Abstract
Vascular endothelial growth factor (VEGF) is important in breast carcinogenesis. However, whether the effect of VEGF expression on survival varies with intrinsic subtypes of breast cancer remains unclear and the prognostic significance of VEGF expression in breast cancer remains controversial. Using immunostaining of tissue microarray sections, VEGF expression was determined in 1,788 primary invasive breast cancers identified from the Nurses’ Health Study cohort. Cox proportional hazards models were used to estimate hazard ratios (HR) of breast cancer-specific and overall mortality and distant recurrence, adjusted for epidemiological, clinicopathological, and related molecular factors, and year of diagnosis. Overall, 72.5% of breast cancers were positive for VEGF. VEGF expression was correlated with intrinsic subtypes (P < 0.0001), with higher frequency in luminal B, HER2, and basal-like types versus luminal A type. Although VEGF expression was not significantly related to worse survival when all cases were considered together, it was significantly associated with increased risks for breast cancer-specific mortality (BCSM) (HR = 1.41, 95% CI = 1.01, 1.97) and distant recurrence (HR = 1.49, 95% CI = 1.07, 2.07) among women with luminal A tumors. In 262 women untreated systemically, VEGF expression was significantly associated with BCSM (HR = 5.58, 95% CI = 1.17, 26.66). In 902 women receiving adjuvant hormonal therapy, VEGF expression did not significantly predict clinical outcomes. The VEGF-associated increased risk of BCSM is limited to luminal A tumors. VEGF expression is a prognostic but not predictive marker of hormonal response in non-metastatic invasive breast cancer.
Keywords: Breast cancer, Prognosis, Survival, Vascular endothelial growth factor, Angiogenesis
Vascular endothelial growth factor (VEGF), a potent angiogenic factor, plays a critical role in tumor growth and metastasis [1, 2]. VEGF signaling in cancer cells is responsible for their resistance to apoptotic stimuli and their migration and invasion [3–5]. VEGF is highly up-regulated in breast cancer. Compared with normal or benign breast tissues, breast cancer showed higher levels of VEGF transcripts [6]. Approximately 72–98% of breast cancer is positive for VEGF by immunohistochemistry (IHC) [7–10]. VEGF expression in breast tumors is correlated with large size, high histologic grade, estrogen receptor (ER) negativity, progesterone receptor (PR) negativity, human epidermal growth factor receptor-2 (HER2) over-expression, and lymph node metastasis [9, 11–14]. In animals, anti-VEGF therapy inhibits the growth of breast tumors, reduces tumor microvessel density, and limits the infiltration of tumor-associated macrophages [15]. Anti-VEGF therapy with bevacizumab, a humanized monoclonal antibody against VEGF, shows an improvement in progression-free survival in combination with chemotherapy for women with metastatic breast cancer [16]. Increased VEGF expression is implicated in acquired anti-estrogen resistance in vitro [17].
Breast cancer is composed of at least five molecular subtypes, as defined through gene expression profiling studies, each with different clinical outcomes, including luminal A, luminal B, HER2, basal-like, and normal-like types. Basal-like tumors account for about 80% of the clinically triple-negative breast cancer (TNBC) which is characterized by the lack of ER and PR immunoreactivity and HER2 over-expression [18]. Although VEGF has been correlated with basal-like tumors and TNBC [14, 19, 20], the effect of VEGF on clinical outcomes in the distinct molecular phenotypes remains unknown.
Prior studies have reported inconsistent results regarding the prognostic and predictive significance of VEGF in breast cancer with some [11–13, 21, 22] supporting and others [7, 9, 23, 24] refuting an adverse effect. The inconsistent findings are likely due in part to molecular heterogeneity in breast cancer. Most studies did not address the question of whether VEGF is a prognostic factor, a predictor of response to adjuvant hormonal therapy, or both.
Using a large number of breast cancer cases identified from the Nurses’ Health Study (NHS), we examined the effects of VEGF on progression of breast cancer by intrinsic subtypes determined by IHC assay as well as its prognostic value in women untreated systemically and predictive significance in women treated with adjuvant hormonal therapy.
Methods
Study population
The NHS included 121,700 female registered US nurses aged 30–55 at enrollment in 1976. At baseline and during each biennial follow-up, participants received a mailed questionnaire to collect information on medical events and risk factors for cancer and cardiovascular diseases. Incident breast cancer cases were ascertained on biennial follow-up questionnaire or by a search of the National Death Index. For self-reported breast cancer cases, permission to review medical records and pathology reports was requested.
Cancer tissue block collection
Since 1993, the NHS has collected archived formalin-fixed paraffin-embedded (FFPE) cancer blocks for participants with primary breast cancer diagnosed between 1976 and 1996. Patients with a prior history of cancer (except non-melanoma skin cancer) at enrollment were excluded from collection. Of the 5,610 eligible cases, pathology specimens were obtained for 3,752 cases. Of these, 855 specimens could not be used in the tissue microarray (TMA) construction due to the unavailability of FFPE blocks or insufficient residual tumor [25]. Hematoxylin and eosin stained slides were reviewed to confirm the diagnosis, classify the cancer according to histological type and grade (Nottingham), and identify the area from which the cores for TMAs would be taken. Three paired 0.6-mm-in-diameter tissue cores were sampled from each FFPE block and were assembled into recipient paraffin blocks. A total of 23 TMA blocks were constructed from 3,093 cancers and positive lymph nodes from 2,897 participants [25]. VEGF was determined in 2,268 cases, of which 1,947 cases had stages I–III breast cancer. We excluded 158 cases with C4 positive lymph nodes and with no information on metastatic work-up at diagnosis and a death case with an unreasonable date of death. Therefore, 1,788 cases were included in the analysis.
Death and distant recurrence
Deaths in the NHS cohort were ascertained by reporting from next-of-kin and/or postal officers or searching the National Death Index. The date and cause of death were obtained from death certificates and/or medical records.
Women were considered to have experienced distant recurrence if they reported a second cancer in the liver, bone, or brain. If lung cancer was reported, medical records were reviewed to distinguish primary lung cancer from breast cancer metastatic to the lung. This self-reported distant recurrence has been validated with both a sensitivity and specificity of 92% [26]. For a woman who died from breast cancer, distant recurrence was assumed to have occurred 2 years before death.
IHC analysis
The immunostaining protocols for ER, PR, HER2, cytokeratin (CK5/6), and epidermal growth factor receptor (EGFR) have been reported elsewhere [27]. Immunostaining for VEGF was performed on TMA sections following deparaffinization and rehydration. After blocking endogenous peroxidase activity, sections were subjected to EDTA antigen retrieval (pH 8.0) for 20 min. The primary monoclonal antibody VEGF (VEGF Ab-7, Clone VG1 from LabVision) was applied to the sections and the slides were incubated overnight at 4°C followed by incubation with the biotinylated universal secondary antibody and the avidin–biotin complex. Visualization was performed using DAKO Envision automated detection system. Tissue sections from samples of angiosarcoma (LabVision VEGF-7 (+) control) were used as both positive and negative controls and were included in all staining runs.
Immunostaining for VEGF, ER, PR, HER2, CK5/6, and EGFR was evaluated in each core by pathologists blinded to clinical outcomes. VEGF immunoreactivity was defined as any positive staining in the cytoplasm of tumor cells. ER, PR, HER2, CK5/6, and EGFR positivity was defined previously [27, 28]. The tumor was considered to be ER positive or PR positive, respectively, if more than 1% of tumor cells showed nuclear staining for ER or PR. ER- and PR-negative tumors were defined as those that showed complete absence of tumor cell staining. HER2 positivity was defined as moderate or strong membranous staining (2+ and 3+) in more than 10% of tumor cells. Tumors were considered positive for CK5/6 or EGFR if any cytoplasmic and/or membranous staining was detected in tumor cells, even if focal.
Definition of intrinsic subtypes
A case was considered positive for a given marker if tumor cells in any of the three cores from that case showed expression of that marker. According to histological grade and the IHC for ER, PR, HER2, EGFR, and CK5/6, breast cancers were classified into five subtypes. Tumors that were ER+ and/or PR+ and HER2− were categorized as luminal A if they displayed low or intermediate histologic grade and as luminal B if their histological grade was high. Luminal B type also included tumors that were ER+ and/or PR+ and HER2+. Tumors that were ER−, PR−, and HER2+ were classified as HER2 type, and tumors that were negative for ER, PR, and HER2 and positive for EGFR and/or CK5/6 were classified as basal-like. Tumors with no staining for all five markers were categorized as “unclassified.”
Statistical analysis
In addition to clinicopathological features, demographic and lifestyle factors that were collected before the self-reported breast cancer diagnosis were used in this study as covariates. The t test and the chi-square test were used respectively to compare continuous variables and categorical variables between cases with VEGF-positive tumors and those with VEGF-negative tumors.
Three survival endpoints were examined, including overall survival (OS) defined as the time from diagnosis to death from any cause, breast cancer-specific survival (BCSS) as the time from diagnosis to death from breast cancer, and recurrence-free survival (RFS) as the time from diagnosis to first metastatic recurrence. Cases without an event and death were censored at the follow-up cut off, June 2008. To analyze BCSS and RFS, death from any other causes were censored. The Kaplan–Meier method was used to develop survival curves in specified patients and the log-rank test to test for equality of survival curves. We used Cox proportional hazards models to calculate hazard ratios (HR) of survival endpoints according to VEGF status, unadjusted and adjusted for variables that were associated with survival in the univariate models at P < 0.1 or of clinical importance. These covariates included age, body mass index, and menopausal status(all at diagnosis), smoking status prior to diagnosis, year of diagnosis, tumor size, nodal status, histological grade, and ER, PR, HER2, EGFR, and CK5/6 status (data not shown). The interaction between VEGF and intrinsic subtypes in survival outcomes was assessed by entering a cross-product term of VEGF status and intrinsic subtypes in multivariable adjusted models to examine if the association between VEGF and survival endpoints varied with intrinsic subtypes. The statistical significance of the interaction term was evaluated using the likelihood ratio test. The survival analysis stratified by intrinsic subtypes was performed. Similarly, we stratified patients according to adjuvant systemic therapy to evaluate the prognostic and predictive values of VEGF expression. To maintain a relatively large sample size, we grouped participants who had missing values for menopausal status (1.9%), smoking status (0.8%), and tumor grade (1.3%) in a separate category. A sensitivity analysis that was conducted by excluding participants who had missing values for any of these variables showed the similar results as those derived from analyses including participants with missing values (data not shown). Because of the limited number of women with other types of breast tumors except luminal A type and 25% of women lacking data on chemotherapy and adjuvant hormonal therapy, the primary analysis of the VEGF-survival relationship stratified by intrinsic subtypes was not adjusted for both therapies to increase the statistical power. However, we conduct secondary analyses with the same model additionally adjusted for both therapies.
Statistical analysis was performed using SAS (version 9.1; SAS Institute, Cary, NC). A P value < 0.05 was considered statistically significant.
Results
VEGF and clinicopathological features
Among the 1,788 tumors, 1,297 (72.5%) were positive for VEGF. Table 1 summarizes epidemiological, clinical, and molecular features by VEGF status. Compared with patients with VEGF-negative tumors, patients with VEGF-positive tumors had higher BMI at diagnosis and were less likely to be current smokers at diagnosis. VEGF immunoreactivity was positively correlated with cancer stage, histological grade, nodal involvement, and expression of HER2, EGFR, and CK5/6 but inversely correlated with expression of ER and PR. VEGF expression was more commonly detected in luminal B, HER2, and basal-like tumors versus luminal A tumors.
Table 1.
Characteristics of patients with non-metastatic invasive breast cancer by VEGF expression at enrollment
| VEGF expression |
P value | ||
|---|---|---|---|
| Positive | Negative | ||
| No. of cases | 1297 | 491 | |
| Age at diagnosis [year, mean (SD)] |
57.7 (8.3) | 57.9 (7.7) | 0.63 |
| BMI at diagnosis [kg/m2, mean (SD)] |
25.0 (7.1) | 24.3 (7.2) | 0.05 |
| Menopausal status at diagnosis [N, (%)] |
0.28 | ||
| Pre-menopause | 271 (21.3) | 92 (19.0) | |
| Post-menopause | 1000 (78.7) | 392 (81.0) | |
| Missing | 26 | 7 | |
| Smoking status prior to diagnosis [N, (%)] |
<0.01 | ||
| Never | 539 (41.9) | 173 (35.5) | |
| Past | 519 (40.4) | 200 (41.1) | |
| Current | 228 (17.7) | 114 (23.4) | |
| Missing | 11 | 4 | |
| Family history of breast cancer among first-degree relatives [N, ( %)] |
0.52 | ||
| No | 1122 (86.5) | 419 (85.3) | |
| Yes | 175 (13.5) | 72 (14.7) | |
| Cancer stage [N, (%)]a,c | <0.0001 | ||
| I | 645 (49.7) | 325 (66.2) | |
| II | 513 (39.6) | 142 (28.9) | |
| III | 139 (10.7) | 24 (4.9) | |
| ER status [N, (%)] | <0.0001 | ||
| Negative | 316 (24.6) | 71 (15.0) | |
| Positive | 968 (75.4) | 401 (85.0) | |
| Missing | 13 | 19 | |
| PR status [N, (%)] | <0.001 | ||
| Negative | 480 (37.3) | 132 (27.6) | |
| Positive | 808 (62.7) | 346 (72.4) | |
| Missing | 9 | 13 | |
| HER2 status [N, (%)] | <0.0001 | ||
| Negative | 1117 (87.3) | 459 (96.4) | |
| Positive | 162 (12.7) | 17 (3.6) | |
| Missing | 18 | 15 | |
| EGFR status [N, (%)] | <0.0001 | ||
| Negative | 989 (77.8) | 431 (90.4) | |
| Positive | 282 (22.2) | 46 (9.6) | |
| Missing | 26 | 14 | |
| CK5/6 status [N, (%)] | <0.01 | ||
| Negative | 1198 (93.5) | 465 (96.9) | |
| Positive | 84 (6.6) | 15 (3.1) | |
| Missing | 15 | 11 | |
| Intrinsic subtypes [N, (%)] | <0.0001 | ||
| Luminal A | 740 (58.9) | 374 (80.1) | |
| Luminal B | 224 (17.8) | 34 (7.3) | |
| HER2 | 86 (6.9) | 5 (1.1) | |
| Basal-like | 158 (12.6) | 29 (6.2) | |
| Unclassified | 48 (3.8) | 25 (5.4) | |
| Missing | 41 | 24 | |
| Type of surgery [N, (%)]b | 0.19 | ||
| No surgery | 3 (0.3) | 1 (0.3) | |
| Breast-conserving surgery |
331 (36.7) | 146 (42.0) | |
| Mastectomy | 568 (63.0) | 201 (57.8) | |
| Unspecified | 395 | 143 | |
| Chemotherapy [N, (%)]b | <0.01 | ||
| No | 572 (58.6) | 252 (67.4) | |
| Yes | 404 (41.4) | 122 (32.6) | |
| Missing | 321 | 117 | |
| Radiotherapy [N, (%)]b | 0.40 | ||
| No | 559 (57.2) | 204 (54.7) | |
| Yes | 418 (42.8) | 169 (45.3) | |
| Missing | 320 | 118 | |
| Adjuvant hormonal therapy [N, (%)]b |
0.70 | ||
| No | 321 (33.1) | 119 (32.0) | |
| Yes | 649 (66.9) | 253 (68.0) | |
| Missing | 327 | 119 | |
| Tumor size [N, (%)]b | <0.0001 | ||
| ≤2 cm | 824 (63.5) | 383 (78.0) | |
| >2 cm | 473 (36.5) | 108 (22.0) | |
| The number of positive nodes [N, (%)]b |
<0.001 | ||
| 0 | 898 (69.2) | 386 (78.6) | |
| 1–3 | 300 (23.1) | 87 (17.7) | |
| 4–9 | 51 (3.9) | 13 (2.7) | |
| ≥10 | 48 (3.7) | 5 (1.0) | |
| Grade of tumor [N, (%)]a |
<0.0001 | ||
| I | 177 (13.9) | 169 (34.6) | |
| II | 721 (56.5) | 269 (55.1) | |
| III | 378 (29.6) | 50 (10.3) | |
| Missing | 21 | 3 | |
Data obtained through central pathology review
Data from medical records
Tumors that were 2 cm or less were grouped as stage I if they had not spread to lymph nodes, or as stage II if 1–3 lymph nodes were positive, or as stage III if 4 or more nodes were positive. Tumors that were larger than 2 cm but were 4 cm or less were grouped as stage II if 3 or less positive nodes were found or as stage III if 4 or more positive nodes were found. Tumors larger than 4 cm were grouped as stage II if they had not spread to lymph nodes or as stage III if positive nodes were found
Overall VEGF-survival relationship
During a median follow-up of 15 years, 689 (38.5%) women died, including 381 (21.3%) breast cancer-specific deaths, and 358 (20.5%) distant recurrences. Ten-year BCSS was 88% among patients with VEGF-negative tumors and 83% among patients with VEGF-positive tumors (log-rank P < 0.001). Ten-year RFS was 87% among patients with VEGF-negative tumors and 82% among patients with VEGF-positive tumors (log-rank P < 0.01); ten-year OS was 81% among patients with VEGF-negative tumors and 77% among those with VEGF-positive tumors (log-rank P = 0.21).
As shown in Table 2, VEGF positivity was associated with significant increases in breast cancer-specific mortality (BCSM; HR = 1.52, 95% CI = 1.19, 1.95) and distant recurrence (HR = 1.52, 95% CI = 1.17, 1.96). Adjustment for covariates attenuated the VEGF-survival association to a non-significant level.
Table 2.
VEGF expression and survival in non-metastatic invasive breast cancer by intrinsic subtypes
| Total | Breast cancer-specific mortality |
Distant recurrence |
Overall mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Death/ Person- years |
Univariate HR (95% CI) |
Multivariatea HR (95% CI) |
Event/ Person- years |
Univariate HR (95% CI) |
Multivariatea HR (95% CI) |
Death/ Person- years |
Univariate HR (95% CI) |
Multivariatea HR (95% CI) |
|
| All patients | ||||||||||
| ******VEGF negative | 491 | 78/7414 | Reference | Reference | 74/7195 | Reference | Reference | 179/7414 | Reference | Reference |
| VEGF positive | 1297 | 303/19015 | 1.52 (1.19, 1.95) |
1.27 (0.98, 1.64) |
284/18204 | 1.52 (1.17, 1.96) |
1.27 (0.97, 1.65) |
510/19016 | 1.12 (0.94, 1.32) |
1.02 (0.86, 1.22) |
| P | <0.001 | 0.07 | <0.01 | 0.08 | 0.21 | 0.82 | ||||
| Patients with luminal A tumors | ||||||||||
| VEGF negative | 374 | 47/5748 | Reference | Reference | 47/5609 | Reference | Reference | 119/5748 | Reference | Reference |
| VEGF positive | 740 | 149/11243 | 1.63 (1.17, 2.26) |
1.41 (1.01, 1.97) |
157/10786 | 1.74 (1.26, 2.41) |
1.49 (1.07, 2.07) |
282/11243 | 1.22 (0.98, 1.51) |
1.19 (0.96, 1.48) |
| P | <0.01 | 0.04 | <0.001 | 0.02 | 0.07 | 0.12 | ||||
| Patients with luminal B tumors | ||||||||||
| VEGF negative | 34 | 15/378 | Reference | Reference | 14/348 | Reference | Reference | 21/378 | Reference | Reference |
| VEGF positive | 224 | 62/3094 | 0.53 (0.30, 0.94) |
0.71 (0.38, 1.32) |
60/2931 | 0.55 (0.31, 0.99) |
0.70 (0.38, 1.32) |
98/3094 | 0.57 (0.35, 0.91) |
0.82 (0.49, 1.37) |
| P | 0.03 | 0.28 | 0.05 | 0.27 | 0.02 | 0.45 | ||||
| Patients with HER2 tumors | ||||||||||
| VEGF negative | 5 | 1/60 | Reference | Reference | 1/58 | Reference | Reference | 3/60 | Reference | Reference |
| VEGF positive | 86 | 28/1204 | 1.86 (0.25, 13.68) |
1.56 (0.18, 13.89) |
20/1153 | 1.38 (0.19, 10.28) |
0.96 (0.09, 9.86) |
38/1204 | 0.70 (0.21, 2.27) |
0.46 (0.12, 1.84) |
| P | 0.54 | 0.69 | 0.75 | 0.98 | 0.55 | 0.27 | ||||
| Patients with basal-like tumors | ||||||||||
| VEGF negative | 29 | 9/426 | Reference | Reference | 6/392 | Reference | Reference | 15/426 | Reference | Reference |
| VEGF positive | 158 | 45/2170 | 0.91 (0.44, 1.87) |
0.54 (0.24, 1.21) |
34/2087 | 0.96 (0.40, 2.29) |
0.57 (0.21, 1.53) |
63/2170 | 0.79 (0.45, 1.40) |
0.50 (0.27, 0.95) |
| P | 0.79 | 0.13 | 0.93 | 0.26 | 0.42 | 0.03 | ||||
| Patients with “unclassified” tumors | ||||||||||
| VEGF negative | 25 | 3/413 | Reference | Reference | 3/405 | Reference | Reference | 9/413 | Reference | Reference |
| VEGF positive | 48 | 17/616 | 3.49 (1.02, 11.93) |
14.09 (2.33, 85.11) |
11/563 | 2.49 (0.69, 8.91) |
10.90 (1.32, 90.25) |
20/616 | 1.43 (0.65, 3.15) |
2.24 (0.81, 6.22) |
| P | 0.05 | <0.01 | 0.16 | 0.03 | 0.37 | 0.12 | ||||
Adjusted for age at diagnosis (continuous), body mass index at diagnosis (continuous), menopausal status at diagnosis (premenopausal, postmenopausal, or unknown), smoking status prior to diagnosis (never smoking, past smoking, current smoking, or unknown), year of diagnosis (continuous), tumor size (>2 or ≤2 cm), histological grade (I/II, III, or unknown), and nodal status (positive or negative)
P for interaction between VEGF and intrinsic subtype was 0.02 in both breast cancer-specific mortality and overall mortality, and was 0.08 in the risk of recurrence
VEGF and survival by intrinsic subtypes
Because of the correlation between VEGF expression and intrinsic subtypes, we further analyzed the VEGF-survival relationship by intrinsic subtypes (Table 2). The association between VEGF and survival outcomes was significantly different among intrinsic subtypes (P for interaction = 0.02 for both BCSM and overall mortality). The multivariate analysis showed that VEGF positivity was significantly associated with increased risks for BCSM (HR = 1.41, 95% CI = 1.01, 1.97) and recurrence (HR = 1.49, 95% CI = 1.07, 2.07) among patients with luminal A tumors. The similar association was also noted in unclassified tumors (for BCSM: HR = 14.09, 95% CI = 2.33, 85.11; for recurrence: HR = 10.90, 95% CI = 1.32, 90.25). VEGF expression did not significantly influence the outcome in patients with luminal B, HER2, and basal-like tumors in terms of BCSM and recurrence. However, VEGF expression was inversely related to overall mortality in basal-like tumors (HR = 0.50, 95% CI = 0.27, 0.95).
Further adjustment for chemotherapy and adjuvant hormonal therapy did not significantly change the overall VEGF-survival associations or the associations among women with luminal A, luminal B, or HER2 type tumors. After controlling for both therapies, the inverse association between VEGF and BCSM among women with basal-like tumors became slightly stronger and statistically significant (HR = 0.42, 95% CI = 0.18, 0.98). In women with unclassified tumors, further adjustment for treatment attenuated the VEGF–BCSM association.
VEGF and survival by adjuvant systemic therapy
Use of adjuvant systemic therapy significantly modified the association between VEGF and BCSM (P for interaction = 0.03) while the interaction between VEGF and adjuvant systemic therapy was not significant for overall survival (P = 0.25) or recurrence (P = 0.14). Among 262 patients (85% stage I and 15% stage II) untreated systemically, 10-year BCSS was 100% among patients with VEGF-negative tumors and 93% among patients with VEGF-positive tumors (log-rank P < 0. 01). VEGF expression was an independent risk factor of BCSM among patients untreated systemically (HR = 5.58, 95% CI = 1.17, 26.66) (Table 3). However, VEGF was not significantly associated with survival outcomes among patients who had chemotherapy and/or adjuvant hormonal therapy.
Table 3.
VEGF expression and survival in non-metastatic invasive breast cancer by adjuvant systemic therapy and expression of ER and PR
| Total | Breast cancer-specific mortality |
Distant recurrence |
Overall mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Death/ Person- years |
Univariate HR (95% CI) |
Multivariate HR (95% CI) |
Event/ Person- years |
Univariate HR (95% CI) |
Multivariate HR (95% CI) |
Death/ Person- years |
Univariate HR (95% CI) |
Multivariate HR (95% CI) |
|
| Patients untreated systemically | ||||||||||
| VEGF negative | 82 | 2/1365 | Reference | Reference | 4/1329 | Reference | Reference | 19/1365 | Reference | Reference |
| VEGF positive | 180 | 22/2877 | 5.49 (1.28, 23.45) |
5.58 (1.17, 26.66)a |
23/2831 | 2.70 (0.94, 7.82) |
1.88 (0.61, 5.78)a |
55/2877 | 1.40 (0.83, 2.36) |
1.36 (0.78, 2.38)a |
| P | 0.02 | 0.03 | 0.07 | 0.27 | 0.21 | 0.28 | ||||
| Patients with adjuvant hormonal therapy | ||||||||||
| VEGF negative | 253 | 30/3663 | Reference | Reference | 31/3581 | Reference | Reference | 73/3663 | Reference | Reference |
| VEGF positive | 649 | 97/9109 | 1.30 (0.87, 1.96) |
0.99 (0.65, 1.53)b |
102/8797 | 1.32 (0.89, 1.98) |
1.07 (0.70, 1.63)b |
207/9109 | 1.15 (0.88, 1.50) |
1.02 (0.77, 1.35)b |
| P | 0.21 | 0.97 | 0.17 | 0.76 | 0.32 | 0.91 | ||||
| Patients with both ER- and PR-positive tumors and adjuvant hormonal therapy | ||||||||||
| VEGF negative | 195 | 19/2804 | Reference | Reference | 20/2744 | Reference | Reference | 56/2804 | Reference | Reference |
| VEGF positive | 451 | 64/6408 | 1.48 (0.89, 2.47) |
1.08 (0.63, 1.86)c |
70/6177 | 1.56 (0.95, 2.56) |
1.22 (0.72, 2.05)c |
136/6408 | 1.06 (0.78, 1.45) |
0.94 (0.67, 1.31)c |
| P | 0.13 | 0.78 | 0.08 | 0.46 | 0.72 | 0.70 | ||||
Adjusted for age at diagnosis (continuous), body mass index at diagnosis (continuous), menopausal status at diagnosis (premenopausal, postmenopausal, or unknown), smoking status prior to diagnosis (never smoking, past smoking, current smoking, or unknown), year of diagnosis (continuous), tumor size (>2 or ≤2 cm), histological grade (I/II, III, or unknown), nodal status (positive or negative), and status of ER, PR, HER2, EGFR, and cytokeratin 5/6 (positive, negative, or unknown)
Adjusted for the above factors as well as adjuvant chemotherapy (yes, no, or unknown)
Adjusted for factors mentioned in b except for ER and PR status
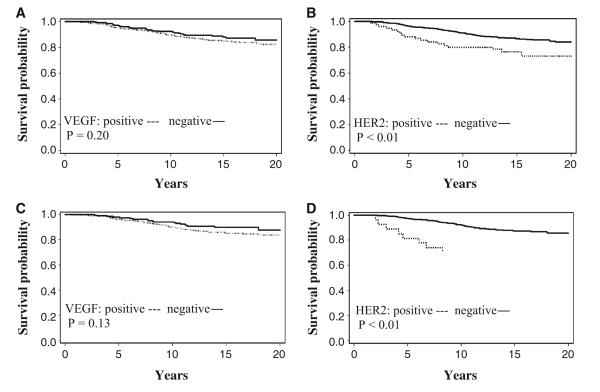
Because in vitro and animal models suggest the involvement of VEGF in hormone resistance, we further evaluated the predictive value of VEGF in response to adjuvant hormonal therapy. The following analyses were restricted to the 902 patients with adjuvant hormonal therapy. VEGF immunoreactivity was not associated with BCSS (log-rank P = 0.20, Fig. 1a), OS (log-rank P = 0.32), or RFS (log-rank P = 0.17); however, patients with HER2-type tumors had shorter BCSS times (log-rank P < 0.01, Fig. 1b) and RFS times (log-rank P = 0.07). When the analysis was restricted to the 646 patients with both ER- and PR-positive tumors receiving adjuvant hormonal therapy, HER2 over-expression was still significantly associated with decreased BCSS (log-rank P < 0.01, Fig. 1d) and RFS (log-rank P = 0.02) and VEGF expression did not significantly influence BCSS (log-rank P = 0.13, Fig. 1c) or RFS (log-rank P = 0.08). Multivariate analyses showed that VEGF was not significantly associated with clinical outcomes, regardless of steroid hormone receptor status (Table 3). While HER2 overexpression was not an independent risk factor predicting clinical outcomes of all patients receiving adjuvant hormonal therapy (data not shown), HER2 over-expression was significantly associated with more than twofold increases in BCSM (HR = 2.64, 95% CI = 1.21, 5.78) and recurrence (HR = 2.32, 95% CI = 1.02, 5.28) in patients with both ER- and PR-positive tumors receiving adjuvant hormonal therapy after adjustment for chemotherapy and other confounders.
Fig. 1.
Breast cancer-specific survival curves by VEGF and HER2 status in breast cancer patients with adjuvant hormonal therapy (a, b) and patients with both ER- and PR-positive tumors and adjuvant hormonal therapy (c, d)
Discussion
The correlation of VEGF with established pathological markers for breast cancer prognosis has been reported [8, 9, 11–14]. However, little is known about whether VEGF is differentially expressed by intrinsic subtypes of breast cancer. We found that VEGF positivity was more frequent in luminal B, HER2, and basal-like tumors versus luminal A types.
Overall, there was a non-significant increased risk of BCSM associated with VEGF expression. However, VEGF positivity was associated with a 40% increased risk of BCSM and recurrence in luminal A tumors. There was also an association between VEGF and BCSM among the tumors negative for all five markers (ER, PR, HER2, EGFR, and CK5/6). VEGF had no obvious effect on clinical outcomes of luminal B, HER2, and basal-like tumors in terms of BCSM and recurrence. Thus, the magnitude of adverse effects of VEGF on breast cancer prognosis may be surpassed by that of co-expressed HER2, EGFR, and/or CK5/6. Due to a limited number of luminal B, HER2, and basal-like tumors negative for VEGF, a larger study is needed to confirm this hypothesis. The differential association between VEGF and survival by intrinsic subtypes of breast cancer may help explain the conflicting results in the literature.
This finding of no effects of VEGF on BCSM in basal-like tumors is similar to that seen in two independent studies among Swedish women with TNBC, one using the cytosol-based method and the other using IHC to determine VEGF status [19, 20]. These results may challenge the use of anti-VEGF therapy for non-metastatic basal-like breast cancer and non-metastatic TNBC, two tumor types with significant overlap. Using IHC to assess VEGF, VEGFR-2, and EGFR expression, Ryden et al. [20] found that only VEGFR-2 was associated with a significant decrease in BCSS in TNBC patients untreated systemically. Thus, components of the VEGF signaling pathway rather than VEGF itself may be involved in the poor prognosis of TNBC. The survival benefit of VEGF-targeted therapy with bevacizumab might not be as high in non-metastatic TNBC as that with immunotherapy directed toward VEGFR-2.
Unexpectedly, we found a VEGF-associated decreased risk of overall mortality in patients with basal-like tumors. The inverse association may be due to nuisance distribution of unmeasured confounders related to death from causes other than breast cancer. Due to the limited power, we could not rule out chance as the basis for this inverse association. Both a limited number of basal-like tumors and the unavailability of treatment data for 25% of patients may account for the inconsistent VEGF–BCSM association between the models adjusted and unadjusted for treatment.
Using a panel of five tissue markers (ER, PR, HER2, EGFR, and CK5/6) to classify breast tumors identified among the NHS participants, Dawood et al. [28] found that 5% of tumors were negative for all five markers and this type of tumors were associated with a 38% increased risk of BCSM as compared with luminal A type. With a small number of cases with unclassified tumors, we had a limited power in this study to evaluate the survival effect of VEGF in this type of tumors.
Consistent with the cytosol-based studies performed in node-negative breast cancer patients untreated systemically [11, 29, 30], we found that VEGF expression was an independent factor associated with increased BCSM of early invasive breast cancer patients untreated systemically. Therefore, VEGF may be a prognostic marker in early invasive breast cancer.
Clinically, not all patients with ER-positive tumors benefit from adjuvant hormonal therapy. In tamoxifentreated mice, induction of VEGF expression in implanted xenografts that were initially tamoxifen responsive caused ER-positive breast tumors to grow and metastasize to the lungs [31]. In patients with both ER- and PR-positive breast cancers, high cytosolic VEGF levels rather than HER2 levels were independently correlated with shorter BCSS and RFS times after adjuvant hormonal therapy [12]. Using IHC to evaluate VEGF and HER2 over-expression, however, we found that despite the high correlation between these two proteins, HER2 rather than VEGF was significantly associated with decreases in BCSS and RFS in patients with both ER- and PR-positive tumors after adjuvant hormonal therapy. The finding is consistent with the results of a randomized trial showing that only IHC-determined HER2 but not IHC-determined VEGF expression was an independent predictor for decreased BCSS after chemotherapy in breast cancer patients [9]. Therefore, cytosolic VEGF levels seem to be more accurate than in situ VEGF expression to predict response to anti-estrogen therapy. Conversely, IHC seems to be superior to cytosol-based method in determining HER2 status to predict hormone resistance.
As a limitation of this study, data on adjuvant systemic therapy are missing in a quarter of the sample. We grouped patients for whom the data on chemotherapy were unavailable in a separate category in the multivariable analysis of predictive value of VEGF after adjuvant hormonal therapy. Sensitivity analyses restricted to patients for whom the data on chemotherapy were available yielded similar results. In addition, year of diagnosis was included in the multivariable models to account for any changes in treatment over time.
Expensive costs limit the use of gene expression profiling in routine clinical practice, although it remains the gold standard to define breast cancer subtypes. Classification of breast cancer by a panel of IHC markers predicts distinct clinical outcomes [32–35]. Our classification scheme was similar, although not identical, to those used in previous epidemiologic studies [27, 28]. The prior studies utilized a panel of IHC markers alone to define molecular subtypes, while we also incorporated histologic grade. It has been suggested that the distinction between luminal A and B tumors can be refined by adding the proliferation marker Ki67 to ER, PR, and HER2 [34]. Due to the lack of Ki67 data for the cases, we used histologic grade as a surrogate for proliferation rate because of the high correlation between them [36]. Intrinsic subtypes defined by our classification scheme have shown different prognosis, with luminal A tumors having better prognosis than other subtypes [28], which was consistent with the results of studies in which molecular subtypes were defined by gene expression patterns [37, 38] or by panels of IHC markers [32–34].
In conclusion, despite the higher frequency of VEGF expression in luminal B, HER2, and basal-like versus luminal A tumors, the VEGF-associated adverse effects on survival were observed only in luminal A breast cancer. VEGF expression was a prognostic factor in invasive breast cancer but not a predictor of hormone resistance in breast cancer.
Acknowledgments
This article supported by GlaxoSmithKline (WE234 (EPI40307)); Public Health Service Grants CA087969, and SPORE in Breast Cancer CA089393, from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services and Breast Cancer Research Fund. Dr. Graham Colditz is supported in part by an American Cancer Society Cissy Hornung Clinical Research Professorship. We thank the Nurses’ Health Study participants for their support.
Contributor Information
Ying Liu, Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, Campus Box 8100, 660 South Euclid Avenue, St. Louis, MO 63110, USA.
Rulla M. Tamimi, Department of Medicine, Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Laura C. Collins, Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Stuart J. Schnitt, Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Hannah L. Gilmore, Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
James L. Connolly, Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA
Graham A. Colditz, Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, Campus Box 8100, 660 South Euclid Avenue, St. Louis, MO 63110, USA; Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA
References
- 1.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 2.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 3.Mercurio AM, Lipscomb EA, Bachelder RE. Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland Biol Neoplasia. 2005;10:283–290. doi: 10.1007/s10911-006-9001-9. [DOI] [PubMed] [Google Scholar]
- 4.Barr MP, Bouchier-Hayes DJ, Harmey JJ. Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. Int J Oncol. 2008;32:41–48. [PubMed] [Google Scholar]
- 5.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13:905–919. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 6.Rice A, Quinn CM. Angiogenesis, thrombospondin, and ductal carcinoma in situ of the breast. J Clin Pathol. 2002;55:569–574. doi: 10.1136/jcp.55.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paola F, Granato AM, Scarpi E, Monti F, Medri L, Bianchi S, Amadori D, Volpi A. Vascular endothelial growth factor and prognosis in patients with node-negative breast cancer. Int J Cancer. 2002;98:228–233. doi: 10.1002/ijc.10118. [DOI] [PubMed] [Google Scholar]
- 8.Fuckar D, Dekanic A, Stifter S, Mustac E, Krstulja M, Dobrila F, Jonjic N. VEGF expression is associated with negative estrogen receptor status in patients with breast cancer. Int J Surg Pathol. 2006;14:49–55. doi: 10.1177/106689690601400109. [DOI] [PubMed] [Google Scholar]
- 9.Kostopoulos I, Arapantoni-Dadioti P, Gogas H, Papadopoulos S, Malamou-Mitsi V, Scopa CD, Markaki S, Karagianni E, Kyriakou V, Margariti A, Kyrkou E, Pavlakis K, Zaramboukas T, Skordalaki A, Bourli A, Markopoulos C, Pectasides D, Dimopoulos MA, Skarlos D, Fountzilas G. Evaluation of the prognostic value of HER-2 and VEGF in breast cancer patients participating in a randomized study with dose-dense sequential adjuvant chemotherapy. Breast Cancer Res Treat. 2006;96:251–261. doi: 10.1007/s10549-005-9062-2. [DOI] [PubMed] [Google Scholar]
- 10.Vogl G, Bartel H, Dietze O, Hauser-Kronberger C. HER2 is unlikely to be involved in directly regulating angiogenesis in human breast cancer. Appl Immunohistochem Mol Morphol. 2006;14:138–145. doi: 10.1097/01.pai.0000168591.58721.a6. [DOI] [PubMed] [Google Scholar]
- 11.Manders P, Beex LV, Tjan-Heijnen VC, Geurts-Moespot J, Van Tienoven TH, Foekens JA, Sweep CG. The prognostic value of vascular endothelial growth factor in 574 node-negative breast cancer patients who did not receive adjuvant systemic therapy. Br J Cancer. 2002;87:772–778. doi: 10.1038/sj.bjc.6600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linderholm B, Bergqvist J, Hellborg H, Johansson U, Linderholm M, von Schoultz E, Elmberger G, Skoog L, Bergh J. Shorter survival-times following adjuvant endocrine therapy in oestrogen- and progesterone-receptor positive breast cancer overexpressing HER2 and/or with an increased expression of vascular endothelial growth factor. Med Oncol. 2009;26:480–490. doi: 10.1007/s12032-008-9157-9. [DOI] [PubMed] [Google Scholar]
- 13.Bando H, Weich HA, Brokelmann M, Horiguchi S, Funata N, Ogawa T, Toi M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br J Cancer. 2005;92:553–561. doi: 10.1038/sj.bjc.6602374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro-Silva A, Ribeiro do Vale F, Zucoloto S. Vascular endothelial growth factor expression in the basal subtype of breast carcinoma. Am J Clin Pathol. 2006;125:512–518. doi: 10.1309/D744-C4NM-15J3-B00D. [DOI] [PubMed] [Google Scholar]
- 15.Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther. 2009;8:1761–1771. doi: 10.1158/1535-7163.MCT-09-0280. [DOI] [PubMed] [Google Scholar]
- 16.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 17.Kim MR, Choi HS, Yang JW, Park BC, Kim JA, Kang KW. Enhancement of vascular endothelial growth factor-mediated angiogenesis in tamoxifen-resistant breast cancer cells: role of Pin1 overexpression. Mol Cancer Ther. 2009;8:2163–2171. doi: 10.1158/1535-7163.MCT-08-1061. [DOI] [PubMed] [Google Scholar]
- 18.Dent SF. The role of VEGF in triple-negative breast cancer: where do we go from here? Ann Oncol. 2009;20:1615–1617. doi: 10.1093/annonc/mdp410. [DOI] [PubMed] [Google Scholar]
- 19.Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtio J, Lewensohn R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20:1639–1646. doi: 10.1093/annonc/mdp062. [DOI] [PubMed] [Google Scholar]
- 20.Ryden L, Jirstrom K, Haglund M, Stal O, Ferno M. Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res Treat. 2010;120:491–498. doi: 10.1007/s10549-010-0758-6. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, Sullivan CA, Zerkowski MP, Molinaro AM, Rimm DL, Camp RL, Chung GG. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. 2008;39:1835–1843. doi: 10.1016/j.humpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linderholm BK, Gruvberger-Saal S, Ferno M, Bendahl PO, Malmstrom P. Vascular endothelial growth factor is a strong predictor of early distant recurrences in a prospective study of premenopausal women with lymph-node negative breast cancer. Breast. 2008;17:484–491. doi: 10.1016/j.breast.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Cimpean AM, Raica M, Suciu C, Tatucu D, Sarb S, Muresan AM. Vascular endothelial growth factor A (VEGF A) as individual prognostic factor in invasive breast carcinoma. Rom J Morphol Embryol. 2008;49:303–308. [PubMed] [Google Scholar]
- 24.Nieto Y, Woods J, Nawaz F, Baron A, Jones RB, Shpall EJ, Nawaz S. Prognostic analysis of tumour angiogenesis, determined by microvessel density and expression of vascular endothelial growth factor, in high-risk primary breast cancer patients treated with high-dose chemotherapy. Br J Cancer. 2007;97:391–397. doi: 10.1038/sj.bjc.6603875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marotti JD, Collins LC, Hu R, Tamimi RM. Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2010;23:197–204. doi: 10.1038/modpathol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, Collins LC. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10:R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawood S, Hu R, Homes MD, Collins LC, Schnitt SJ, Connolly J, Colditz GA, Tamimi RM. Defining breast cancer prognosis based on molecular phenotypes: results from a large cohort study. Breast Cancer Res Treat. 2011;126:185–192. doi: 10.1007/s10549-010-1113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coradini D, Boracchi P, Daidone MG, Pellizzaro C, Miodini P, Ammatuna M, Tomasic G, Biganzoli E. Contribution of vascular endothelial growth factor to the Nottingham prognostic index in node-negative breast cancer. Br J Cancer. 2001;85:795–797. doi: 10.1054/bjoc.2001.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasparini G, Toi M, Biganzoli E, Dittadi R, Fanelli M, Morabito A, Boracchi P, Gion M. Thrombospondin-1 and -2 in node-negativeg breast cancer: correlation with angiogenic factors, p53, cathepsin D, hormone receptors and prognosis. Oncology. 2001;60:72–80. doi: 10.1159/000055300. [DOI] [PubMed] [Google Scholar]
- 31.Qu Z, Van Ginkel S, Roy AM, Westbrook L, Nasrin M, Maxuitenko Y, Frost AR, Carey D, Wang W, Li R, Grizzle WE, Thottassery JV, Kern FG. Vascular endothelial growth factor reduces tamoxifen efficacy and promotes metastatic colonization and desmoplasia in breast tumors. Cancer Res. 2008;68:6232–6240. doi: 10.1158/0008-5472.CAN-07-5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H, Akslen LA, Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10, 159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 34.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 35.Mulligan AM, Pinnaduwage D, Bull SB, O’Malley FP, Andrulis IL. Prognostic effect of basal-like breast cancers is time dependent: evidence from tissue microarray studies on a lymph node-negative cohort. Clin Cancer Res. 2008;14:4168–4174. doi: 10.1158/1078-0432.CCR-07-4543. [DOI] [PubMed] [Google Scholar]
- 36.Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, Coates AS, Collins J, Castiglione-Gertsch M, Gusterson BA. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors—a surrogate marker? Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 37.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]