Abstract
Objective
Few studies have compared prevalence rates of metabolic abnormalities in antipsychotic-treated patients with different psychiatric disorders, including posttraumatic stress disorder (PTSD). In this study, we examined components of metabolic syndrome among middle-aged and older patients with psychiatric disorders.
Method
In the study, 203 outpatients older than 40 years and with psychotic symptoms that needed antipsychotic treatment were enrolled. Among them, 65 had a diagnosis of schizophrenia, 56 had dementia, 49 had mood disorder, and 33 had PTSD. Clinical evaluations included medical history, use of psychotropic and other medications, adverse effects, physical examination, and clinical laboratory tests for metabolic profiles.
Results
Overall, the prevalence rates of metabolic syndrome were 72% in patients with PTSD, 60% in those with schizophrenia, 58% in those with mood disorder, and 56% in those with dementia. There were significant differences in body mass index, diastolic blood pressure, waist circumference, and high-density lipoprotein cholesterol among the 4 diagnostic groups. Posttraumatic stress disorder, schizophrenia, and mood disorder groups had significantly higher body mass indexes compared with the dementia group. The PTSD group also had significantly higher diastolic blood pressure compared with the dementia and mood disorder groups.
Conclusions
Posttraumatic stress disorder may be associated with worsened metabolic profile. The overall frequency of metabolic syndrome and its components in patients with PTSD taking antipsychotics seemed to be at least equivalent, if not slightly worse, compared with that in patients with schizophrenia, dementia, or a mood disorder.
Metabolic syndrome is a significant risk factor for cardiovascular disease and diabetes.1,2 The prevalence of metabolic syndrome is higher in people older than 40 years than in those younger than 40 years, with the highest rates being among women older than 60 years.3 Metabolic syndrome and its components have also been shown to be more common in individuals with schizophrenia, 4–6 bipolar disorder, 4,6–8 and major depressive disorder.4,6,9 In recent years, there has been considerable focus on the role of atypical antipsychotics in elevating the risk of metabolic syndrome.10,11 Although patients with posttraumatic stress disorder (PTSD) are often prescribed atypical antipsychotics off-label,12–17 metabolic syndrome has only recently been studied in this population.
There is some evidence that PTSD is associated with increased rates of metabolic syndrome 6,18–20 and its components, including being overweight and obese 21–24 as well as dyslipidemia.21,25 Jakovljevic et al 18 examined the prevalence of metabolic syndrome among 47 Croatian war veterans with PTSD and found that 31.9% met criteria for it, far exceeding the rate in a middle-aged Croatian nonclinical comparison group (8.9%). In contrast, a larger sample of 100 male Croatian combat veterans with PTSD had a metabolic syndrome prevalence rate of 35%, compared with 41.8% in an at-risk sample of 79 Croatian male patients needing medical care.20 These investigators also found that the prevalence of metabolic syndrome was significantly related to severity of PTSD; the rate in veterans with severe PTSD was 66.7%, compared with 23.3% in veterans with low-intensity PTSD. Similarly, Violanti et al 19 reported a study on police officers and found that people with the most severe PTSD symptoms had a significantly higher prevalence of metabolic syndrome (50%) than those with subclinical symptoms (15.1%). Vieweg et al 24 noted that the high prevalence of being overweight and obese in their sample of veterans with PTSD could not be explained by psychotropic medications typically associated with weight gain. Diabetes has also been shown to be more prevalent in patients with PTSD 21,26–28; however, one study reported that trauma was associated with increased likelihood of diabetes, but PTSD did not mediate this relationship.29
A higher frequency of diseases related to metabolic syndrome has been linked to PTSD. For example, stroke was significantly more common in female veterans with PTSD.22 The results for heart disease have been mixed, with some studies reporting PTSD to be associated with higher rates of heart disease,21,30 but not others.22,26 Although previous studies show that individuals with a severe mental illness had a higher prevalence of metabolic syndrome than individuals who have no mental illness, to our knowledge, no studies have compared rates of metabolic syndrome across mental illness diagnostic groups in older adults.
In the present investigation, we sought to compare the risk of metabolic abnormalities across diagnostic groups among middle-aged and older patients receiving antipsychotics for schizophrenia, PTSD, mood disorder, and dementia with psychosis. We hypothesized that patients with schizophrenia and PTSD would have a higher prevalence of metabolic syndrome including each of its components than patients with mood disorder or dementia. We should stress the fact that the antipsychotics are approved by the Food and Drug Administration primarily for schizophrenia and bipolar disorder, and their use in other conditions is considered off-label. Furthermore, there are black-box warnings issued by the Food and Drug Administration regarding an increased risk of strokes and mortality with these drugs in elderly patients with dementia.31,32 The decision to prescribe antipsychotics to the patients in this study was made by the respective clinical psychiatrists treating those individuals.
METHODS
Here we present an analysis of baseline data from an ongoing National Institute of Mental Health (NIMH)-funded investigation examining metabolic, cardiovascular, and cerebrovascular effects in patients older than 40 years who had psychotic symptoms that, according to the patients’ own treating psychiatrists, needed treatment with antipsychotics. This research is being conducted at the NIMH-funded Advanced Center for Innovation in Services and Intervention Research for the study of older patients with psychosis at the University of California, San Diego (UCSD), and Veterans Affairs San Diego Healthcare System. The study has been approved by the UCSD institutional review board, and all the participants have provided written informed consent. Participants enrolled in this investigation completed a baseline evaluation and will have follow-up assessments at 6 and 12 weeks and every 3 months thereafter. The present paper is restricted to baseline data from the available sample, which include the following: (1) medical history and use of psychotropic and other medications, as well as neurologic and other physical examination results; (2) anthropomorphic measurements for obesity; (3) psychopathology, medication adverse effects, and everyday functioning; and (4) clinical laboratory results for metabolic profiles.
Subjects
The patients were recruited from psychiatric clinics at UCSD and the Veterans Affairs San Diego Healthcare System as well as from nursing homes and board-and-care homes in San Diego County. All the patients had their conditions diagnosed by their primary psychiatrists, many of whom are on the clinical faculty of the UCSD Department of Psychiatry. The patients met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for schizophrenia/schizoaffective disorder, psychosis associated with mood disorder, dementia, PTSD, or psychotic disorder not otherwise specified.33 Patients with active substance abuse in the past 30 days or unstable medical conditions were excluded. Twelve patients with a diagnosis of psychotic disorder not otherwise specified were excluded from the following analyses in view of the small number of patients in this group.
Assessment
Medical History, Use of Psychotropic and Other Medications, and Neurologic and Other Physical Examination
Two trained physician assistants obtained detailed medical history about medical illnesses, known risk factors for metabolic abnormalities, and treatment for these conditions. Physical comorbidity was evaluated with the Cumulative Illness Rating Scale for geriatrics.34 In addition to having their vital signs and blood pressure recorded, each patient had a neurologic and other physical examination, including a specific assessment for stroke using the National Institutes of Health Stroke Scale.35 For the blood pressure assessment, subjects were seated quietly for at least 5 minutes before the measurement was made. Two blood pressure readings were recorded at least 30 seconds apart using a sphygmomanometer. The mean of the 2 readings for both systolic and diastolic pressures was computed and used as the record of blood pressure.36
Information regarding past and present treatment with antipsychotic and other medications potentially impacting metabolic conditions was also obtained. Specifically, the study physician assistants went through a medication checklist with each patient, including all categories of relevant medications such as antipsychotics, antidiabetes medications, lipid-lowering agents, and antihypertensives, and carefully recorded past and current use.
Anthropomorphic Measurements for Obesity
Height was measured using a wall-mounted stadiometer, and weight, by a western digital indicator to the nearest tenth of a kilogram. Body mass index (BMI), a standardized method to estimate overall obesity, was determined from these values (kg/m2). The waist, hip, and thigh circumferences in centimeters were assessed using a measuring tape. The maximum waist circumference was measured at the level of the umbilicus, and the hip, at the maximum horizontal circumference of the hips. All the measurements were taken with the subject in a standing position (the arms at rest along the body) and in apnea fixed at the mid respiratory phase.
Psychopathology and Medication Adverse Effects
We used standardized rating scales to evaluate psychopathology, movement disorders, cognitive impairment, and everyday functioning. Psychopathology was assessed with the Brief Psychiatric Rating Scale 37 and the Hamilton Depression Rating Scale.38 Movement disorders were rated according to a modified Simpson-Angus Scale 39 for extrapyramidal symptoms and the Abnormal Involuntary Movement Scale 40 for tardive dyskinesia. In patients with dementia, the Mini-Mental State Examination 41 and Dementia Rating Scale of Mattis 42 were used to assess level of cognitive impairment.
Before the enrollment of subjects, raters were trained on the administration of assessments. With regard to interrater reliability, an intraclass correlation coefficient of 0.80 or higher was established. All raters hired after the study was initiated were trained and passed reliability training tests before evaluating the subjects.
Clinical Laboratory Examinations for Metabolic Measures
All blood samples were collected by a nurse working in the General Clinical Research Center at UCSD, and the laboratory testing was done at the UCSD Medical Center-certified clinical laboratory. The blood for the chemistry panel that included fasting plasma glucose and the lipid panel (total, high-density lipoprotein [HDL], and low-density lipoprotein cholesterol, and triglycerides) was drawn in the early morning, after at least 12 hours of fasting.43,44 A diagnosis of metabolic syndrome was made on the basis of the following 5 conditions, namely, waist circumference, blood pressure, fasting blood glucose, triglycerides, and HDL cholesterol, according to criteria of the American Heart Association (AHA).45 The cutoff criteria for each component of metabolic syndrome are as follows: waist circumference, greater than 40 in (>102 cm) in men and greater than 35 in (>88 cm) in women; blood pressure, 130/85 mm Hg or higher; fasting glucose level, 100 mg/dL or higher; triglyceride level, 150 mg/dL or higher; and HDL cholesterol level, less than 40 mg/dL in men and less than 50 mg/dL in women. If patients endorsed using antidiabetic, antihypertensive, or antilipemic medications, we counted them as meeting the respective criteria even if their glucose, blood pressure, or lipid levels were normal.45 Anyone having 3 or more conditions meeting the AHA cutoff criteria was considered to have metabolic syndrome.
Statistical Analysis
Continuous variables were assessed for normality of distribution, and appropriate transformations were performed when necessary. The raw percentages of rates for metabolic syndrome and its components were compared between the different psychiatric diagnostic groups. These rates were then adjusted for age, sex, and treatment duration in view of significant differences in these variables between the diagnostic groups. The adjusted percentages of metabolic syndrome for each diagnosis were computed by creating logistic regression models adjusting for age, sex, and treatment duration, and then, using the sample means for each of the covariates, the model was used to predict proportions of patients in each of the 4 diagnostic groups. Between-group comparisons of categorical variables were made using [chi]2 tests. Group comparisons were performed on continuous variables with Welch analysis of variance (ANOVA), and post hoc analyses between groups were carried out on the variables having significant differences on ANOVA. The comparisons between groups adjusting for age, sex, and treatment duration were performed using linear regression with robust tests of heteroscedastic consistent covariance matrix.46 All comparisons were 2-tailed, with P < 0.05 considered statistically significant.
RESULTS
The first 203 patients who completed the baseline evaluation are included in this analysis. The demographic and clinical characteristics of the 4 diagnostic groups (schizophrenia, mood disorder, dementia, and PTSD) are presented in Table 1. The mean age of entire sample was 65.9 years (range, 40–94 years); 67% were men, and 85% were currently taking antpsychotics. The dementia group was significantly older and had a higher percentage of women than the other diagnostic groups. The schizophrenia group had a lower level of education and a lower percentage of white patients. The proportion of patients currently taking antipsychotics was lower in the dementia group than in patients with mood disorder or PTSD. The mean duration of prior antipsychotic use was shorter in the patients with dementia compared with the other groups. Among the patients taking antipsychotics, the percentages of patients on specific atypical agents (including olanzapine) were not different across the 4 diagnostic groups. None of the participants was on clozapine. The proportions of patients currently taking antidiabetic, cholesterol-lowering, and antihypertensive drugs did not differ among the 4 diagnostic groups.
Table 1.
Demographic and Clinical Characteristics of Middle-Aged and Older Patients with 4 Different Psychotic Disorders
| Characteristic | Diagnostic Groups
|
P* | Post hoc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dementia With Psychosis (n=56)
|
Mood Disorder With Psychosis (n=49)
|
Schizophrenia (n=65)
|
PTSD (n=33)
|
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age, yr | 77.2 | 9.8 | 67.1 | 13.2 | 58.3 | 10.6 | 59.7 | 10.5 | <0.001 | D> M> S, P |
| Education level, yr | 13.7 | 2.8 | 14.0 | 3.1 | 12.4 | 2.8 | 13.7 | 2.5 | <0.05 | D, P, M >S |
| Duration of antipsychotic treatment, mo | 44.4 | 36.1 | 129.4 | 133.1 | 253.3 | 162.9 | 173.4 | 160.6 | <0.001 | S>P, M>D |
| Number of metabolic syndrome conditions | 2.8 | 1.3 | 3.0 | 1.4 | 2.9 | 1.5 | 3.3 | 1.4 | 0.459 | — |
| BMI | 26.1 | 5.0 | 29.5 | 7.8 | 29.8 | 6.8 | 30.4 | 4.3 | <0.001 | D<M, S, P |
| Diastolic blood pressure, mm Hg | 69.4 | 9.7 | 69.3 | 8.6 | 72.8 | 9.1 | 75.1 | 9.7 | 0.015 | D, M <P |
| Systolic blood pressure, mm Hg | 126.2 | 18.6 | 126.4 | 15.8 | 123.4 | 19.7 | 132.7 | 15.2 | 0.089 | — |
| Waist circumference, in | 36.9 | 5.2 | 40.3 | 7.2 | 41.0 | 6.4 | 39.9 | 7.0 | 0.002 | D<M, S, P |
| Fasting glucose level, mg/dL | 100.0 | 23.1 | 109.9 | 44.6 | 119.7 | 78.5 | 114.2 | 41.3 | 0.088 | — |
| HDL cholesterol level, mg/dL | 51.9 | 18.7 | 44.8 | 12.6 | 44.3 | 18.5 | 41.4 | 10.3 | 0.012 | D>M, S, P |
| Triglyceride level, mg/dL | 123.5 | 115.1 | 140.5 | 87.6 | 149.6 | 120.6 | 127.3 | 61.1 | 0.558 | — |
| Categorical variables | n | % | n | % | n | % | n | % | ||
|
| ||||||||||
| Sex | ||||||||||
| Male | 31 | 55 | 30 | 61 | 48 | 74 | 29 | 88 | <0.01 | D<S, P; M<P |
| Female | 25 | 45 | 19 | 39 | 17 | 26 | 4 | 12 | (% male) | |
| Ethnicity | ||||||||||
| White | 47 | 84 | 40 | 82 | 42 | 64 | 21 | 64 | <0.05 | D, M > S, P |
| Nonwhite | 9 | 16 | 9 | 18 | 23 | 37 | 12 | 36 | (% White) | |
| On antidiabetic drugs | 6 | 11 | 11 | 22 | 16 | 25 | 7 | 21 | 0.351 | — |
| On statins | 20 | 34 | 22 | 45 | 15 | 23 | 15 | 45 | 0.058 | — |
| On antihypertensive drugs | 30 | 54 | 29 | 59 | 28 | 43 | 22 | 67 | 0.234 | — |
| Currently on antipsychotics† | 42 | 75 | 46 | 94 | 55 | 89 | 30 | 91 | <0.01 | M, P>D |
Welch ANOVA for continues variables and χ2 for categorical variables.
The percentage of patients taking atypical antipsychotics or rate of olanzapine use was not significantly different between the diagnostic groups.
D indicates dementia with psychosis; M, mood disorder with psychosis; P, PTSD; S, schizophrenia.
Rates of the Metabolic Syndrome and Each of Its Abnormal Components
Using the AHA criteria,45 72% of patients with PTSD, 60% of those with schizophrenia, 58% of those with mood disorder, and 56% of those with dementia were classified as having metabolic syndrome (Fig. 1). Because older age and female sex have been associated with an increased risk of metabolic syndrome in the general population,3 and age and sex were significantly different among the 4 diagnostic groups, we adjusted the rates of metabolic syndrome and its components by age, sex, and duration of prior antipsychotic treatment. The adjusted rates of metabolic syndrome in patients with PTSD, schizophrenia, mood disorder, and dementia were 73%, 61%, 58%, and 54%, respectively. Pairwise comparison showed a significant difference between patients with PTSD and those with dementia.
Figure 1.
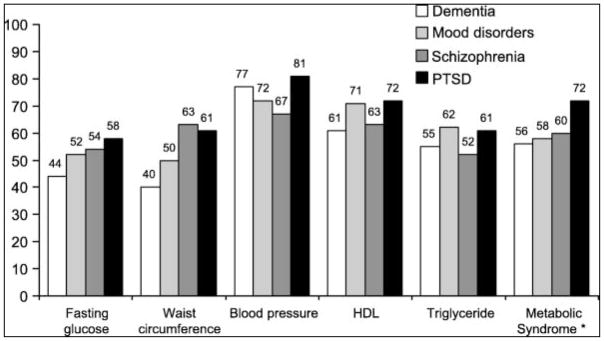
Percentage of patients with metabolic syndrome and with each component above cutoff point among 4 diagnostic groups. The AHA criterion for blood glucose level of 100 mg/dL or higher was used. Patients taking antidiabetic, antihypertensive, and antilipemic medications were also considered to meet criteria even if they had normal glucose, blood pressure, or lipid levels.
There were significant differences in BMI (P < 0.001), diastolic blood pressure (P = 0.033), waist circumference (P = 0.002), and HDL cholesterol (P = 0.012) among the 4 diagnostic groups. The PTSD, schizophrenia, and mood disorder groups had higher BMIs than the patients with dementia (P < 0.001). The PTSD group had higher diastolic blood pressure compared with the dementia (P = 0.011) and mood disorder groups (P = 0.008). The waist circumference in patients with schizophrenia, mood disorder, and PTSD was greater than in those with dementia. The HDL cholesterol level was lower in the participants with PTSD (P < 0.001), schizophrenia (P = 0.033), and mood disorder (P = 0.026) compared with that of the dementia group, with the PTSD participants having the lowest HDL level of all the groups.
To eliminate the possible confounding effects of age, sex, and duration of antipsychotic treatment on the prevalence of metabolic syndrome, we further analyzed the data after adjusting for these variables and found that the group differences in BMI and diastolic blood pressure remained significant. Adjusting for treatment duration alone, we found that the group differences in BMI, diastolic blood pressure, waist circumference, and HDL cholesterol remained significant. Adjusting for sex alone resulted in BMI, waist circumference, and diastolic blood pressure being different between the groups, whereas an adjustment for age alone resulted in all the group differences becoming nonsignificant.
Although we did not specifically assess PTSD severity, we examined correlations between the Brief Psychiatric Rating Scale total and psychosis subscale scores 37,47 and each component of metabolic syndrome in these patients. No significant correlations were found between any of the metabolic outcomes and severity of symptoms in patients with PTSD.
DISCUSSION
Metabolic syndrome was common in middle-aged and older patients with psychotic disorders, especially in those with PTSD. The rates of metabolic syndrome and some of its components in patients with PTSD taking antipsychotics were statistically equivalent to (and numerically worse than) those in patients with schizophrenia, although the schizophrenia group had a longer duration of prior neuroleptic exposure.
The mean prevalence of metabolic syndrome in the US population in the National Health and Nutrition Examination Survey was 44% in people older than 40 years, with the rate being higher in women than in men.3 The 60% rate of metabolic syndrome in our patients with schizophrenia was higher compared with the 42.7% rate reported in the Clinical Antipsychotic Trials of Intervention Effectiveness.5 However, the Clinical Antipsychotic Trials of Intervention patient sample was younger, and that study used the Adult Treatment Panel III criteria for metabolic syndrome with a higher glucose cutoff.
The 72% prevalence rate of metabolic syndrome in our PTSD group is not only higher than that in the general US population but also higher than the previously reported rates of 16% to 67% among patients with PTSD.18–20 However, the prior investigations used the National Cholesterol Education Program/Adult Treatment Panel III criteria,48 and 2 of the groups were from Croatia, which has a lower (8.6%) rate of metabolic syndrome in the general population 18 than the 24% to 27% rate in the United States.3 We did not find significant correlations between the metabolic outcomes and severity of symptoms in patients with PTSD, although they all were deemed to need antipsychotics, suggesting that they probably had severe illness.
Possible mechanisms explaining the link between PTSD and metabolic abnormalities include lifestyle factors, medications, and physiologic dysregulation due to long-term overactivation of the body’s stress-response pathways. Posttraumatic stress disorder, schizophrenia, and mood disorders are all associated with an increased risk of having lifestyle behaviors that can predispose to metabolic syndrome, such as smoking, unhealthy diets, sedentary habits, and alcohol and drug abuse. Among the atypical antipsychotics, clozapine and olanzapine have the highest risk of causing weight gain and metabolic abnormalities.49–51 However, the rates of olanzapine use were equivalent among the 4 diagnostic groups, and no patient was on clozapine. Therefore, it is unlikely that differences in prior antipsychotic use could fully explain the higher rates of metabolic abnormalities seen among patients with PTSD. It is possible, however, that differences in antipsychotic dosages could have contributed to variations in metabolic risk across the groups.
Potential physiologic explanations associating chronic stress with metabolic syndrome include allostatic load, hypothalamic-pituitary-adrenal axis dysregulation, and autonomic nervous system imbalance.52,53 Examples of allostatic state in PTSD include exaggerated startle response, sleep disturbance, and heightened sympathetic tone indicated by higher resting heart rates, blood pressure, and cardiovascular, electromyographic, and skin conductance responses to reminders of the traumatic event.30,54 Hypothalamic-pituitary-adrenal axis activation may result in increased glucocorticoid levels, which have been linked to abdominal obesity, which in turn leads to increased insulin resistance.55,56 Through such mechanisms, PTSD itself could be associated with increased likelihood of metabolic syndrome, and antipsychotics might further increase that risk.
Our study has several limitations. First, our PTSD sample size was relatively small, and all diagnoses were based on clinical assessment rather than on a structured interview such as the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.33 Second, women were underrepresented. Third, we did not have detailed information on prior antipsychotic use including types and dosages of medications. Finally, this study used a convenience sample referred from clinics, and the prescription of antipsychotics had been based on the individual treating psychiatrist’s decision rather than on randomization. It is thus possible that the prescribers chose medications such as olanzapine for patients at lower risk of developing metabolic syndrome.
Despite these limitations, our results suggest that PTSD carries liability for metabolic complications, along with a significant mental health burden, and indicates a need for caution in using antipsychotics in this patient population. Further study is needed to confirm these findings and to guide the development of optimally tailored prevention and intervention efforts targeting metabolic changes in patients with PTSD and other disorders associated with psychotic symptoms.
References
- 1.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Alexander CM, Landsman PB, Teutsch SM, et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 4.Casey DE. Metabolic issues and cardiovascular disease in patients with psychiatric disorders. Am J Med. 2005;118(suppl 2):15S–22S. doi: 10.1016/j.amjmed.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Jakovljevic M, Crncevic Z, Ljubicic D, et al. Mental disorders and metabolic syndrome: a fatamorgana or warning reality? Psychiatr Danub. 2007;19:76–86. [PubMed] [Google Scholar]
- 7.Fagiolini A, Frank E, Scott JA, et al. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas J, Frye MA, Marusak SL, et al. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:91–97. doi: 10.1016/j.jad.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Heiskanen TH, Niskanen LK, Hintikka JJ, et al. Metabolic syndrome and depression: a cross-sectional analysis. J Clin Psychiatry. 2006;67:1422–1427. doi: 10.4088/jcp.v67n0913. [DOI] [PubMed] [Google Scholar]
- 10.Wirshing DA, Boyd JA, Meng LR, et al. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63:856–865. doi: 10.4088/jcp.v63n1002. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, Meyer JM, Jeste DV. Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophr Res. 2004;71:195–212. doi: 10.1016/j.schres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield MI, Becker ME, Connor KM, et al. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int Clin Psychopharmacol. 2001;16:197–203. doi: 10.1097/00004850-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hamner MB, Deitsch SE, Brodrick PS, et al. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psychopharmacol. 2003;23:15–20. doi: 10.1097/00004714-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Petty F, Brannan S, Casada J, et al. Olanzapine treatment for post-traumatic stress disorder: an open-label study. Int Clin Psychopharmacol. 2001;16:331–337. doi: 10.1097/00004850-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry. 2002;159:1777–1779. doi: 10.1176/appi.ajp.159.10.1777. [DOI] [PubMed] [Google Scholar]
- 16.Lambert MT. Aripiprazole in the management of post-traumatic stress disorder symptoms in returning Global War on Terrorism veterans. Int Clin Psychopharmacol. 2006;21:185–187. doi: 10.1097/01.yic.0000185021.48279.00. [DOI] [PubMed] [Google Scholar]
- 17.Kozaric-Kovacic D. Psychopharmacotherapy of posttraumatic stress disorder. Croat Med J. 2008;49:459–475. doi: 10.3325/cmj.2008.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakovljevic M, Saric M, Nad S, et al. Metabolic syndrome, somatic and psychiatric comorbidity in war veterans with post-traumatic stress disorder: preliminary findings. Psychiatr Danub. 2006;18:169–176. [PubMed] [Google Scholar]
- 19.Violanti JM, Fekedulegn D, Hartley TA, et al. Police trauma and cardiovascular disease: association between PTSD symptoms and metabolic syndrome. Int J Emerg Ment Health. 2006;8:227–237. [PubMed] [Google Scholar]
- 20.Babic D, Jakovljevic M, Martinac M, et al. Metabolic syndrome and combat post-traumatic stress disorder intensity: preliminary findings. Psychiatr Danub. 2007;19:68–75. [PubMed] [Google Scholar]
- 21.David D, Woodward C, Esquenazi J, et al. Comparison of comorbid physical illnesses among veterans with PTSD and veterans with alcohol dependence. Psychiatr Serv. 2004;55:82–85. doi: 10.1176/appi.ps.55.1.82. [DOI] [PubMed] [Google Scholar]
- 22.Dobie DJ, Kivlahan DR, Maynard C, et al. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;164:394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- 23.Vieweg WV, Julius DA, Bates J, et al. Posttraumatic stress disorder as a risk factor for obesity among male military veterans. Acta Psychiatr Scand. 2007;116:483–487. doi: 10.1111/j.1600-0447.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 24.Vieweg WV, Julius DA, Fernandez A, et al. Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: psychotropic, antihypertensive, and metabolic medications. Prim Care Companion J Clin Psychiatry. 2006;8:25–31. doi: 10.4088/pcc.v08n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maia DB, Marmar CR, Mendlowicz MV, et al. Abnormal serum lipid profile in Brazilian police officers with post-traumatic stress disorder. J Affect Disord. 2008;107:259–263. doi: 10.1016/j.jad.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisberg RB, Bruce SE, Machan JT, et al. Nonpsychiatric illness among primary care patients with trauma histories and posttraumatic stress disorder. Psychiatr Serv. 2002;53:848–854. doi: 10.1176/appi.ps.53.7.848. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Prev Med. 2005;40:570–574. doi: 10.1016/j.ypmed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Trief PM, Ouimette P, Wade M, et al. Post-traumatic stress disorder and diabetes: co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29:411–418. doi: 10.1007/s10865-006-9067-2. [DOI] [PubMed] [Google Scholar]
- 29.Norman SB, Means-Christensen AJ, Craske MG, et al. Associations between psychological trauma and physical illness in primary care. J Trauma Stress. 2006;19:461–470. doi: 10.1002/jts.20129. [DOI] [PubMed] [Google Scholar]
- 30.Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21:227–234. doi: 10.1007/BF02884839. [DOI] [PubMed] [Google Scholar]
- 31.Jeste DV, Blazer D, Casey DE, et al. ACNP White Paper: update on the use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33:957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889–898. doi: 10.4088/jcp.v69n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer RL, Williams JB, Gibbon M, et al. Structured Clinical Interview for the DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 34.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale (CIRS) Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 35.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 37.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 38.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 39.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 40.National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS) Early Clinical Drug Evaluation Unit Intercom. 1975;4:3–6. [Google Scholar]
- 41.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 42.Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources, Inc; 1976. [Google Scholar]
- 43.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 44.Megnien JL, Denarie N, Cocaul M, et al. Predictive value of waist-to-hip ratio on cardiovascular risk events. Int J Obes Relat Metab Disord. 1999;23:90–98. doi: 10.1038/sj.ijo.0800764. [DOI] [PubMed] [Google Scholar]
- 45.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 46.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- 47.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97–99. [PubMed] [Google Scholar]
- 48.National Institutes of Health. NIH publication no 01–3670. Vol. 2008 Bethesda, MD: National Institute of Health; 2001. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP/ATP III): Executive Summary. [Google Scholar]
- 49.Hagg S, Soderberg S, Ahren B, et al. Leptin concentrations are increased in subjects treated with clozapine or conventional antipsychotics. J Clin Psychiatry. 2001;62:843–848. [PubMed] [Google Scholar]
- 50.Melkersson KI, Dahl ML. Relationship between levels of insulin or triglycerides and serum concentrations of the atypical antipsychotics clozapine and olanzapine in patients on treatment with therapeutic doses. Psychopharmacology. 2003;170:157–166. doi: 10.1007/s00213-003-1529-4. [DOI] [PubMed] [Google Scholar]
- 51.Melkersson KI, Hulting AL. Insulin and leptin levels in patients with schizophrenia or related psychoses-a comparison between different antipsychotic agents. Psychopharmacology. 2001;154:205–212. doi: 10.1007/s002130000639. [DOI] [PubMed] [Google Scholar]
- 52.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 53.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 54.Blanchard EB, Kolb LC, Prins A. Psychophysiological responses in the diagnosis of posttraumatic stress disorder in Vietnam veterans. J Nerv Ment Dis. 1991;179:97–101. doi: 10.1097/00005053-199102000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 56.Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci. 2006;1083:110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]


