Abstract
Aging demonstrates deleterious effects upon the skeleton which can predispose an individual to osteoporosis and related fractures. Despite the well-documented evidence that aging decreases bone formation, there remains little understanding whereby cellular aging alters skeletal homeostasis. We, and others, have previously demonstrated that gap junctions—membrane-spanning channels that allow direct cell-to-cell conductance of small signaling molecules—are critically involved in osteoblast differentiation and skeletal homeostasis. We examined whether the capacity of rat osteoblastic cells to form gap junctions and respond to known modulators of gap junction intercellular communication (GJIC) was dependent on the age of the animal from which they were isolated. We observed no effect of age upon osteoblastic Cx43 mRNA, protein or GJIC. We also examined age-related changes in PTH-stimulated GJIC. PTH demonstrated age-dependent effects upon GJIC: osteoblastic cells from young rats increased GJIC in response to PTH, whereas there was no change in GJIC in response to PTH in osteoblastic cells from mature or old rats. PTH-stimulated GJIC occurred independently of changes in Cx43 mRNA or protein expression. Cholera toxin significantly increased GJIC in osteoblastic cells from young rats compared to those from mature and old rats. These data demonstrate an age-related impairment in the capacity of osteoblastic cells to generate functional gap junctions in response to PTH, and suggest that an age-related defect in G protein-coupled adenylate cyclase activity at least partially contributes to decreased PTH-stimulated GJIC.
Keywords: osteoblast, aging, connexion 43, gap junction, parathyroid hormone
INTRODUCTION
Gap junctions are membrane-spanning channels that facilitate intercellular communication by allowing passive flux of intracellular signaling molecules, such as Ca2+, cyclic AMP (cAMP), and ATP, to pass from the cytosol of one cell directly into the cytosol of another [1]. Gap junction channels are composed of protein subunits termed connexins (Cx), at least 20 of which have been identified in mammalian species [2]. Hexamerization of connexins generates a connexon, which can function unapposed in the plasmalemma (Cx hemichannel), or can bind to another connexon in a neighboring cell to generate a functional gap junction. In adult bone, the predominant gap junction protein expressed is connexin 43 (Cx43) but Cx45 and 46 are also expressed [3], although Cx46 is retained in monomeric form in the Golgi network and thus does not present itself within the plasmalemma [4].
The role of gap junctions and gap junction intercellular communication (GJIC) in skeletal metabolism and homeostasis is well-established. Several studies have presented compelling evidence that GJIC is critical for osteoblastic cell differentiation into mature bone-forming cells both in vitro and in vivo [5-9]. Additionally, previous studies demonstrate that GJIC contributes to the responsiveness of osteoblastic cells in vitro to diverse anabolic signals including parathyroid hormone [10], electromagnetic fields [11], and fluid shear stress [12-15]. Gap junction channels also allow osteocytes to communicate mechanical signals they detect to osteoblastic cells [16]. The critical importance of connexons, especially Cx43, are noted in murine models and in human disease [17]. In mice, targeted Cx43 mutagenesis elicits neonatal lethality because of severe cardiovascular malformation[18]; nonetheless, these mice demonstrate hypomineralization of craniofacial bones, as well as delayed ossification of appendicular and axial skeleton [6]. Similar results are observed in mice with osteoblast and osteocyte-specific deletion of Cx43 [19]. These mice also display altered bone adaptation to mechanical load [20]. In humans, mis-sense mutations in Cx43 produce the rare genetic disease oculodentodigital dysplasia (ODDD; OMIM 164200) [21], involving, among other pathologies, cranial hyperostosis (reviewed in [22]). Taken together these studies indicate that GJIC involving Cx43 significantly contributes to skeletal homeostasis.
Bone formation rates decrease with increasing age [23, 24], suggesting that decreased osteoblastic activity may contribute to age-related osteopenia [25]. Osteoblast activity is determined to a large extent by the capacity of osteoprogenitors and osteoblasts to adapt to changes in their extracellular environment. The ability of osteoblastic cells to adapt to hormonal or biophysical signals appears to decrease with advanced age[26-28]. Since GJIC contributes to bone cell responsiveness to extracellular signals, we postulated that GJIC may change as a function of age. Furthermore, we have previously reported an age-related decrease in PTH-stimulated cAMP accumulation in osteoblastic cells [27] and since PTH stimulation of GJIC is at least partially dependent on cAMP [27, 29, 30], we also postulated that PTH-stimulated GJIC decreases as a function of osteoblast age.
We examined GJIC in primary cultures of rat osteoblastic cells isolated from young (4 month old), mature (12 month old), and old (24-28 month old) rats. In order to examine the mechanisms underlying any age-related changes we found in GJIC, we also examined PTH-stimulated Cx43 mRNA and protein expression, and PTH and cholera toxin (CTX)-stimulated GJIC in ROB. We observed that PTH stimulated GJIC in young ROBs, but not mature or old ROBS, and this occurred independently of PTH-stimulated changes in Cx43 mRNA or protein expression. In agreement with out previous work upon age-related changes in cAMP induction, we observed impaired CTX-stimulated GJIC in osteoblastic cells from mature and old rats compared to those from young rats, suggesting that aging may alter the mechanisms whereby GJIC communication is regulated.
METHODS
Reagents
Rat parathyroid hormone fragment 1-34 (rPTH[1-34]) was purchased from Bachem. RNeasy RNA isolation kits were purchased from Qiagen. Reagents for real time RT-PCR were purchased from Applied Biosystems. Calcein-A M a n d 1, 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) were purchased from Molecular Probes. Dulbecco’s modified Eagle’s media (DMEM), and penicillin/streptomycin were purchased from Gibco. Fetal bovine serum (FBS) was purchased from Hyclone Laboratories. CTX was purchased from Calbiochem. All other reagents were purchased from Fisher Scientific and were of tissue culture grade.
Primary culture of rat osteoblastic cells
Rat osteoblastic cells were isolated from the tibiae and femorae of 4, 12 and 24-28 month old male Fisher 344 rats as previously described [27, 31]. Briefly, sub-periosteal osteoblastic cells were isolated by sequential collagenase digestion at 37°C. Second digestion cells were collected by centrifugation and placed into T-25 tissue culture flasks and cultured in DMEM supplemented with 20% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin. Cells were allowed to reach confluence and then subcultured into culture dishes or glass slides for each experiment. We have previously demonstrated that cells isolated in this manner express phenotypic characteristics of osteoblastic cells [31].
Assessment of GJIC
GJIC between osteoblastic cells was assessed by a double fluorescent labeling technique [32]. ‘Acceptor’ cells were plated at a density of 4 × 104 cells/cm2 on 25mm glass and were allowed to reach 90% confluence, over a period of 24-48 hours. Simultaneously, ‘donor’ cells were plated at a density of 4 × 104 cells/cm2 in 35mm tissue culture dishes and maintained at 37°C over the same time period. To measure GJIC, donor cells were washed twice with phosphate-buffered saline (PBS), incubated with 10μM calcein-AM, 10μM DiI, 1% pluronic acid, and 20mg/mL BSA in Hank’s Balanced Salt Solution, and incubated for 20min at 37°C. Following incubation, donor cells were washed with PBS, collected via trypsin, and 500 donor cells were added to confluent monolayers of unlabeled acceptor cells and incubated for 20min at 37°C. Thereafter, coverslips were removed, washed in PBS and inverted onto microscope slides; GJIC was evaluated using a Nikon epifluorescence microscope (Nikon EFD-3, Optical Apparatus Co., Ardmore, PA) and visualized to locate the calcein and DiI loaded cells, respectively. Calcein-AM, because of its small size (MW 994.87), can be transferred to neighboring cells when functional (open) gap junctions are established between donor and acceptor cells. The fluorescent dye DiI intercalates within cell membranes and does not transfer to neighboring cells via GJIC and is used to identify donor cells.
Both donor cells and acceptor cells were exposed for 20 minutes at 37°C to vehicle control, 10−10 - 10−6 M rPTH [1-34] or 10−10 - 10−6 M CTX. Each vehicle or drug was also present during the formation of GJIC between donor and acceptor cells. For each condition, the number of calcein-positive acceptor cells coupled to each of 40 DiI positive donor cells was recorded. The average number of acceptor cells per donor cell was then calculated. The number of donor cells counted was 40 as it was the maximum number of coupled donor cells under any condition.
Western Blotting and Quantitative Real Time RT-PCR
Osteoblastic cells were plated at 5,000/cm2 in 10cm2 tissue culture dishes and cultured to 80% confluence (3-4 days). The cells were then exposed for 2 hours at 37°C to rPTH [1-34] (10−10-10−6 M) or CTX (10−10 – 10−7 M) or vehicle control, after which whole cell protein lysate or RNA was isolated. Western immunoblotting and qtPCR was subsequently performed as described previously [33].
Statistical Analysis
Basal Gja1 and Cx43 protein expression and basal GJIC (Figure 1A-C) are reported as mean ± SEM. The remaining data are presented as fold-change in coupled cells compared to age-matched, vehicle control in order to minimize inter-animal variability and are presented as mean ± SEM. Data were analyzed by ANOVA followed by Tukey or Dunnet’s test. p < 0.05 was considered statistically significant. For CTX studies, osteoblastic cells from one young animal showed no responsiveness to CTX, and were deemed suitable for eliminated from analysis by Q-test [34].
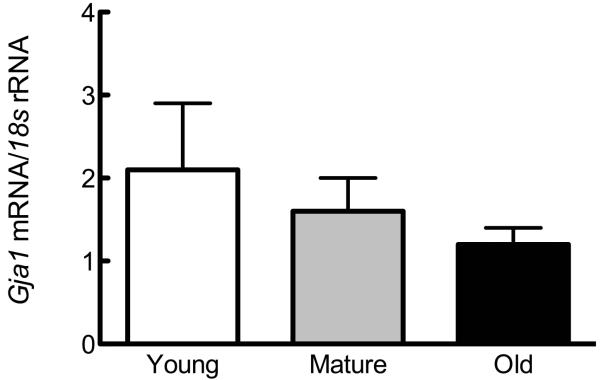
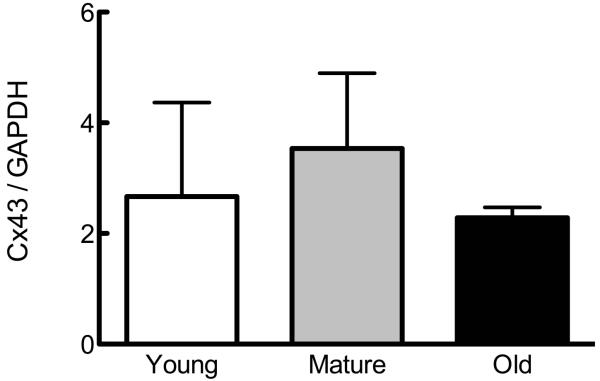
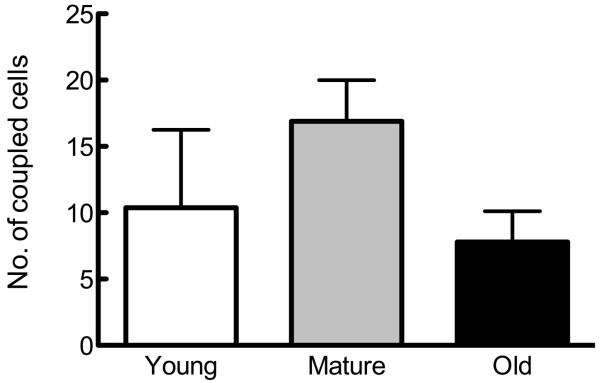
Figure 1. Osteoblastic cells from young, mature, and old rats demonstrate no differences in Cx43 expression or GJIC.
Osteoblastic cells were isolated as described in Materials and Methods and plated. (A) Basal Gja1 mRNA expression relative to 18s rRNA was examined by qPCR, and is not altered as a function of age of donor animal. (B) Basal Cx43 protein expression relative to GAPDH was examined by western immunoblotting, and also demonstrated no age-related differences. (C) Formation of GJIC was assessed by parachute assay, and demonstrated no age-related differences. n=3-5 per age group.
RESULTS
Basal Cx43 expression and GJIC is not altered as a function of age of animal from which osteoblastic cells were isolated
We first examined the influence of donor animal age on Cx43 mRNA, protein and GJIC expression in osteoblastic cells isolated from young, mature, and old rats. We found no significant influence of donor animal age on basal Gja1 (Cx43 mRNA; Figure 1A) or protein (Figure 1B). Similarly, we observed no age-related change basal GJIC in osteoblastic cells (Figure 1C).
Age of donor animal influences PTH-stimulated GJIC in osteoblastic cells
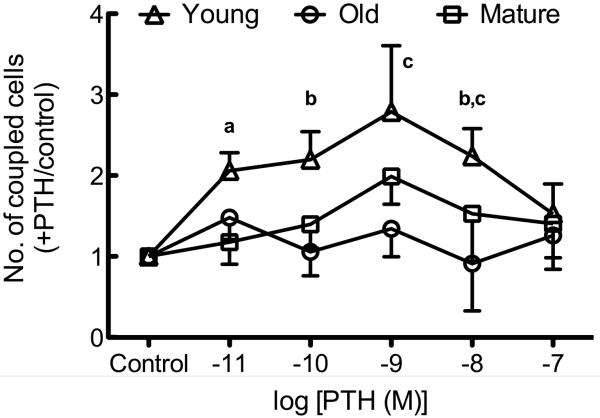
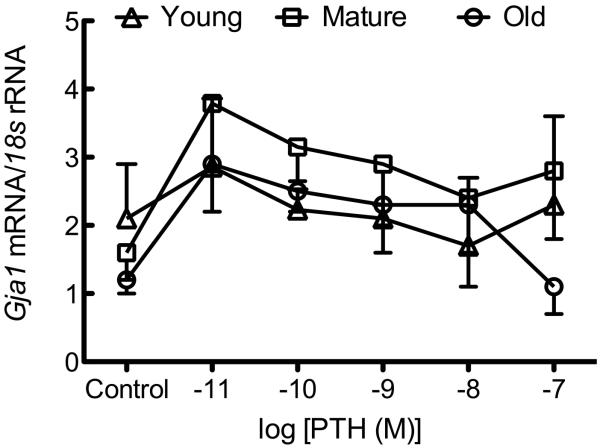
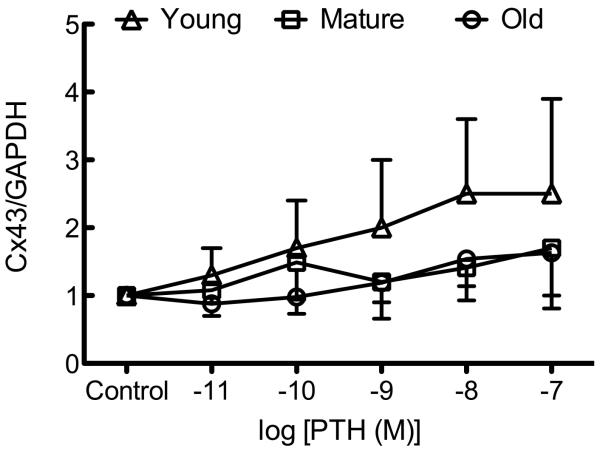
Osteoblastic cells isolated from young rats demonstrated a statistically significant increase in the number of coupled cells in response to PTH treatment (Figure 2A). Exposure to PTH (10−11 – 10−8 M) revealed a trend for increased GJIC in osteoblastic cells isolated from young rats, which reached statistical significance at 10−9 and 10−8 M PTH relative to vehicle controls. In contrast, PTH at any dose used had no effect upon GJIC in osteoblastic cells isolated from mature or old rats (Figure 2A). We found no effect of PTH upon Cx43 mRNA (Figure 2B) or protein (Figure 2C), indicating that PTH increases GJIC via post-translational mechanisms in osteoblastic cells from young rats.
Figure 2. PTH-induced GJIC is age-related and independent of de novo Gja1 or Cx43 expression.
Osteoblastic cells were isolated and GJIC was evaluated as described in Materials and Methods. (A) Recombinant PTH(1-34) dose-dependently increased the degree of GJIC in osteoblastic cells isolated from young, but not mature or old, rats. (B) Recombinant PTH(1-34) (10−11–10−7 M) had no effect on Gja1 mRNA expression in osteoblastic cells from young, mature, or old rats. (C) Recombinant PTH(1-34) (10−11–10−7 M) had no effect on Cx43 protein expression in osteoblastic cells from young, mature, or old rats.. a indicates p < 0.05 compared to osteoblastic cells from mature rats; b indicates p < 0.05 versus osteoblastic cells from old rats; c indicates p < 0.05 young treated versus control. n=2-4 animals per age group per treatment.
Increasing cAMP accumulation does not stimulate GJIC in osteoblastic cells isolated from older animals
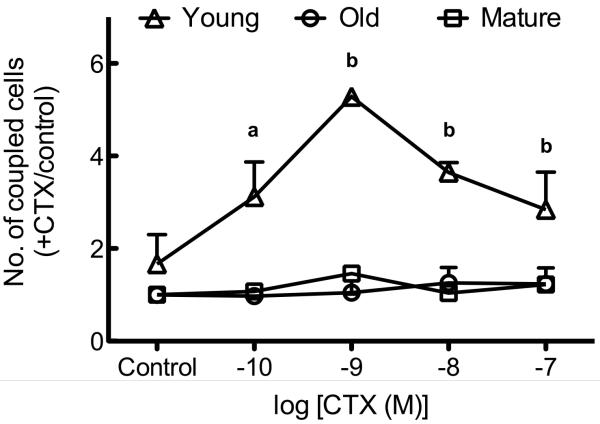
We have previously demonstrated an age-related impairment in cAMP synthesis in response to CTX [27]. CTX exerted a dose-dependent increase in GJIC in osteoblastic cells from young animals, but had no effect upon osteoblastic cells from mature or old rats (Figure 3).
Figure 3. CTX increases GJIC in osteoblastic cells from young, but not mature or old, rats.
Osteoblastic cells from young rats exposed to CTX demonstrated a dose-dependent increase in GJIC whereas those from mature or old rats do not. a indicates p < 0.05 compared to vehicle in osteoblastic cells from young, mature, or old rats; b indicates p < 0.001 versus osteoblastic cells from young rats exposed to vehicle or osteoblastic cells from mature and old rats at the same dose of CTX. n=2-4 per age group per treatment.
DISCUSSION
Gap junctions are critical regulators of cell-cell communication and are involved in diverse developmental and homeostatic function. Within cardiac muscle, they rapidly propagate action potentials generated in the sinoatrial (SA) node to enable the myocardium to generate synchronous myocardial contraction [35]. Within the vasculature, GJIC between the endothelium and the underlying smooth muscle contributes to endothelium-dependent dilation [36]. Since the discovery of gap junctions within the skeleton [37], tremendous effort has been exerted to understand the role and importance of connexins, GJIC, and hemichannels in skeletal development. Ample data have demonstrated a role for gap junctions and connexins, particularly Cx43, in osteoblast differentiation and adult bone homeostasis. Chemical inhibition of GJIC promotes transdifferentiation of osteoblasts into adipocytes [9] and attenuates osteoprogenitor differentiation in vitro [7, 38]. Similar findings are reported in vivo [6]. What has been studied less extensively, however, is the influence of aging upon Cx43 expression and GJIC in osteoblastic cells. Within, we examined Cx43 expression in osteoblastic cells derived from young, mature, and old rats, and further examined the capacity for GJIC in response to hormonal and chemical signals.
We observed no influence of donor animal age upon Gja1 or Cx43 protein expression, nor was basal GJIC significantly different among these age groups (Figure 1A-C). In contrast, age-related decreases in Cx43 expression have been observed in the SA node [39], endothelium[40], fibroblasts [41], dental pulp [42], and the bladder [43]. While we observed no significant difference in basal Cx43 expression or GJIC among the groups, we did find that PTH was able to stimulate GJIC only in osteoblastic cells from young animals. (Figure 2A). PTH stimulates GJIC by increasing Cx43 mRNA and protein expression, although this occurs over a longer time-scale (hours rather than minutes). We examined whether there was an age-related impairment of Cx43 mRNA or protein in response to PTH. While there was an age-related impairment in rapid activation of GJIC by PTH (likely independent of de novo protein synthesis; Figure 2A), no cells from any population demonstrated changes in Cx43 (Gja1) mRNA (Figure 2B) or protein (Figure 2C) after 2hrs of PTH treatment.
Increasing cAMP positively correlates with increased GJIC, and it is through cAMP signaling that PTH acutely stimulates GJIC [44]. We have previously demonstrated that osteoblastic cells from mature and old rats are less capable of generating cAMP in response to PTH or CTX compared to osteoblastic cells from young rats [27]. Therefore, we examined whether an age-related inability to generate cAMP in response to hormonal or chemical signals also prevents formation of gap junctions and GJIC in mature and aged mice. PTH dose-dependently increased GJIC in osteoblastic cells from young, but not mature or old, rats. These populations of cells have similar levels of PTH/PTHrP receptor mRNA and numbers of PTH receptors per cell [27], indicating that age-related defects in PTH signaling, but not receptor number or binding affinity, are a likely cause. Since PTH-stimulated cAMP accumulation decreases as a function of age [27] and cAMP mediates PTH stimulation of GJIC [45], the age-related decrease in PTH-stimulate GJIC reported could result from defective cAMP accumulation. CTX, which ADP-ribosylates Gαs to synthesize cAMP, mimicked the capacity of PTH to promote GJIC in young, but, as was the case with PTH, did not increase GJIC in osteoblastic cells from mature and old rats. This suggests that an age-related defect in Gαs protein coupled adenylate cyclase activity at least partially contributes to decreased PTH-stimulated GJIC.
In summary, our results suggest osteoblastic cells isolated from mature (12 month) and old (24-28 month) old rats display decreased PTH- and CTX-stimulated GJIC. The mechanism underlying this decreased responsiveness to PTH involves uncoupling of Gs. protein to adenylate cyclase. Given the critical role GJIC plays in osteoblastic differentiation and bone adaptation to mechanical load, this age-related defect in PTH regulation of cAMP may contribute to age-related bone loss.
ACKNOWLEDGEMENTS
This work was supported by Award Number R03AR057547 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (DCG) and Award Number AG013087 from the National Institute on Aging (HJD).
REFERENCES
- 1.Civitelli R, et al. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91(5):1888–96. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda S, Tsukihara T. Structure of the gap junction channel and its implications for its biological functions. Cell Mol Life Sci. 2011;68(7):1115–29. doi: 10.1007/s00018-010-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473(2):188–92. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koval M, et al. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137(4):847–57. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecanda F, et al. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9(8):2249–58. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lecanda F, et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151(4):931–44. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiller PC, et al. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28(4):362–9. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- 8.Gramsch B, et al. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res. 2001;264(2):397–407. doi: 10.1006/excr.2000.5145. [DOI] [PubMed] [Google Scholar]
- 9.Schiller PC, et al. Inhibition of gap-junctional communication induces the trans-differentiation of osteoblasts to an adipocytic phenotype in vitro. J Biol Chem. 2001;276(17):14133–8. doi: 10.1074/jbc.M011055200. [DOI] [PubMed] [Google Scholar]
- 10.Vander Molen MA, et al. Gap junctional intercellular communication contributes to hormonal responsiveness in osteoblastic networks. J Biol Chem. 1996;271(21):12165–71. doi: 10.1074/jbc.271.21.12165. [DOI] [PubMed] [Google Scholar]
- 11.Vander Molen MA, et al. Osteoblastic networks with deficient coupling: differential effects of magnetic and electric field exposure. Bone. 2000;27(2):227–31. doi: 10.1016/s8756-3282(00)00315-x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng B, et al. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142(8):3464–73. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 13.Cheng B, et al. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16(2):249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 14.Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33(1):64–70. doi: 10.1016/s8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 15.Cherian PP, et al. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278(44):43146–56. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AF, et al. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- 17.Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest. 2011;41(1):103–16. doi: 10.1111/j.1365-2362.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 18.Reaume AG, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267(5205):1831–4. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 19.Chung DJ, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119(Pt 20):4187–98. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6(8):e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson RJ, et al. A nonsense mutation in the first transmembrane domain of connexin 43 underlies autosomal recessive oculodentodigital syndrome. J Med Genet. 2006;43(7):e37. doi: 10.1136/jmg.2005.037655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Civitelli R, Donahue HJ. Connexins in Skeletal Biology. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; New York: 2009. pp. 371–386. [Google Scholar]
- 23.Nishimoto SK, et al. The effect of aging on bone formation in rats: biochemical and histological evidence for decreased bone formation capacity. Calcif Tissue Int. 1985;37(6):617–24. doi: 10.1007/BF02554919. [DOI] [PubMed] [Google Scholar]
- 24.Tsuboyama T, et al. Decreased endosteal formation during cortical bone modelling in SAM-P/6 mice with a low peak bone mass. Bone Miner. 1989;7(1):1–12. doi: 10.1016/0169-6009(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 25.Parfitt AM, et al. Relationship between bone formation rate and osteoblast surface in aging and osteoporosis: evidence for impaired osteoblast recruitment in pathogenesis. J Bone Miner Res. 1992;7(Suppl 1):S116. [Google Scholar]
- 26.Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int. 1992;50(4):306–13. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- 27.Donahue HJ, et al. Age-related decreases in stimulatory G protein-coupled adenylate cyclase activity in osteoblastic cells. Am J Physiol. 1997;273(4 pt 1):E776–781. doi: 10.1152/ajpendo.1997.273.4.E776. [DOI] [PubMed] [Google Scholar]
- 28.Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;281(5):C1635–41. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- 29.Schiller PC, et al. Hormonal regulation of intercellular communication: parathyroid hormone increases connexin 43 gene expression and gap-junctional communication in osteoblastic cells. Mol Endocrinol. 1992;6(9):1433–40. doi: 10.1210/mend.6.9.1331776. [DOI] [PubMed] [Google Scholar]
- 30.Schiller PC, Roos BA, Howard GA. Parathyroid hormone up-regulation of connexin 43 gene expression in osteoblasts depends on cell phenotype. J Bone Miner Res. 1997;12(12):2005–13. doi: 10.1359/jbmr.1997.12.12.2005. [DOI] [PubMed] [Google Scholar]
- 31.Donahue HJ, et al. Cell-to-cell communication in osteblastic networks: cell line-dependent hormonal regulation of gap junction function. J Bone Miner Res. 1995;10:881–889. doi: 10.1002/jbmr.5650100609. [DOI] [PubMed] [Google Scholar]
- 32.Yellowley CE, et al. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15(2):209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Zhou Z, Donahue HJ. Alterations in Cx43 and OB-cadherin affect breast cancer cell metastatic potential. Clin Exp Metastasis. 2008;25(3):265–72. doi: 10.1007/s10585-007-9140-4. [DOI] [PubMed] [Google Scholar]
- 34.Dean RB, Dixon WJ. Simplified Statistics for Small Numbers of Observations. Analytical Chemistry. 1951;23(4):636–638. [Google Scholar]
- 35.Kimura H, et al. Reversible inhibition of gap junctional intercellular communication, synchronous contraction, and synchronism of intracellular Ca2+ fluctuation in cultured neonatal rat cardiac myocytes by heptanol. Exp Cell Res. 1995;220(2):348–56. doi: 10.1006/excr.1995.1325. [DOI] [PubMed] [Google Scholar]
- 36.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86(3):341–6. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 37.Doty SB. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int. 1981;33(5):509–12. doi: 10.1007/BF02409482. [DOI] [PubMed] [Google Scholar]
- 38.Donahue HJ, et al. Differentiation of human fetal osteoblastic cells and gap junctional intercellular communication. Am J Physiol. 2000;278(2):C315–322. doi: 10.1152/ajpcell.2000.278.2.C315. [DOI] [PubMed] [Google Scholar]
- 39.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol. 2004;560(Pt 2):429–37. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh HI, et al. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J Histochem Cytochem. 2000;48(10):1377–89. doi: 10.1177/002215540004801008. [DOI] [PubMed] [Google Scholar]
- 41.Statuto M, et al. Drop of connexin 43 in replicative senescence of human fibroblasts HEL-299 as a possible biomarker of senescence. Exp Gerontol. 2002;37(8-9):1113–20. doi: 10.1016/s0531-5565(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 42.Muramatsu T, et al. Reduction of connexin 43 expression in aged human dental pulp. Int Endod J. 2004;37(12):814–8. doi: 10.1111/j.1365-2591.2004.00880.x. [DOI] [PubMed] [Google Scholar]
- 43.Suadicani SO, et al. Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int. 2009;103(12):1686–93. doi: 10.1111/j.1464-410X.2008.08337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Civitelli R, et al. Regulation of connexin43 expression and function by prostaglandin E2 (PGE2) and parathyroid hormone (PTH) in osteoblastic cells. J Cell Biochem. 1998;68(1):8–21. doi: 10.1002/(sici)1097-4644(19980101)68:1<8::aid-jcb2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 45.Romanello M, et al. Effects of cAMP on intercellular coupling and osteoblast differentiation. Biochem Biophys Res Commun. 2001;282(5):1138–44. doi: 10.1006/bbrc.2001.4710. [DOI] [PubMed] [Google Scholar]