an Olympus Provis AX70 microscope with the indicated objective magnification.
Analysis by Flow Cytometry
After chromate exposure, mice were euthanized and cells were collected from lungs by BAL. Red blood cells were first lysed using a 5 minute treatment with RBC lysis buffer (0.3 M NH4Cl, 20 mM KHCO3, 1 mM EDTA), and the remaining live cells were determined by hemacytometer using Trypan Blue exclusion. Next, BAL cells were stained for FACS analysis to detect neutrophils (Gr1), eosinophils (FcεRIα), T lymphocytes (CD3, CD4 and CD8), B cells (B220), and alveolar macrophages (CD11c); antibodies were obtained from BD Biosciences (Franklin Lakes, NJ). Data were collected on a FACSCalibur flow cytometer, and analyzed using CellQuest software (BD Biosciences).
Analysis by Cytospin
BAL was performed on female BALB/c mice 24 hours following intranasal treatment with either 1.2 mg/mL Cr(VI) or saline control. After lysing red blood cells, approximately 2 × 105 total cells in 100 µL volume were loaded into the cytospin apparatus, and centrifuged for 5 min at 800 rpm on a Shandon Cytospin3 (Thermo Scientific, Waltham, MA). The resulting slides were fixed and stained with H&E. Differential cell counts of 100 or more cells were then performed in duplicate to assess the proportion of leukocyte subsets.
Cytokine Analysis
BAL fluid was centrifuged at 1800 rpm for 8 min to remove leukocytes and the supernatant was used for cytokine analysis. Enzyme-linked immunosorbant assays (ELISAs) were performed following manufacturer’s instructions to assess the production of the cytokines IL-6, GROα (mouse IL-8 homologue), and TNF-α (R&D Systems, Minneapolis, MN).
Statistical analysis
To assess significant differences among experimental groups, statistical analyses were performed using GraphPad Prism version 4.00 for Windows (San Diego, California). Two tailed, unpaired t tests were completed when comparing two experimental groups, while one-way analysis of variance and a Tukey or Dunnetts post test was completed for multiple sample comparisons. In all experiments results are presented as the mean ± standard error of the mean (SEM) and significance was accepted at P < 0.05.
Results
Intranasal exposure to particulate Cr(VI)
To examine the injury and inflammatory response as a result of Cr(VI) exposure, we exposed female BALB/c mice intranasally to 50 µL of basic zinc chromate suspended in saline at 1.2 mg/mL. BALB/c mice are an established intranasal model of asthma and are advantageous for lung cancer studies because they are susceptible to tumors with various drug treatments and have a moderate spontaneous lung tumor frequency (Gwinn et al., 2006;Saini et al., 2008). The 50 µL volume was used for intranasal exposures as it has been shown to give the best distribution of particles to the lungs (Eyles et al., 2001). Furthermore, the concentration of 1.2 mg/mL of basic zinc chromate in saline was employed because it has previously been shown to increase alveolar adenomas in AJ mice chronically exposed by intratracheal administration in a long term carcinogenesis biosassay (Steffee C.H.and Baettjer A.M., 1965).
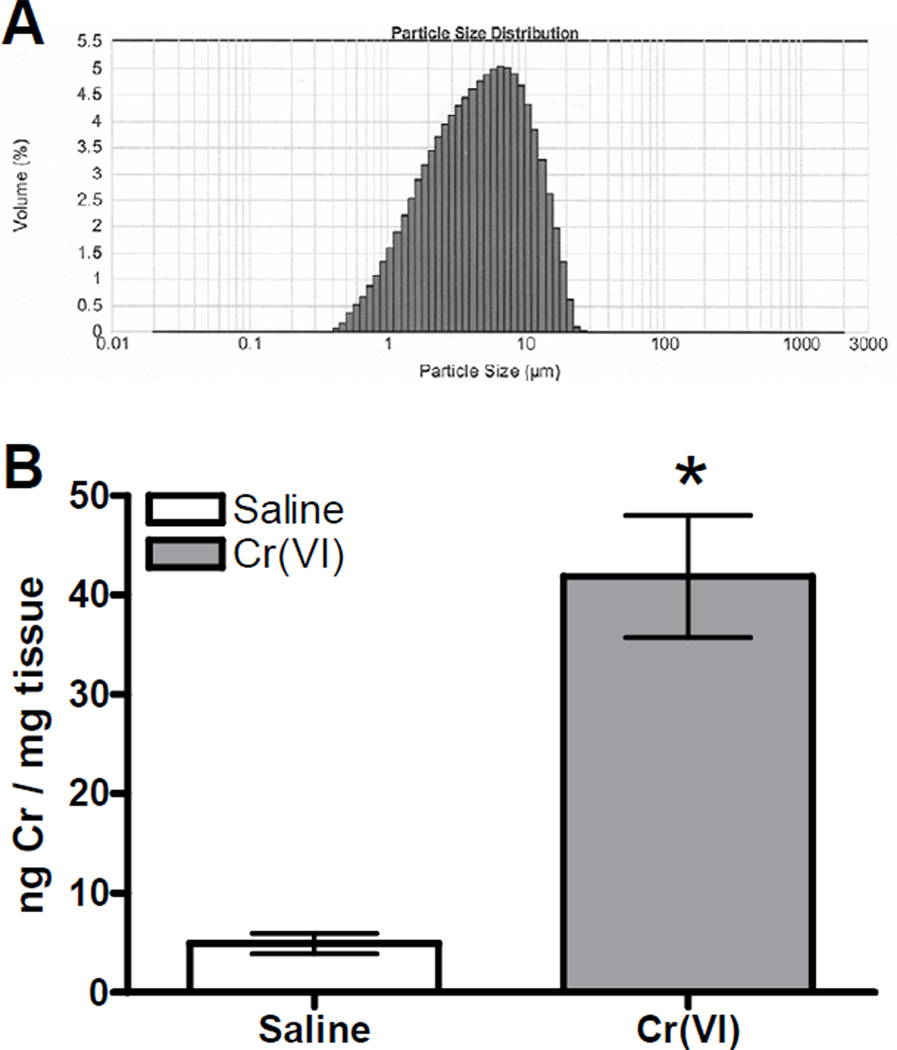
The size of the basic zinc chromate particles was determined by laser diffraction and found to be an average size of 4.73 microns (Figure 1A). Using a hemacytometer we estimated the number of basic zinc chromate particles contained in a 50 µL volume to be around 286,500 single particles and 215,500 particle aggregates. The pH of the basic zinc chromate and saline suspension was observed to be approximately 7.39, indicating that any resulting cell toxicity or tissue damage was not due to pH effects. Cr content in the lungs was determined by ICP-AES following intranasal treatment with 50 µL of saline or basic zinc chromate (1.2 mg/mL saline). The average amount of Cr detected in the lungs of chromate treated animals was 41.88 ng/mg lung tissue, which is within the range of concentrations reported in lungs of chromate workers upon autopsy (Figure 1B) (Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994a). Cr(VI) treated animals had approximately 10-fold higher levels of Cr in their lungs as compared to saline controls, which illustrates that the intranasal method of particulate Cr(VI) treatment was effective at delivering Cr to the lungs.
Figure 1. Characterization of Basic Zinc Chromate.
A) Particle size distribution of basic zinc chromate particles suspended in saline at a concentration of 2.4 mg/mL. Analysis was completed on a Malvern Multisizer 2000 laser diffractor at Particle Technology Labs, Ltd (Downers Grove, IL). The average particle size is 4.73 µm. B) BALB/c mice were exposed to 50 µL of 1.2 mg/mL basic zinc chromate or saline. The Cr content of the right portion of the lung was quantified by ICP-AES. Data are the mean ± SEM for 4 animals. * indicates a statistically significant difference, as compared to saline at p=0.001.
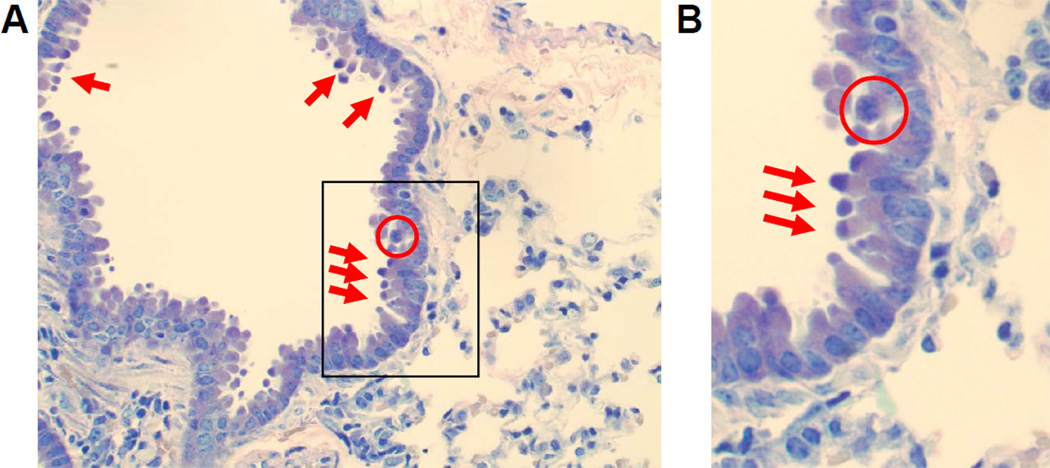
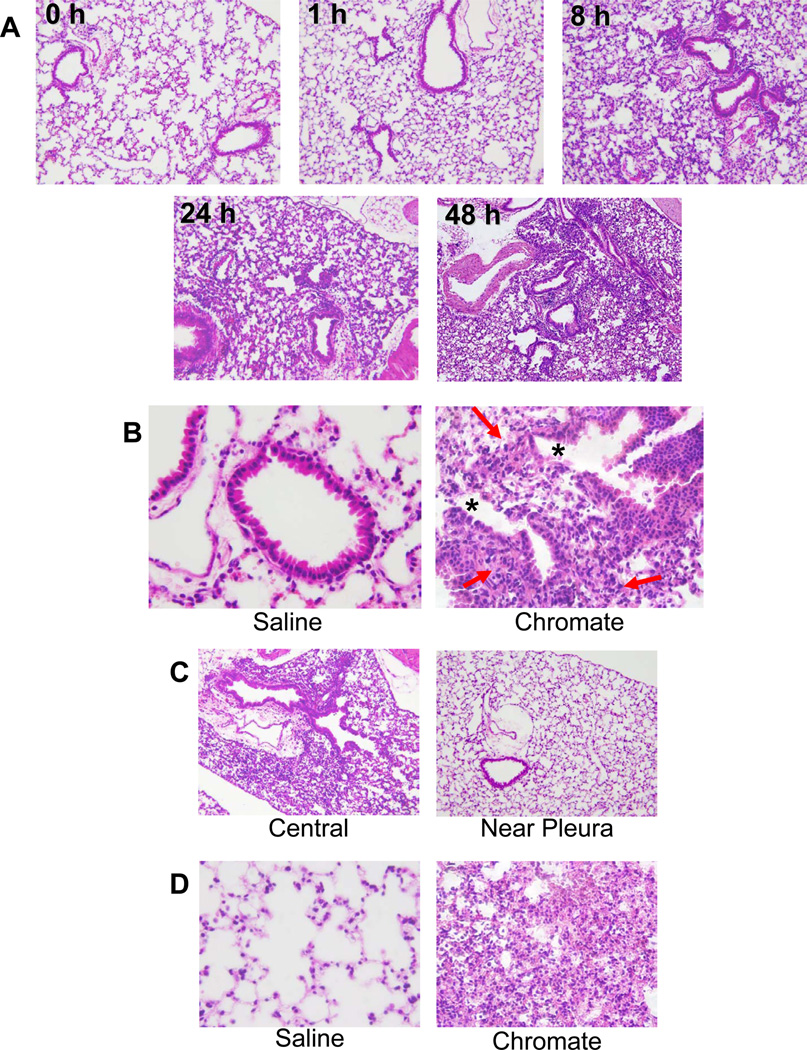
In order to assess if the chromate dose was injuring the epithelial lining of the airways, lung sections were examined from mice 24 hours after particulate chromate exposure by light microscopy using standard H&E and Giemsa staining. Clear evidence of Cr-induced bronchiolar epithelial cell death was visible, including the presence of apoptotic cells and bodies (Figure 2). Furthermore, cells undergoing karyorrhexis were identified by the fragmentation of the nucleus and irregularly distributed chromatin in the cytoplasm (Figure 2, circles). These apoptotic features were predominantly centrally located in the lung and not detected in animals examined 1 and 8 hours after Cr(VI) exposure (data not shown). Taken together, these data provide clear evidence of chromate-induced cell death in epithelial cells lining the airways.
Figure 2. Chromate Induces Apoptosis and Karyorrhexis.
Bright field microscopy pictures taken of lung sections obtained from mice at 24 hrs post-intranasal exposure to 50 µL of 1.2 mg/mL basic zinc chromate suspended in saline. Sections were stained with Giemsa and images were taken at an original magnification of 40×. A) Cells in the airways of chromate-exposed animals exhibit apoptosis (red arrows) and karyorrhexis (circled). B) Cropped 40× original magnification of the boxed region in A.
Particulate Cr(VI)-Induced Acute Inflammatory Response
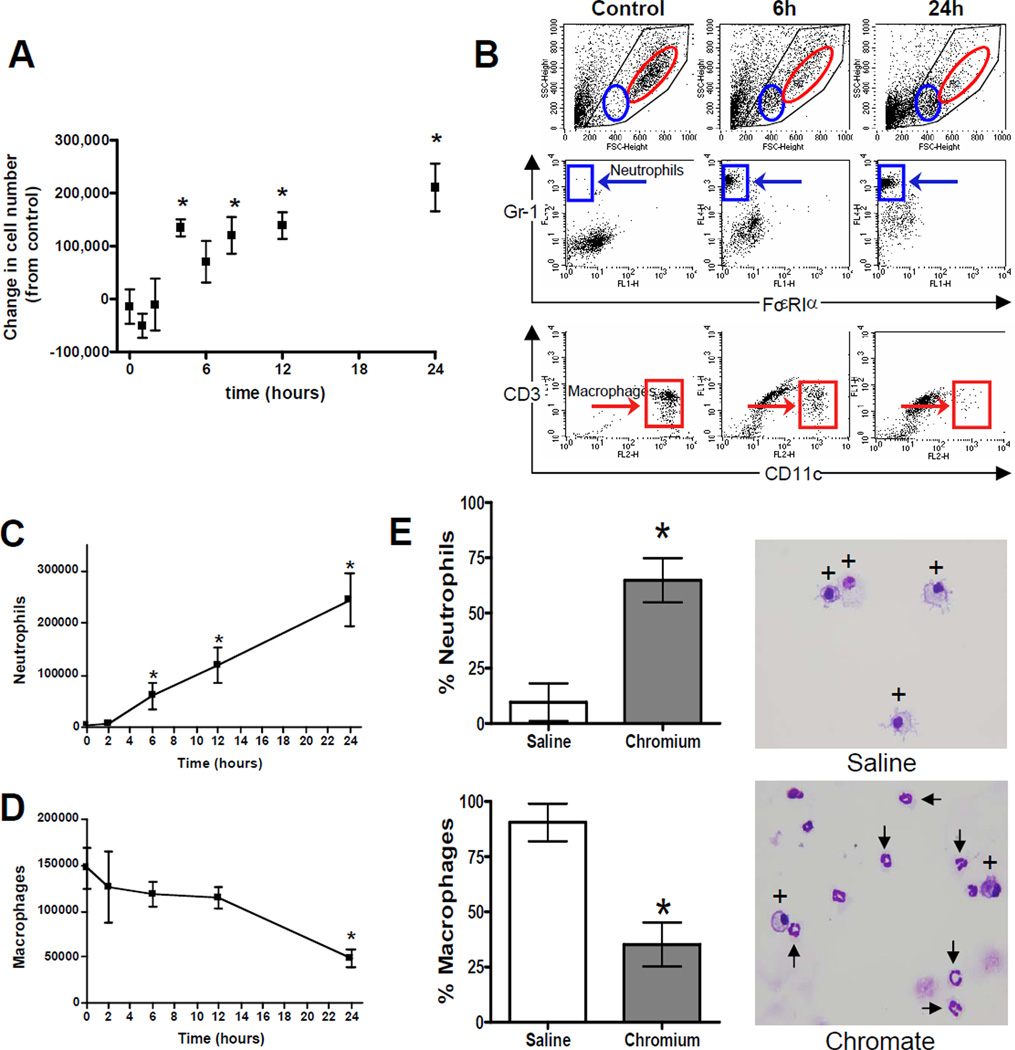
The number of cells in BAL fluid was evaluated as an initial measure of inflammation in the lungs. We observed a significant influx in the total number of viable cells in lung airways beginning 4 hours after intranasal chromate exposure (Figure 3A). This elevated cell number persisted through the final time point measured at 24 hours. The cells recovered from BAL fluid were then characterized and quantified by flow cytometry. Cells were stained for markers indicative of several lineages: Gr-1 (neutrophils); FcεR1α (high-affinity IgE receptor, eosinophils); CD3 (T lymphocytes); and CD11c (alveolar macrophages). Figure 3B shows representative dot plots of FSC/SSC (upper panels), Gr1/FcεR1α staining (middle panels), and CD3/CD11c staining (lower panels), at 3 time points. Red and blue regions correspond to macrophage and neutrophil populations, respectively. A time course analysis revealed a significant recruitment of neutrophils into lung airways, beginning 6 hours after exposure to Cr(VI) (Figure 3C). The marked increase in neutrophils following Cr(VI) exposure was accompanied by a gradual, but significant, decrease in the number of macrophages in airways (Figure 3D). Neither T lymphocytes nor eosinophils were observed at any time (data not shown).
Figure 3. Chromium Induces Changes in Leukocyte Subsets after Acute Cr(VI) Inhalation.
BALBc mice were exposed to 50 µL of 1.2 mg/mL basic zinc chromate over a period of 24 hrs. A) The number of living cells present in BAL fluid were quantified for 5 animals in each experiment, and the change in cell number from the 0 hr time point was determined. Data are the mean ± SEM of two independent experiments, n=5–10 animals/time point. * indicates a statistically significant difference from time 0 at p<0.05. B) Representative dot plots of forward scatter/side scatter (FSC/SSC), GR-1/FcεRIα staining, and CD3/CD11c staining from cells in the BAL fluid collected at indicated time points. Blue and red regions on FSC/SSC plots highlight the location of neutrophils and macrophages, respectively. Blue and red boxes corresponding to these same two populations are shown on the fluorescence plots. C) Changes in total neutrophil and D) macrophage cell numbers at 0–24 hrs post-Cr(VI) exposure. Data are the mean ± SEM of 5 mice from a representative experiment. * denotes a statistically significant difference from time 0 at p<0.01. E) Quantification and representative images (40× original magnification) of cells in the BAL fluid and stained with H&E. Macrophages and neutrophils are indicated be * and arrows respectively. Data are the mean ± SEM of 3 Cr(VI)-treated and 2-saline treated animals 24 hrs after exposure * denotes a statistically significant difference from saline at p<0.05.
Airway leukocyte subsets were also examined by cytospin and H&E staining at 24 hours after Cr(VI) exposure (Figure 3E). In concurrence with our results using FACS analysis, the proportion of neutrophils in the Cr(VI)-treated animals significantly increased to 64.8% of the cells examined, as compared to 9.6% of the cells in saline-treated animals. Likewise, the proportion of macrophages declined to 35.2% following Cr(VI) exposure as compared to 90.4% in control mice. No other cell type was consistently observed by cytospin analysis.
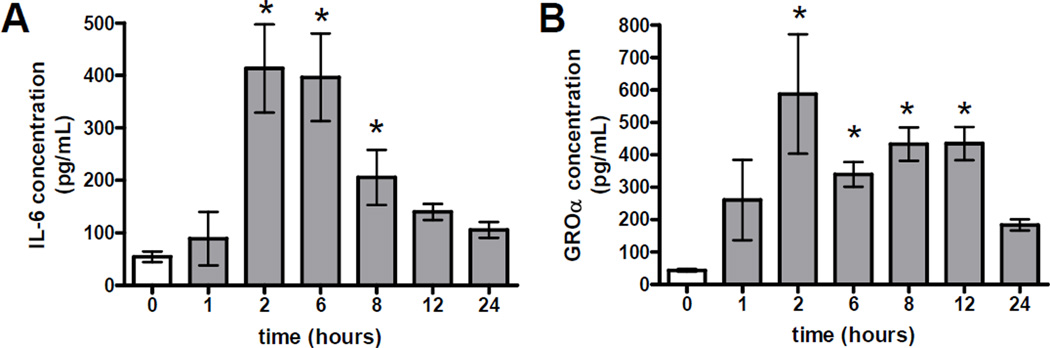
Cytokines and chemokines play an important role in recruiting inflammatory mediators into sites of injury. In order to gain an understanding of the mechanism by which the neutrophils were recruited to the lung, we analyzed BAL fluid for the presence of the chemokine GRO-α (IL-8 homolog) and the cytokines IL-6 and TNF-α. These cytokines were measured by ELISA at various time points up to 24 hours after intranasal exposure to Cr(VI) (Figure 4). Significant increases in both IL-6 and GRO-α levels were detected following particulate Cr(VI) exposure. These cytokine levels peaked 2 hours after chromate treatment and subsequently declined to near baseline levels by 24 hours (Figure 4). Although a slight increase in TNF-α was detected at the mRNA level in Cr(VI) exposed mice, TNF-α protein was undetectable at all time point examined (data not shown).
Figure 4. Increases in Cytokine Levels After Acute Cr(VI) Exposure.
A)IL-6 and B)GROα levels were measured in BAL fluid at 0–24 hrs post-Cr(VI) exposure. Data are the mean ± SEM of two independent experiments, n=5–10 animals/time point. * indicates a statistically significant difference from time 0 at p<0.05.
Particulate Cr(VI)-induced changes in lung pathology
To further understand the extent of injury and inflammation induced by a single particulate Cr(VI) exposure, gross changes in lung tissue were examined at 0, 1, 8, 24 and 48 hour time points following treatment. A first observation was that the lungs of Cr(VI)-exposed mice were markedly redder in color relative to the lungs of control animals (data not shown). Whole lungs were fixed, sectioned and stained by H&E, and examined for both injury to the airways and infiltration of immune cells. Most striking was the marked increase in leukocytic infiltrates that occurred over time (Figure 5A). Foci of inflammation were clearly seen at 8 hours post Cr(VI) exposure and were most abundant at 48 hours. Cr(VI) exposure induced proximal and mid-proximal toxic bronchiolar mucosal injury characterized by sloughing of epithelial cells, degenerative changes in the epithelium and peribronchiolar inflammation (Figure 5B).
Figure 5. Chromium Induces Injury and Inflammation in the Lung.
Bright field microscopy pictures taken of lung sections obtained from mice up to 48 hrs post-Cr(VI) exposure to 50 µL of 1.2 mg/mL basic zinc chromate suspended in saline. Sections were stained with H&E and images were taken at 10× (A, C) and 40× (B, D) original magnification. B) Chromate-exposed animals exhibit proximal and mid-proximal toxic airway mucosal injury with sloughing of airway epithelial cells (*), degenerative changes, and periductal inflammation (red arrows). C) Injury in chromate-exposed animals is primarily located in the central region of the lungs while near-pleural lung regions show less response to chromate treatment at 24 hrs. D) Chromate-exposed animals exhibit parenchymal inflammation with features of alveolar and interstitial pneumonitis and in the central regions of the lungs at 48 hrs post-chromate exposure.
The most abundant leukocyte subset detected in Cr(VI) exposed lung tissue was lymphoid cells, although monocytes, macrophages, and microfoci of neutrophil abscesses were also detected. These histological observations were confirmed by FACS analysis of cell suspensions generated from lung tissue. Specifically a 10-fold higher number of lymphoid cells was present in the lungs 24 hours post-Cr(VI) treatment, as compared to that of neutrophils. These lymphoid cells consisted of an equal proportion of B cells (B220+) and helper T cells (CD4+), and a smaller proportion of cytotoxic T cells (CD8+) (data not shown). Further histological analysis revealed that the airway injury was primarily centrally located in the lung while near-pleural regions remained healthy (Figure 5C). This observation is consistent with the occupational studies on the lungs of chromate industry workers in which the highest concentrations of Cr were detected at the bifurcations of the bronchi (Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994a). Alveolar and interstitial inflammation was also observed at 48 hours after Cr(VI) treatment and was also more centrally located in the lung (Figure 5D). Overall, the pathology illustrates that a single intranasal exposure to particulate Cr(VI) induces an injury and inflammation that evolves into a pneumonitis in mouse lungs.
Particulate Cr(VI)-Induced activation of Akt
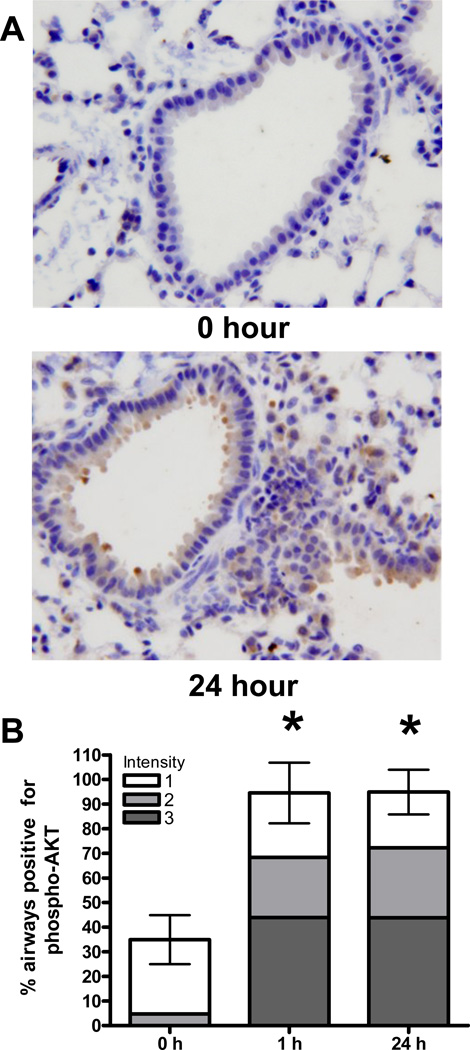
In experiments performed on cultured human cells we found that exposure to Cr(VI) lead to subsequent dysregulation of Akt, and that overexpression of Akt resulted in inappropriate checkpoint bypass (manuscripts in preparation). We therefore hypothesized that particulate Cr(VI) exposure could induce a survival signaling mechanism in airway epithelial cells in vivo. To test this hypothesis we examined the level of Akt activity, as measured by phosphorylation of Akt at ser-473 (Figure 6). The percentage of airways containing epithelial cells immunoreactive for phospho-Akt ser-473 was scored, and the intensity of staining was noted on a scale from 1–3. Within the first hour following Cr(VI) exposure, a 2.4-fold increase in the number of airways positive for Akt was detected. Furthermore, an increase in the intensity of staining was also observed within this short time period. No airways stained at the highest intensity of staining in the control lungs but an average of 40 airways / lung section stained at this highest intensity at 1 h post Cr(VI) exposure. It is important to note that this acute activation of Akt clearly precedes the visible injury and inflammatory response that was produced due to exposure. The elevated level of Akt activity persisted for at least 24 hours following Cr(VI) treatment. Additionally, an increase in phospho-Akt ser-473 was also detected in some inflammatory cells that were present in the Cr(VI) treated lungs at this time.
Figure 6. Akt Phosphorylation Increases in Airways Exposed to Cr(VI).
A) Bright field microscopy pictures taken of lung sections obtained from mice at 0–24 hrs post-intranasal exposure to 50 µL of 1.2 mg/mL basic zinc chromate suspended in saline. Lung sections were stained by immunohistochemistry with phosphospecific serine-473 AKT antibody and representative images were taken at 40×, original magnification. B) Quantification of phospho-Akt staining frequency and intensity in lung airways. Staining intensity was scored on a scale of 1–3, where 1 is a low level of staining and 3 is strong staining. Data are the mean ± SEM of 4 mice and * denotes a statistically significant difference from time 0 at p<0.01.
Discussion
The goal of this study was to gain insight into the disease process by which an occupational and possibly environmental human carcinogen induces lung injury, inflammation, and survival signaling. The work presented here demonstrates that inhalation of a particulate chromate induces an immediate injury and inflammatory response in the lung which is associated with upregulation of Akt phosphorylation. These results are based on the use of basic zinc chromate and an intranasal route of exposure, which were used for several reasons. Intranasal administration of Cr(VI) avoids a systemic inflammatory response that is generated following the surgery that is necessary for intratracheal instillations (O'Hara et al., 2006). Particulate basic zinc chromate was specifically chosen because it is a moderately insoluble form of Cr(VI) which yields a tumor responses in mice and has been associated with cancer in humans (Dalager, Mason, Fraumeni, Jr., Hoover, and PAYNE, 1980;Steffee C.H. and Baettjer A.M., 1965). Furthermore, studies on zinc compounds in cell culture or inhalation exposure demonstrate that zinc by itself has little toxicological or immunological consequence to the lungs of animals (Wallenborn et al., 2008). By employing this new mouse model of particulate Cr(VI) exposure we showed that airway injury was primarily centrally located in the lung while near-pleural regions remained healthy. This observation is consistent with the regions of the lungs where the highest concentrations of Cr were detected among workers in the chromate industry and suggests that the intranasal delivery method is effective for modeling human exposure (Ishikawa, Nakagawa, Satoh, Kitagawa, Sugano, Hirano, and Tsuchiya, 1994a).
Histological examination of lung tissue revealed that Cr(VI) exposure induced toxic mucosal injury, with apoptosis, sloughing, and degenerative changes of the epithelium in the central regions of the lung. While epithelial cell apoptosis has not previously been shown in vivo 24 hours following particulate Cr(VI) inhalation, the observed injury and apoptosis is expected on a cellular level due to the genotoxic properties of Cr(VI) which produces many different DNA damaging events (O'Brien, Ceryak, and Patierno, 2003). This Cr-induced damage has been shown to lead to functional changes in cells including mutagenesis, chromosomal aberrations, apoptosis, terminal growth arrest, and selection for death-resistant clonogenic survivors (O'Brien, Ceryak, and Patierno, 2003). No decline in cell viability was detected among cells in the BAL fluid of rats exposed to soluble Cr(VI) or particulate barium chromate, although this study did not examine apoptosis in epithelial cells of exposed airways (Cohen, Zelikoff, Chen, and Schlesinger, 1998). A study that examined epithelial cell cells of C57BL/6 mice following soluble Cr(VI) exposure did not observe any signs of apoptosis but the lungs were not examined until 21 days after Cr(VI) inhalation (O'Hara, Nemec, Alam, Klei, Mossman, and Barchowsky, 2006). Lung injury and cell death have been shown in mice and rats following welding fume and soluble Cr(VI) exposure, as determined by the presence and activity of albumin and lactate dehydrogenase in BAL fluid respectively (Antonini et al., 2007;Solano-Lopez et al., 2006;Taylor et al., 2003;Zeidler-Erdely et al., 2008).
Our results indicate that a single chromate exposure results in an acute inflammatory response that evolved into a diffuse pneumonitis of the lung. The first inflammatory response to particulate chromate was an induction of IL-6 and GRO-α, at 2 hours post exposure. The detection of IL-6 in vivo is consistent with Cr(VI) induced upregulation of IL-6 transcription in cultured human bronchial epithelial cells (O'Hara et al., 2007). The release of cytokines was followed by the recruitment of neutrophils into the airways and infiltration of lymphocytes into the peribronchiolar regions of the airways beginning at 6 and 8 hours after particulate Cr(VI) exposure respectively. Subsequent alveolar interstitial and alveolar inflammation was also observed at 48 hours after the Cr(VI) treatment. Our findings demonstrate that neutrophils were the predominant cells at the site of mucosal injury, while lymphoid cells were the predominant type in the interstitium. This finding is likely due to the presence of GRO-α (IL-8 homolog) in the airways which has been shown to stimulate neutrophils to leave lung tissue and enter alveolar spaces (Reutershan et al., 2004).
Our finding of an influx of neutrophils into the airways 6 hours after particulate Cr(VI) challenge is consistent with previous reports of soluble Cr(VI), welding fume and particulate air pollutant exposure (Solano-Lopez, Zeidler-Erdely, Hubbs, Reynolds, Roberts, Taylor, Young, Castranova, and Antonini, 2006;Antonini, Stone, Roberts, Chen, Schwegler-Berry, Afshari, and Frazer, 2007;Cohen et al., 1998). It is important to note that other metals, including iron, manganese, and nickel, as well as many reactive organic compounds, were present in the welding fume studies and likely contributed to the welding fume and air pollutant lung injury observed. Here we show that particulate Cr(VI) alone is capable of inducing the neutrophil response and show that the influx occurs earlier than previously suggested (Antonini, Stone, Roberts, Chen, Schwegler-Berry, Afshari, and Frazer, 2007;Solano-Lopez, Zeidler-Erdely, Hubbs, Reynolds, Roberts, Taylor, Young, Castranova, and Antonini, 2006;Taylor, Roberts, Leonard, Shi, and Antonini, 2003). Inhalation of comparable size and number of latex beads resulted in a slight increase in the number of neutrophils in the airways but the maximum increase in the amount of neutrophils was ~10 fold lower than observed following Cr(VI) exposure (data not shown). These results suggest that the observed neutrophilc response was induced due to the presence of basic zinc chromate rather than a general response to inhaled particles. This result with Cr(VI) is in contrast to the paper by Cohen et al. wherein a highly insoluble form of particulate Cr(VI), barium chromate, did not cause an increase in neutrophils in the airways 24 hours after exposure (Cohen, Zelikoff, Chen, and Schlesinger, 1998). This difference in neutrophil response may possibly be due to the varying solubility’s of the particulate Cr(VI) compounds.
Our findings that particulate Cr(VI)-exposed animals have a significant decline in macrophages in the airways is different than welding fume reports, where either no change or a significant increase in macrophages was detected (Antonini, Stone, Roberts, Chen, Schwegler-Berry, Afshari, and Frazer, 2007;Solano-Lopez, Zeidler-Erdely, Hubbs, Reynolds, Roberts, Taylor, Young, Castranova, and Antonini, 2006;Taylor, Roberts, Leonard, Shi, and Antonini, 2003). The observed decline in the number of macrophages in the airways is also different from another Cr(VI) study where no differences in macrophage number were observed (Cohen, Zelikoff, Chen, and Schlesinger, 1998). This difference in observations could be due to the varied metal content and dosage schedule to which these animals were exposed. The only reports regarding alveolar macrophages number changing with Cr(VI) inhalation involved chronic exposure to low levels of soluble sodium chromate. In these latter studies, repeated exposure to soluble Cr(VI) induced cellular inflammatory foci containing macrophages and induced macrophage activation (Taylor, Roberts, Leonard, Shi, and Antonini, 2003). It remains to be determined if chronic basic zinc chromate exposure will induce an increase in alveolar macrophages in the lung.
It has been suggested that chronic exposure to Cr(VI) can lead to hypersensitivity reactions including contact dermatitis and allergic/asthma-like responses (World Health Organization International Agency for Research on Cancer, 1990;Shrivastava et al., 2002;Hansen et al., 2003;Iyer et al., 2002). Thus far, we have not observed signs of eosinophilia or mast cells, which are characteristics of classic extrinsic asthmatic responses. It is possible that a switch to an asthmatic immune response will occur only during chronic exposure to particulate chromate. Alternatively, it is also possible that Cr(VI) alone does not induce an asthma-like response. Most studies linking chromate exposure and asthma/allergy were based on observations in occupationally exposed workers, who were likely exposed to other metals concurrently (Malkinson, 2005;Shrivastava, Upreti, Seth, and Chaturvedi, 2002). The development of asthma in these workers could also be an independent and underlying factor that is not directly regulated by Cr(VI) exposure.
Like Cr(VI), asbestos is an occupational and environmental agent associated with increased inflammation, fibrosis, and increased lung cancer risk. We show here that Cr(VI) particles induce a pneumonitis in the lung that has some similarities to asbestos-mediated lung inflammation (Mossman et al., 1998). Exposure to both of these environmental agents results in IL-8 production and recruitment of neutrophils and lymphocytes to the respiratory tract. TNF-α and macrophages also play a critical role in the context of chronic injury produced by asbestos exposure, and contribute to fibrosis of the lung (Mossman and Churg, 1998;Chapman et al., 2003;Wagner, 1997). In contrast to asbestos, no TNF-α protein or increases in macrophage numbers were observed in response to a single exposure of particulate Cr(VI). Studies are currently underway to investigate if repetitive particulate Cr(VI) exposure might induce a chronic injury and inflammatory response that is associated with TNF-α production and infiltration of macrophages, eosinophils, and/or mast cells in the lung. It should be noted that inhalation of latex particles of a similar size to the zinc chromate particles used in the current study resulted in <10% of the neutrophilia induced by inhaled hexavalent chromate (data not shown), supporting the idea that the type and composition of the inhaled particles is likely to have a significant influence on the phenotype of lung inflammatory responses.
At a molecular level, changes in survival signaling are critical alterations that contribute to cellular transformation. Akt is a well characterized survival signaling protein that is commonly upregulated in a variety of cancers (Altomare and Testa, 2005). We observed that particulate Cr(VI) inhalation induced an increase in the phosphorylation of Akt at serine 473. Nicotine and the carcinogenic chemicals found in cigarette smoke, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo(a)pyrene, also upregulate Akt activity in the lung through interactions with the nicotinic acetylcholine receptors (West et al., 2003;West et al., 2004;Lee et al., 2005). Activation of Akt following Cr(VI) inhalation presumably occurs through a different mechanism, as Cr(VI) is not known to interact with the nicotinic acetylcholine receptors. Soluble Cr(VI) has previously been reported to directly activate the Src family of protein tyrosine kinases in cultured lung cancer cells (O'Hara et al., 2003). Interestingly, we found that tyrosine phosphorylation of p85 was enhanced in cultured human bronchial epithelial cells treated with Cr(VI) (data not shown). Tyrosine phosphorylation removes an inhibitory signal on p85 which is the regulatory subunit of PI3K. Thus, a Cr(VI)-induced increase in p85 phosphorylation is consistent with activation of PI3K and could facilitate Akt activation (Altomare and Testa, 2005). Future studies will explore the hypothesis that particulate Cr(VI) induces a similar upregulation of p85 and/or Src family kinases in vivo, which may in turn upregulate phospho-Akt, but a detailed examination of this pathway is beyond the scope of this study.
Our data illustrating the immediate and persistent upregulation of the survival signaling molecule Akt in the bronchial epithelium may seem contradictory to the simultaneous widespread presence of cells undergoing apoptosis. Examination of the literature reveals that increased Akt activity has previously been correlated with LPS and ventilation induced lung injury, and associated with neutrophil migration to the affected region (Li et al., 2007;Wang et al., 2006). Notably, upregulation of Akt phosporylation at serine 473 was the initial event we observed following exposure to particulate Cr(VI). Akt has been shown to regulate COX-2 expression, via NFқB, in lung cancer cells (Schroeder et al., 2006;Shishodia et al., 2004;Albini and Sporn, 2007). This molecular pathway may provide an underlying mechanism by which upregulation of Akt may participate in the induction of inflammation in the Cr(VI)-exposed lung. Additionally, increased Akt activity has been associated with in vivo protection against oxidant and mechanical-induced lung injury, tissue protection and repair (Lai et al., 2007;Ray et al., 2003;Lu et al., 2001). Taken together, these data suggest that upregulation of Akt activity can influence many physiologically relevant responses following lung injury including inflammation, cell survival and repair of airways. We hypothesize that the ability of Akt to promote cell survival in the environment of genotoxic Cr(VI)-induced injury may enhance the selection of cells that have acquired growth characteristics that predispose them towards neoplastic progression. Further chronic Cr(VI) exposure studies will be needed to test this hypothesis.
Acknowledgments
We thank Ifeanyi Okwumabua for her technical assistance and Drs. Travis O’Brien and Dongsoon Bae for critical discussion of experimental methodology and data interpretation. This work was supported by NIH grants R01-AI067254 to SLC, R01-CA107972 to SMC, and R01-ES05304 and R01-ES09961 to SRP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.The Chromium Problem: Research Needs and Risk Assessment. Environ Health Perspect. 1991:92. [Google Scholar]
- 2.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat.Rev.Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 3.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 4.Antonini JM, Stone S, Roberts JR, Chen B, Schwegler-Berry D, Afshari AA, Frazer DG. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol.Appl.Pharmacol. 2007;223:234–245. doi: 10.1016/j.taap.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu.Rev.Med. 2000;51:511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 8.Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin.Cancer Res. 2004;10:4266s–4269s. doi: 10.1158/1078-0432.CCR-040014. [DOI] [PubMed] [Google Scholar]
- 9.Chapman SJ, Cookson WO, Musk AW, Lee YC. Benign asbestos pleural diseases. Curr.Opin.Pulm.Med. 2003;9:266–271. doi: 10.1097/00063198-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MD, Zelikoff JT, Chen LC, Schlesinger RB. Immunotoxicologic effects of inhaled chromium: role of particle solubility and co-exposure to ozone. Toxicol.Appl.Pharmacol. 1998;152:30–40. doi: 10.1006/taap.1998.8502. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J.Exp.Med. 2001;193:F23–F26. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalager NA, Mason TJ, Fraumeni JF, Jr, Hoover R, PAYNE WW. Cancer mortality among workers exposed to zinc chromate paints. J.Occup.Med. 1980;22:25–29. [PubMed] [Google Scholar]
- 13.Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Curr.Opin.Pulm.Med. 2005;11:431–437. doi: 10.1097/01.mcp.0000170521.71497.ba. [DOI] [PubMed] [Google Scholar]
- 14.Eyles JE, Spiers ID, Williamson ED, Alpar HO. Tissue distribution of radioactivity following intranasal administration of radioactive microspheres. J.Pharm.Pharmacol. 2001;53:601–607. doi: 10.1211/0022357011775929. [DOI] [PubMed] [Google Scholar]
- 15.Fishbein L. Sources, transport and alterations of metal compounds: an overview. I. Arsenic, beryllium, cadmium, chromium, and nickel. Environ.Health Perspect. 1981;40:43–64. doi: 10.1289/ehp.814043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J.Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen MB, Johansen JD, Menne T. Chromium allergy: significance of both CR(III) and CR(VI) Contact Dermatitis. 2003;49:206–212. doi: 10.1111/j.0105-1873.2003.0230.x. [DOI] [PubMed] [Google Scholar]
- 19.Hold GL, El Omar ME. Genetic aspects of inflammation and cancer. Biochem.J. 2008;410:225–235. doi: 10.1042/BJ20071341. [DOI] [PubMed] [Google Scholar]
- 20.HUEPER WC, PAYNE WW. Experimental cancers in rats produced by chromium compounds and their significance to industry and public health. Am.Ind.Hyg.Assoc.J. 1959;20:274–280. doi: 10.1080/00028895909343716. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. "Hot spots" of chromium accumulation at bifurcations of chromate workers' bronchi. Cancer Res. 1994a;54:2342–2346. [PubMed] [Google Scholar]
- 22.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers' cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br.J.Cancer. 1994b;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer VJ, Banerjee G, Govindram CB, Kamath V, Shinde S, Gaikwad A, Jerajani HR, Raman G, Cherian KM. Role of different valence states of chromium in the elicitation of allergic contact dermatitis. Contact Dermatitis. 2002;47:357–360. doi: 10.1034/j.1600-0536.2002.470608.x. [DOI] [PubMed] [Google Scholar]
- 24.Khuder SA, Herial NA, Mutgi AB, Federman DJ. Nonsteroidal antiinflammatory drug use and lung cancer: a metaanalysis. Chest. 2005;127:748–754. doi: 10.1378/chest.127.3.748. [DOI] [PubMed] [Google Scholar]
- 25.Lai JP, Dalton JT, Knoell DL. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br.J.Pharmacol. 2007;152:1172–1184. doi: 10.1038/sj.bjp.0707501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HY, Oh SH, Woo JK, Kim WY, Van Pelt CS, Price RE, Cody D, Tran H, Pezzuto JM, Moriarty RM, Hong WK. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J.Natl.Cancer Inst. 2005;97:1695–1699. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 27.Levy LS, Venitt S. Carcinogenicity and mutagenicity of chromium compounds: the association between bronchial metaplasia and neoplasia. Carcinogenesis. 1986;7:831–835. doi: 10.1093/carcin/7.5.831. [DOI] [PubMed] [Google Scholar]
- 28.Li LF, Liao SK, Lee CH, Huang CC, Quinn DA. Involvement of Akt and endothelial nitric oxide synthase in ventilation-induced neutrophil infiltration: a prospective, controlled animal experiment. Crit Care. 2007;11:R89. doi: 10.1186/cc6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Parkyn L, Otterbein LE, Kureishi Y, Walsh K, Ray A, Ray P. Activated Akt protects the lung from oxidant-induced injury and delays death of mice. J.Exp.Med. 2001;193:545–549. doi: 10.1084/jem.193.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malkinson AM. Role of inflammation in mouse lung tumorigenesis: a review. Exp.Lung Res. 2005;31:57–82. doi: 10.1080/01902140490495020. [DOI] [PubMed] [Google Scholar]
- 31.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am.J.Respir.Crit Care Med. 1998;157:1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat.Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 33.O'Hara KA, Klei LR, Barchowsky A. Selective activation of Src family kinases and JNK by low levels of chromium(VI) Toxicol.Appl.Pharmacol. 2003;190:214–223. doi: 10.1016/s0041-008x(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 34.O'Hara KA, Nemec AA, Alam J, Klei LR, Mossman BT, Barchowsky A. Chromium (VI) inhibits heme oxygenase-1 expression in vivo and in arsenic-exposed human airway epithelial cells. J.Cell Physiol. 2006;209:113–121. doi: 10.1002/jcp.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Hara KA, Vaghjiani RJ, Nemec AA, Klei LR, Barchowsky A. CR(VI)-stimulated STAT3 tyrosine phosphorylation and nuclear translocation in human airway epithelial cells requires Lck. Biochem.J. 2007;402:261–269. doi: 10.1042/BJ20061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PHS US Department of Health and Human Services, A. f. t. s. a. d. r. Toxigological Profile for Chromium. 2000 [Google Scholar]
- 37.Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang XF, Ray A. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc.Natl.Acad.Sci.U.S.A. 2003;100:6098–6103. doi: 10.1073/pnas.1031851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome - how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saini RK, Sanyal SN. Pulmonary carcinogenesis in mice with a single intratracheal instillation of 9, 10-dimethyl benz[a]anthracene. Drug Chem.Toxicol. 2008;31:459–471. doi: 10.1080/01480540802390544. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder CP, Kadara H, Lotan D, Woo JK, Lee HY, Hong WK, Lotan R. Involvement of mitochondrial and Akt signaling pathways in augmented apoptosis induced by a combination of low doses of celecoxib and N-(4-hydroxyphenyl) retinamide in premalignant human bronchial epithelial cells. Cancer Res. 2006;66:9762–9770. doi: 10.1158/0008-5472.CAN-05-4124. [DOI] [PubMed] [Google Scholar]
- 41.Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J.Immunol. 2004;173:2011–2022. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- 42.Shrivastava R, Upreti RK, Seth PK, Chaturvedi UC. Effects of chromium on the immune system. FEMS Immunol.Med.Microbiol. 2002;34:1–7. doi: 10.1111/j.1574-695X.2002.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 43.Solano-Lopez C, Zeidler-Erdely PC, Hubbs AF, Reynolds SH, Roberts JR, Taylor MD, Young SH, Castranova V, Antonini JM. Welding fume exposure and associated inflammatory and hyperplastic changes in the lungs of tumor susceptible a/j mice. Toxicol.Pathol. 2006;34:364–372. doi: 10.1080/01926230600815122. [DOI] [PubMed] [Google Scholar]
- 44.Staal SP, Hartley JW, Rowe WP. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc.Natl.Acad.Sci.U.S.A. 1977;74:3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steffee CH, Baettjer AM. Histopathologic Effects of Chromate Chemicals. Arch.Environ Health. 1965;11:66–75. [Google Scholar]
- 46.Taylor MD, Roberts JR, Leonard SS, Shi X, Antonini JM. Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol.Sci. 2003;75:181–191. doi: 10.1093/toxsci/kfg173. [DOI] [PubMed] [Google Scholar]
- 47.Tsurutani J, Fukuoka J, Tsurutani H, Shih JH, Hewitt SM, Travis WD, Jen J, Dennis PA. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J.Clin.Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 48.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular Adult Aspirin Use Decreases the Risk of Non-Small Cell Lung Cancer among Women. Cancer Epidemiol.Biomarkers Prev. 2008;17:148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner GR. Asbestosis and silicosis. Lancet. 1997;349:1311–1315. doi: 10.1016/S0140-6736(96)07336-9. [DOI] [PubMed] [Google Scholar]
- 50.Wallenborn JG, Evansky P, Shannahan JH, Vallanat B, Ledbetter AD, Schladweiler MC, Richards JH, Gottipolu RR, Nyska A, Kodavanti UP. Subchronic inhalation of zinc sulfate induces cardiac changes in healthy rats. Toxicol.Appl.Pharmacol. 2008;232:69–77. doi: 10.1016/j.taap.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 51.Wang XQ, Bdeir K, Yarovoi S, Cines DB, Fang W, Abraham E. Involvement of the urokinase kringle domain in lipopolysaccharide-induced acute lung injury. J.Immunol. 2006;177:5550–5557. doi: 10.4049/jimmunol.177.8.5550. [DOI] [PubMed] [Google Scholar]
- 52.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J.Clin.Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3'-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–451. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel and Welding. Lyon France: 1990. [PMC free article] [PubMed] [Google Scholar]
- 55.Zeidler-Erdely PC, Kashon ML, Battelli LA, Young SH, Erdely A, Roberts JR, Reynolds SH, Antonini JM. Pulmonary inflammation and tumor induction in lung tumor susceptible A/J and resistant C57BL/6J mice exposed to welding fume. Part Fibre.Toxicol. 2008;5:12–12. doi: 10.1186/1743-8977-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]