Abstract
Cancer is the second leading cause of death in the United States. Conventional therapies cause widespread systemic toxicity and lead to serious side effects which prohibit their long term use. Additionally, in many circumstances tumor resistance and recurrence is commonly observed. Therefore, there is an urgent need to identify suitable anticancer therapies that are highly precise with minimal side effects. Curcumin is a natural polyphenol molecule derived from the Curcuma longa plant which exhibits anticancer, chemo-preventive, chemo- and radio-sensitization properties. Curcumin’s widespread availability, safety, low cost and multiple cancer fighting functions justify its development as a drug for cancer treatment. However, various basic and clinical studies elucidate curcumin’s limited efficacy due to its low solubility, high rate of metabolism, poor bioavailability and pharmacokinetics. A growing list of nanomedicine(s) using first line therapeutic drugs have been approved or are under consideration by the Food and Drug Administration (FDA) to improve human health. These nanotechnology strategies may help to overcome challenges and ease the translation of curcumin from bench to clinical application. Prominent research is reviewed which shows that advanced drug delivery of curcumin (curcumin nanoformulations or curcumin nanomedicine) is able to leverage therapeutic benefits by improving bioavailability and pharmacokinetics which in turn improves binding, internalization and targeting of tumor(s). Outcomes using these novel drug delivery systems have been discussed in detail. This review also describes the tumor-specific drug delivery system(s) that can be highly effective in destroying tumors. Such new approaches are expected to lead to clinical trials and to improve cancer therapeutics.
Keywords: Nanotechnology, curcumin nanomedicine, drug delivery, cancer therapy, chemo-prevention, and tumor targeting
1. INTRODUCTION
Cancer is the most prevalent disease not only in the United States but worldwide. An estimated 1,596,670 new cancer cases and 571, 950 cancer deaths occurred in 2011 in the United States [1]. Conventional therapeutic approaches such as chemotherapy, radiation, combinational chemotherapy, and surgical treatments are widely accepted to treat or eradicate tumor(s). While chemotherapy remains a highly successful weapon to treat cancer, it is often associated with limitations and major side effects. There is always a possibility of recurrence and these cancers can develop resistance to chemo- and radiation therapies. A drug or combinational drug(s) may not work in all types of cancers since therapeutic agents work on single or dual mechanisms which control growth or induce apoptosis in fast growing cells. Therefore, developing new treatment modalities is essential to precisely treat tumors and prevent progression of cancer to the metastatic stage. In order to overcome common major obstacles in conventional cancer therapeutics, scientists are searching for effective treatments within alternative medicine, complementary medicine, and supplements. The National Cancer Institute at the National Institutes of Health (USA) recognizes complementary and alternative medicine (CAM) as prevention/treatment options (http://www.cancer.gov/cancertopics/cam). Natural herbal compounds or daily food ingredients are widely studied to learn their specific roles in anticancer activities [2, 3]. Unlike first line cancer therapeutic drugs, herbal and natural compounds are capable of targeting cancer(s) via several pathways and are therefore, more valuable and reliable in producing superior therapeutic effects in a disease condition (multiple pathogenic factors) [4, 6]. It is believed that herbal medicine brings a new hope for cancer prevention due to the safety of herbs and lack of discernible toxicity to normal cells. This alternative approach has been used to treat a wide spectrum of cancers.
Curcumin (CUR) is a hydrophobic polyphenolic compound derived from the rhizomes of Curcuma longa. This natural compound has a long history of use as curry (turmeric) in East Asian countries. Commercially available curcumin consists of a mixture of three curcuminoids [diferuloylmethane (~77%), demethoxycurcumin (~18%), and bisdemethoxycurcumin (~5%)]. Curcumin exhibits keto-enol tautomerism, having a predominant keto form in acidic and neutral solutions and a stable enol form in alkaline media. Curcumin is “Generally Recognized as Safe (GRAS)” by the Food and Drug Administration (FDA). Curcumin is characterized by a wide range of antibacterial, antifungal, antiviral, antioxidative, antiinflammatory, and antiproliferative activities [5, 7, 9]. Curcumin has demonstrated strong cancer preventive activity, including prevention of tumor initiation, promotion, metastasis, and angiogenesis in experimental animal systems, against a wide range of tumor cells [8, 10, 11]. Curcumin has pleiotropic properties that modulate numerous targets including proteins (thioredoxin reductase, cyclooxygenase 2 (COX-2), protein kinase C (PKC), 5-lipoxygenase, and tubulin), transcription factors, growth factors and their receptors, cytokines, enzymes, and gene regulating cell proliferation and apoptosis [12–14]. Because of this multi-targeted behavior, curcumin can perform a wide spectrum of actions while smart drugs or therapeutic drugs have only one target and are eliminated from the cells if they do not reach the right compartment [9].
Several in vitro investigations demonstrate that curcumin inhibits cancer cells growth (IC50,50% cell growth inhibition) at concentration of 5–30 μM [3, 8, 12, 14, 15], resembling cisplatin and gem-citabine (chemotherapeutic drug) concentrations. Because of its exceptional medicinal value, a total of 68 clinical trials have been registered with clinicaltrials.gov (as of May 3, 2012) in which the majority of them are targeting cancer. Curcumin has an extremely safe profile in both animals and humans [16, 17]. A detailed discussion about the bioavailability and safety profiles of native curcumin can be found in many review articles [18–20]. So far all the preclinical and clinical results from oral administration of curcumin have revealed very poor bioavailability, typically in nanomolar concentrations [18–20]. A classic example is a pharmacokinetic study involving healthy humans which found that only 2.30 ± 0.26 and 1.73 ± 0.19 μg.mL−1 of curcumin (Cmax) was present in serum levels even after a high oral dose of 10 and 20 g curcumin, respectively, was given [21]. This suggests curcumin undergoes extensive metabolic changes in the intestine and liver. Additionally, a clinical study comprised of 15 patients with colorectal cancer showed the cancer was nonresponsive to curcumin at a daily dose of 3.6 g (4 months) [22]. This study suggested there was no change in tumorigenesis or tumor markers. Overall, the studies concluded that while curcumin exhibits anti-cancer effects at a concentration of 5–30 μM for 1 or 2 days, achieving these concentrations at the tumor site in humans not been accomplished due to curcumin’s low bioavailability and higher metabolic activity. Therefore, curcumin must be formulated in such a way that it can overcome these critical issues. Various pharmaceutical and generic industries have developed and customized curcumin formulations with the aim of improving solubility and bioavailability (Fig. 1). Adjuvant, cyclodextrin, and some patented technologies were initially used to overcome this issue [23–25]. For instance, piperine is highly recommended because of its inhibitory effects of hepatic and intestinal glucuronidation, which promoted 154% and 2000% bioavailability in rats and humans, respectively [27, 28]. While these attempts can improve bioavailability they are unable to target the fate of curcumin to tumors. Therefore, this manifests the necessity of curcumin encapsulation in nanoparticles for cancer therapy with possible targeting moieties [15, 18, 20]. This review discusses and offers potential new avenues that are appropriate to translate curcumin into nanomedicine for cancer therapeutics. This review also presents a comprehensive list of important approaches that have been undertaken to make efficient cancer therapeutic curcumin nanoformulations.
Fig. 1.
Commercially available and proposed curcumin formulations to improve bioavailability and activity.
2. LITERATURE SURVEY OF CURCUMIN AND CURCUMIN NANOFORMULATIONS
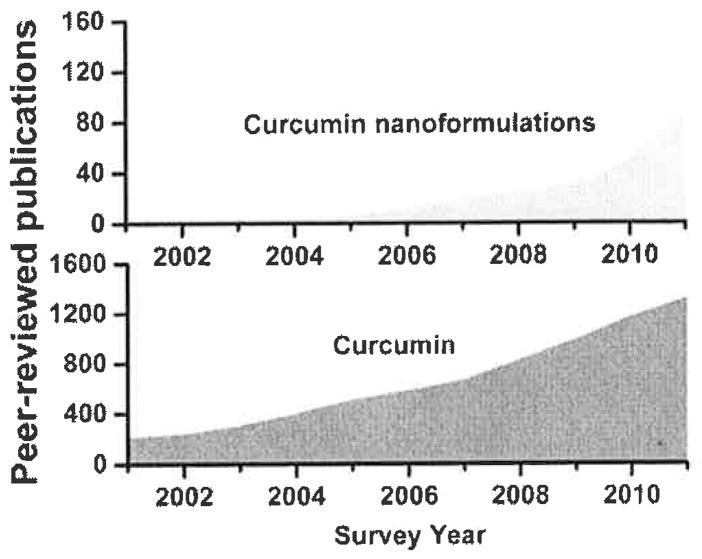
Curcumin is a widely studied molecule for a number of medicinal applications. A PubMed (http://www.ncbi.nlm.nih.gov/pub-med/) search justifies curcumin’s clinical importance and provides a rational why curcumin nanoformulations are needed for further investigation (Fig. 2). The database covering Jan. 2001 to Dec. 2011 with key words ‘curcumin’ in “title and abstract” demonstrates an exponential growth of investigations, i.e., over 4000 studies. The search results for curcumin based nanoparticle, liposome, nanotechnology, and nanomedicine, reveals very little. A total of ~ 220 reports delineate various aspects of curcumin medicinal benefits. These investigations suggest nanotechnology mediated delivery of curcumin is in the early stages of development. As per our knowledge, there are only 4 review articles primarily covering curcumin and its nanoformulations. The first review article by Aggarwal group [20] explains bioavailability of curcumin, drug delivery associated issues, and possible adjuvant and conjugate analogues and nanotechnology promises. Our review focuses on various types of nanoformulations based on their structural variability, function, and improved activity [15]. At the same time, Bansal et al. [18] present an outstanding review primarily focused on the chemo-preventive aspect of nanoformulations and curcumin stent technologies. Another review article describes curcumin induced mechanisms in cancer and implications of curcumin nanoformulations in chemoprevention and treatment [29]. In our opinion, to date there is no specific, detailed review documenting the important synthetic routes for preparation of curcumin nanoformulations, drug loading phenomenon and the critical role of nanoparticle uptake by cancer cells, anticancer activities, tissue and bioavailability, and blood compatibility. Therefore, this review aims to provide up-to-date contributions of curcumin nanoformulations to cancer therapeutics; and further, to discuss how novel trends benefit cancer therapeutics.
Fig. 2.
Basic and clinical significance of curcumin and nanocurcumin formulations in the field of medicine over a ten-year period. The number of peer-reviewed publications was collected using PubMed (data was collected for the 10 year period from Jan. 2001–Dec. 2011).
3. CURCUMIN NANOFORMULATIONS
For the past few decades, nanoparticle technology has been widely employed in medicine, including for cancer therapy [30–32]. As drug nanocarriers, nanoparticles possess several attractive features: (i) improved encapsulation or solubilization of therapeutic drugs for protective and targeted delivery, (ii) high surface to volume ratio enable modifications to surface functional groups in order to obtain extensive stabilization and internalization, (iii) biocompatibility, superior pharmacokinetics and minimal clearance from body, and (iv) controlled, stimuli responsive, remote actuation and on demand drug release properties. A large number of anticancer drug nanoformulations are currently in clinical or preclinical development. Some of the nanoformulations have been approved by the FDA are currently available in the market. A detailed list of approved formulations is available [33, 35]. Among these, the albumin-bound paclitaxel (PTX) poly(lactide-co-glycolide) (PLGA) nanoformulation (Abraxane™, http://www.abraxane.com/dtc/) is highly successful in increasing the specificity and treatment efficiency of various cancer(s). Nanoparticle formulations of nutritional ingredients such as carotenoids, co-enzyme Q10, vitamins (A, D, E, K), phytosterols, minerals, and natural extracts are not new and have been available since 1960 [36].
The main principle in effective cancer therapy is to achieve the desired concentration of therapeutic agents at the tumor site to destroy specific cancerous cells while minimizing toxicity to normal cells [37, 40]. In our view, it is essential to develop curcumin nano-formulations that exhibit superior anticancer activity compared to native curcumin. Our recent review documented that various types of nanocarriers were being used to achieve superior properties with implications for cancer therapeutics [15]. Each method of preparation determines the formulation stability, efficacy and specificity in cancer therapeutics. In our opinion, poly(lactide-co-glycolide) (PLGA) or poly(caprolactone) (PCL) nanoparticles, liposomal and self-assembly formulations of curcumin should be given the highest priority for cancer therapeutic applications due to their biocompatibility. The majority of important curcumin nanoformulations will be discussed. Table 1 provides a number of curcumin nanoformulations and their preparative methods which regulate particle size of formulations.
Table 1.
Various approaches to Prepare Curcumin Nanoformulations, their Composition, and Particles Evaluation
| Curcumin Nanoformulation | Method/Technique of Preparation | Composition | Particle Size (nm) and Zeta Potential (mV) | Reference |
|---|---|---|---|---|
| PLGA | Solid/oil/water (S/O/W) technique | 30 mg of PLGA polymer, 2% poly(vinyl alcohol) (PVA) and ethanol (1:1) solution, and curcumin 0.5–2 mg | 30–50 nm (TEM) ~ 100 nm (Confocal microscopy) |
[42, 43] |
| PLGA | Single emulsion–solvent evaporation | 200 mg of PLGA in 2 ml of ethyl acetate, 20 mg of curcumin, 4 ml of PVA (5%w/v), and 100 ml of PVA (0.3%w/v) | 150–200 nm (TEM and SEM) −30 to −20 mV (DLS) |
[79] |
| PLGA | Nanoprecipitation | PLGA–PEG (100 mg), drug (5 mg), and acetonitrile (10 mL) in the presence of 0.1% Pluronic F68 | 25–75 nm (SEM) 80.9 nm (DLS) −42.4 mV (DLS) |
[44] |
| PLGA | Single-emulsion/solvent-evaporation method | 20 mg of curcumin, 4 ml of 5% w/v of PVA solution, and 100 mL of 0.3% w/v PVA solution | 77±16 nm (SEM) | [80] |
| PLGA | Single emulsion (o/w)/solvent evaporation | 100 mg of PLGA and 10 mg of curcumin in dichloromethane and acetone (w/v, 10:1) in the presence of 1% (w/v) PVA aqueous solution. | 129.7±9.6nm (SEM) 0.194±0.09 (PDI) |
[81] |
| poly(lactide)-vitamin E TPGS (PLA-TPGS) copolymer |
Ring-opening polymerization | Curcumin solution in methanol was added to the solution of PLA-TPGS in dichloromethane in a polymer ratio of 1: 100 | 100 to 400 nm (SEM) The small particles are 20–40 nm in size but micrometer-sized group of several clusters |
[82] |
| Alginate nanoparticles | Alginate pre-gel nanoparticle hardening | Calcium chloride 7.5 ml of 18 mM and 0.063% of sodium alginate and chitosan 0.05% in the presence of Pluronic F127 | 100 ± 20 nm (SEM and AFM) | [83] |
| Soy protein nanoparticles | Isoelectric precipitation and diffusion | Soy protein isolate (SPI) (60 mg/ml) and curcumin (3 mg/mL) stock solution and curcumin/SPI ratio of 1:20, 1:50, or 1:100 (w/w) | 200–1000 nm (DLS) depending on the ethanol and glutaraldehyde concentrations | [84] |
| Poly(vinyl pyrrolidone) (PVP) conjugate micellae | Chemical conjugation | 1.5 g of PVP, 0.5 g of 4-dimethylaminopyridine, 1 mL of triethyl amine, and 100 mg of curcumin | 22.4 nm and 20 mV (DLS) 18.94±4.35 nm (TEM) |
[85] |
| α-cyclodextrin (α-CD) derivatives | Chemical conjugation | CD derivatives and their 2:l and 4:1-complexes with Curcumin | In between 268±16 nm and 692±53 nm depending on the ratios of conjugates and curcumin | [86] |
| β-cyclodextrin-self assembly | Inclusion complexation and self-assembly | 5, 10, 20 and 30 wt.% of curcumin in β-cyclodextrin | 50 nm small clusters to 500 nm self-assemblies (TEM) | [87] |
| Poly(β-cyclodextrin)-self assembly | Inclusion complexation and self-assembly | 5,10,20 and 30 wt.% of curcumin in poly(P-cyclodextrin) | Individual complex or assembly about 50 nm and clusters can reach up to 1 μm (TEM) | [88] |
| Casein micelle | Micelle or complexation | Casein (10 μM) in the presence of 0, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 μM curcumin | 166.3±33.1 nm (DLS) and the same was verified with SEM and AFM | [60] |
| Dextrin nanogels | Self-assembly process at 50 °C | DexC16 is composed of a hydrophilic dextrin backbone with grafted acrylate groups, which are partially substituted with long alkyl chains (SC16). DexC16 (0.008 mg/ml) and the curcumin (10, 30, 50 μM) | 61.1 nm in water and 59.2 in PBS solution (DLS) (freshly prepared samples) Size does not change much in 12 days in water (58.7 nm) but in PBS it increases to 100 nm |
[89] |
| Thermosensitive polymer nanoparticles | Redox-free radical polymerization | 1.8 g monomer, cross-linker (N′, N′-methylene bisacrylamide), 100 mg PEG-ester, initiator/activator and curcumin 20 wt.% loading | ~ 132 nm and −1.46 mV (DLS) | [50, 90] |
| Thermosensitive polymer nanoparticles | Free-radical polymerization | Curcumin (5 mg in 0.1 ml ethanol) and polymer (chitosan-PNIPAM, 50 mg in 5 ml 1% acetic acid) with 100 μl 0.05% TPP solution | 100–300 nm (DLS) SEM analysis of curcumin loaded TRC-NPs revealed a size range of 180–220 nm |
[51] |
| O/W nanoemulsions | High-pressure homogenization | Medium chain triacylglycerols (oil), tween 20, and curcumin | 79.5–174.3 nm(DLS) | [57] |
| Sub-micrometer dispersions | Moschwitzer’s method by high-speed homogenization | Curcumin suspensions in water (1%) were subjected to premilling treatments to reduce curcumin particle sizes to the micrometer range according to Moschwitzer’s method by high-speed homogenization at pressure levels ranging from 50 to 200 MPa and for up to 40 HPH cycles | 2000, 1000–600 nm (SEM) | [91] |
| Self-emulsifying drug delivery system | Self-emulsification | 57.5% surfactant (emulsifier OP: Cremorphor EL, 1:1), 30% co-surfactant (PEG 400) and 12.5% oil (ethyl oleate). It improves curcumin solubility to 21 mg/g | ~ 3.3 nm (DLS) | [56] |
| Nanoprecipitation | Syringe driven filter nanoprecipitation | Curcumin/ethanol solution with antisolvent water was done in a micromixer [poly(methyl methacrylate)] | The nanoprecipitate first formed as amorphous 30–40 nm nanoparticles, then their amorphous aggregates (~140 nm after 10 min and ~ 200 nm after 90 min), and finally became dendritic aggregates of needle-shaped curcumin crystals (SEM) | [71] |
| Nanoprecipitation | Droplet controlled nanoprecipitation | Curcumin/ethanol solution (0.2, 0.4, 0.8, 1.6, and 2.0 gl−1) | 450–210 nm (SEM) | [70] |
| Lipid nanospheres | Vesicle formation | Soybean oil (10 mg/ml) and DMPC:PEG-DSPE (10/1/0.06 molar ratio) | 187±53 to 217±93 nm (DLS) | [58] |
| Liposomal formulation | Curcumin decoration on liposomes using click chemistry | Dipalmitoylphosphatidylcholine/Chol(2:l) liposomes incorporating 10–20% curcumin conjugate | 52.8±5.5 to 207.2 ± 8.0 with zetapotential between −7.6±1.7 and −24.3±1.7mV depending on the liposome modifications (DLS) | [59] |
| Superparamagnetic silica reservoirs | Composite | Fe3O4, nanoparticles (37% wt) and curcumin (30% wt) into the porous silica matrix | Fe3O4 core diameter 7.13 nm (variance = l.89 nm) curcumin shell 2.59±0.07 nm (SAXS) Curcumin and Fe3O4, nanoparticle containing silica particles were ellipsoidal in shape and the size of the particles ranged from 200 nm to 1 μm. |
[92] |
| Magnetic nanoparticles | Nanoparticle coating with stabilizer or polymers | Fe3+/Fe2+ ratio of 2:1, chitosan or oleic acid | 300 nm and 500 nm (DLS and TEM/SEM). | [93] |
| Magnetic poly(lactic acid) microspheres | Oil-in-water emulsion | 1% (w/v, 50 ml) of PVA, Fe3O4 nanoparticles (5 mg), PLA (50 mg), PEG (20 mg), and curcumin (5 mg) | 0.55 to 0.75 (μm (DLS and SEM) | [94] |
| Hollow capsules | Layer by layer assembly | Melamine formaldehyde templates coated with six double layers of poly(sodium 4-styrene sulfonic acid) and poly(ethylene imine) and 4.5 mg/mg of microcapsules | 2.2 to 2.8 μm (DLS) | [95] |
| Silk fibroin and chitosan blend | Capillary microdot technique | Silk fibroin: chitosan with compositions of 100:0; 25:75; 50:50; 75:25) | <100 nm(TEM) 50:50 SFCS (130 ± 4.2 nm) (TEM) |
[96] |
| Dendrasome | Diffusion | Dendrosome and curcumin ratio 25:1 | 200–500 nm (UV-microscope) | [47] |
| Albumin nanosuspension | Solvent evaporation | Not available | 245.2 nm (DLS) | [97] |
AFM – Atomic force microscopy; DLS - Dynamic light scattering method; DMPC - l,2-Dimyristoyl-sn-glycero-3-phosphochlorine; PEG-DSPE - l,2-distearoyl-sn-glycero-3-phosphoehanolamine-N-[monomethoxy poly(ethylene glycol); PLA - Poly(lactic acid); PVA - Poly(vinyl alcohol); SA - L-glutamic acid, N-(3-carboxyl-l-oxopropyl)-, 1,5-dihexadecyl ester; SAXS – Side angle X-ray spectroscopy; SEM – Scanning electron microscopy; TEM - Transmission electron microscopy.
Polymer nanoparticles based on PLGA, a biodegradable and biocompatible polymer, can produce curcumin nanoparticles of approximately 100–200 nm size [41–44]. This particle size range is small enough to allow intracapillary passage while suitable surface coating allows escape from macrophage uptake [45]. These curcumin nanoformulations are widely studied in cell culture and animal models. Dendrimers have been employed to prepare curcumin nanoformulations, resulting in a globular macromolecule defined by core and branched units and surface groups [46, 47], This type of curcumin derivative formulation retains biological activity and has the ability to destroy human neurotumor cells in a selective manner. Such methodology provides a general strategy for attaching curcumin to various functional macromolecular chains. Gel nanoparticles (nanogels) offer an excellent depot for drug(s) and protein delivery [48]. Poly(N-isopropyl acrylamide)-curcumin (PNIPAM-CUR) gel nanoparticle formulations exist with different compositions [49–52]. Because of a hydrophilic-hydrophobic interchange opportunity within the structure, they make a unique model drug delivery systems. Additionally, high water retention, low interfacial tension with biological fluids and stimuli responsive drug release suggest pharmaceutical implications. Hydroxypropyl methylcellulose (HPMC) and poly(vinyl pyrrolidone) (PVP) alone or in combination with six different surfactants were utilized to screen for the best composition for achieving a low particle size formulation [53]. Pluronic F68 stabilized PVP-curcumin formulation exhibited the lowest particle size of 100 nm. The order of various PVP-curcumin formulations with surfactants is followed as: Pluronic F68 (~ 100 nm) < Pluronic F127 (500 nm) < Cremophor RH 40 (720 nm) < D-a-Tocopheryl polyethylene glycol 1000 succinate (TPGS) (~ 800 nm) < tween 80 (~ 1.75 μm) < Tween 20 (3.75 μm). Pluronic F68’s particle size is smaller than F127 due to its amphiphilic nature. The oil in water emulsion method is also a very popular method of producing curcumin nanoparticles [54–56]. This method strictly relies on the homogenization speed, number of cycles and pressure. A recent method subjected medium chain triacylglycerols (MCT) as oil and Tween 20 as emulsifier to high speed homogenization at 24,000 rpm, pressure of HP1500 for 40 cycles, and produced a mean droplet size of ~ 79.5 nm from 618.6 nm [57].
Micelles (di- or tri-block copolymers), liposomes and phospholipids are considered to be excellent drug delivery carriers because of their hydrophilic and hydrophobic units which bind curcumin through self-assembly process [56, 58–61]. Sou et al. [62] coined a new technique to assemble 40 mol% curcumin together with poly(ethylene glycol) -cholesteryl ether (PEG-Chol) (10 nm) which creates a highly stable formulation that can used in an injectable form. Curcumin and these micelles can cooperatively damage the cancer cells. Such type of carriers can improve the gastrointestinal absorption of curcumin and thus increase the curcumin levels in plasma [63, 64]. A recent study designed novel nanosized liposomes functionalized with curcumin through a conventional click chemistry technique [59]. A liposomal patented technology with various compositions has been identified for cancer therapeutics which exhibits very similar anticancer potential as free curcumin in BxPC-3, Capan-1, Capan-2, and HS766-T cells [65]. Phospholipid bilayers loaded with a curcumin formulation stabilized by apolipoproteins form a disk shape nanostructure (nanodisk) with a diameter <50 nm. This disk formulation showed enhanced cell growth inhibition in HepG2 and mantle cell lymphoma cells [66, 67]. Nanoplex is another type of self-assembly method to insert curcumin molecules in polyelectrolyte layers through electrostatic interactions. Biocompatible poly(allylamine hydrochloride) and dextran sulfate can form ternary phase diagrams of the curcumin-polymer salt complexes with ~80–85% curcumin loading (~300–500 nm). These complexes remain in an amorphous form and increase solubility up to 9-fold [68]. A soft gel-like formulation has been developed in a similar fashion as a layer by layer approach to embed natural polyphenols [69]. However, this sandwich approach involves the use of several polymers including poly(styrene sulfonate), poly(allylamine hydrochloride), poly(glutamic acid), poly(L-lysine), dextran sulfate, protamine sulfate, carboxymethyl cellulose, and gelatin.
Recently, approaches to prepare curcumin nanoformulations based on nanoprecipitation have received much attention [70, 71]. Such methods utilize three steps, i.e., nucleation, condensation, and coagulation, to achieve smaller particles. Curcumin nanoprecipitates with uniform size can be achieved by adjusting initial drug concentrations. For example, curcumin/ethanol solution (0.2–2 g/l) nanoprecipitation quickly formulates with particle size ranging from ~ 450 to 210 nm [70]. Using higher concentrations of curcumin in ethanol solution procures lower particle size. Anionic copolymers based on mefhacrylic acid and methyl methacrylate (EU-DRAGIT® S 100, Evonik Industries) modified particles have been widely investigated to improve colon-specific drug delivery of curcumin [72–76]. Dev et al. [77] developed a scalable process to obtain curcumin nanoparticles smaller than 50 nm using a continuous flow microfluid rotating tube processor. These formulations are stabilized by dimethyldioctadecyl ammonium bromide (DDAB) and Pluronic F127 polymer which enhances penetration through their higher cationic nature into cancer cells. In another method, 60–100 nm curcumin nanocrystals were obtained by the addition of polyelectrolyte under ultrasonic condition [78]. These polyelectrolyte layers control the drug release from nanoparticles. Many other important curcumin nanoformulations are provided in Table 1.
3.1. Curcumin Encapsulation and Release Characteristics
Curcumin drug loading is highly associated with the type of nanoparticle and preparative method used (Table 2). The drug loading can be determined by encapsulation efficiency which provides the percentage of drug added to the formulation that exists within the nanoformulations. Some estimation methods involve separating the curcumin nanoparticles from the medium and then quantifying the un-entrapped or unbound fraction of curcumin, giving an indirect quantification of curcumin encapsulated in the nanoparticles [52]. The most frequently utilized estimation method is breaking nanoparticles in organic solvent which results in an accurate curcumin encapsulated quantification [41, 50, 98]. It is interesting to note that many curcumin nanoformulations previously reported has achieved a loading capacity up to 25 wt./wt.% with 70–99% encapsulation efficiency. Based on the polymer structure, drug nature and their interactions by 3D molecular modeling drug loading information can be quickly predicted, minimizing not only the cost of the entire process but also reducing the time required for the optimization process [99]. The amount of curcumin released from nanoparticle formulations is important since the amount of curcumin release in its active form is responsible for the therapeutic effect. Therefore, complete drug loading information and release profiles of curcumin nanoformulations has been presented in Table 2. A curcumin encapsulated solid lipid nanoparticle demonstrates a simple Higuchi’s square root model up to 12 h [100]. The release profile of poly(butylcyanoacrylate) nanoparticles illustrate 34.74% in 2 h followed by a sustained release. According to the calculated two phases kinetics equation: 100 − Q = 4.5235e(−0.1724t) + 4.1641e(−0.0114t) [101]. Most of the nanoformulations follow in vitro release of curcumin in a biphasic pattern. Curcumin sustained release profile may vary depending on the type of nanoformulation, composition, location of entrapment and amount. One study suggests that the micro environment (in artificial gastric juice at pH 2.0 and in artificial intestinal juice at pH 7.4) drastically affects the release profile [102]. Although, initial release in 8 h does not vary much but 7 day sustained release from the nanoparticles found ~77% in the intestinal juice and 48% in artificial gastric juice. In some cases, the mechanism of release depends on various physical and chemical environmental conditions. Ultrasound is a powerful noninvasive modality for biomedical imaging, and holds great promise for noninvasive drug delivery enhancement and targeting. The release of curcumin entrapped in microemulsion droplets (20–35 nm) can be regulated by ultrasound frequency (40 kHz) and curcumin loading capacity [94]. Similarly, curcumin release from nanogel formulations can be controlled by diffusion and stimuli-responsive approaches [15, 52, 103, 104]. In addition, a magnetic field also promotes the release of curcumin from the formulations and thereby exhibits immediate therapeutic effects [105]. The controlled release of curcumin nanoformulations can promote accumulation of the drug in tumor(s) and slow release enhances the therapeutic outcome.
Table 2.
Curcumin Loading, Release and Uptake Behavior of Various Curcumin Nanoformulations
| Curcumin Nanoformulations | Curcumin Loading | Curcumin Release | Uptake/Internalization | Reference |
|---|---|---|---|---|
| PLGA | 2 mg loading in 30 mg batch of PLGA formulation with 90.88±0.14% encapsulation efficiency | 10–13% release was observed within 1 hour and then a sustained curcumin release of about 65% was noted for 10 days | Robust uptake in DU145, PC-3, and LNCaP cells | [42] |
| PLGA | 4 μg/mg of particles encapsulated with 97.5% encapsulation efficiency | Not available | 4.5 to 1 fold change with curcumin and 4.9 to 1.4 fold change with nano-curcumin formulation in 0 to 60 min incubation in KBM-5 cells | [44] |
| PLGA | 7.6 w/w% loading | A biphasic release profile is observed with an initial burst release during the first several hours followed by a sustained uniform release (~65% of curcumin release in 20 days) | [80] | |
| Dendrosome | 4 w/w% loading | Not available | 6 fold increased uptake found in A431 cancer cells by dendrosomal curcumin formulation | [47] |
| Lauroyl sulphated chitosan | Encapsulation efficiency and drug loading content were 50.3% and 9.31%, respectively | 16 mg release in 30 days and 82% stable curcumin in chitosan-CUR formulation for 30 day | Uptake similar to free curcumin in Caco-2 cells was observed | [111, 112] |
| Alginate-chitosan-pluronic composite nanoparticles | 5–10 fold increase in encapsulation in the presence of Pluronic polymer | 36% in 12 h, 51% in 24 h and 96 h about 75% of curcumin determined | No significant difference in uptake detected between curcumin and curcumin nanoformulation | [83] |
| Curcumin/ mono-methoxy poly(ethylene glycol)-poly(ε-caprolactone) (MPEG-PCL) | 5–25 % by weight loading with > 97% encapsulation efficiency | About 54.6% curcumin release found in 9 days | Not available | [61] |
| β-cyclodextrin self-assembly | Loading content in the range of 6.17–26.21 by weight of formulation | 80% of curcumin retention in 6 days in the formation | 2–9 fold increase was noticed in DU145 prostate cancer cells | [87] |
| Poly(β-cyclodextrin)-self assembly | The order of loading capacity (μg of CUR per mg of PCD) is PCD5 (48.5) < PCD10 (115.2) < PCD20 (163.4) < PCD30 (223.2) | The order of stability for 72 h found to be PCD30 (~88.7%) > PCD20 (~82.5%) > PCD10 (~77.4%) > PCD5(~71.2%). | 3–4 fold increased uptake of CUR was noticed in PCD20 or PCD30 treated prostate cancer cells | [88] |
| Albumin nanoemulsions | The encapsulation efficiency was up to 42.39 ±0.91% depending on the ratio albumin to curcumin | 96% curcumin release in 72 h | Not available | [97] |
| Superparamagnetic Silica Reservoirs | A high loading of Fe3O4 nanoparticles (37% wt) and curcumin (30% wt) into the porous silica matrix | The curcumin entrapped inside the silica capsules diffuses out through passive diffusion processes | Not available | [92] |
| Hollow capsules | 4.5 mg of curcumin/mg of microcapsules | Only 1.11% (0.26 μg/ml) of curcumin release was observed in 24 h, followed by a sustained release for about 1 week | Not available | [95] |
| Silk fibroin | Up to 96% encapsulation efficiency depending on the ratio of chitosan/silk fibroin/curcumin ratios | SF formulations released > 0.3 and 0.6 μg of curcumin in 6 days while chitosan blend composion released only < 0.1 μg | Chitosan-SF-curcumin formulation exhibited superior uptake in MCF-7 and MDA-MB-231 breast cancer cells | [96] |
| Thermosensitive nanoparticles | Higher loading efficiency and higher affinity of curcumin noticed between curcumin and thermosensitive nanoparticles | About 15–25% of the drug is released in about 10 h. Then, a much slower and almost constant release rate is observed. | Up to 2 fold increase in accumulation of curcumin nanoparticles in PC-3 and L929 cells | [51] |
3.2. Cellular Uptake
There is a clear correlation that increased blood circulation time and accumulation of nanomedicine in target tissues improve therapeutic effects compared with free drugs. A stable nanoformulation can be determined by its cellular uptake which is one of the important parameters for drug delivery applications. Like any other nanoparticle mediated drug delivery system, curcumin nanoformulations also promote the uptake of tumor or cancer cells in passive targeting due to “Enhanced Permeation and Retention” (EPR) effect. Table 2 illustrates the preferential uptake of curcumin nanoformulations in various cancer cells. Similarly, some of these formulations show decreased uptake in macrophage or normal cells which suggests the reticuloendothelial system (RES) clearance of nanoparticles is avoided [51, 98, 106, 107]. Such a selective and improved intracellular accumulation or uptake of curcumin nanoformulations in cancer cells is an indication for a higher therapeutic index.
The extent of cellular uptake of curcumin depends on the type of nanocarrier, particle size, surface charge, and cell line. For example, polyvinyl alcohol) (PVA) coated PLGA nanoformulations of curcumin whose particle size varied between ~ 560 to 76 nm (6 formulations) have shown distinctly different uptake patterns [41]. The uptake is continuously increased with a decrease in particle size. This is evidence that low particle size is more easily and highly endocytosized than higher particle size. Additional coating of poly(L-lysine) (PLL) on these nanoparticles further increase the uptake due to positive charge which helps in penetrating inside the cells. Numerous reports of various drug nanoformulations support this phenomenon. Researchers [51, 107] have demonstrated that a chitogen based nanocarrier enhances the internalization in MCF-7 and PC-3 cancer cells as time increased from 1 h to 48 h. The curcumin levels are increased from 0.2 to 0.8% absorbance in UV-vis spectral study. We also learn that the uptake by various cancer cells is quite different and vary formulation to formulation [50, 108]. In a comparative study, PLGA, cellulose, β-cyclodextrin (β-CD), nanogel and dendrimer nanoformulations of curcumin were evaluated for uptake in SKBR-3, MDA-MB-231 (breast), and HPAF-II (pancreatic) cancer cells [50]. The order of uptake was found as MDA-MB-231 > SKBR-3 > HPAF-II. It is important to note that curcumin uptake through nanoformulations is at least 2–3 fold greater than free curcumin. In another comparative cellular uptake study, free curcumin diffuse across the melanoma cell membrane and observed fluorescence presence in cytoplasm, localization in the peri-nuclear region and microfilament displacement suggests curcumin interaction with cytoskeleton proteins [109]. When these melanoma cells were treated with magnetite nanoparticle the fluorescence intensity was lower. The reason was that curcumin inside the hydrophobic bilayers of the nanoparticles quench the fluorescence property. However, the targeting specificity of curcumin nanoformulations towards cancer cells can be improved via antibody, peptide, penetrating ligand or aptamer conjugation [15, 110].
After exposure of curcumin nanoformulations to cancer cells, many nanoparticles were localized in the cytoplasm and inside or around the nucleus [41, 51, 98, 107, 108]. Longer periods of exposure drastically changed the morphology (e.g., cell lysis and loss of spindle shape) of cancer cells and observed cell debris. This type of behavior is highly dependent upon typical physico-chemical properties such as amorphous, crystalline, particle size and morphology and surface charge of the nanoformulations. To study an effective internalization process of curcumin nanoformulations fluorescence or confocal microscope is commonly employed. These methods utilize the inherent fluorescence property of curcumin, however, it is interfered with some type of nanoparticles in some cases. Recent investigations also rely on transmission electron microscopy to further validate curcumin nanoformulations cellular uptake (internalization) [41, 51, 98, 107, 108]. These investigations provide clear evidence of the presence of nanoparticle internalization at higher magnification. Another convenient method established for the determination of cellular uptake is Prussian blue staining. This method provides both the qualitative and quantitative uptake of iron oxide based curcumin nanoformulations. The uptake of magnetic nanoparticles-curcumin (MNP-CUR) can be viewed via an accumulation pattern of nanoparticles: the accumulation increases as the MNP-CUR concentration increases. The internalized particles are localized in almost every cell and throughout the cell components. This type of nanoformulation showed very minimal uptake by macrophages which supports increased circulation time for a more effective therapy [98, 106]. It is also possible to improve the internalization capacity of this type of nanoparticle by a ligand/antibody/penetrating peptide [110].
3.3. Anticancer Properties
Anticancer properties of each curcumin nanoformulation depend on the mechanism of specific accumulation or affinity of released curcumin in cancer cells [15, 20, 44, 113]. The activity of the same formulation may vary in different cancer cell lines. For instance, the mPEG2000–curcumin conjugate formulation is active against Caco-2 (colon), KB (oral cavity), MCF-7 (breast), and NCI-H187 (lung) with IC50 values in the range of 1–6 μM, similar to that observed for curcumin itself [114]. The treated cells were much smaller in size when compared with untreated cells and had lost intercellular adhesion.
Maitra and collaborators have developed a promising formulation (NanoCurc™) that can be implemented for future clinical translation in pancreatic and brain cancer(s) [52, 115–117]. Their initial study suggests NanoCurc™ in vitro efficacy and mechanisms of actions mirror that of free curcumin [52]. NanoCurc™ not only increases the bioavailability of curcumin in plasma and tissues but inhibits tumor growth in xenograft and athymic mice models of human pancreatic cancer [117]. It is interesting to note that tumor growth stops completely when NanoCurc™ was injected along with gemcitabine. These superior inhibiting effects were attributed to the attenuation of nuclear factor-kappaB (NF-κB) activity, reduction in expression of matrix metalloproteinase-9 and cyclin D1. This formulation has also shown a dose-dependent decrease in growth of multiple brain tumor cell lines: DAOY, D283Med (embryonic), HSR-GBM1, and JHH-GBM14 (glioblastoma neurosphere) [116]. The inhibition of cell growth is related to down-regulation of the insulin-like growth factor pathway and loss of STAT3. NanoCurc™ (5 mg/kg) localized up to 0.5% of formulation which is similar to when PLGA-curcumin or liposomal curcumin (20 mg/kg) is employed [115].
Nanocarriers made from ethylcellulose (EC) and methylcellulose (MC)/EC [ECMC] (a blend carrier) readily release curcumin into blood circulation by adhering to stomach mucosa. This property was initially detected using scanning electron microscopy analysis with in vivo experiments. These formulations have shown dose-dependent activity in MCF-7 and HepG2 hepatoblastoma cells [118]. Further, these curcumin nanoformulations were also applied in the form of lotions (oil in water, water in oil) which preferentially penetrated into porcine skin better than the water nanosuspensions [119]. Jayakumar group has established a novel formulation of curcumin loaded thermoresponsive chitosan-g-poly (N-vinylcaprolactam) nanoparticles which exhibit extreme toxicity to MCF-7, KB and PC-3 cancer cells [51]. These specific attributions are related to the loss of mitochondrial membrane potential. It was noticed in many studies that nanocurcumin formulations are effective in down regulating pro-survival proteins, up regulating pro-apoptosis, activating casepases (3,7,8, and 9), and cleaving Poly (ADP-ribose) polymerase (PARP). This implies induction of apoptosis in cancer cells. Recent studies published from our group have demonstrated similar apoptosis characteristics with PLGA, CD assembly, cellulose, magnetic and dendrimer nanoformulations of curcumin [41, 50, 87, 106, 108, 120]. Excessive lysosomal activity or production of vacuoles is responsible for active apoptosis induction in cancer cells by curcumin nanoformulations as demonstrated by transmission electron microscopy (TEM) analysis [50, 108]. This activity is infrequently observed with free curcumin. The primary reason for greater apoptosis is that curcumin nanoformulations internalize in cancer cells by endocytosis and escape from the phagocytosis, which may result in the release of curcumin in active form which then efficiently acts on cancer cells. Chen et al, [121] have also verified this phenomenon with magnetoplasmonic nanoparticle-loaded drug formulations for such biological activity in HL60 cells. Their TEM results suggest clear characteristics of apoptosis such as blebbing, pyknosis, and damage of cell structure. This phenomenon most likely occurred due to the transport of drug to the nucleus of the cell which induced the activity of the telomerase. A core-shell curcumin-loaded nanoparticle generated by amphilic methoxy polyethylene glycol-poly(caprolactone) (mPEG-PCL) block copolymers has shown similar effects in a rat C6 glioma cell line [122]. Curcumin encapsulation in a chitosan (CS) and silk fibroin (SF) blend polymer showed significantly lower IC50 than SF-encapsulated curcumin Her2/neu (low and high) expressing breast cancer cells [96].
Numerous curcumin nanoformulations have exhibited very similar anticancer potential compared to free curcumin [42, 60, 62, 122, 123]. This can be explained by the release property of nanoformulations. Many formulations release curcumin in a sustained manner over a period of 15–30 days. In vitro cytotoxicity studies investigate the proliferation of cells in 2, 4 or 5 days. During this time curcumin release from the formulation is 1/3 that of free curcumin, yet nanoformulations still exhibit equivalent or slightly greater anticancer potentials. However, their improved efficacy can be observed in long term experiments (such as colony formation) [41, 62, 87, 88, 98] as well as in animal models [61, 117, 124–126]. Cationic poly(butyl) cyanoacrylate nanoparticles coated with chitosan mediated the release of curcumin efficiently which inhibited tumor growth and tumor angiogensis [127]. Similarly, dendrosomal curcumin significantly reduced the tumor burden in BALB/c mice models in comparison with void curcumin and control samples. Additionally, this formulation increased the splenocyte proliferation and IFN-γ production and decreased IL-4 production [47]. Liposomal-cyclodextrin formulation of curcumin promoted autophagic cell death and is highly suitable to treat mesenchymal and epithelial origin cancers [128]. A complex of human serum albumin and curcumin not only transports 7.7-fold more curcumin than free curcumin but also confirms greater therapeutic effect, i.e., up to 66% tumor growth inhibition [129]. A recent treatment modality using curcumin nanoparticles enhanced the tumor reduction of HepG2 tumor xenografts, via decreased expression of Vascular endothelial growth factor (VEGF) as well as COX-2 [127]. Curcumin nano-disks (disk-shaped phospholipid bilayer formulations) demonstrated a dose-dependent increase in apoptosis through enhanced Fox03a and p27 expression, caspase-3, -9, PARP cleavage, and decreased cyclin D1, pAkt, and Bcl2 protein [67]. A recent formulation composed of cationic liposome, PEG and PEI complex exhibited 5 and 20-fold increases in the cytotoxic potential against curcumin-sensitive cells and curcumin-resistant cells, respectively [130]. This formulation is capable of inhibiting tumor growth 60–90% in mice bearing CT-26 or B16F10 cells.
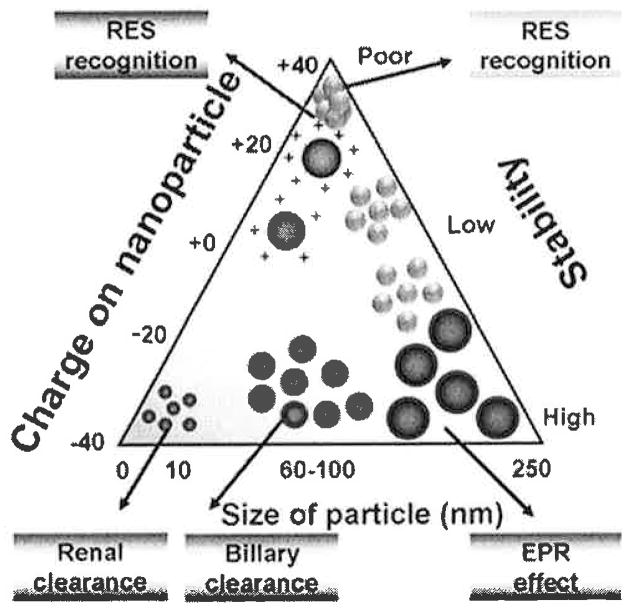
Most of the tabulated formulations report that curcumin nanoparticles follow the passive targeting mechanism (Table 3) rather than the active targeting. Passive targeting is a key property of curcumin nanoparticles and this property promotes the accumulation in tumor(s). Passive targeting may depend on a few important parameters such as particle size, zeta potential, and solubility or dispersion of nanoparticles (Fig. 3). Nanoformulations with an optimal size only exhibit EPR effect which in turn increase levels of accumulation in tumor. Additionally, a hydrophilic coating with poly(ethylene glycol) reduces the protein-protein/cells interaction and thereby minimizes the opsonization process.
Table 3.
In Vitro and In Vivo Anticancer Potential and Mechanism of Action of Various Curcumin Nanoformulations
| Curcumin Nanoformulations | In Vitro Cytotoxicity Profile | Molecular Mechanism | In Vivo Results | Reference |
|---|---|---|---|---|
| PLGA | IC50 (50% cell growth inhibitory concentration) of curcumin-loaded PLGA nanoparticles was between 20 μM and 22.5 μM while free curcumin ranged from 32 μM to 34 μM, in LNCaP, PC-3, and DU145 cancer cell lines | Inhibition of NF-κB function | Not available | [42] |
| PLGA | IC50 of curcumin nanoparticles was less than 5 μM in human leukemia (KBM-5 and Jurkat), prostate (DU145), breast (MDA-MB-231), colon (HCT116) and esophageal (SEG-1) cancer cells | Do not induce NF-κB activation expression of cyclin D1, MMP-9, and VEGF | Half-life of curcumin NPs (2.5 mg/Kg mice) was 1.75 longer than that of curcumin | [44] |
| PLGA | Nanocurcumin is as effective as curcumin in HeLa cells, SKBr3, and A549 cells | Increased Annexin V staining Cleaved PARP expression Down regulation of the activation of NF-κB |
Not available | [81] |
| MPEG-PCL micelle | IC50 of free curcumin and Cur-MPEG-PCL micelles was 3.95 mg mL−1 and 5.78 mg mg mL−1, respectively | Not available | Up to 2-fold increase in CUR concentration was observed in plasma of rats Inhibited the growth of subcutaneous C-26 colon carcinoma in xenograft mouse model |
[61] |
| β-cyclodextrin self-assembly | IC50 of self-assemblies of curcumin was 16.8 μM and 17.6 μM(C4-2 cells and DU145 cells, respectively) which is slightly lower than free curcumin | Increased cleaved PARP expression | Improved CUR levels serum concentrations up to 2-fold (Unpublished data with Subhash Chauhan Lab) | [87] |
| Poly(β-cyclodextrin) self-assembly | Very close IC50 values for both self-assembly and free curcumin in C4-2, DU145 and PC3 cancer cells | The PARP cleavage caused by PCD30 is much greater than free curcumin | Not available | [88] |
| poly(butyl cyanoacrylate) nano-particles | IC50 was observed approximately 15_μg/mL for HepG2, Bel7402 and Huh7 cells | Down regulation of COX-2 and VEGF expression | 2.2 fold decrease in tumor volume in HepG2 xenograft-bearing mice | [127] |
| Dendrosome | 2-fold reduction in IC50 with dendrosome curcumin in WEHI-164 (16.8 μM and 7.5 μM) and A431 cells (19.2 and 14.3 μM) in 24 and 48 h time | Increased Annexin V stain Cleaved PARP (apoptosis) | Tumor growth was significantly suppressed in mice treated with dendrosomal curcumin | [47] |
| Thermo-sensitive nanocarrier | Formulation showed a specific toxicity cancer cell lines (MCF-7, KB, and PC-3) and non toxic to L929. | Increase apoptosis (PI and Annexin-A binding) Loss of mitochondrial membrane potential |
Not available | [51] |
| Folate-modified self-microemulsifying drug delivery system | 18.27, 36.69, 30.4 μM and 20.57, 38.59, 25.62 μM in Hela and HT-29 cancer cells for folate CUR-nanoemulsion, CUR-emulsion and free curcumin, respectively | Not available | In situ colon perfused rats showed absorption of curcumin increased from 58.41% to 73.38% in 6 h with folate conjugated formulation. | [131] |
| NanoCurc™ | IC50 ranged between 10–15 μM for BxPC3, ASPC-1, PL-11 and XPA-1 | Blocks the activation of NF-κB Downregulation of steady state transcripts of multiple pro-inflammatory cytokines |
5 fold increased concentration was observed in pancreas. 3-fold or no growth in tumor was observed in mice with NanoCurc™ in combination with gemcitabine |
[52, 117] |
| PEG-chlorestrol | Cm/PEG-cholesterol based curcumin system showed IC50 1 μM more than free curcumin | Not available | Not available | [62] |
| NanoCurc™ | Almost no growth was observed in DAOY and D283Med, and the glioblastoma neurosphere lines HSR-GBM1 andJHH-GBM14 | Blocked the STAT3 and Hedgehog signaling G(2)/M arrest and apoptotic induction |
0.5% of the injected material was localized in the brain | [115, 116] |
| Amphiphilic mPEG-palmitic acid polymer | IC50 of curcumin, 14.32 μM, and nanocurcumin, 15.58 μM, were observed in HeLa cells | In vitro enzyme-catalyzed drug release enhances the anticancer activity | Not available | [132] |
Fig. 3.
Various clearance mechanisms of curcumin nanoformulations based on their physico-chemical properties. Note: Reticuloendothelial system (RES) is an older term for mononuclear phagocyte system.
Folic acid (FA) is a well-known small molecule that binds to folate-receptors and facilitates receptor-mediated endocytosis in a variety of cancer cells and tumors. An optimized formulation of folate conjugated microemulsion (31.1 ± 0.99 nm) comprised of 57.5% Cremophor EL, 32.5% Transcutol, and 10% Capryol 90, increases the percentage of curcumin absorption from 58.41±7.26 to 73.38 ± 3.12 in the colon of rats [131]. Furthermore, this formulation efficiently targets HeLa and HT-29 cancer cells compared to plain curcumin and curcumin loaded microemulsions. A curcumin-loaded magnetic nanoparticle formulation with transferrin ligand exhibits active targeting of K562 cancer cells (myeloid leukemia) [131]. The active targeting of these curcumin nanoparticles results in significant down-regulation of the Bcr-Abl protein that effectively operates an intrinsic apoptotic mechanism in myeloid leukemia cancer cells. Transferrin-mediated solid lipid nanoparticles demonstrate selective enhanced anticancer activity against MCF-7 breast cancer cells. This increased activity is due to increased cellular uptake, loss of mitochondrial membrane potential, and generation of excessive reactive oxygen species (ROS) [134]. A composite of PVP and hyaluronic acid (HA) curcumin formulation (six double layers) increased the hyaluronic acid receptor-mediated endocytosis to target cancer cells (glioma cells and Caco-2 cells) [135]. Additionally, this strategy also utilizes magnetic property to enhance the internalization. Manju and Sreenivasan [136] demonstrated enhanced efficacy of HA-conjugated curcumin with folate conjugated gold nanoparticles in HeLa cells, glioma and Caco 2 cells. Similarly, curcumin nanoformulations conjugated with Tet-1 peptide [79], apotransferrin [137], and apolipoprotein E (ApoE)-derived peptide [138] have improved the therapeutic value of curcumin.
A recent pre-clinical study reported for the first time using a targeted Prostate-specific membrane antigen (PSMA) nanoparticle containing the chemotherapeutic docetaxel in patients with solid tumors [139]. This formulation was developed from a combinatorial library of more than 100 compositions varying in particle size, drug loading and release, targeting efficiency and surface modifications. This further supports the premise that effective curcumin targeted nanoformulations can be developed for treatment of prostate cancer [15, 20]. Monoclonal antibody mediated delivery would improve targeting and binding efficacy to cancer cells which would significantly improve the curcumin anticancer activity. A number of monoclonal antibody conjugation techniques already exist for this purpose [32, 41, 98, 106, 133, 134, 140]. It is anticipated that by selectively choosing particle size, zeta potential, stability and targeting moiety, curcumin nanoformulations can be targeted to specific cancer cells (Fig. 4).
Fig. 4.
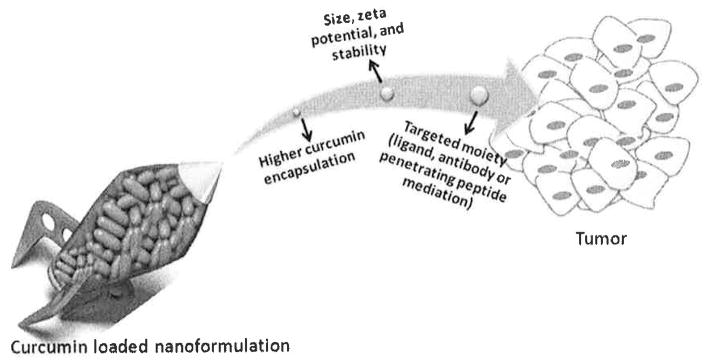
Schematic representation of targeted approach of curcumin nanoformulations for cancer treatment.
3.4. Reversal of Multi Drug Resistance
Drug resistance or multidrug resistance is a phenomenon whereby tumor cells become resistant to primary anticancer drugs. Curcumin is known to sensitize cancer cells to chemo/ radiation therapies [141]. Therefore, curcumin nanoformulations will have great therapeutic impact in cancer treatment. In our study, curcumin pre-treatment effectively induced chemo/radio-sensitization and considerably reduced the effective dose of cisplatin and radiation to inhibit the growth of cisplatin resistant ovarian cancer cells (A2780CP) [120]. This property can be induced more effectively using Nano-CUR with antibody conjugation capability. Co-encapsulated curcumin (CUR) and doxorubicin (DOX) in poly(butyl cyanoacrylate) nanoparticles prompted the highest drug resistance reversal and down-regulation of P-glycoprotein expression in MCF-7/ADR cell lines [142]. A new attempt has been made to leverage the therapeutic benefits of PLGA-CUR formulation in rats [143]. This study illustrates that a hypoxia condition considerably reduces the particle endocytosis and localization thereby lower tissue levels of curcumin are required compared to normoxic conditions. Such phenomenon can be altered by surface modification of nanoparticles. Similarly, curcumin and doxorubicin co-encapsulation in a lisosomal formulation supports the greater in vitro anti-tumor activities against A549 cells compared with that of free DOX [126]. A combined CUR/DOX nanoformulation would also facilitate the retention of DOX in the nucleus for a longer period of time as well as inhibit the expression of MDR1 and BCL-2 at the mRNA level in K562 cells. It is also true that when co-administered, curcumin and paclitaxel nanoformulations open up the drug resistance in cancer cells [144–146]. SKOV-3(TR) human ovarian adenocarcinoma cells showed less growth with combination treatment and this co-therapy successfully inhibited the NF-κB activity and down regulated P-glycoprotein [144]. These study results demonstrate an enhancement of paclitaxel available for therapy (5.2-fold) and such approach results in no acute toxicity with better therapeutic outcomes in ovarian adenocarcinoma [145]. Another group proved that this approach greatly inhibits the cervical cancer growth in a xenograft mouse model. This higher therapeutic index was achieved due to down regulating the antiapoptotic factors downstream signaling of survival signals [146].
3.5. Pharmacokinetics and Bioavailability
Bioavailability is one of the key pharmacokinetic properties of a drug molecule. This behavior mainly depends on the solubility, stability, metabolism, and degradation of drug molecules. Drug bioavailability follows the administration route: intravenous > intramuscular > subcutaneous > oral > rectal > inhalation [147]. Bioavailability of curcumin indicates the extent of active compound that reaches the systemic circulation which is readily available at the site of action. Extensive research on in vivo investigations of curcumin nanoformulations is still limited. For the first time, Shaikh et al. proved that PLGA-curcumin nanoparticles are safe and beneficial in several ways. In vivo pharmacokinetics demonstrated a 9-fold increase in oral bioavailability compared to a curcumin combination (curcumin with piperine) [148]. Curcumin oral bioavailability was significantly improved with different compositions of various formulations using stabilizers or adjuvant [149]. For this experiment, 250 mg/kg equivalent curcumin was administered using oral gavages to male SD rats. The order of bioavailability was found to be: curcumin formulation of milk > aqueous suspension > micronized suspension > piperine > nanosuspension ≥ amorphous solid dispersion > inclusion complex (HP-β-CD). 200–500% enhancement of the curcumin area under the curve (AUC0-t) and maximum concentration (Cmax) was observed with the nano-suspension and inclusion complex (HP-β-CD). A brief summary of important formulations that significantly improved the pharmacokinetics and bioavailability is available in Table 4.
Table 4.
Pharmacokinetics Properties and Bioavailability of Various Curcumin Nanoformulations
| Curcumin Nanoformulations | Comment | Reference |
|---|---|---|
| MPEG-PCL micelles | Tmax (min): 5 and 5 t(l/2) (time): 34.2 and 19.6 AUC(0/t) (mg L−1 min−1): 47642.1 and 7933.2 AUC(0/N) (mg L−1 min−1): 47864.6 and 7944.6 Cmax (mg mL−1): 430.5 and 305.7 for curcumin and micelle-curcumin, respectively | [61] |
| Dibenzoylmethane (DBM) nanoemulsion | 3-fold increase in oral bioavailability | [19] |
| Curcurain-loaded solid lipid nanoparticles (C-SLNs) | Curcumin levels in plasma were significantly increased i.e., 39 times at 50 mg/kg; 155 times at 1 mg/kg; and, 59 at 12.5 and 32 times at 25 mg/kg, respectively | [150] |
| PLGA | Nanoformulation significantly increased the retention time of curcumin by 96% in the cerebral cortex and 83% in the hippocampus | [151] |
| Theracurcumin | C (max) for 150 and 210 mg: 189 ±48 and 275 ± 67 ng/ml AUC (24 h) for 150 and 210 mg: 2,649 ± 350 and 3,649 ± 430 ng/ml × h t(l/2) for 150 and 210 mg: 9.7 ± 2.1 h and 13.0 ± 3.3 h | [152] |
| Nanosuspension | Area under the curve in plasma: 3.8-fold greater than curcumin the mean residence time: 11.2-fold longer than curcumin | [153] |
| (PLGA-PEG-PLGA) copolymer nanoparticles | AUC((0-infinity): 1.31 fold greater than curcumin t(l/2α), t(l/2β): 2.48 and 4.54 fold increase than curcumin Mean residence time: 2.67 fold longer than curcumin | [125] |
AUC: Area under the curve
Cmax: Peak concentration
(Tmax): Time to peak concentration
(tlag): Absorption lag time
AUC(0–t) and AUC(0–∞) of the test (e.g. generic formulation) to reference (e.g. innovator brand formulation)
The hemo-compatibility is an index for therapeutic formulations that are immediately exposed upon administration in blood. Accessing their hemo-compatibility in animal or human blood would enhance translation of curcumin formulations from “bench to bed site”. A recent study suggests that PLGA, CD, cellulose, nano-gel, and dendrimer based curcumin formulations did not show any erythrocytes damage or occurrence of thrombus [50]. Similar observations were made with intravenous PLGA nanosuspensions, curcumin conjugated nanoparticles [149–153], gold- curcumin nanoparticles, and a layer-by-layer self-assembly curcumin formulation [115, 135, 136, 154]. Rejinold et al. [155] demonstrated the biocompatibility nature of a thermosensitive curcumin formulation by hemolysis assay. The hemolytic ratio of this formulation was determined to be 2.155% which is in the acceptable range for therapeutic applications. Approximately 5% is considered as the maximum safe hemolytic ratio for bio-materials according to ISO/TR 7406.
3.6. Challenges to Curcumin Nanopharmaceuticals
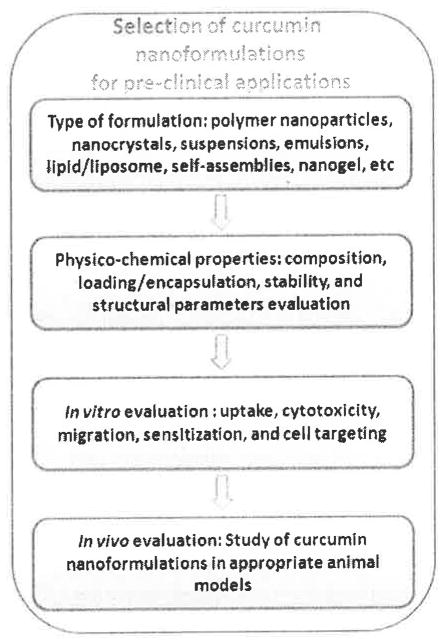
This section provides some key information on how curcumin nanoformulations can be utilized as pharmaceuticals. The first logical question to ask is whether the pharma industry has an interest to expand curcumin nanoformulations since curcumin is very inexpensive. The next question is which type of preparative method should be followed to obtain a more effective formulation with ensured reproducibility. We provide a schematic layout which proposes the basis upon which curcumin nanoformulations can be selected for future clinical application or clinical trials (Fig. 5). Liposomal formulations of drugs (Doxil, Myocet, Ambisome, and Depocyt), contrast imaging agents (gadolinium and iron oxide nanoparticles), PLGA formulations of paclitaxel (Abraxane), nanocrystal technology, nanomorph, nanoedge, nanopure, crititech and nanocochleate technologies are currently available in the market. Curcumin formulations developed by following these principle technologies would benefit from obtaining early approval from the FDA provided evident appropriate science, characterization tools, purity, stability, toxicity, safety profiles along with benefit to human health. However, curcumin formulations are considered to be as Abbreviated New Drug Applications (ANDAs) or New Drug Applications (NDAs). FDA is also authorized to inspect and examine records to nanotechnology, monitor the post-market safety and identify adverse events reporting. Based on such criteria FDA can pose ban if it is necessary.
Fig. 5.
Schematic flow chart delineates the step by step process for the selection of curcumin nanoformulations for clinical applications.
Our laboratory is interested in identifying hybrid nanocurcumin formulations that can be applied for multi-functional applications in cancer therapeutics. Currently, we have developed theranostic curcumin nanoparticles that combine therapy and diagnosis in one platform [98, 105]. Such type of nanoformulation allows loading therapeutic drug(s), biomacromolecule(s) and diagnostic agents and provide not only real-time monitoring of therapeutic outcome but also offers stimuli therapeutic strategies (Fig. 6). Overall this review highlights important contributions and issues associated with curcumin nanoformulation translations for clinical use in the future. It also provides enormous opportunity for implementation of nanotechnology in curcumin delivery to cancer cells efficiently. Evidence of superior anticancer properties exist for all the strategies but further developments of these curcumin nanoformulations should follow commonly employed good laboratory and manufacturing practice (cGLP and GMP) using FDA approved compounds. Suitable curcumin nanoformulations can then be chosen based on appropriate priorities established for both the development of nanotechnology and subsequent therapeutic application.
Fig. 6.
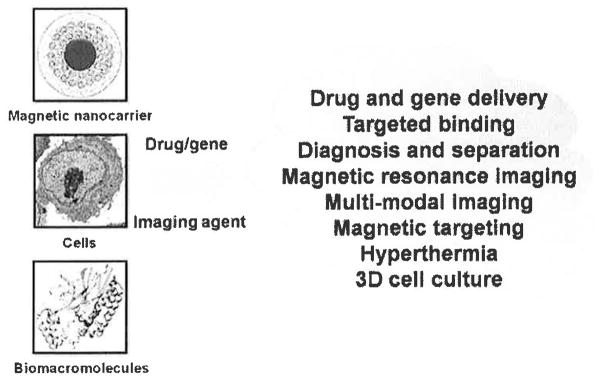
Magnetic curcumin nanoformulations for theranostic and multi-functional applications.
4. CONCLUSION
Curcumin showed excellent anticancer properties yet its inherent poor solubility, higher metabolic activity and poor pharmacokinetics properties hamper its ability to emerge as a potent medicine for cancer. In addition, since curcumin is a natural compound, there would be some regulatory and intellectual property right issues in regard to using curcumin as a drug. However, through developing proper formulations, i.e., nanoformulations are possible to get approval. Nanoparticle technology of curcumin is one of the frontier areas in medicine which will improve human health care. Interest in this area has been emerging worldwide over the last few years. Curcumin nanoformulations may offer numerous advantages including improved efficacy, tumor targeting, reduced systemic toxicity, compliance and convenience. A simplified and standardized approach is necessary to obtain curcumin nanoformulations. The process should not be extensive which would make the formulation costs minimal. In this perspective, curcumin nanoformulations based on cyclodextrin assembly, PLGA and magnetic nanoparticle formulations are highly appropriate. Oral and intraperitonial dosage nanoformulations are more preferred which reduces patient visits and also the cost burn enormously. Future pre-clinical and clinical investigations are required to gain in depth information about curcumin nanoformulations to translate as drug candidates to treat cancer(s) alone or in combination with other therapeutic modalities.
Acknowledgments
The authors thank Cathy Christopherson and Jenna Hultgren (Sanford Research/University of South Dakota) for editorial assistance. This work was partially supported by grants from Department of Defense (PC073887, PC073643), Governor’s Cancer 2010, the National Institutes of Health Research Project Grant Program (RO1) (CA142736), and the Centers of Biomedical Research Excellence (P20 RR024219).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
DISCLOSURE
Declared none.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Tan W, Lu J, Huang M, et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treasure J. Herbal medicine and cancer: an introductory overview. Semin Oncol Nurs. 2005;21:177–83. doi: 10.1016/j.soncn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad A, Sakr WA, Rahman KM. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front Biosci (Elite Ed) 2012;4:410–25. doi: 10.2741/e388. [DOI] [PubMed] [Google Scholar]
- 5.Gullett NP, Ruhul Amin AR, Bayraktar S, et al. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–81. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–78. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Visioli F, De La Lastra CA, Andres-Lacueva C, et al. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. 2011;51:524–46. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17:190–7. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 9.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–7. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altem Med Rev. 2009;14:141–53. [PubMed] [Google Scholar]
- 12.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–98. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teiten MH, Eifes S, Dicato M, Diederich M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins (Basel) 2010;2:128–62. doi: 10.3390/toxins2010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17:71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar TN, Shantha NV, Ramesh HP, Murthy IA, Murthy VS. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guineapigs & monkeys. Indian J Exp Biol. 1980;18:73–5. [PubMed] [Google Scholar]
- 17.Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1158–71. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75:R50–7. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 20.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 21.Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–7. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 23.Parkkinen J, inventor; Novokion Oy., assignee. Soluble complexes of curcumin. 20110034564. United States patent US. 2011 Feb;:A1.
- 24.Desai K, inventor. Curcumin cyclodextrin combination for preventing or treating various diseases. 20100179103. United States patent US. 2010 Jul;:A1.
- 25.Akhtar F, Ray G, Pandey AK, inventors; Kar SK, assignee. Curcumin nanoparticles and methods of producing the same. 20110190399. United States patent US. 2011 Aug;:A1.
- 26.Ranjan AP, Mukarjee A, Vishwanatha JK, inventors. University of North Texas Health Science Center at Fort Worth, assignee. Solid in oil/evaporation formulation for preparing curcumin-loaded PLGA nanoparticles. 20100290982. United States patent US. 2010 Nov;:A1.
- 27.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 28.Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–64. [PubMed] [Google Scholar]
- 29.Mimeault M, Batra SK. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chin Med. 2011;6:31. doi: 10.1186/1749-8546-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao ZG, Jin ZH, Yin XZ, Tian L. Multifunctional nanoparticles for targeting cancer therapy. J Nanosci Nanotechnol. 2010;10:7743–6. doi: 10.1166/jnn.2010.2818. [DOI] [PubMed] [Google Scholar]
- 32.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6:865–78. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui IA, Adhami VM, Christopher J, Chamcheu, Mukhtar H. Impact of nanotechnology in cancer: emphasis on nanochemoprevention. Int J Nanomedicine. 2012;7:591–605. doi: 10.2147/IJN.S26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16:354–60. doi: 10.1016/j.drudis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Bawa R. Nanoparticle-based therapeutics in humans: A survey. Nanotech Law Bus. 2008;5:135–155. [Google Scholar]
- 36.http://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/NanotechnologyTaskForce/ucm117221.htm
- 37.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010;144:3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–63. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- 39.Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 40.Dodd MJ. Side effects of cancer chemotherapy. Annu Rev Nurs Res. 1993;11:77–103. [PubMed] [Google Scholar]
- 41.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–75. [PubMed] [Google Scholar]
- 43.Braden ARC, Vishwanatha JK, Kafka E, inventors. University of North Texas Health Science Center at Fort Worth, assignee. Formulation of Active Agent Loaded Activated PLGA Nanoparticles for Targeted Cancer Nano-Therapeutics. 20080253961. United States patent US. 2008 Oct;:A1.
- 44.Anand P, Nair HB, Sung B, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330–8. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Shi W, Dolai S, Rizk S, et al. Synthesis of monofunctional curcumin derivatives, clicked curcumin dimer, and a PAMAM dendrimer curcumin conjugate for therapeutic applications. Org Lett. 2007;9:5461–4. doi: 10.1021/ol702370m. [DOI] [PubMed] [Google Scholar]
- 47.Babaei E, Sadeghizadeh M, Hassan ZM, Feizi MA, Najafi F, Hashemi SM. Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int Immunopharmacol. 2012;12:226–34. doi: 10.1016/j.intimp.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Liechty WB, Peppas NA. Expert opinion: Responsive polymer nanoparticles in cancer therapy. Eur J Pharm Biopharm. 2012;80:241–6. doi: 10.1016/j.ejpb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yallapu MM, Vasir JK, Jain TK, Vijayaraghavalu S, Labhasetwar V. Synthesis, Characterization and Antiproliferative Activity of Rapamycin-Loaded Poly(N-Isopropylacrylamide)-Based Nanogels in Vascular Smooth Muscle Cells. J Biomed Nanotechnol. 2008;4:16–24. [Google Scholar]
- 50.Yallapu MM, Ebeling MC, Chauhan N, Jaggi M, Chauhan SC. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int J Nanomedicine. 2011;6:2779–90. doi: 10.2147/IJN.S25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rejinold NS, Sreerekha PR, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int J Biolog Macromol. 2011;49:161–172. doi: 10.1016/j.ijbiomac.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dandekar PP, Jain R, Patil S, et al. Curcumin-loaded hydrogel nanoparticles: application in anti-malarial therapy and toxicological evaluation. J Pharm Sci. 2010;99:4992–5010. doi: 10.1002/jps.22191. [DOI] [PubMed] [Google Scholar]
- 54.Liu CH, Chang FY, Hung DK. Terpene microemulsions for transdermal curcumin delivery: effects of terpenes and cosurfactants. Colloids Surf B Biointerfaces. 2011;82:63–70. doi: 10.1016/j.colsurfb.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Lee MH, Lin HY, Chen HC, Thomas JL. Ultrasound mediates the release of curcumin from microemulsions. Langmuir. 2008;24:1707–13. doi: 10.1021/la7022874. [DOI] [PubMed] [Google Scholar]
- 56.Cui J, Yu B, Zhao Y, et al. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–55. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Jiang Y, Wang YW, Huang MT, Ho CT, Huang Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108:419–24. doi: 10.1016/j.foodchem.2007.10.086. [DOI] [PubMed] [Google Scholar]
- 58.Sou K, Inenaga S, Takeoka I, Tsuchida E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int J Pharm. 2008;352:287–93. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Mourtas S, Canovi M, Zona C, et al. Curcumin-decorated nanoliposomes with very high affinity for amyloid-betal-42 peptide. Biomaterials. 2011;32:1635–45. doi: 10.1016/j.biomaterials.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Sahu A, Kasoju N, Bora U. Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells. Biomacromolecules. 2008;9:2905–12. doi: 10.1021/bm800683f. [DOI] [PubMed] [Google Scholar]
- 61.Gou M, Men K, Shi H, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3:1558–67. doi: 10.1039/c0nr00758g. [DOI] [PubMed] [Google Scholar]
- 62.Sou K, Oyajobi B, Goins B, Phillips WT, Tsuchida E. Characterization and cytotoxicity of self-organized assemblies of curcumin and amphiphatic poly(ethylene glycol) J Biomed Nanotechnol. 2009;5:202–8. doi: 10.1166/jbn.2009.1025. [DOI] [PubMed] [Google Scholar]
- 63.Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006;40:720–7. doi: 10.1016/j.jpba.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 64.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–5. [PubMed] [Google Scholar]
- 65.Kurzrock R, Li L, Mehta K, Aggarwal BB, Helson L, inventors. Board of Regents, The University of Texas Systems, assignee. Liposomal curcumin for treatment of diseases. 20080138400. United States patent US. 2008:A1.
- 66.Ghosh M, Singh AT, Xu W, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011;7:162–7. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh AT, Ghosh M, Forte TM, Ryan RO, Gordon LI. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk Lymphoma. 2011;52:1537–43. doi: 10.3109/10428194.2011.584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheow WS, Hadinoto K. Green amorphous nanoplex as a new supersaturating drug delivery system. Langmuir. 2012;28:6265–75. doi: 10.1021/la204782x. [DOI] [PubMed] [Google Scholar]
- 69.Shutava TG, Balkundi SS, Vangala P, et al. Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano. 2009;3:1877–85. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Huang Y, Jin Y, Cheng Y. Mixing intensification by chaotic advection inside droplets for controlled nanoparticle preparation. Microfluid Nanofluid. 2010;9:773–86. [Google Scholar]
- 71.He Y, Huang Y, Cheng Y. Structure Evolution of Curcumin Nano-precipitation from a Micromixer. Crystal Growth Design. 2010;10:1021–4. [Google Scholar]
- 72.Zhang L, Cao F, Ding B, Li Q, Xi Y, Zhai G. Eudragit(R) S100 coated calcium pectinate microspheres of curcumin for colon targeting. J Microencapsul. 2011;28:659–67. doi: 10.3109/02652048.2011.604436. [DOI] [PubMed] [Google Scholar]
- 73.Ghorab DM, Amin MM, Khowessah OM, Tadros MI. Colon-targeted celecoxib-loaded Eudragit(R) S100-coated poly-epsilon-caprolactone microparticles: preparation, characterization and in vivo evaluation in rats. Drug Deliv. 2011;18:523–35. doi: 10.3109/10717544.2011.595841. [DOI] [PubMed] [Google Scholar]
- 74.Rawat M, Saraf S. Formulation optimization of double emulsification method for preparation of enzyme-loaded Eudragit S100 microspheres. J Microencapsul. 2009;26:306–14. doi: 10.1080/02652040802319767. [DOI] [PubMed] [Google Scholar]
- 75.Raffin RP, Colome LM, Haas SE, Jornada DS, Pohlmann AR, Guterres SS. Development of HPMC and Eudragit S100 blended microparticles containing sodium pantoprazole. Pharmazie. 2007;62:361–4. [PubMed] [Google Scholar]
- 76.Jain D, Panda AK, Majumdar DK. Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS Pharm Sci Tech. 2005;6:E100–7. doi: 10.1208/pt060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dev S, Prabhakaran P, Filgueira L, Iyer KS, Raston CL. Microfluidic fabrication of cationic curcumin nanoparticles as an anti-cancer agent. Nanoscale. 2012;4:2575–9. doi: 10.1039/c2nr11502f. [DOI] [PubMed] [Google Scholar]
- 78.Zheng Z, Zhang X, Carbo D, Clark C, Nathan C, Lvov Y. Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir. 2010;26:7679–81. doi: 10.1021/la101246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathew A, Fukuda T, Nagaoka Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One. 2012;7:e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cartiera MS, Ferreira EC, Caputo C, Egan ME, Caplan MJ, Saltzman WM. Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol Pharm. 2010;7:86–93. doi: 10.1021/mp900138a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425:44–52. doi: 10.1016/j.ijpharm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Ha PT, Nguyet Tran TM, Pham HD, Nguyen QH, Nguyen XP. The synthesis of poly(lactide)-vitamin E TPGS (PLA-TPGS) copolymer and its utilization to formulate a curcumin nanocarrier. Adv Nat Sci Nanosci Nanotechnol. 2010;1:7pp. [Google Scholar]
- 83.Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2010;6:153–60. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Teng Z, Luo Y, Wang Q. Nanoparticles synthesized from soy protein: preparation, characterization, and application for nutraceutical encapsulation. J Agric Food Chem. 2012;60:2712–20. doi: 10.1021/jf205238x. [DOI] [PubMed] [Google Scholar]
- 85.Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J Pharm Sci. 2011;100:504–11. doi: 10.1002/jps.22278. [DOI] [PubMed] [Google Scholar]
- 86.Chirio D, Gallarate M, Trotta M, Carlotti ME, Gaudino EC, Cravotto G. Influence of a- and c- cyclodextrin lipophilic derivatives on curcumin-loaded SLN. J Incl Phenom Macrocycl Chem. 2009;65:391–402. [Google Scholar]
- 87.Yallapu MM, Jaggi M, Chauhan SC. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79:113–25. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 88.Yallapu MM, Jaggi M, Chauhan SC. Poly(beta-cyclodextrin)/curcumin self-assembly: a novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol Biosci. 2010;10:1141–51. doi: 10.1002/mabi.201000084. [DOI] [PubMed] [Google Scholar]
- 89.Goncalves C, Pereira P, Schellenberg P, Coutinho PJ, Gama FM. Self-Assembled Dextrin Nanogel as Curcumin Delivery System. J Biomat Nanobiotech. 2012;3:178–84. [Google Scholar]
- 90.Reddy MK, Vasir JK, Sahoo SK, Jain TK, Yallapu MM, Labhasetwar V. Inhibition of apoptosis through localized delivery of rapamycin-loaded nanoparticles prevented neointimal hyperplasia and reendothelialized injured artery. Circ Cardiovasc Interv. 2008;1:209–16. doi: 10.1161/CIRCINTERVENTIONS.108.830018. [DOI] [PubMed] [Google Scholar]
- 91.Donsi F, Wang Y, Li J, Huang Q. Preparation of curcumin sub-micrometer dispersions by high-pressure homogenization. J Agric Food Chem. 2010;58:2848–53. doi: 10.1021/jf903968x. [DOI] [PubMed] [Google Scholar]
- 92.Chin SF, Iyer KS, St Saunders M, et al. Encapsulation and Sustained Release of Curcumin using Superparamagnetic Silica Reservoirs. Chem Eur J. 2009;15:5661–5. doi: 10.1002/chem.200802747. [DOI] [PubMed] [Google Scholar]
- 93.Tran LD, Hoang NMT, Mai TT, et al. Nanosized magnetofluorescent Fe304–curcumin conjugate for multimodal monitoring and drug targeting. Colloids and Surfaces A: Physicochem Eng Aspects. 2010;371:104–12. [Google Scholar]
- 94.Li F, Li X, Li B. Preparation of magnetic polylactic acid microspheres and investigation of its releasing property for loading curcumin. J Mag Mang Mater. 2011;323:2770–5. [Google Scholar]
- 95.Manju S, Sreenivasan K. Hollow microcapsules built by layer by layer assembly for the encapsulation and sustained release of curcumin. Colloids Surf B Biointerfaces. 2011;82:588–93. doi: 10.1016/j.colsurfb.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 96.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine. 2009;4:115–22. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang H, Zhang L, Yuan P, Wang C. Preparation and in vitro release characteristics of curcumin in nanosuspensions. Zhongguo Zhong Yao Za Zhi. 2011;36:132–5. [PubMed] [Google Scholar]
- 98.Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, Chauhan SC. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32:1890–905. doi: 10.1016/j.biomaterials.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costache AD, Sheihet L, Zaveri K, Knight DD, Kohn J. Polymer-drug interactions in tyrosine-derived triblock copolymer nanospheres: a computational modeling approach. Mol Pharm. 2009;6:1620–7. doi: 10.1021/mp900114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiyaboonchai W, Tungpradit W, Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm. 2007;337:299–306. doi: 10.1016/j.ijpharm.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 101.Zhu XQ, Sun M, Zhu FP, Ding TT, Zhai YJ, Zhai GX. Preparation and characterization of curcumin polybutylcyanoacrylate nanoparticles. Zhong Yao Cai. 2010;33:797–801. [PubMed] [Google Scholar]
- 102.Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59:9280–9. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 103.Patel NA, Patel NJ, Patel RP. Formulation and evaluation of curcumin gel for topical application. Pharm Dev Technol. 2009;14:80–9. doi: 10.1080/10837450802409438. [DOI] [PubMed] [Google Scholar]
- 104.Iwunze MO, McEwan D. The characterization of the sol-gel encapsulated curcumin as a possible sensor for small biologically important molecules. Cell Mol Biol (Noisy-le-grand) 2007;53:81–7. [PubMed] [Google Scholar]
- 105.Yallapu MM, Othman SF, Curtis ET, et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine. 2012;2012:1761–79. doi: 10.2147/IJN.S29290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yallapu MM, Foy SP, Jain TK, Labhasetwar V. PEG-functionalized magnetic nanoparticles for drug delivery and magnetic resonance imaging applications. Pharm Res. 2010;27:2283–95. doi: 10.1007/s11095-010-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anitha A, Maya S, Deepa N, et al. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydrate Polymers. 2011;83:452–61. [Google Scholar]
- 108.Yallapu MM, Dobberpuhl MR, Maher DM, Jaggi M, Chauhan SC. Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr Drug Metab. 2012;13:120–8. doi: 10.2174/138920012798356952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Souza FF, dos Santos MC, dos Passos DC, Lima EC, Guillo LA. Curcumin associated magnetite nanoparticles inhibit in vitro melanoma cell growth. J Nanosci Nanotechnol. 2011;11:7603–10. doi: 10.1166/jnn.2011.5124. [DOI] [PubMed] [Google Scholar]
- 110.Utreja P, Jain S, Tiwary AK. Novel drug delivery systems for sustained and targeted delivery of anti- cancer drugs: current status and future prospects. Curr Drug Deliv. 2010;7:152–61. doi: 10.2174/156720110791011783. [DOI] [PubMed] [Google Scholar]
- 111.Shelma R, Sharma CP. Submicroparticles composed of amphiphilic chitosan derivative for oral insulin and curcumin release applications. Colloids Surf B Biointerfaces. 2011;88:722–8. doi: 10.1016/j.colsurfb.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 112.Shelma R, Sharma CP. Development of lauroyl sulfated chitosan for enhancing hemocompatibility of chitosan. Colloids Surf B Biointerfaces. 2011;84:561–70. doi: 10.1016/j.colsurfb.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 113.Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta. 2008;1780:673–9. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 114.Wichitnithad W, Nimmannit U, Callery PS, Rojsitthisak P. Effects of different carboxylic ester spacers on chemical stability, release characteristics, and anticancer activity of mono-PEGylated curcumin conjugates. J Pharm Sci. 2011;100:5206–18. doi: 10.1002/jps.22716. [DOI] [PubMed] [Google Scholar]
- 115.Chiu SS, Lui E, Majeed M, et al. Differential distribution of intravenous curcumin formulations in the rat brain. Anticancer Res. 2011;31:907–11. [PMC free article] [PubMed] [Google Scholar]
- 116.Lim KJ, Bisht S, Bar EE, Maitra A, Eberhart CG. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11:464–73. doi: 10.4161/cbt.11.5.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bisht S, Mizuma M, Feldmann G, et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther. 2010;9:2255–64. doi: 10.1158/1535-7163.MCT-10-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suwannateep N, Banlunara W, Wanichwecharungruang SP, Chiablaem K, Lirdprapamongkol K, Svasti J. Mucoadhesive curcumin nanospheres: biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J Control Release. 2011;151:176–82. doi: 10.1016/j.jconrel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 119.Suwannateep N, Wanichwecharungruang S, Fluhr J, Patzelt A, Lademann J, Meinke MC. Comparison of two encapsulated curcumin particular systems contained in different formulations with regard to in vitro skin penetration. Skin Res Technol. 2012 doi: 10.1111/j.1600-0846.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 120.Yallapu MM, Maher DM, Sundram V, Bell MC, Jaggi M, Chauhan SC. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J Ovarian Res. 2010;3:11. doi: 10.1186/1757-2215-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen W, Xu N, Xu L, et al. Multifunctional Magnetoplasmonic Nanoparticle Assemblies for Cancer Therapy and Diagnostics (Theranostics) Macromol Rapid Commun. 2010;31:228–36. doi: 10.1002/marc.200900793. [DOI] [PubMed] [Google Scholar]
- 122.Shao J, Zheng D, Jiang Z, et al. Curcumin delivery by methoxy polyethylene glycol-poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim Biophys Sin (Shanghai) 2011;43:267–74. doi: 10.1093/abbs/gmr011. [DOI] [PubMed] [Google Scholar]
- 123.Rejinold NS, Muthunarayanan M, Chennazhi KP, Nair SV, Jayakumar R. Curcumin loaded fibrinogen nanoparticles for cancer drug delivery. J Biomed Nanotechnol. 2011;7:521–34. doi: 10.1166/jbn.2011.1320. [DOI] [PubMed] [Google Scholar]
- 124.Mazzarino L, Silva LF, Curta JC, et al. Curcumin-loaded lipid and polymeric nanocapsules stabilized by nonionic surfactants: an in vitro and In vivo antitumor activity on B16-F10 melanoma and macrophage uptake comparative study. J Biomed Nanotechnol. 2011;7:406–14. doi: 10.1166/jbn.2011.1296. [DOI] [PubMed] [Google Scholar]
- 125.Song Z, Feng R, Sun M, et al. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci. 2011;354:116–23. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 126.Wang H, Hu L, Li C, Zhang J, Zhang T. Increase of therapeutic activity of doxorubicin by long circulating liposomes in combination with curcumin. Pharmazie. 2011;66:871–4. [PubMed] [Google Scholar]
- 127.Duan J, Zhang Y, Han S, et al. Synthesis and in vitro/in vivo anticancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2010;400:211–20. doi: 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 128.Dhule SS, Penfornis P, Frazier T, et al. Curcumin-loaded gamma-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8:440–51. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim TH, Jiang HH, Youn YS, et al. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403:285–91. doi: 10.1016/j.ijpharm.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 130.Lin YL, Liu YK, Tsai NM, et al. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine. 2012;8:318–327. doi: 10.1016/j.nano.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 131.Zhang L, Zhu W, Yang C, et al. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int J Nanomedicine. 2012;7:151–62. doi: 10.2147/IJN.S27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752–61. doi: 10.1016/j.actbio.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 133.Dilnawaz F, Singh A, Sahoo SK. Transferrin-conjugated curcumin-loaded superparamagnetic iron oxide nanoparticles induce augmented cellular uptake and apoptosis in K562 cells. Acta Biomater. 2012;8:704–19. doi: 10.1016/j.actbio.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 134.Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J Pharm. 2010;398:190–203. doi: 10.1016/j.ijpharm.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 135.Manju S, Sreenivasan K. Enhanced drug loading on magnetic nanoparticles by layer-by-layer assembly using drug conjugates: blood compatibility evaluation and targeted drug delivery in cancer cells. Langmuir. 2011;27:14489–96. doi: 10.1021/la202470k. [DOI] [PubMed] [Google Scholar]
- 136.Manju S, Sreenivasan K. Gold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: blood compatibility evaluation and targeted drug delivery onto cancer cells. J Colloid Interface Sci. 2012;368:144–51. doi: 10.1016/j.jcis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 137.Gandapu U, Chaitanya RK, Kishore G, Reddy RC, Kondapi AK. Curcumin-loaded apotransferrin nanoparticles provide efficient cellular uptake and effectively inhibit HIV-1 replication in vitro. PLoS One. 2011;6:e23388. doi: 10.1371/journal.pone.0023388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Re F, Cambianica I, Zona C, et al. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomedicine. 2011;7:551–9. doi: 10.1016/j.nano.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 139.Hrkach J, Von Hoff D, Ali MM, et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci Transl Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 140.Thamake SI, Raut SL, Ranjan AP, Gryczynski Z, Vishwanatha JK. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology. 2011;22:035101. doi: 10.1088/0957-4484/22/3/035101. [DOI] [PubMed] [Google Scholar]
- 141.Goel A, Aggarwal B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 142.Duan J, Mansour HM, Zhang Y, et al. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2012;426:193–201. doi: 10.1016/j.ijpharm.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 143.Devadasu VR, Wadsworth RM, Ravi Kumar MN. Tissue localization of nanoparticles is altered due to hypoxia resulting in poor efficacy of curcumin nanoparticles in pulmonary hypertension. Eur J Pharm Biopharm. 2012;80:578–584. doi: 10.1016/j.ejpb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 144.Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6:928–39. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 145.Ganta S, Devalapally H, Amiji M. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J Pharm Sci. 2010;99:4630–41. doi: 10.1002/jps.22157. [DOI] [PubMed] [Google Scholar]
- 146.Sreekanth CN, Bava SV, Sreekumar E, Anto RJ. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene. 2011;30:3139–52. doi: 10.1038/onc.2011.23. [DOI] [PubMed] [Google Scholar]
- 147. [Accessed on July 2, 2012]; http://howmed.net/pharmacology/bioavailability-of-drugs/
- 148.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–30. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 149.Munjal B, Pawar YB, Patel SB, Bansal AK. Comparative oral bioavailability advantage from curcumin formulations. Drug Del Trans Res. 2011;1:322–331. doi: 10.1007/s13346-011-0033-3. [DOI] [PubMed] [Google Scholar]
- 150.Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2011;55:495–503. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- 151.Tsai YM, Chien CF, Lin LC, Tsai TH. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharm. 2011;416:331–8. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 152.Kanai M, Imaizumi A, Otsuka Y, et al. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother Pharmacol. 2012;69:65–70. doi: 10.1007/s00280-011-1673-1. [DOI] [PubMed] [Google Scholar]
- 153.Gao Y, Li Z, Sun M, et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36:1225–34. doi: 10.3109/03639041003695139. [DOI] [PubMed] [Google Scholar]
- 154.Sindhu K, Indra R, Rajaram A, Sreeram KJ, Rajaram R. Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J Biomed Nanotechnol. 2011;7:56. doi: 10.1166/jbn.2011.1199. [DOI] [PubMed] [Google Scholar]
- 155.Rejinold NS, Sreerekha PR, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly(N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Inter J Biol Macromol. 2011;49:161–72. doi: 10.1016/j.ijbiomac.2011.04.008. [DOI] [PubMed] [Google Scholar]