Abstract
Okadaic acid (OA) and its derivatives, which are produced by dinoflagellates of the genera Prorocentrum and Dinophysis, are responsible for diarrhetic shellfish poisoning in humans. In laboratory animals, these toxins cause epithelial damage and fluid accumulation in the gastrointestinal tract, and at high doses, they cause death. These substances have also been shown to be tumour promoters, and when injected into the brains of rodents, OA induces neuronal damage reminiscent of that seen in Alzheimer’s disease. OA and certain of its derivatives are potent inhibitors of protein phosphatases, which play many roles in cellular metabolism. In 1990, it was suggested that inhibition of these enzymes was responsible for the diarrhetic effect of these toxins. It is now repeatedly stated in the literature that protein phosphatase inhibition is not only responsible for the intestinal effects of OA and derivatives, but also for their acute toxic effects, their tumour promoting activity and their neuronal toxicity. In the present review, the evidence for the involvement of protein phosphatase inhibition in the induction of the toxic effects of OA and its derivatives is examined, with the conclusion that the mechanism of toxicity of these substances requires re-evaluation.
Keywords: okadaic acid toxicity, protein phosphatase inhibition
1. Introduction
Okadaic acid (OA, Figure 1, R1 = CH3, R2 = H, R3 = H) is produced by dinoflagellates of the genera Prorocentrum and Dinophysis, which are of worldwide distribution [1]. These organisms also produce the isomeric compounds dinophysistoxin 2 (DTX-2, Figure 1, R1 = H, R2 = H, R3 = CH3) and 19-epi-okadaic acid, together with the methylated derivative, dinophysistoxin 1 (DTX-1, Figure 1, R1 = CH3, R2 = CH3, R3 = H). Many esters formed from OA and the dinophysistoxins by conjugation of the terminal carboxylic acid group with poly-hydroxylated, sulphated or unsaturated alcohols have also been isolated from these organisms [2]. When ingested by shellfish, a proportion of the toxins present in the dinoflagellates are acylated at the C-7 hydroxyl group with long-chain fatty acids, forming derivatives collectively known as DTX-3 [3].
Figure 1.
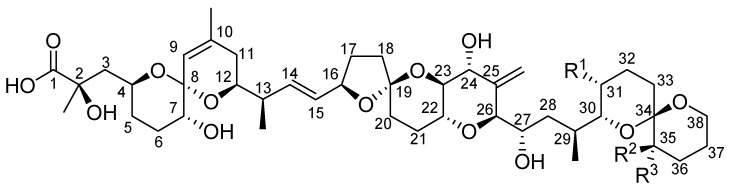
General structure of okadaic acid and derivatives.
Shellfish contaminated with OA and its derivatives are responsible for Diarrhetic Shellfish Poisoning (DSP) in humans. DSP was first reported in Japan in the late 1970s [4], but since that time, it has been recorded in many other parts of the world, including Europe, Scandinavia, North and South America and New Zealand [5,6]. The predominant symptoms associated with DSP are nausea, vomiting, diarrhoea and abdominal pain, observed soon after ingestion of contaminated shellfish. Symptoms generally resolve within 2–3 days, and no deaths associated with DSP have been reported.
2. Toxicity of Okadaic Acid and Derivatives to Experimental Animals
2.1. Acute Toxicity of Okadaic Acid and Derivatives
OA itself is highly toxic to mice by intraperitoneal injection, with LD50 values between 192 and 225 µg/kg being reported [7,8,9,10]. Although no LD50 data on DTX-1 are available, it would appear that this compound, with a reported minimum lethal dose of 160 µg/kg [11], is of similar toxicity to OA. DTX-2, DTX-3 and DTX-4 are somewhat less acutely toxic, with LD50 values between 352 and 600 µg/kg [7,12,13]. Acylation of the 7-hydroxyl group with a saturated fatty acid, to form 7-O-palmitoyl-OA or a di-unsaturated fatty acid, giving 7-O-linoleoyl-OA, greatly decreases toxicity, and these compounds are approximately 20-times less toxic than OA. In contrast, the polyunsaturated 7-O-docosahexaenoyl-OA was reported to be 10-times more toxic than the saturated or di-unsaturated esters, with a minimum lethal dose of 550 µg/kg [14], indicating that this substance is of similar toxicity to DTX-2, DTX-3 and DTX-4 and approximately half as toxic as OA.
OA is less toxic by oral administration than by intraperitoneal injection, although the data that are available are inconsistent. The median lethal dose of OA by gavage was reported as 400 µg/kg [15] and 880 µg/kg [16], while Tubaro et al. [9] observed no deaths at 1,000 µg/kg. Even within the same laboratory, wide variations in estimates of the median lethal dose occur. Le Hégarat [17] found 100% mortality in mice dosed with OA at 300 µg/kg in one experiment, but none at 610 µg/kg in a subsequent one. The reason or reasons for these disparate results are unknown, but it would appear that OA is between 2- and 5-fold less toxic by oral administration than by injection. Few data are available on the OA derivatives, but it would appear that DTX-1 is only slightly less toxic when administered orally than when given by intraperitoneal injection [18,19].
Severe damage to the epithelium of villi in the duodenum and upper jejunum, with separation and desquamation of epithelial cells from the lamina propria, was seen after intraperitoneal injection of OA in mice, although little effect was seen in the crypts. Pronounced oedema of the lamina propria was observed, attributed to increased permeability of vessels of the intestinal villi [20]. Erosion of the intestinal epithelium was recorded in animals injected with OA and DTX-1, while little effect on the gastrointestinal tract was observed in animals receiving the same dose of DTX-3 [18,21]. The toxic changes induced in the small intestine of mice by intraperitoneal injection of OA and its derivatives were also observed after oral administration of these substances, and some epithelial damage was also observed in the caecum and large intestine of these animals [9,15,18,21]. Oral administration of OA also caused oedema and mucosal erosion in the stomach of mice, accompanied by acute inflammatory changes in the submucosa [9,15,22]. Oral administration of OA to rats induced changes in the gastrointestinal tract similar to those seen in mice [23].
The cause of death following administration of lethal doses of OA is presently unclear. After intraperitoneal injection, mice showed hypothermia and muscular paralysis (particularly in the hind legs) and respiratory paralysis [8], and the latter may have been responsible for the death of the animals. In contrast, Ito and Terao [18] attributed death after intraperitoneal injection to hypovolaemic shock following haemorrhage and congestion in the liver. Congestion of blood in the liver, associated with dissociation of biliary canalicular actin sheaths, was also observed in rats following intravenous administration of OA [23]. Other authors, however, have reported relatively minor hepatic effects (isolated necrosis, lipidosis or vacuolation of hepatocytes) after injection of OA [9]. No liver damage was observed in mice or rats dosed orally with OA at lethal doses [15,21,23].
2.2. Diarrhoeagenicity of Okadaic Acid
After intraperitoneal injection of OA in mice, distension of the duodenum and upper jejunum was observed, associated with fluid accumulation in the lumen [9,20]. In a repeated-dose experiment with OA, five mice were dosed by gavage at a dose of 1,000 µg/kg/day for seven days. Diarrhoea was observed in all of the mice. In three animals, this ceased within a few hours, but in two of the mice, the diarrhoea was profuse and persistent, and these animals died after the fifth dose of the test compound. The surviving mice were killed on the eighth day of the experiment. At necropsy, the small intestines of the animals were observed to be full of fluid [24].
2.3. Toxicity of Okadaic Acid and Derivatives through Dermal Application
OA and DTX-1 have been shown to cause severe irritation when applied to mouse skin [25,26].
2.4. Tumour Promotion by Okadaic Acid and Derivatives
Repeated application of OA or DTX-1 to mouse skin was shown to promote tumour formation following initiation with 7,12-dimethylbenz[a]anthracene (DMBA) [25,26,27]. OA also acted as a tumour promoter in the rat glandular stomach after initiation with N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) [28].
2.5. Neurotoxicity of Okadaic Acid after Intra-Cerebral Injection
Injection of OA into the brain of rats causes neuronal cell death [29,30,31,32,33,34,35] and memory impairment [35,36,37,38,39]. In contrast, DTX-1 caused no neuronal death when injected into the brain at a dose equivalent to that at which OA caused severe neurodegeneration [31].
It has been reported that the neuronal damage induced by OA is accompanied by hyperphosphorylation of tau protein, increased formation of neurofibrillary tangles and deposits of β-amyloid [35,40,41,42,43], although other authors have been unable to replicate such effects [29,34].
3. Inhibition of Protein Phosphatases by Okadaic Acid and Derivatives
In 1988, Bialojan and Takai [44] reported that OA inhibited certain protein phosphatases in vitro at nanomolar concentrations. The inhibitory action of OA is greater against PP2A than against PP1 [45]. It is also a potent inhibitor of PP4 and PP5 [46]. This compound has proved valuable in the study of the functions of protein phosphatases in cells in vitro [46,47].
DTX-1 is a somewhat more potent inhibitor of protein phosphatases than OA [45,48,49,50], while DTX-2 is half as active [7]. DTX-4 is much less effective (~500-times less active than OA) [51], while 7-O-palmitoyl-OA is a very weak inhibitor, more than 3,000-times less active than OA [45]. 7-O-Docosahexaenoyl-OA is a little more effective than the palmitoyl derivative, showing 64% inhibition at a concentration of 1 mg/ml, compared to 40% for the palmitoyl derivative at the same concentration, but it is clearly still a very weak inhibitor [50].
The ability of OA and derivatives to inhibit protein phosphatases has been utilised in the development of sensitive assays for DSPs in shellfish [52,53,54,55].
4. Involvement of Protein Phosphatase Inhibition in the Toxic Effects of Okadaic Acid
In 1990, Cohen et al. [56] published a review of the use of OA in the study of the biological processes involving protein phosphatases and stated that OA “probably” causes diarrhoea by stimulating the phosphorylation of proteins controlling sodium secretion by intestinal cells, although no evidence for this suggestion was given. This statement has been repeated many times in the literature, often with omission of the word “probably”, and, despite publications questioning the association between protein phosphatase inhibition and toxicity [57,58], it is often implied or stated, without supporting evidence, that inhibition of protein phosphatases is responsible not only for the diarrhoeagenicity of OA, but also for its acute toxic effects, its tumour-promoting activity and its neurotoxicity [2,5,15,23,24,31,48,50,52,53]. As discussed below, however, there is conflicting evidence for the validity of this suggestion, and the possible role of protein phosphatase inhibition in the toxic effects of OA and its derivatives requires re-evaluation.
Support for a causal relationship between protein phosphatase inhibition and toxicity would be gained from a number of pieces of evidence:
Demonstration of protein phosphatase inhibition in vivo, at the sites at which toxicity has been observed.
Proportionality between the efficacy of OA derivatives in inhibition of protein phosphatases and the severity of the toxic effects that they induce.
The induction of toxic effects similar to those observed with OA by other inhibitors of protein phosphatases.
A defined pathway from protein phosphatase inhibition to the observed toxic effects.
4.1. Protein Phosphatase Inhibition and Acute Toxicity of Okadaic Acid
Comparisons between the relative acute toxicities of OA and its derivatives, as measured by intraperitoneal injection in mice, and the relative activities of these substances in inhibiting PP2A and PP1 are shown in Table 1.
Table 1.
Relative toxicities of OA and derivatives to mice by intraperitoneal injection and relative inhibitory activities toward protein phosphatases.
| Compound | Relative toxicity to mice (i.p. injection) | Relative inhibition of PP2A | Relative inhibition of PP1 |
|---|---|---|---|
| OA | 1.0 | 1.0 | 1.0 |
| DTX-1 | 1.0 [11] | 1.6–2.4 [45,48,49] | 0.4–0.9 [45,48] |
| DTX-2 | 0.6 [7] | 0.5 [7] | - |
| DTX-4 | 0.3 [13] | 0.002 [51] | - |
| 7-O-palmitoyl-OA | 0.05 [14] | <0.0003 [45] | <0.00005 [45] |
| 7-O-docosahexaenoyl-OA | 0.5 [14] | <0.0003 [45,50] | - |
The equivalent relative potency of OA and DTX-2 with regard to toxicity and enzyme inhibition is consistent with the involvement of protein phosphatases in the acute toxicity of these substances. Similarly, the relatively non-toxic 7-O-palmitoyl derivative is a weak inhibitor of the protein phosphatases. However, a rather higher acute toxicity of DTX-1 might have been expected in view of its relatively high potency against PP2A, and while DTX-4 is ~500-times less effective as a protein phosphatase inhibitor than OA, it is only three-times less toxic to mice when injected intraperitoneally. Similarly, 7-O-docosahexaenoyl-OA is a very weak inhibitor of protein phosphatases, yet it is highly toxic to mice.
It could be argued that the relatively high acute toxicities of DTX-4 and 7-O-docosahexaenoyl-OA reflect saponification of the ester function within the peritoneal cavity, yielding OA. While esters of OA are known to be hydrolysed under acid or alkaline conditions or through the action of esterases or lipases within the intestine [59], saponification of injected esters is unlikely under the neutral conditions pertaining in the peritoneum and in the absence of the hydrolytic enzymes. Furthermore, if the esters are converted to OA in the peritoneum, it would be expected that 7-O-palmitoyl-OA would be of similar toxicity to 7-O-docosahexaenoyl-OA, since it is difficult to see why the toxic unsaturated ester should be saponified more readily than the relatively non-toxic saturated ester.
The possibility that OA-induced death is due to hypovolaemic shock following haemorrhage in the liver [18] is of interest, since other compounds known to inhibit protein phosphatases, including microcystin-LR [60,61,62], cantharidin and cantharidic acid [63,64] and acetaminophen [65,66], produce similar hepatic damage. However, such hepatotoxicity is not necessarily attributable to protein phosphatase inhibition. Inhibition of protein phosphatases has not been demonstrated in the livers of animals dosed with OA or its derivatives, and similar effects in the liver are induced by phalloidin [67] and saponin [68], which are not known to inhibit these enzymes. Furthermore, it must also be noted that the hepatic haemorrhage has not been seen in all studies involving injection of OA, and it is not seen after oral administration, even though OA causes death by this route and is taken up into the liver after administration by gavage [15].
The results of other studies [8] suggest that death may be attributable to respiratory paralysis, and OA has been shown to inhibit the twitch response in the mouse phrenic nerve hemidiaphragm preparation [69]. It may be that OA causes death through inhibition of neuromuscular transmission, as has been demonstrated with many other natural toxins [70]. In this context, the report of Valdiglesias et al. [71] that OA has significant effects on genes concerned with synaptic transmission in SHSY5Y neuroblastoma cells is of interest.
More work on the acute toxicity of OA is required, particularly with regard to the mechanism of death and the association between toxicity and protein phosphatase inhibition in vivo. At present, it must be concluded that the suggestion that OA-induced death is due to protein phosphatase inhibition is not proven.
4.2. Protein Phosphatase Inhibition and Diarrhoeagenicity of Okadaic Acid
Protein phosphatase activity was significantly decreased in homogenates of the intestines of mice 3 and 6 hours after oral administration of OA at 750 µg/kg [72], an observation that would satisfy the first criterion for an association between enzyme inhibition and toxicity. However, it should be noted that sloughing of intestinal villi was also observed at these time intervals, and it is possible that cellular loss could have contributed to the observed change in protein phosphatase activity.
The often-cited suggestion that OA causes diarrhoea by mediating hyperphosphorylation of proteins controlling sodium secretion [56] was disproved by subsequent studies by Tripuraneni et al. [73], who showed that OA has no significant effect on ion currents in intestinal cell monolayers. It does, however, increase paracellular permeability in such monolayers in vitro [73,74] and in the rat colon in vivo [75], and it was suggested that such an effect would lead to fluid accumulation in the intestine and, hence, to diarrhoea. The observed increase in paracellular permeability could reflect disruption of tight junctions (TJs), which are important in the regulation of paracellular permeability in epithelial cells. TJs form the physical barrier to diffusion through the paracellular space, and the structure of these junctions may be indirectly regulated by the adjacent adherens junctions (AJs) [76]. TJs consist of assemblies of trans-membrane proteins, including claudins and occludin (an important sealing protein of TJs [77]), scaffolding proteins of the zona occludens family and signalling proteins, such as protein kinases [78] and protein phosphatases [79]. AJs in epithelial cells are formed by interaction between E-cadherin and catenins [80]. There is also evidence that peri-junctional actin [77] and myosin light chain [81] are involved in the regulation of the barrier function of TJs.
Phosphorylation and dephosphorylation of components of the epithelial barrier have profound effects on paracellular permeability, and PP2A has been shown to play a crucial role in the formation and patency of tight junctions [82,83]. Formation of the E-cadherin/catenin complex is regulated by protein phosphatases [84], in this case, primarily by tyrosine phosphatase 1B [80]. The role of protein phosphatase inhibition and activation in the increase in paracellular permeability induced by OA and other protein phosphatase inhibitors therefore needs to be considered.
Phosphorylation of occludin plays a crucial role in the regulation of TJ integrity, and this protein undergoes dephosphorylation on Ser and Thr residues and hyperphosphorylation of Tyr residues during the disruption of TJs induced by various pharmacological and toxic substances, and enhanced PP2A activity, which induces dephosphorylation of occludin, is associated with increased paracellular permeability [79,85]. In this situation, therefore, inhibition of Ser/Thr phosphatases by OA would be expected to protect against increased paracellular permeability, rather than inducing such an effect. The increased paracellular permeability in Caco-2 cell monolayers induced by hydrogen peroxide and by acetaldehyde was indeed shown to be ameliorated by OA and/or fostriecin, another potent protein phosphatase inhibitor, or by knockdown of PP2A [79,86]. Under the conditions of the above experiments, therefore, OA-induced increases in paracellular permeability do not appear to be due to effects on occludin. Furthermore, OA and other protein phosphatase inhibitors or knockdown of PP2A or PP1 enhanced the integrity of tight junctions and accelerated the calcium-induced reassembly of tight junctions in Caco-2 cell monolayers [82]. OA also increased the calcium-induced biogenesis of TJs in Madin-Darby canine kidney (MDCK) cells through promotion of the phosphorylation and recruitment of proteins into TJs [83], and no increase in paracellular permeability was observed at OA concentrations of up to 250 nM. Such an effect was seen only at very high concentrations of OA, at which the cells became rounded, indicating gross cytotoxicity [83].
Hyperphosphorylation of myosin light chain (MLC) increases tight junction permeability [81]. Treatment of cultured human intestinal epithelial monolayers with OA at a concentration of 1 µM caused no increase in the phosphorylation of MLC [73], although higher levels (10 µM) did lead to an increase in the phosphorylation of this protein in MDCK cells [87].
Disruption of the F-actin cytoskeleton by OA has been observed in several cell types in vitro [58,74,88,89], and it was suggested that such a change could decrease the patency of TJs [74] and, thus, lead to diarrhoea in vivo. It has been shown, however, that methyl okadaate, which has little or no effect on the activities of PP2A and PP1, was of similar activity to OA in disrupting the F-actin cytoskeleton in hepatocytes [58] and in neuroblastoma cells [90]. It was suggested [58] that methyl okadaate may inhibit protein phosphatases other than PP1 and PP2A or that this compound and OA may have targets other than the protein phosphatases for their effects on the actin cytoskeleton.
It has been suggested that effects on E-cadherin could contribute to the increased paracellular permeability induced by OA [91]. In one study [92], OA was shown to induce hyperphosphorylation of β-catenin exclusively on Ser/Thr residues in keratinocytes, although no effect of OA on the phosphorylation state of β-catenin was seen in human intestinal epithelial T84 monolayers [73]. OA has been reported to decrease the expression of E-cadherin in MCF-7 breast cancer cells [93], although the mechanism of this effect is presently unknown.
It has been concluded that the mechanisms underlying the ability of OA to enhance paracellular permeability are not fully understood [94]. There appear to be marked differences in the effect of OA on different cell types in vitro, and the evidence for the involvement of protein phosphatase inhibition in the induction of increased paracellular permeability is inconclusive.
Alternative pathways to increased paracellular permeability have been proposed. A proteomic study by Wang et al. [72] revealed that 58 proteins in the mouse intestine were altered in abundance after oral administration of OA. These proteins were identified as those involved in macromolecular metabolism, cytoskeleton reorganisation, signal transduction, molecular chaperoning and oxidative stress, and the authors concluded that multiple proteins, other than the protein phosphatases, could be involved in the diarrhoeagenicity of OA. OA induces TNFα release from cells in vitro [95], and this cytokine has been shown to increase paracellular permeability in cultured epithelial monolayers [96]. OA has also been shown to generate highly oxidising active oxygen species in cells in vitro [89,97,98], and oxidative stress is known to increase paracellular permeability [86,99]. Hosokawa et al. [75] demonstrated increased paracellular permeability in the rat colon in vivo, which was associated with the formation of micro-thrombi in the submucosal venules, followed by mucosal oedema. The change in paracellular permeability was attributed to increased permeability of the venule and subsequent mucosal damage.
The epithelial barrier is known to be severely compromised when epithelial cells are lost, as occurs in intestinal erosion or ulceration [81]. Indeed, diarrhoea could simply result from the structural damage induced by OA in the intestine. Secretion of fluid occurs in the crypts, while absorption is mediated by the cells at the tips of the villi [100,101]. The crypts are largely undamaged by OA [20], so secretion will continue unabated, but loss of cells at the tips of villi will prevent fluid absorption, thus resulting in fluid accumulation in the intestinal lumen and, hence, diarrhoea. Reversible structural damage to intestinal epithelial cells, similar to that induced by OA, has been observed in animals following administration of irritant purgatives used in human medicine, such as castor oil [102,103], dioctyl sodium sulphosuccinate [104], bisacodyl [105], phenolphthalein [106] and sennosides and other anthranoid cathartics [107,108], none of which are known to inhibit protein phosphatases.
4.3. Protein Phosphatase Inhibition and Tumour Promotion by Okadaic Acid
The initial studies by Fujiki and co-workers on tumour promotion by OA and DTX-1 were followed by a series of experiments on the promoting activity of calyculin A, microcystin LR and nodularin, which, like OA, are potent inhibitors of protein phosphatases. Repeated application of calyculin A was as effective as OA in promoting DMBA-initiated skin carcinogenesis [109], while repeated intraperitoneal injections of microcystin LR or nodularin promoted tumour formation in the liver of rats following initiation with diethylnitrosamine [110,111]. These findings led to the conclusion that inhibition of protein phosphatases is a key factor in the mechanism of action of certain tumour promoters, classified as the “okadaic acid type promoters”, as distinct from promoters of the 12-O-tetradecanoylphorbol-13-acetate (TPA) type, and that promoting activity could be predicted on the basis of inhibitory effects on these enzymes.
In 1995, however, the Fujiki group reported on results with tautomycin, another inhibitor of PP1 and PP2A. Unlike the other protein phosphatase inhibitors, tautomycin had no effect on the incidence or multiplicity of skin tumours induced by DMBA, and this substance actually protected against cancer of the glandular stomach induced by MNNG, rather than increasing carcinogenic activity, as was expected. It was therefore concluded that inhibition of protein phosphatases is not a sufficient condition for tumour promotion [112].
Later studies showed the importance of irritant potential for tumour promotion in the skin of mice. OA, DTX-1 and calyculin A caused severe irritation when applied to the mouse ear, but tautomycin did not [25,26,109]. Similarly, oedema, inflammation and mucosal erosion were recorded in the stomach of mice after oral administration of OA or calyculin A, while no such effect was seen with tautomycin [22].
An ability to induce irritation and inflammation is a common property of tumour promoters [113,114,115], and the importance of irritation in tumour promotion is consistent with the suggestion [116,117] that pro-inflammatory cytokines, notably TNF-α, are of critical importance in this process. OA increased TNF-α gene expression in mouse skin and induced TNF-α release from cultured cells in vitro [95], as do many other irritant materials, including the “classical” tumour promoter TPA [117,118,119,120]. In contrast, tautomycin had no effect on cytokine production, and the inability of this substance to act as a tumour promoter was attributed to its failure to induce TNF-α [112]. It is possible that irritation and TNF-α induction are downstream effects of protein phosphatase inhibition, although this appears unlikely in view of tumour promotion by other irritant materials, such as TPA, lyngbyatoxin and palytoxin [121,122], which have not been reported to inhibit protein phosphatases. It is possible, therefore, that the tumour promoting ability of OA is simply due to its irritant potential, not to its ability to inhibit protein phosphatases.
4.4. Protein Phosphatase Inhibition and Neurotoxicity of Okadaic Acid
Changes in the cerebral activity of protein phosphatases have been implicated in the pathogenesis of Alzheimer’s disease. Individuals suffering from this disease suffer memory loss, and histological examination of the brains of such individuals has revealed neuronal neurodegeneration and the presence of neurofibrillary tangles within neurons and extracellular deposits of β-amyloid [123,124,125]. Neurofibrillary tangles result from accumulation of paired helical filaments within neurons, and such filaments consist largely of hyperphosphorylated tau protein [124]. Hyperphosphorylation of tau has been suggested to be caused by an increase in kinase activity or by a decrease in phosphatase activity within the neurons during the development of Alzheimer’s disease [126,127], and Arendt et al. [32] reasoned that injection of OA into the brain would increase tau phosphorylation by inhibiting PP2A, since this enzyme is predominantly responsible for the dephosphorylation of this protein [128]. Arendt et al. showed inhibition of PP1 and PP2A in the brain of rats following intracerebral injection [32,40,42], although others found no such effect [35]. As noted above, intracerebral injection of OA causes tau hyperphosphorylation, formation of neurofibrillary tangles and deposits of β-amyloid, together with memory loss and neurodegeneration. It was therefore suggested that intracerebral injection of OA, through its ability to inhibit protein phosphatases, would provide a useful model of Alzheimer’s disease [32].
There is evidence for and against this suggestion. The observation that calyculin A and microcystins, which, like OA, are protein phosphatase inhibitors, cause memory deficit and/or tau hyperphosphorylation and neuronal degeneration when injected into the rat brain [129,130,131] is evidence in support. However, DTX-1 did not induce neuronal death when injected into the hippocampus of rats [31], even though this substance is of similar potency to OA in inhibiting protein phosphatases. Furthermore, other cytotoxic compounds that are not known to inhibit protein phosphatases, such as kainic acid [132,133], domoic acid [134], isoproterenol [135] and the peroxynitrite generator, SIN-1 [136] cause similar changes after intracerebral injection. Furthermore, tau hyperphosphorylation is not dependent upon the administration of a protein phosphatase inhibitor, since this has been observed after injection of saline into the brain [32,137] and after transient cerebral ischaemia [138,139], and physical damage to the brain leads not only to hyperphosphorylation of tau, but also other cerebral changes characteristic of Alzheimer’s disease [140]. The weight of evidence is therefore against the hypothesis that the primary event in OA-induced cerebral toxicity involves protein phosphatase inhibition, and it has been argued that the observed changes may simply reflect a cellular response to neuronal injury [29,33,137].
5. Conclusion
Although it is widely accepted that the toxic effects of OA and derivatives are caused through inhibition of protein phosphatases, the evidence in support of such an association is very limited at the present time. In no case has a pathway from enzyme inhibition to toxic effect been identified, and the severity of the toxic effects of the OA derivatives are not in proportion to their inhibitory activity. Furthermore, the toxic effects induced by OA and derivatives are replicated by substances that are not protein phosphatase inhibitors, and known protein phosphatase inhibitors do not consistently exert the same toxic effects in animals as OA and derivatives. More work on the mechanism(s) of toxicity of these substances is required.
Acknowledgment
This work was supported by the New Zealand Foundation for Research, Science and Technology, Contract CAWX0703.
Conflict of Interest
The author declares no conflict of interest.
References
- 1.Reguera B., Velo-Suárez L., Raine R., Park M.G. Harmful Dinophysis species: a review. Harmful Algae. 2012;14:87–106. doi: 10.1016/j.hal.2011.10.016. [DOI] [Google Scholar]
- 2.Hu W., Xu J., Sinkkonen J., Wu J. Polyketides from marine dinoflagellates of the genus Prorocentrum, biosynthetic origin and bioactivity of their okadaic acid analogues. Mini-Rev. Med. Chem. 2010;10:51–61. doi: 10.2174/138955710791112541. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T., Ota H., Yamasaki M. Direct evidence of transformation of dinophysistoxin-1 to 7-O-acyl-dinophysistoxin-1 (dinophysistoxin-3) in the scallop Patinopecten yessoensis. Toxicon. 1999;37:187–198. doi: 10.1016/S0041-0101(98)00182-2. [DOI] [PubMed] [Google Scholar]
- 4.Yasumoto T., Oshima Y., Yamaguchi M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1978;44:1249–1255. doi: 10.2331/suisan.44.1249. [DOI] [Google Scholar]
- 5.James K.J., Carey B., O'Halloran J.O., van Pelt F.N.A.M., Škrabáková Z. Shellfish toxicity: human health implications of marine algal toxins. Epidemiol. Infect. 2010;138:927–940. doi: 10.1017/S0950268810000853. [DOI] [PubMed] [Google Scholar]
- 6.Picot C., Nguyen T.A., Roudot A.C., Parent-Massin D. A preliminary risk assessment of human exposure to phycotoxins in shellfish: a review. Hum. Ecol. Risk Assess. 2011;17:328–366. doi: 10.1080/10807039.2011.552393. [DOI] [Google Scholar]
- 7.Aune T., Larsen S., Aasen J.A.B., Rehmann N., Satake M., Hess P. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon. 2007;49:1–7. doi: 10.1016/j.toxicon.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Dickey R.W., Bobzin S.C., Faulkner D.J., Bencsath F.A., Andrzejewski D. Identification of okadaic acid from a Caribbean dinoflagellate, Prorocentrum concavum. Toxicon. 1990;28:371–377. doi: 10.1016/0041-0101(90)90074-H. [DOI] [PubMed] [Google Scholar]
- 9.Tubaro A., Sosa S., Carbonatto M., Altinier G., Vita F., Melato M., Satake M., Yasumoto T. Oral and intraperitoneal acute toxicity studies of yessotoxin and homoyessotoxins in mice. Toxicon. 2003;41:783–792. doi: 10.1016/S0041-0101(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 10.Tachibana K., Scheuer P.J., Tsukitani Y., Kikuchi H., Van Engen D., Clardy J., Gopichand Y., Schmitz F.J. Okadaic acid, a cytotoxic polyether from two marine sponges of the genus Halichondria. J. Am. Chem. Soc. 1981;103:2469–2471. [Google Scholar]
- 11.Murata M., Shimatani M., Sugitani H., Oshima Y., Yasumoto T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1982;48:549–552. [Google Scholar]
- 12.Yasumoto T., Murata M., Oshima Y., Sano M., Matsumoto G.K., Clardy J. Diarrhetic shellfish toxins. Tetrahedron. 1985;41:1019–1025. [Google Scholar]
- 13.Hu T., Curtis J.M., Walter J.A., Wright J.L.C. Identification of DTX-4, a new water-soluble phosphatase inhibitor from the toxic dinoflagellate Prorocentrum lima. J. Chem. Soc. Chem. Comm. 1995:597–599. [Google Scholar]
- 14.Yanagi T., Murata M., Torigoe K., Yasumoto T. Biological activities of semisynthetic analogs of dinophysistoxin-3, the major diarrhetic shellfish toxin. Agric. Biol. Chem. 1989;53:525–529. doi: 10.1271/bbb1961.53.525. [DOI] [Google Scholar]
- 15.Ito E., Yasumoto T., Takai A., Imanishi S., Harada K. Investigation of the distribution and excretion of okadaic acid in mice using immunostaining method. Toxicon. 2002;40:159–165. doi: 10.1016/S0041-0101(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 16.Aune T., Espenes A., Aasen J.A.B., Quilliam M.A., Hess P., Larsen S. Study of possible combined toxic effects of azaspiracid-1 and okadaic acid in mice via the oral route. Toxicon. 2012;60:895–906. doi: 10.1016/j.toxicon.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Le Hégarat L., Jacquin A.-G., Bazin E., Fessard V. Genotoxicity of the marine toxin okadaic acid, in human Caco-2 cells and in mice gut cells. Environ. Toxicol. 2006;21:55–64. doi: 10.1002/tox.20154. [DOI] [PubMed] [Google Scholar]
- 18.Ito E., Terao K. Injury and recovery process of intestine caused by okadaic acid and related compounds. Nat. Toxins. 1994;2:371–377. [PubMed] [Google Scholar]
- 19.Ogino H., Kumagai M., Yasumoto T. Toxicologic evaluation of yessotoxin. Nat. Toxins. 1997;5:255–259. doi: 10.1002/(SICI)1522-7189(1997)5:6<255::AID-NT6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Terao K., Ito E., Yanagi T., Yasumoto T. Histopathological studies on experimental marine toxin poisoning. I. Ultrastructural changes in the small intestine and liver of suckling mice induced by dinophysistoxin-1 and pectenotoxin-1. Toxicon. 1986;24:1141–1151. doi: 10.1016/0041-0101(86)90140-6. [DOI] [PubMed] [Google Scholar]
- 21.Terao K., Ito E., Ohkusu M., Yasumoto T. A comparative study of the effects of DSP-toxins on mice and rats. In: Smayda T.J., Shimizu Y., editors. Toxic Phytoplankton Blooms in the Sea. Elsevier; New York, NY, USA: 1993. pp. 581–586. [Google Scholar]
- 22.Yuasa H., Yoshida K., Iwata H., Nakanishi H., Suganuma M., Tatematsu M. Increase of labeling indices in gastrointestinal mucosae of mice and rats by compounds of the okadaic acid type. J. Cancer Res. Clin. Oncol. 1994;120:208–212. doi: 10.1007/BF01372558. [DOI] [PubMed] [Google Scholar]
- 23.Berven G., Sætre F., Halvorsen K., Seglen P.O. Effects of the diarrhetic shellfish toxin, okadaic acid, on cytoskeletal elements, viability and functionality of rat liver and intestinal cells. Toxicon. 2001;39:349–362. doi: 10.1016/S0041-0101(00)00137-9. [DOI] [PubMed] [Google Scholar]
- 24.Tubaro A., Sosa S., Altinier G., Soranzo M.R., Satake M., Della Loggia R., Yasumoto T. Short-term oral toxicity of homoyessotoxins, yessotoxin and okadaic acid in mice. Toxicon. 2004;43:439–445. doi: 10.1016/j.toxicon.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Fujiki H., Suganuma M., Suguri H., Yoshizawa S., Takagi K., Uda N., Wakamatsu K., Yamada K., Murata M., Yasumoto T., Sugimura T. Diarrhetic shellfish toxin, dinophysistoxin-1, is a potent tumor promoter on mouse skin. Jpn. J. Cancer Res. 1988;79:1089–1093. doi: 10.1111/j.1349-7006.1988.tb01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiki H., Suganuma M., Suguri H., Yoshizawa S., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Induction of ornithine decarboxylase activity in mouse skin by a possible tumor promoter, okadaic acid. Proc. Jpn. Acad. Ser. B. 1987;63:51–53. doi: 10.2183/pjab.63.51. [DOI] [Google Scholar]
- 27.Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl Acad. Sci. USA. 1988;85:1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suganuma M., Tatematsu M., Yatsunami J., Yoshizawa S., Okabe S., Uemura D., Fujiki H. An alternative theory of tissue specificity by tumor promotion of okadaic acid in glandular stomach of SD rats. Carcinogenesis. 1992;13:1841–1845. doi: 10.1093/carcin/13.10.1841. [DOI] [PubMed] [Google Scholar]
- 29.Mudher A.K., Perry V.H. Using okadaic acid as a tool for the in vivo induction of hyperphosphorylated tau. Neuroscience. 1998;85:1329–1332. doi: 10.1016/S0306-4522(97)00695-7. [DOI] [PubMed] [Google Scholar]
- 30.He J., Yamada K., Zou L.-B., Nabeshima T. Spatial memory deficit and neurodegeneration induced by the direct injection of okadaic acid into the hippocampus in rats. J. Neural Transm. 2001;108:1435–1443. doi: 10.1007/s007020100018. [DOI] [PubMed] [Google Scholar]
- 31.Arias C., Becerra-García F., Arrieta I., Tapia R. The protein phosphatase inhibitor okadaic acid induces heat shock protein expression and neurodegeneration in rat hippocampus in vivo. Exp. Neurol. 1998;153:242–254. doi: 10.1006/exnr.1998.6900. [DOI] [PubMed] [Google Scholar]
- 32.Arendt T., Hanisch F., Holzer M., Brückner M.K. In vivo phosphorylation in the rat basal nucleus induces PHF-like and APP immunoreactivity. NeuroReport. 1994;5:1397–1400. [PubMed] [Google Scholar]
- 33.Cummings B.J., Hayward N., Stoltzner S., Molineaux S., Nixon R.A. Intraventricular infusion of okadaic acid induces mild changes in tau and APP, but fails to produce AD-like neuropathology in adult rats. Soc. Neurosci. Abstracts. 1997;232:1639. [Google Scholar]
- 34.Van Dam A.M., Bol J.G.J.M., Binnekade R., van Muiswinkel F.L. Acute or chronic administration of okadaic acid to rats induces brain damage rather than Alzheimer-like neuropathology. Neuroscience. 1998;85:1333–1335. doi: 10.1016/s0306-4522(97)00696-9. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z., Simpkins J.W. An okadaic acid-induced model of tauopathy and cognitive deficiency. Brain Res. 2010;1359:233–246. doi: 10.1016/j.brainres.2010.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He J., Yang Y., Xu H., Zhang X., Li X.-M. Olanzapine attenuates the okadaic acid-induced spatial memory impairment and hippocampal cell death in rats. Neuropsychopharmacology. 2005;30:1511–1520. doi: 10.1038/sj.npp.1300757. [DOI] [PubMed] [Google Scholar]
- 37.Kamat P.K., Tota S., Rai S., Swarnkar S., Shukla R., Nath C. A study on neuroinflammatory marker in brain areas of okadaic acid (ICV) induced memory impaired rats. Life Sci. 2012;90:713–720. doi: 10.1016/j.lfs.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Yin Y.-Y., Liu H., Cong X.-B., Liu Z., Wang Q., Wang J.-Z., Zhu L.-Q. Acetyl-L-carnitine attenuates okadaic acid induced tau hyperphosphorylation and spatial memory impairment in rats. J. Alzheimer's Dis. 2010;19:735–746. doi: 10.3233/JAD-2010-1272. [DOI] [PubMed] [Google Scholar]
- 39.Costa A.P., Tramontina A.C., Biasibetti R., Batassini C., Lopes M.W., Wartchow K.M., Bernardi C., Tortorelli L.S., Leal R.B., Gonçalves C.-A. Neuroglial alterations in rats submitted to the okadaic acid-induced model of dementia. Behav. Brain Res. 2012;226:420–427. doi: 10.1016/j.bbr.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 40.Arendt T., Holzer M., Fruth R., Brückner M.K., Gärtner U. Paired helical filament-like phosphorylation of tau, deposition of β/A4-amyloid and memory impairment in rat induced by chronic inhibition of phosphatase 1 and 2A. Neuroscience. 1995;69:691–698. doi: 10.1016/0306-4522(95)00347-L. [DOI] [PubMed] [Google Scholar]
- 41.Lee J.-H., Hong H.-N., Im J.-O., Byun H.-S., Kim D. The formation of PHF-1 and SMI-31 positive dystrophic neurites in rat hippocampus following acute injection of okadaic acid. Neurosci. Lett. 2000;282:49–52. doi: 10.1016/S0304-3940(00)00863-6. [DOI] [PubMed] [Google Scholar]
- 42.Arendt T., Holzer M., Fruth R., Brückner M.K., Gärtner U. Phosphorylation of tau, Aβ-formation, and apoptosis after in vivo inhibition of PP-1 and PP-2A. Neurobiol. Aging. 1998;19:3–13. doi: 10.1016/S0197-4580(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 43.Arendt T., Holzer M., Brückner M.K., Janke C., Gärtner U. The use of okadaic acid in vivo and the induction of molecular changes typical for Alzheimer's disease. Neuroscience. 1998;85:1337–1340. doi: 10.1016/S0306-4522(97)00697-0. [DOI] [PubMed] [Google Scholar]
- 44.Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takai A., Murata M., Torigoe K., Isobe M., Mieskes G., Yasumoto T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992;284:539–544. doi: 10.1042/bj2840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swingle M., Ni L., Honkanen R.E. Small-molecule inhibitors of Ser/Thr protein phosphatases. In: Moorhead G., editor. Methods in Molecular Biology, Volume 365: Protein Phosphatase Protocols. Springer; New York, NY, USA: 2007. pp. 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheppeck J.E., Gauss C.-M., Chamberlin A.R. Inhibition of the Ser-Thr phosphatases PP1 and PP2A by naturally occurring toxins. Bioorg. Med. Chem. 1997;5:1739–1750. doi: 10.1016/S0968-0896(97)00146-6. [DOI] [PubMed] [Google Scholar]
- 48.Holmes C.F.B., Luu H.A., Carrier F., Schmitz F.J. Inhibition of protein phosphatases-1 and -2A with acanthifolicin. Comparison with diarrhetic shellfish toxins and identification of a region on okadaic acid important for phosphatase inhibition. FEBS Lett. 1990;270:216–218. doi: 10.1016/0014-5793(90)81271-o. [DOI] [PubMed] [Google Scholar]
- 49.Rivas M., García C., Liberona J.L., Lagos N. Biochemical characterization and inhibitory effects of dinophysistoxin-1, okadaic acid and microcystine l-r on protein phosphatase 2a purified from the mussel Mytilus chilensis. Biol. Res. 2000;33:197–206. doi: 10.4067/s0716-97602000000300005. [DOI] [PubMed] [Google Scholar]
- 50.Nishiwaki S., Fujiki H., Suganuma M., Furuya-Suguri H., Matsushima R., Iida Y., Ojika M., Yamada K., Uemura D., Yasumoto T., Schmitz F.J., Sugimura T. Structure-activity relationship within a series of okadaic acid derivatives. Carcinogenesis. 1990;11:1837–1841. doi: 10.1093/carcin/11.10.1837. [DOI] [PubMed] [Google Scholar]
- 51.Hu T., Curtis J.M., Walter J.A., McLachlan J.L., Wright J.L.C. Two new water-soluble DSP toxin derivatives from the dinoflagellate Prorocentrum maculosum: possible storage and excretion products. Tetrahedron Lett. 1995;36:9273–9276. doi: 10.1016/0040-4039(95)02010-M. [DOI] [Google Scholar]
- 52.Albano C., Ronzitti G., Rossini A.M., Callegari F., Rossini G.P. The total activity of a mixture of okadaic acid-group compounds can be calculated by those of individual analogues in a phosphoprotein phosphatase 2A assay. Toxicon. 2009;53:631–637. doi: 10.1016/j.toxicon.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 53.Garibo D., Dàmaso E., Eixarch H., de la Iglesia P., Fernández-Tejedor M., Diogène J., Pazos Y., Campàs M. Protein phosphatase inhibition assays for okadaic acid detection in shellfish: matrix effects, applicability and comparison with LC-MS/MS analysis. Harmful Algae. 2012;19:68–75. doi: 10.1016/j.hal.2012.06.001. [DOI] [Google Scholar]
- 54.Ikehara T., Imamura S., Yoshino A., Yasumoto T. PP2A inhibition assay using recombinant enzyme for rapid detection of okadaic acid and its analogs in shellfish. Toxins. 2010;2:195–204. doi: 10.3390/toxins2010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vilariño N., Louzao M.C., Vieytes M.R., Botana L.M. Biological methods for marine toxin detection. Anal. Bioanal. Chem. 2010;397:1673–1681. doi: 10.1007/s00216-010-3782-9. [DOI] [PubMed] [Google Scholar]
- 56.Cohen P., Holmes C.F.B., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 57.Kikuchi K., Shima H., Mitsuhashi S., Suzuki M., Oikawa H. The apoptosis-inducing activity of the two protein phosphatase inhibitors, tautomycin and thyrsiferyl 23-acetate, is not due to the inhibition of protein phosphatases PP1 and PP2A. Int. J. Mol. Med. 1999;4:395–401. doi: 10.3892/ijmm.4.4.395. [DOI] [PubMed] [Google Scholar]
- 58.Espiña B., Louzao M., Cagide E., Alfonso A., Vieytes M.R., Yasumoto T., Botana L.M. The methyl ester of okadaic acid is more potent than okadaic acid in disrupting the actin cytoskeleton and metabolism of primary cultured hepatocytes. Br. J. Pharmacol. 2010;159:337–344. doi: 10.1111/j.1476-5381.2009.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doucet E., Ross N., Quilliam M. Enzymatic hydrolysis of esterified diarrhetic shellfish poisoning toxins and pectenotoxins. Anal. Bioanal. Chem. 2007;389:335–342. doi: 10.1007/s00216-007-1489-3. [DOI] [PubMed] [Google Scholar]
- 60.Runnegar M.T., Kong S., Berndt N. Protein phosphatase inhibition and in vivo hepatotoxicity of microcystins. Am. J. Physiol. 1993;265:G224–G230. doi: 10.1152/ajpgi.1993.265.2.G224. [DOI] [PubMed] [Google Scholar]
- 61.Falconer I.R., Jackson A.R.B., Langley J., Runnegar M.T. Liver pathology in mice in poisoning by the blue-green alga Microcystis aeruginosa. Aust. J. Biol. Sci. 1981;34:179–188. [Google Scholar]
- 62.Seawright A.A., Cain K., Nolan C.C., Dinsdale D., Codd G.A. The lesion caused by microcystin-LR in the liver of the rat. Is it a model for apoptosis in vivo in the liver? Phycologia. 1996;35(Suppl. 6):172–176. [Google Scholar]
- 63.Graziano M.J., Casida J.E. Comparison of the acute toxicity of endothal and cantharidic acid on mouse liver in vivo. Toxicol. Lett. 1987;37:143–148. doi: 10.1016/0378-4274(87)90150-0. [DOI] [PubMed] [Google Scholar]
- 64.Bagatell F.K., Dugan K., Wilgram G.F. Structural and biochemical changes in tissues isolated from the cantharidin-poisoned rat with special emphasis upon hepatic subcellular particles. Toxicol. Appl. Pharmacol. 1969;15:249–261. doi: 10.1016/0041-008X(69)90024-6. [DOI] [PubMed] [Google Scholar]
- 65.Ito Y., Abril E.R., Bethea N.W., McCuskey R.S. Inhibition of matrix metalloproteinases minimizes hepatic microvascular injury in response to acetaminophen in mice. Toxicol. Sci. 2005;83:190–196. doi: 10.1093/toxsci/kfh291. [DOI] [PubMed] [Google Scholar]
- 66.DeLeve L.D., Wang X., Kaplowitz N., Shulman H.M., Bart J.A., van der Hoek A. Sinusoidal endothelial cells as a target for acetaminophen toxicity: direct action versus requirement for hepatocyte activation in different mouse strains. Biochem. Pharmacol. 1997;53:1339–1345. doi: 10.1016/S0006-2952(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 67.Wieland T., Faulstich H., Fiume L. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushroo. CRC Crit. Rev. Biochem. Mol. Biol. 1978;5:185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura S.-I., Mori K.J. Effects of reticuloendothelial blockade on acute saponin poisoning in mice. Toxicology. 1984;29:235–242. doi: 10.1016/0300-483X(84)90024-6. [DOI] [PubMed] [Google Scholar]
- 69.Hong S.J. Inhibition of mouse neuromuscular transmission and contractile function by okadaic acid and cantharidin. Br. J. Pharmacol. 2000;130:1211–1218. doi: 10.1038/sj.bjp.0703418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munday R., Selwood A.I., Rhodes L. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon. 2012;60:995–999. doi: 10.1016/j.toxicon.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Valdiglesias V., Fernández-Tajes J., Pásaro E., Méndez J., Laffon B. Identification of differentially expressed genes in SHSY5Y cells exposed to okadaic acid by suppression subtractive hybridization. BMC Genomics. 2012;13:46 doi: 10.1186/1471-2164-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J., Wang Y.-Y., Lin L., Gao Y., Hong H.-S., Wang D.-Z. Quantitative proteomic analysis of okadaic acid treated mouse small intestines reveals differentially expressed proteins involved in diarrhetic shellfish poisoning. J. Proteomics. 2012;75:2038–2052. doi: 10.1016/j.jprot.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Tripuraneni J., Koutsouris A., Pestic L., De Lanerolle P., Hecht G. The toxin of diarrheic shellfish poisoning, okadaic acid, increases intestinal epithelial paracellular permeability. Gastroenterology. 1997;112:100–108. doi: 10.1016/S0016-5085(97)70224-5. [DOI] [PubMed] [Google Scholar]
- 74.Okada T., Narai A., Matsunaga S., Fusetani N., Shimizu M. Assessment of the marine toxins by monitoring the integrity of human intestinal Caco-2 cell monolayers. Toxicol. in Vitro. 2000;14:219–226. doi: 10.1016/S0887-2333(00)00014-X. [DOI] [PubMed] [Google Scholar]
- 75.Hosokawa M., Tsukada H., Saitou T., Kodama M., Onomura M., Nakamura H., Fukuda K., Seino Y. Effects of okadaic acid on rat colon. Dig. Dis. Sci. 1998;43:2526–2535. doi: 10.1023/A:1026658921369. [DOI] [PubMed] [Google Scholar]
- 76.Rao R.K., Basuroy S., Rao V.U., Karnaky K.J., Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson J.M., van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 78.Andreeva A.Y., Piontek J., Blasig I.E., Utepbergenov D.I. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int. J. Biochem. Cell Biol. 2006;38:222–233. doi: 10.1016/j.biocel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Dunagan M., Chaudhry K., Samak G., Rao R.K. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am. J. Physiol. 2012;303:G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheth P., Seth A., Atkinson K.J., Gheyi T., Kale G., Giorgianni F., Desiderio D.M., Li C., Naren A., Rao R.K. Acetaldehyde dissociates the PTP1B-E-cadherin-β-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem. J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turner J.R. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am. J. Path. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seth A., Sheth P., Elias B.C., Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- 83.Nunbhakdi-Craig V., Machleidt T., Ogris E., Bellotto D., White C.L., Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-L. [DOI] [PubMed] [Google Scholar]
- 85.Rao R.K. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N.Y. Acad. Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheth P., Samak G., Shull J.A., Seth A., Rao R. Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem. J. 2009;421:59–70. doi: 10.1042/BJ20081951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shasby D.M., Kamath J.M., Moy A.B., Shasby S.S. Ionomycin and PDBU increase MDCK monolayer permeability independently of myosin light chain phosphorylation. Am. J. Physiol. 1995;269:L144–L150. doi: 10.1152/ajplung.1995.269.2.L144. [DOI] [PubMed] [Google Scholar]
- 88.Vale C., Botana L.M. Marine toxins and the cytoskeleton: okadaic acid and dinophysistoxins. FEBS J. 2008;275:6060–6086. doi: 10.1111/j.1742-4658.2008.06711.x. [DOI] [PubMed] [Google Scholar]
- 89.Martín-López A., Gallardo-Rodríguez J.J., Sánchez-Mirón A., García-Camacho F., Molina-Grima E. Cytotoxicity of yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicon. 2012;60:1049–1056. doi: 10.1016/j.toxicon.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Vilariño N., Ares I.R., Cagide E., Louzao M.C., Vieytes M.R., Yasumoto T., Botana L.M. Induction of actin cytoskeleton rearrangement by methyl okadaate–comparison with okadaic acid. FEBS J. 2008;275:926–934. doi: 10.1111/j.1742-4658.2008.06256.x. [DOI] [PubMed] [Google Scholar]
- 91.Rossini G.P., Hess P. Phycotoxins: chemistry, mechanisms of action and shellfish poisoning. In: Luch A., editor. Molecular, Clinical and Environmental Toxicology. Volume2: Clinical Toxicology. Birkhäuser; Basel, Switzerland: 2010. pp. 65–122. [DOI] [PubMed] [Google Scholar]
- 92.Serres M., Grangeasse C., Haftek M., Durocher Y., Duclos B., Schmitt D. Hyperphosphorylation of β-catenin on seine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp. Cell Res. 1997;231:163–172. doi: 10.1006/excr.1996.3443. [DOI] [PubMed] [Google Scholar]
- 93.Malaguti C., Rossini G.P. Recovery of cellular E-cadherin precedes replenishment of estrogen receptor and estrogen-dependent proliferation of breast cancer cells rescued from a death stimulus. J. Cell. Physiol. 2002;192:171–181. doi: 10.1002/jcp.10123. [DOI] [PubMed] [Google Scholar]
- 94.Sontag J.-M., Sontag E. Regulation of cell adhesion by PP2A and SV40 small tumor antigen: an important link to cell transformation. Cell. Mol. Life Sci. 2006;63:2979–2991. doi: 10.1007/s00018-006-6300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujiki H., Sueoka E., Komori A., Suganuma M. Tumor promotion and TNF-α gene expression by the okadaic acid class tumor promoters. Environ. Carcinogen. Ecotox. Rev. 1997;15:1–40. [Google Scholar]
- 96.Marano C.W., Lewis S.A., Garulacan L.A., Peralta Soler A., Mullin J.M. Tumor necrosis factor-α increases sodium and chloride conductance across the tight junction of CACO-2 BNE, a human intestinal cell line. J. Membrane Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- 97.Kamat P.K., Tota S., Shukla R., Ali S., Najmi A.K., Nath C. Mitochondrial dysfunction: a crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacol. Biochem. Behav. 2011;100:311–319. doi: 10.1016/j.pbb.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt K.N., Traenckner E.B.-M., Meier B., Baeuerle P.A. Induction of oxidative stress by okadaic acid is required for activation of transcription factor NF-κB. J. Biol. Chem. 1995;270:27136–27142. doi: 10.1074/jbc.270.45.27136. [DOI] [PubMed] [Google Scholar]
- 99.Rao R.K., Baker R.D., Baker S.S., Gupta A., Holycross M. Oxidant-induced disruption of intestinal barrier function: role of protein tyrosine phosphorylation. Am. J. Physiol. 1997;273:G812–G823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 100.Ewe K. Intestinal transport in constipation and diarrhoea. Pharmacology. 1988;36(Suppl. 1):73–84. doi: 10.1159/000138424. [DOI] [PubMed] [Google Scholar]
- 101.Gaginella T.S., Bass P. Laxatives: an update on mechanism of action. Life Sci. 1978;23:1001–1009. doi: 10.1016/0024-3205(78)90659-8. [DOI] [PubMed] [Google Scholar]
- 102.Cline W.S., Lorenzsonn V., Benz L., Bass P., Olsen W. The effects of sodium ricinoleate on small intestinal function and structure. J. Clin. Invest. 1976;58:380–390. doi: 10.1172/JCI108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynell P.C., Spray G.H. Chemical gastroenteritis in the rat. Gastroenterology. 1958;34:867–873. [PubMed] [Google Scholar]
- 104.Saunders D.R., Sillery J., Rachmilewitz D. Effect of dioctyl sodium sulfosuccinate on structure and function of rodent and human intestine. Gastroenterology. 1975;69:380–386. [PubMed] [Google Scholar]
- 105.Saunders D.R., Sillery J., Rachmilewitz D., Rubin C., Tytgat G. Effect of bisacodyl on the structure and function of rodent and human intestine. Gastroenterology. 1977;72:849–856. [PubMed] [Google Scholar]
- 106.Surawicz C., Saunders D.R., Rubin C.E., Tytgat G.N. Pharmacology of laxatives: effects of phenolphthalein (PHE) on structure and function of intestinal mucosa. Gastroenterology. 1977;72:A-114. [Google Scholar]
- 107.Van Gorkom B.A.P., de Vries E.G.E., Karrenbeld A., Kleibeuker J.H. Review article: anthranoid laxatives and their potential carcinogenic effects. Aliment. Pharmacol. Ther. 1999;13:443–452. doi: 10.1046/j.1365-2036.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 108.Van Gorkom B.A.P., Karrenbeld A., van der Sluis T., Koudstaal J., de Vries E.G.E., Kleibeuker J.H. Influence of a highly purified senna extract on colonic epithelium. Digestion. 2000;61:113–120. doi: 10.1159/000007743. [DOI] [PubMed] [Google Scholar]
- 109.Suganuma M., Fujiki H., Furuya-Suguri H., Yoshizawa S., Yasumoto S., Kato Y., Fusetani N., Sugimura T. Calyculin A, an inhibitor of protein phosphatases, a potent tumor promoter on CD-1 mouse skin. Cancer Res. 1990;50:3521–3525. [PubMed] [Google Scholar]
- 110.Ohta T., Sueoka E., Iida N., Komori A., Suganuma M., Nishiwaki R., Tatematsu M., Kim S.J., Carmichael W.W., Fujiki H. Nodularin, a potent inhibitor of protein phosphatases 1 and 2A, is a new environmental carcinogen in male F344 rat liver. Cancer Res. 1994;54:6402–6406. [PubMed] [Google Scholar]
- 111.Nishiwaki-Matsushima R., Ohta T., Nishiwaki S., Suganuma M., Kohyama K., Ishikawa T., Carmichael W.W., Fujiki H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992;118:420–424. doi: 10.1007/BF01629424. [DOI] [PubMed] [Google Scholar]
- 112.Suganuma M., Okabe S., Sueoka E., Nishiwaki R., Komori A., Uda N., Isono K., Fujiki H. Tautomycin: an inhibitor of protein phosphatases 1 and 2A but not a tumor promoter on mouse skin and in rat glandular stomach. J. Cancer Res. Clin. Oncol. 1995;121:621–627. doi: 10.1007/BF01197780. [DOI] [PubMed] [Google Scholar]
- 113.Kuraishy A., Karin M., Grivennikov S.I. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slaga T.J. Antiinflammatory steroids: potent inhibitors of tumor promotion. In: Slaga T.J., editor. Carcinogenesis, Volume 5: Modifiers of Chemical Carcinogenesis. Vol. 5. Raven Press; New York, NY, USA: 1980. pp. 111–126. [PubMed] [Google Scholar]
- 115.Fürstenberger G., Csuk-Glänzer B.I., Marks F., Keppler D. Phorbol ester-induced leukotriene biosynthesis and tumor promotion in mouse epidermis. Carcinogenesis. 1994;15:2823–2827. doi: 10.1093/carcin/15.12.2823. [DOI] [PubMed] [Google Scholar]
- 116.Fujiki H., Suganuma M. Unique features of the okadaic acid activity class of tumor promoters. J. Cancer Res. Clin. Oncol. 1999;125:150–155. doi: 10.1007/s004320050257. [DOI] [PubMed] [Google Scholar]
- 117.Suganuma M., Okabe S., Marino M.W., Sakai A., Sueoka E., Fujiki H. Essential role of tumor necrosis factor α (TNF-α) in tumor promotion as revealed by TNF-α-deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- 118.Fujiki H., Suganuma M., Okabe S., Sueoka E., Suga K., Imai K., Nakachi K. A new concept of tumor promotion by tumor necrosis factor-α, and cancer preventive agents (-)-epigallocatechin gallate and green tea - a review. Cancer Detect. Prev. 2000;24:91–99. [PubMed] [Google Scholar]
- 119.Corsini E., Galli C.L. Cytokines and irritant contact dermatitis. Toxicol. Lett. 1998;102-103:277–282. doi: 10.1016/S0378-4274(98)00323-3. [DOI] [PubMed] [Google Scholar]
- 120.Lewis R.W., McCall J.C., Botham P.A., Kimber I. Investigation of TNF-α release as a measure of skin irritancy. Toxicol. in Vitro. 1993;7:393–395. doi: 10.1016/0887-2333(93)90034-3. [DOI] [PubMed] [Google Scholar]
- 121.Fujiki H., Suganuma M., Hakii H., Bartolini G., Moore R.E., Takayama S., Sugimura T. A two-stage mouse skin carcinogenesis study of lyngbyatoxin A. J. Cancer Res. Clin. Oncol. 1984;108:174–176. doi: 10.1007/BF00390993. [DOI] [PubMed] [Google Scholar]
- 122.Fujiki H., Suganuma M., Nakayasu M., Hakii H., Horiuchi T., Takayama S., Sugimura T. Palytoxin is a non-12-O-tetradecanoylphorbol-13-acetate type tumor promoter in two-stage mouse skin carcinogenesis. Carcinogenesis. 1986;7:707–710. doi: 10.1093/carcin/7.5.707. [DOI] [PubMed] [Google Scholar]
- 123.Gong C.-X., Liu F., Grundke-Iqbal I., Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer's disease: a therapeutic target. J. Biomed. Biotechnol. 2006 doi: 10.1155/JBB/2006/31825. Article ID 31825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blennow K., de Leon M.J., Zetterberg H. Alzheimer's Disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 125.Gómez-Isla T., Hollister R., West H., Mui S., Growdon J.H., Petersen R.C., Parisi J.E., Hyman B.T. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann. Neurol. 2004;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 126.Lovestone S., Reynolds C.H. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309–324. doi: 10.1016/S0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- 127.Martin L., Latypova X., Wilson C.M., Magnaudeix A., Perrin M.-L., Terro F. Tau protein phosphatases in Alzheimer's disease: The leading role of PP2A. Ageing Res. Rev. 2013;12:39–49. doi: 10.1016/j.arr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 128.Liu F., Grundke-Iqbal I., Iqbal K., Gong C.-X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005;22:1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 129.Sun L., Liu S., Zhou X., Wang X., Liu R., Wang Q., Wang J. Inhibition of protein phosphatase 2A-and protein phosphatase 1-induced tau hyperphosphorylation and impairment of spatial memory retention in rats. Neuroscience. 2003;118:1175–1182. doi: 10.1016/S0306-4522(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 130.Maidana M., Carlis V., Galhardi F.G., Yunes J.S., Geracitano L.A., Monserrat J.M., Barros D.M. Effects of microcystins over short- and long-term memory and oxidative stress generation in hippocampus of rats. Chem.-Biol. Interact. 2006;159:223–234. doi: 10.1016/j.cbi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 131.Li G., Yan W., Cai F., Li C., Chen N., Wang J. Spatial learning and memory impairment and pathological change in rats induced by acute exposure to microcystin-LR. Environ. Toxicol. 2012 doi: 10.1002/tox.21754. [DOI] [PubMed] [Google Scholar]
- 132.Stein-Behrens B., Mattson M., Chang I., Yeh M., Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 1994;14:5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Q., Yu S., Simonyi A., Sun G.Y., Sun A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- 134.Antequera D., Bolos M., Spuch C., Pascual C., Ferrer I., Fernandez-Bachiller M.I., Rodríguez-Franco M.I., Carro E. Effects of a tacrine-8-hydroxyquinoline hybrid (IQM-622) on Aβ accumulation and cell death: involvement in hippocampal neuronal loss in Alzheimer's disease. Neurobiol. Dis. 2012;46:682–691. doi: 10.1016/j.nbd.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 135.Sun L., Wang X.C., Liu S., Wang Q., Wang J.Z., Bennecib M., Gong C.-X., Sengupta A., Grundke-Iqbal I., Iqbal K. Bilateral injection of isoproterenol into hippocampus induces Alzheimer-like hyperphosphorylation of tau and spatial memory deficit in rat. FEBS Lett. 2005;579:251–258. doi: 10.1016/j.febslet.2004.11.083. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y.-J., Xu Y.-F., Liu Y.-H., Yin J., Li H.-L., Wang Q., Wang J.-Z. Peroxynitrite induces Alzheimer-like tau modifications and accumulation in rat brain and its underlying mechanisms. FASEB J. 2006;20:1431–1442. doi: 10.1096/fj.05-5223com. [DOI] [PubMed] [Google Scholar]
- 137.Janke C., Gärtner U., Holzer M., Arendt T. Reversible in vivo phosphorylation of tau induced by okadaic acid and by unspecific brain lesion in rat. J. Brain Res. 1998;39:143–153. [PubMed] [Google Scholar]
- 138.Wen Y., Yang S., Liu R., Brun-Zinkernagel A.M., Koulen P., Simpkins J.W. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer's disease-like tauopathy in female rats. J. Biol. Chem. 2004;279:22684–22692. doi: 10.1074/jbc.M311768200. [DOI] [PubMed] [Google Scholar]
- 139.Wen Y., Yang S., Liu R., Simpkins J.W. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004;1022:30–38. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- 140.King C.E., Adlard P.A., Dickson T.C., Vickers J.C. Neuronal response to physical injury and its relationship to the pathology of Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2000;27:548–552. doi: 10.1046/j.1440-1681.2000.03292.x. [DOI] [PubMed] [Google Scholar]


