Abstract
Polymethoxyflavones (PMF) isolated from citrus peel have potent anti-cancer activity, however their utilization as functional ingredients in foods is currently limited because of their high melting point and poor water-solubility. The influence of oil type and concentration, hydrophilic polymer addition, and simulated intestinal conditions on PMF (5-hydroxytangeretin) solubility in solutions and nanoemulsions was examined. The saturation concentration of PMF in water was relatively low (0.93 µM), but could be increased appreciably by adding certain hydrophilic polymers: polyethylene glycol (PEG) and β-cyclodextrin (CD) were ineffective at increasing solubility, but poly(vinyl alcohol) (PVA) and hydroxypropyl methylcellulose (HPMC) greatly enhanced solubility (e.g., > 6 µM for 0.5 % polymer). PMF was more soluble in medium chain triglycerides (MCT, 6.1 mM) than long chain triglycerides (LCT, 4.2 mM). The encapsulation efficiency of PMF in oil-in-water nanoemulsions was higher when MCT was used as the oil phase rather than LCT, and could be increased by increasing the oil droplet content. The solubility of PMF in simulated small intestinal fluids was increased by solubilization in bile micelles and mixed micelles formed during lipid digestion. These results have important implications for the development of functional foods fortified with bioactive hydrophobic components aimed at improving human health and wellness.
Keywords: Nutraceuticals, Pharmaceuticals, Flavonoids, Bioavailability, Nanoemulsions, Nanoparticles
Introduction
Many bioactive agents intended for oral ingestion (e.g., drugs and nutraceuticals) are non-polar compounds with high melting points, low water-solubilities, and poor oral bioavailabilities.1–3 It is therefore difficult to incorporate many of these bioactive compounds into commercial products, such as functional foods and beverages, due to their tendency to precipitate and sediment. The formation of crystals adversely impacts the appearance, texture, or mouthfeel of food products.4 The sedimentation of crystals to the bottom of a product reduces the amount of bioactive consumed, thereby decreasing its potential biological activity. The poor absorption of crystalline compounds in the gastrointestinal tract (GIT) compared to soluble ones reduces the amount of bioactive compound that reaches the blood circulation and target tissues.1,5,6 Many of these problems can be overcome by ensuring that the bioactive component remains in a soluble state within the product during storage, as well as during its passage throughout the GIT after ingestion.
The food industry may be able to overcome some of these potential problems by adopting strategies developed within the pharmaceutical industry to increase the aqueous solubility of crystalline hydrophobic drugs so as to improve their biopharmaceutical properties.1, 7, 8 A number of technological approaches have been investigated by pharmaceutical researchers to achieve this goal such as converting the drug into nanocrystals 9, solubilizing the drug within colloidal delivery systems 10, 11, and complexing the drug with various polymers.7, 12 Some of these approaches aim to increase the equilibrium solubility of drugs, whereas others aim to increase their kinetic solubility. The maximum amount of solute that can be solubilized within a particular system under equilibrium conditions is referred to as the equilibrium saturation concentration. Nevertheless, it is often possible to create supersaturated solutions where the concentration of the solute is considerably higher than the equilibrium saturation concentration by introducing a kinetic energy barrier to nucleation and/or crystal growth.5, 13–17 Various water-soluble polymers may inhibit the precipitation of solutes from aqueous solutions based on either the equilibrium or kinetic approaches, such as hydroxypropyl methylcellulose (HPMC), polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), cellulose derivatives, and cyclodextrin.1, 8, 18, 19 If an aqueous drug solution can be made to exist in a supersaturated state within the GIT for a sufficiently long time, then the amount of drug that is absorbed through the intestinal wall can be increased.1
The purpose of the current study was to investigate the influence of various factors on the solubility of crystalline hydrophobic bioactive compounds within nanoemulsion-based delivery systems under storage and simulated GIT conditions. In particular, we focused on the addition of hydrophilic polymers, carrier oil type and concentration, and simulated small intestinal conditions on the solubility of a polymethoxyflavone (PMF). PMFs can be isolated from the peels of citrus fruits (such as oranges and tangerines), which have traditionally been used as herbal medicines in Eastern societies to prevent several diseases.20, 21 A number of studies have shown that PMFs have strong biological activity, including anti-inflammatory, anti-carcinogenic, anti-viral, anti-oxidant, anti-thrombogenic, and antiatherogenic properties.22–27 Nevertheless, PMFs are highly hydrophobic molecules that have high melting points,28 poor water-solubilities, and low oral bioavailabilities.23 There is therefore a need to develop effective food-grade delivery systems to incorporate this kind of crystalline hydrophobic bioactive compound into aqueous-based functional food products, such as beverages, sauces, dips, dressings, and deserts.
In a previous study, we showed that oil-in-water nanoemulsions could be utilized to encapsulate PMFs.4 If the PMF concentration was kept below the equilibrium saturation concentration, then it remained soluble throughout storage. However, if the PMF concentration exceeded this level, then PMF crystals formed in the nanoemulsions during storage and sedimented. In the present study, we examined whether various hydrophilic polymers could be utilized to inhibit the precipitation of PMF (5-hydroxytangeretin) from nanoemulsions during storage and passage through a simulated GIT. The four polymers tested were hydroxypropyl methylcellulose (HPMC), poly(vinyl alcohol) (PVA), polyethylene glycol (PEG), and β-cyclodextrin (CD), which are generally recognized as safe (GRAS) for use in foods. The knowledge gained from this study should facilitate the rational design of nanoemulsion-based delivery systems for encapsulating highly hydrophobic pharmaceuticals and nutraceuticals.
Materials and Methods
Materials
PMF powder (5-hydroxy-6,7,8,4'-tetramethoxy-flavone or 5-hydroxytangeretin) was provided by Dr. Hang Xiao’s laboratory at the University of Massachusetts.29 Medium chain triglyceride (MCT) oil was purchased from Sasol Germany Gmbh (Miglyol 812 N, Witten, Germany). Corn oil was purchased from a local supermarket and was used as an example of a long chain triglyceride (LCT) oil. Tween20 (T20) was purchased from MP Biomedicals LLC (Solon, OH). Poly (vinyl alcohol) (PVA, Mw089,000-98,000), β-cyclodextrin (CD), hydroxypropyl methylcellulose (HPMC, viscosity080-120 cP for 2 % in H2O at 20 °C), Polyethylene glycol (PEG, average Mn0400 Da), bile extract (porcine, B8613) and lipase from porcine pancreas (Type II, triacylglycerol hydrolase E.C. 3.1.1.3, PPL) were purchased from Sigma-Aldrich (St Louis, MO). Analytical grade sodium monophosphate, sodium diphosphate, hydrochloric acid (HCl) and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (St Louis, MO). Dimethyl sulfoxide (DMSO), chloroform, sodium chloride (NaCl) and calcium chloride (CaCl2.2H2O) were purchased from Fisher Scientific (USA). Distilled and deionized water from a water purification system (Nanopure Infinity, Barnstead International, Dubuque, IA) was used for the preparation of all solutions and nanoemulsions.
Determination of Organic Phase Solubility of PMF
The influence of oil type on the organic phase solubility of PMF was established using a thermal quenching method. A weighed amount of PMF powder was added to 10 mL oil in a glass test tube and then stirred for 1 h at 90 to 100 °C by placing the test tube in a temperature-controlled water bath. After 1 h, the PMF/oil system had a transparent yellowish appearance, indicating that the PMF had fully dissolved at this elevated temperature. The samples were then cooled to 25 °C and stored at this temperature for up to 72 h, which led to the formation of visible PMF crystals in some of the systems. The general appearance of the systems was recorded using a digital camera (PowerShot SD1300IS, Canon, Lake Success, NY, USA), while the microstructure of the samples was determined by optical microscopy (Nikon Eclipse E400, Nikon Corp., Japan). Samples were then centrifuged (3500 rpm, 15 min) and 0.01 mL of the supernatant was collected and mixed with 9.99 mL of chloroform using a vortex. The amount of PMF in this supernatant solution was then measured using a UV-visible spectrometer.
Determination of Aqueous Phase Solubility of PMF
The influence of polymer type and concentration on the aqueous solubility of PMF was established using a solvent injection method. Aqueous solutions containing a fixed amount of PMF (56 µM) were prepared by injecting 0.01 mL of 1 % PMF in DMSO solution into 4.99 mL of aqueous polymer (0–5 %) solution. These mixtures were then stored at 25 °C with continuous stirring (120 rpm) for 72 h. Samples (3 mL) were collected and then centrifuged (15 min, 4000 rpm) to remove any crystalline material. The concentration ofPMF remaining in the supernatant was then determined using a UV-visible spectrometer. Preliminary experiments were also carried out to establish the time required to reach a steady state by periodically collecting samples from the mixtures and analyzing the amount of soluble PMF present. These initial experiments indicated that the systems reached steady state after about 24–48 h storage, and so an incubation time of 72 h was used in all subsequent experiments.
Nanoemulsion Preparation
In some studies, we examined the formation of PMF crystals in oil-in-water nanoemulsions, and their influence on nanoemulsion properties. These nanoemulsions were prepared using a high-energy homogenization method.
Oil Phase Preparation
PMF powder (1 wt%) was added to a heated carrier oil (90–100 °C) and the system was continuously stirred until the PMF was completely dissolved. Two kinds of carrier oil were used in this study: a long chain triglyceride (LCT) and medium chain triglyceride (MCT).
Aqueous Phase Preparation
An aqueous surfactant solution (2 wt% Tween 20 in 5 mM phosphate buffer) was prepared and then warmed prior to utilization to avoid PMF crystallization when the oil and aqueous phases were brought into contact.
Emulsion Preparation
A stock emulsion was prepared by homogenizing 20 wt% oil phase (0 or 1 wt% PMF in carrier oil) with 80 wt% aqueous phase (2 wt% surfactant in buffer solution, pH 7.0) with a high-speed blender for 2 min (M133/1281-0, Biospec Products, Inc., ESGC, Switzerland) followed by five passes at 12,000 psi through a high pressure homogenizer (Microfluidics M-110Y, F20Y 75 µm interaction chamber, Newton, MA). The oil and aqueous phases were kept warm (> 50 °C) throughout the mixing and homogenization process, otherwise the PMF would crystallize and the whole system would gel. Emulsions with different oil phase concentrations were prepared using the same method but varying the initial ratios of oil and aqueous phase.
Determination of Encapsulation of PMF in Nanoemulsions
In a previous study, we found that PMF formed crystals in emulsions during storage when it was incorporated at levels above its saturation concentration, and that these crystals sedimented to the bottom of the samples.4 We therefore measured the amount of PMF that remained dispersed throughout the bulk of the emulsions over time. 4 mL of emulsion was placed in a glass test tube immediately after preparation. The emulsions were then stored at ambient temperature for different times and small aliquots (0.02 mL) of sample were collected from a position located 1 cm below their surface. DMSO (4.98 mL) was then added to this aliquot and mixed using a vortex. This sample was taken to represent the PMF still dispersed throughout the nanoemulsion. The total PMF concentration present was determined by mixing the nanoemulsion to make it homogeneous (20 min, 3000 rpm) and then analyzing the PMF using the same method as described earlier. The PMF concentration was measured using a UV-Vis spectrometer.
In Vitro Digestion Model
An in vitro digestion model was used to determine the lipolysis of the triacylglycerol carrier oils, and the bioavailability of the bioactive compound. This digestion model was based on that described earlier 30 with some minor adjustments. A total of 192.5 mL simulated small intestinal fluid (SSIF) was placed in a beaker. PMF-loaded nanoemulsion (7.5 mL) with or without PMF was added to the SSIF followed by an equilibration period of 10 min prior to allow the sample to reach 37 °C. If necessary, this solution was adjusted to pH 7.00 by adding NaOH or HCl solution prior to adding the lipase. Lipolysis was initiated by addition of 100 mL pancreatic extract solution. During lipolysis the media was maintained at pH 7.00 by addition of a 2 M NaOH solution. The compositions of the SSIFmedia (fasted and fedmodels) in the reaction chamber are shown in Table 1 unless stated otherwise.
Table 1.
Composition of simulated small intestinal fluids used in the in vitro digestion models: Fasted-SSIF and Fed-SSIF
| System | NaCl (mmol/L) |
CaCl2 (mmol/L) |
Bile salt extract (mg/mL) |
Pancreatic lipase (mg/mL) |
Phosphate buffer (mmol/L) |
|---|---|---|---|---|---|
| Fa-SSIF | 100 | 5 | 5 | 0.6 | 5 |
| Fe-SSIF | 150 | 10 | 15 | 2.4 | 5 |
A control in vitro lipolysis experiment was carried out in the absence of nanoemulsion to determine the influence of other possible compounds that could alter the pH during digestion (such as proteins and phospholipids). These values were then subtracted from the values for the nanoemulsion samples. All lipolysis experiments were carried out in triplicate. 20 wt% oil-in-water nanoemulsions were prepared. The initial concentration of PMF in the oil phase was 0.5 wt%, which corresponds to 0.1 wt% PMF in a 20 wt% nanoemulsion. The lipolysis reaction was stopped by adding 4-bromobenzene boronic acid (4-BBBA), which is a recognized lipase inhibitor.31 Samples (4 mL) were withdrawn at different time points and lipolysis was inhibited with 36 µL of 5 M 4-BBBA. The collected samples were centrifuged at 4000 rpm for 30 min at room temperature (CL10 centrifuge, Thermofisher Scientific, Germany). For determination of non-sedimented PMF, the supernatants were collected, mixed, and diluted 1:10 with DMSO and analyzed by UV-visible spectrometry.
PMF Analysis
The PMF concentration in the samples was measured using a UV/visible spectrometer at 330 nm (Ultra-spec 3000 pro, Biochrom Ltd Cambridge, UK). The samples were contained within quartz cuvettes with a path length of 1.0 cm. Calibration curves were prepared using either DMSO or chloroform as the solvent. 1 wt% PMF stock solution was prepared by dissolving PMF powder in DMSO or chloroform. Samples (n = 12) were then diluted to fit in the range of the linear calibration curve. As chloroform can evaporate quickly, all samples were covered immediately after preparation.
Emulsion Microstructure & Visual Observations
The microstructures of selected nanoemulsions were observed using an optical microscope (Nikon Eclipse E400, Nikon Corp., Japan). Emulsion samples were thoroughly mixed in a glass test tube before analysis, except when images of the sediment were acquired. A drop of nanoemulsion was placed on a microscope slide, and covered by a cover slip. An image of the sample was acquired using digital image processing software (Micro Video Instruments Inc., Avon, MA) and stored on a personal computer. Nanoemulsions were characterized by both bright field and polarized light microscopy. The general appearance of the emulsions was recorded by taking images using a digital camera (PowerShot SD1300IS, Canon, Lake Success, NY, USA).
Statistical Analysis
All measurements were performed in duplicate or triplicate, and the results are plotted as means and standard deviations in the figures, and the results are plotted as means and standard deviations in the figures.
Results and Discussion
In a recent study, we reported that PMF has relatively low solubilities in both water and oil phases.4 PMF therefore tends to precipitate from both aqueous solutions and nanoemulsions even when it is incorporated at low amounts, because these levels exceed the equilibrium solubility concentration. In the current study, we therefore examined the influence of various factors on the solubility and crystallization of PMF in oil phases, aqueous phases, and nanoemulsions under storage and simulated gastrointestinal tract (GIT) conditions.
Solubility of PMF in Oil Phases
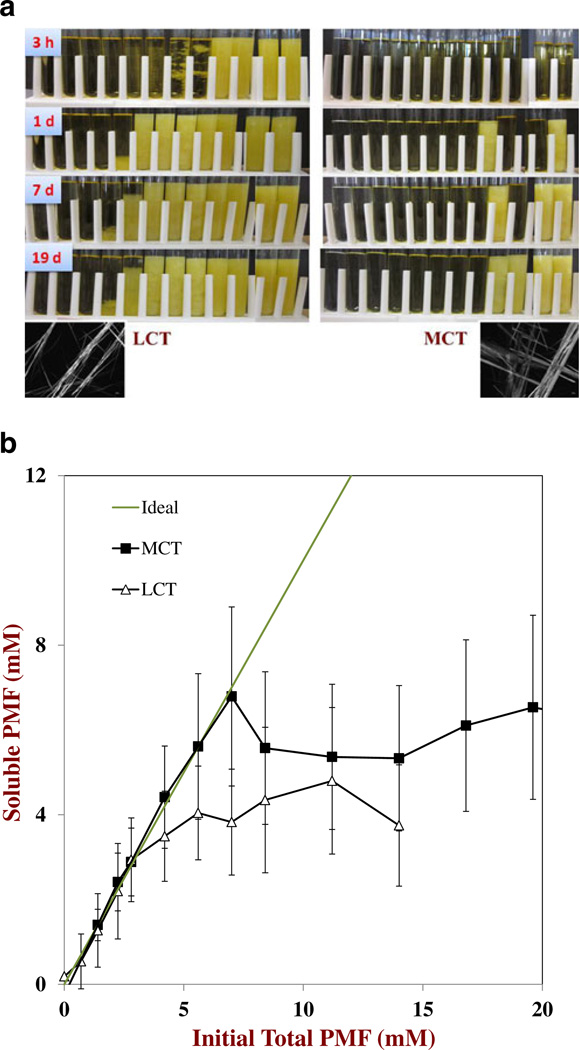
Initially, we examined the influence of oil type on the solubility of PMF in the oil phase used to prepare the nanoemulsions. Different amounts of crystalline PMF were dispersed into carrier oils and then the resultant mixtures were heated to dissolve the PMF. These mixtures were then cooled to ambient temperature to induce supersaturation, and the change in appearance of the samples was monitored over time (Figure 1a). After 3 h storage, we observed that some of the samples containing the highest PMF concentrations became cloudy, whereas the ones with the lower PMF concentrations remained transparent. After formation, crystals either sedimented to the bottom of the tubes (low crystal contents) or remained dispersed throughout the oil (high crystal contents) presumably due to the formation of a three-dimensional network that inhibited crystal movement. Initially, the number of samples that appeared cloudy increased as the incubation time increased indicating that nucleation and crystal growth continued throughout storage. However, after about 48 h the appearance of the samples did not change appreciably, indicating that the crystallization process was complete. Interestingly, crystal formation occurred earlier in some samples with low PMF concentrations than in samples with higher PMF concentrations (e.g., MCT after 1 day), which highlights the stochastic nature of the nucleation/crystallization process. The rate and extent of crystal formation depended on the nature of the carrier oil. For example, after 72 h storage more samples contained PMF crystals when LCT was used than when MCT was used (Figure 1a). The morphology of the crystals formed in the oil phases was determined by optical microscopy (Figure 1a). In both oils, PMF tended to form long needle-like crystals that tended to associate with each other and form clusters. The length of the crystals was over 500 µm, which may account for their ability to form three-dimensional gel networks at sufficiently high concentrations.
Fig. 1.
a. Visual appearance of PMF – oil mixtures during storage after they had been heated to dissolve the crystalline PMF and then cooled to ambient temperature. The mixtures contained increasing amounts of PMF (from left to right: 0, 0.7, 1.4, 2.2, 2.8, 4.2, 5.6, 7.0, 8.4, 11.2, 14.0, and 16.8 mM) dispersed in two different carrier oils (LCT or MCT). Pictures were taken after 3 h, 1 day, 7 days and 19 days storage. Photomicrograph images were taken by optical microscopy, and have dimensions of 514 by 389 mm. b. Influence of carrier oil type on the relationship between soluble PMF remaining in the oil phases after centrifugation and the total amount of PMF initially added to the oil phases. The PMF was dissolved in the oil phases by heating and then the PMF-oil mixtures were cooled to ambient temperature and stored for 72 h, prior to centrifugation to remove any crystals
The amount of soluble PMF in the oil phases after 72 h storage was measured by centrifuging them to remove any crystals, and then analyzing using UV-visible spectrophotometry (Figure 1b). At relatively low concentrations, the soluble PMF concentration increased linearly with the total PMF concentration with a slope close to unity, indicating that all of the added PMF was soluble. This finding was supported by the fact that these mixtures all appeared optically transparent prior to centrifugation. However, once a critical PMF concentration was exceeded the mixtures appeared cloudy before centrifugation, and the amount of soluble PMF decreased less than expected, eventually reaching a relatively constant level (Figure 1b). The amount of soluble PMF measured after prolonged storage times at high initial PMF concentrations was taken to be the saturation concentration in the oil phase (CS,O*). This value was determined for the two carrier oils by taking the average of the four measurements made at the highest PMF concentrations: CS,O*04.2±0.4 mM and 6.1±0.5 mM for LCT and MCT, respectively. In this study, we did not find an appreciable degree of supersaturation of PMF in the oil phases after 72 h storage, i.e., the PMF concentration where the ideal and measured solubility curves deviated (Figure 1b) was fairly close to CS,O* for the two oils. This effect can be attributed to the fact that most of the nucleation and crystal growth had already occurred over this extended time frame (72 h).
Solubility of PMF in Aqueous Phases
Our earlier study showed that PMF has a relatively low water-solubility, which could limit its application in certain aqueous-based food products.32 We therefore examined the influence of adding various water-soluble polymers on the aqueous phase solubility of PMF: polyethylene glycol (PEG); β-cyclodextrin (CD); poly(vinyl alcohol) (PVA); and, hydroxypropyl methylcellulose (HPMC). Studies in the pharmaceutical industry have shown that certain water-soluble polymers are able to inhibit the precipitation of crystalline hydrophobic compounds from aqueous solutions and therefore increase the amount that can be solubilized.8
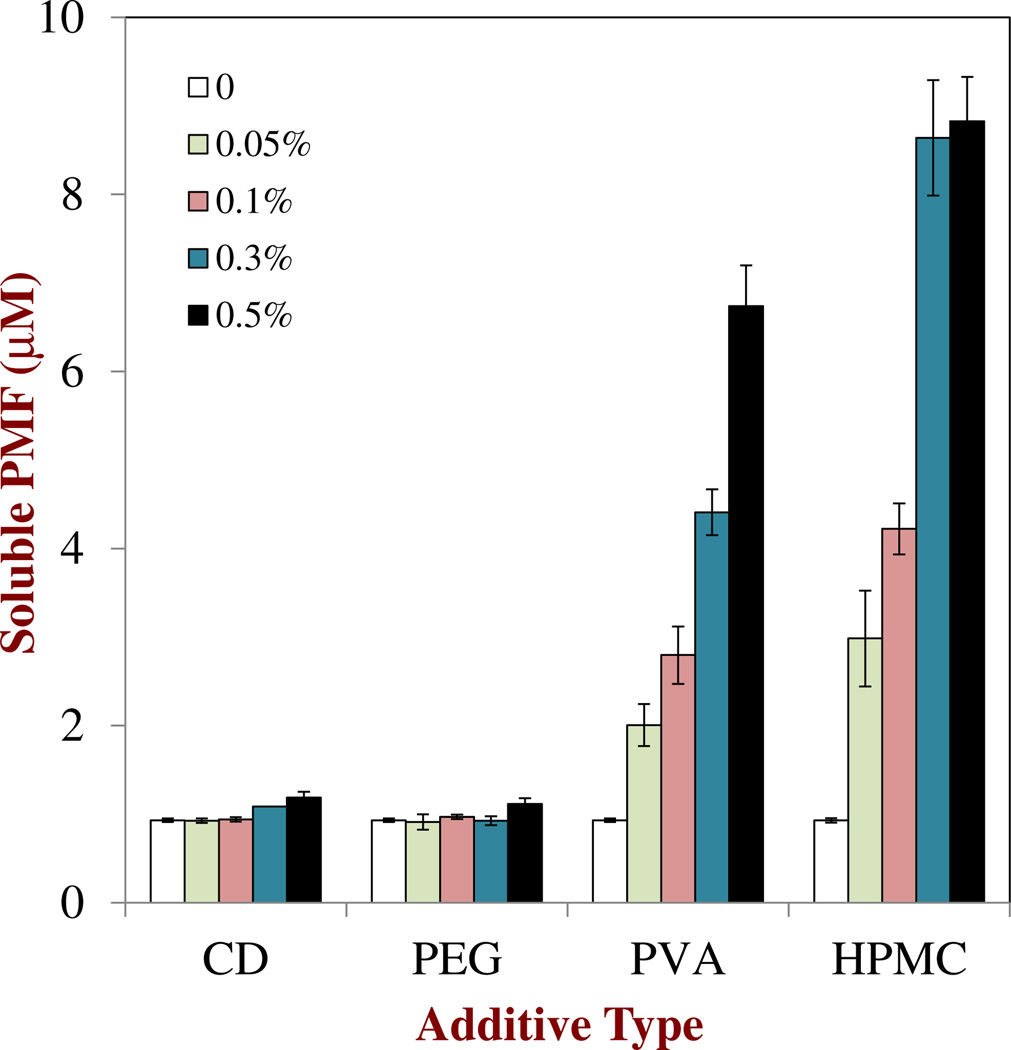
Initially, we examined the influence of polymer type and concentration on the solubility of PMF in aqueous solutions after 72 h storage. This incubation time was selected because an initial study showed that a constant level of water-soluble PMFwas reached in all the samples after about 48 h.PMF was initially dissolved in DMSO and then aliquots of the resulting solution were injected into aqueous buffer solutions containing different types and concentrations of polymer. The initial PMF concentration in the aqueous solutions was 56 µM, which was well above the reported saturation level of polymethoxyflavones in pure water, i.e., ≈ 0.9 µM.4 After storage, some of the samples were observed to contain crystals that sedimented to the bottom of the test tubes. These crystals had similar morphologies (long thin crystals) as those observed in the oil phase by optical microscopy (data not shown). The morphology of the crystals was fairly similar for all types and concentrations of polymers added to the aqueous solutions. The concentration of water-soluble PMF in the samples after 72 h storage was measured after they were centrifuged to remove any crystals formed.
The saturation level of PMF in buffer solution (no polymer) was measured to be ≈ 0.93 µM, which is close to the value reported in our previous study.4 The concentration of PMF remaining soluble in the aqueous solutions after 72 h depended strongly on polymer type and concentration (Figure 2). CD and PEG had little effect on the soluble PMF concentration, indicating that they did not play an important role in inhibiting nucleation and crystal growth. On the other hand, both HPMC and PVA caused an appreciably increase in PMF solubility, with the amount of soluble PMF remaining after 72 h increasing with increasing polymer concentration (Figure 2). These two polymers may therefore be useful for inhibiting the precipitation of PMF in aqueous-based products. It is difficult to say whether the improvement in water-solubility by these two polymers was due to a kinetic or a thermodynamic effect. The presence of these polymers may have created an activation energy to PMF nucleation or crystal growth, which slowed down the rate of crystal precipitation. On the other hand, the polymers may have formed hydrophobic structures (analogous to the interiors of surfactant micelles) that solubilized non-polar PMF and thereby increased its equilibrium saturation concentration. A number of studies using hydrophobic drugs have reported that the addition of HPMC can inhibit their precipitation in aqueous solutions.19, 33–35 Recent molecular dynamics simulations suggest that certain water-soluble polymers (including HPMC) can inhibit crystal precipitation due to their ability to bind to crystal surfaces and prevent solute molecules from attaching.36 PVA has been shown to be useful in controlling the nucleation and crystallization of ice.37, 38 Overall, these results suggest that almost a 10-fold increase in PMF concentration in an aqueous phase is possible by adding certain types and amounts of water-soluble polymer, which may be important for the development of aqueous-based functional food or beverage products that contain little or no oil.
Fig. 2.
Influence of water soluble type and concentration on the amount of soluble PMF remaining in the aqueous phases after centrifugation. Samples were prepared by injecting a 1 % PMF in DMSO solution into aqueous polymer solutions (final PMF concentration056 µM), storing them for 72 h, and then centrifuging to remove any crystals
Encapsulation Efficiency of PMF-loaded Nanoemulsions
The research reported in the previous sections indicates that PMF ismuchmore soluble in an oil phase (≈ 4 – 6mM) than in an aqueous phase (≈ 1 – 10 µM). It may therefore be more practical to incorporate highly hydrophobic bioactive components into foods and beverages in the form of emulsions or nanoemulsions.39–41 For this reason, we examined the factors influencing the solubility and crystallization of PMF in O/W nanoemulsions.
Initially, we estimated the maximum amount of PMF that could theoretically be solubilized within a nanoemulsionbased delivery system at saturation. At equilibrium, PMF will be distributed between the oil and water phases according to its oil-water partition coefficient (KOW). The maximum amount of a solute that can be dissolved in an oil-water system at equilibrium can be calculated from the following expression:32
| (1) |
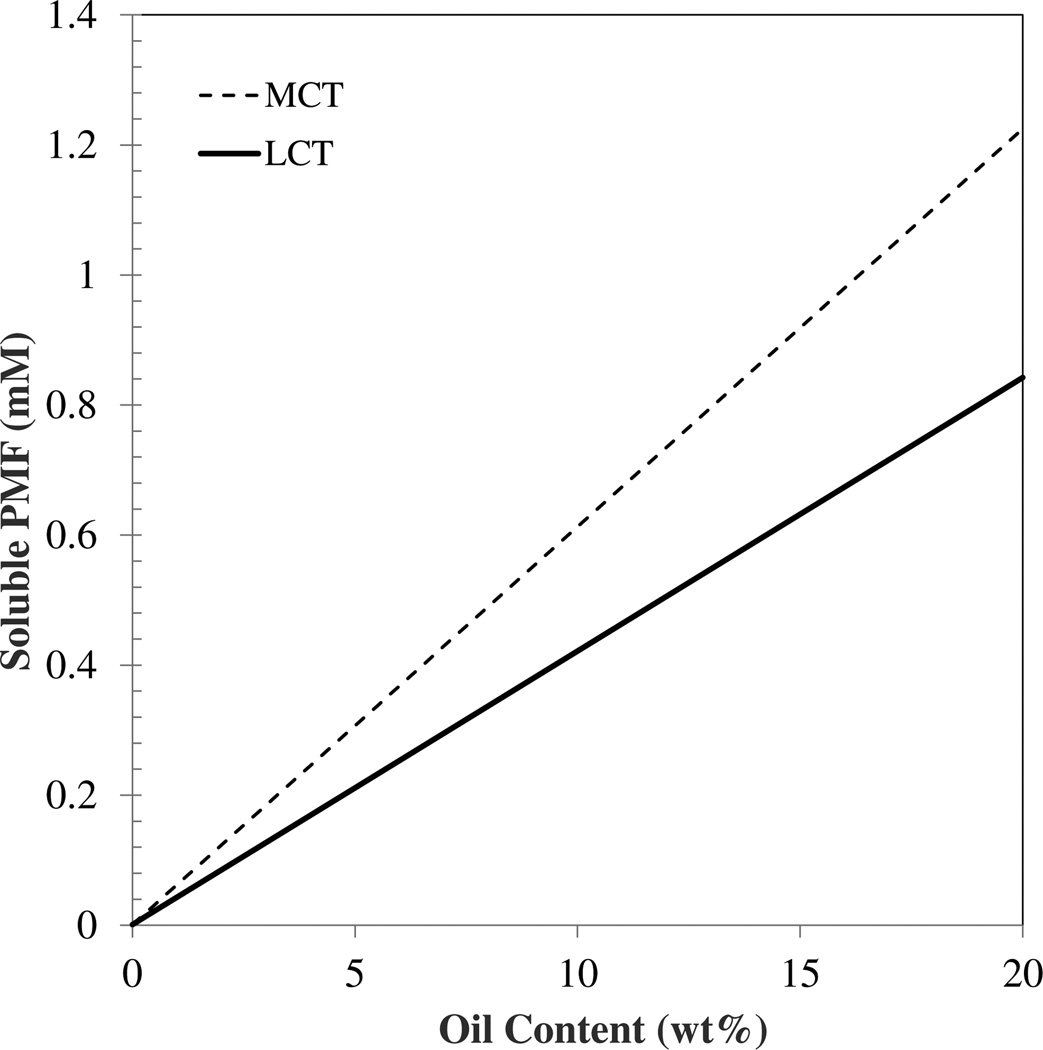
Here, CSO* (= MSO* / [MSO* + MO]) and CSW* (= MSW* / [MSW* + MW]) are the equilibrium saturation concentrations of the solute in the oil and water phases (expressed as mass fractions), ΦO is the mass fraction of oil in the overall system (= MO / [MS + MW + MO]), and KOW is the oil-water partition coefficient. MSO and MSW are the masses of the solute dissolved in the oil and water phases, while MS, MO and MW refer to the masses of the solute, oil and water, respectively. The superscript “*” refers to saturation conditions. In our study, the saturation concentration of PMF in the aqueous phase (CWO*) was determined to be 0.33 µg/mL (0.93 µM) for pure buffer solutions, while the saturation concentration of PMF in the different oils (CSO*) was around 1.55 and 2.2 mg/mL (4.2 and 6.1 mM) for LCT and MCT, respectively. These values were then used to estimate the KOW (=CSO* / CSW*) for the oil-water system used in this study to be about 4,520 and 6,560 (i.e. logP=3.65 and 3.82). The total amount of PMF that could be solubilized in the nanoemulsions at equilibrium was then estimated as a function of oil content using Eq. 1. The amount of PMF that could be solubilized within the nanoemulsions at saturation was higher for MCT than LCT at all oil contents (Figure 3). These calculations also indicated that the amount of PMF solubilized within the nanoemulsions at saturation should increase almost linearly as the total fat content increases because PMF is much more soluble in oil than in water.
Fig. 3.
Theoretical prediction of the maximum amount of PMF that can be fully dissolved in an oil-in-water nanoemulsion at saturation. The predictions were made using Eq. 1 and the experimentally measured equilibrium saturation concentrations of PMF in oils and buffer solution
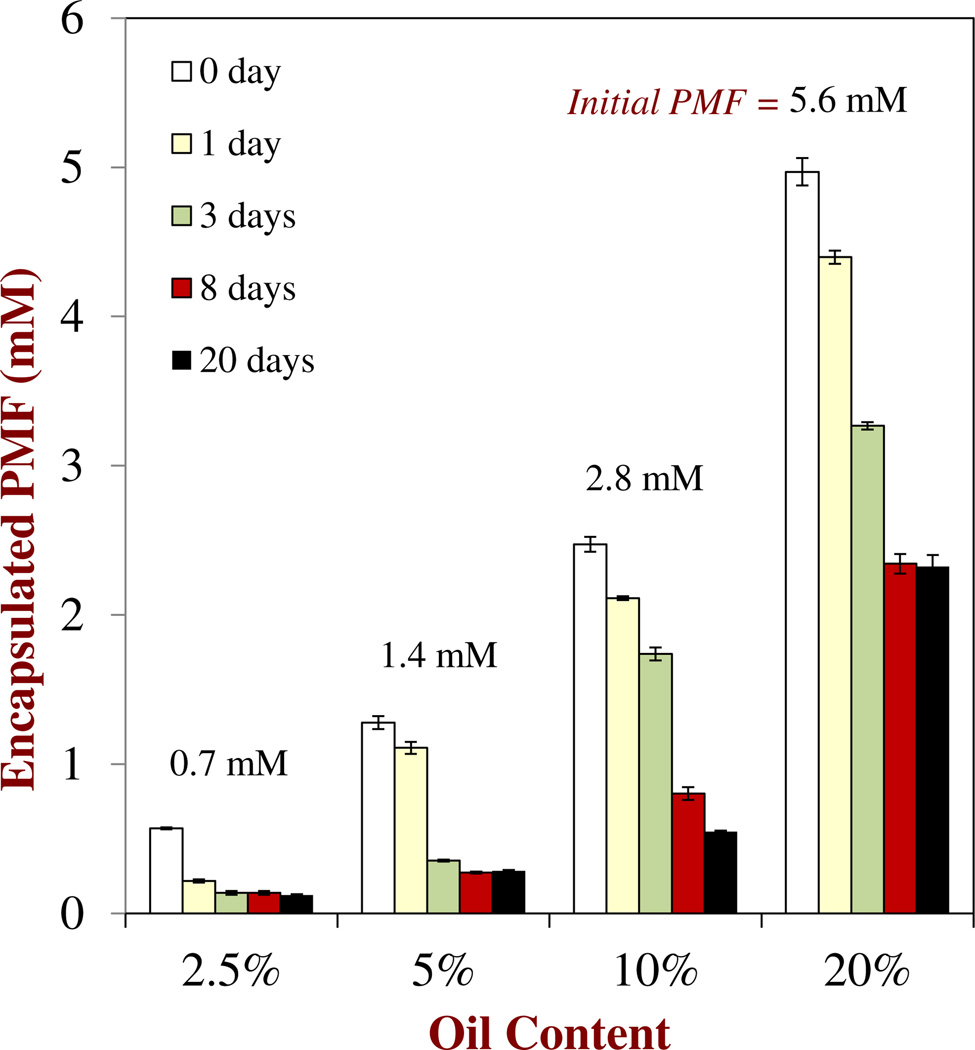
Experiments were carried out to determine the concentration of soluble PMF in oil-in-water nanoemulsions. A series of nanoemulsions containing different amounts of oil (2.5 to 20 % MCT) but similar PMF-to-oil ratios (1:100) were prepared, stored for different times, and then analyzed to determine the amount of encapsulated PMF remaining. The initial PMF concentration in these nanoemulsions therefore depended on the fat content: 0.56, 1.4, 2.8 and 5.6 mM PMF for 2.5, 5, 10 and 20 % fat, respectively. The encapsulated PMF content was defined as the amount which remained dispersed throughout the bulk of the nanoemulsion (rather than sedimented). The influence of oil content and storage time on the amount of encapsulated PMF present in the nanoemulsions was determined (Figure 4). Soon after they were prepared (≈ 3 h) all of the nanoemulsions contained encapsulated PMF levels that were slightly lower than the amount of PMF initially incorporated into the system, which suggests that some crystallization and sedimentation had already occurred. During storage the amount of encapsulated PMF remaining in the nanoemulsions decreased, which indicated that PMF crystals had formed and sedimented to the bottom of the samples. This was confirmed through visual observations of the nanoemulsions, which indicated that a small yellow precipitate formed at the bottomof the test tubes. At the end of 20 days storage, the amount of encapsulated PMF in the nanoemulsions was ≈ 0.12, 0.29, 0.55 and 2.33 mM for 2.5, 5, 10 and 20 % fat, respectively (Figure 4), which correspond to encapsulation efficiencies of 21 %, 21 %, 20 % and 42 %, respectively. The values for the amount of soluble PMF at saturation predicted using Eq. 1 for similar fat contents were 0.15, 0.31, 0.61 and 1.22 mMfor 2.5, 5, 10 and 20 % fat, respectively (Figure 3). Thus, it seems that the amount of encapsulated PMF is fairly similar to the saturation level for nanoemulsions containing low oil contents (2.5, 5 and 10 %), but that the amount of encapsulated PMF was somewhat higher than expected for the nanoemulsions with the highest oil content (20 %). The reason that the measured encapsulated PMF level was higher than the predicted value may have been an experimental artifact associated with the settling of PMF crystals. The nanoemulsions with the highest oil content also had the highest PMF concentration, which may have led to the formation of a three-dimensional network of aggregated PMF crystals in the aqueous phase that inhibited their downward movement. Similarly, the viscosity of a nanoemulsion increases as the droplet concentration increases 42, which may also have slowed down any settling of the PMF crystals at high oil contents. Consequently, the encapsulated PMF measurement may have contained contributions from PMF crystals that remained dispersed within the upper part of the nanoemulsions, as well as from soluble PMF. Indeed, in our previous study of nanoemulsions containing PMF crystals we did find some crystals dispersed throughout the system using optical microscopy.4
Fig. 4.
Influence of oil content and initial PMF concentration on the amount of encapsulated PMF remaining in oil-in-water nanoemulsions after 72 h storage. The initial PMF-to-fat content ratio was kept constant (1:100) in all systems
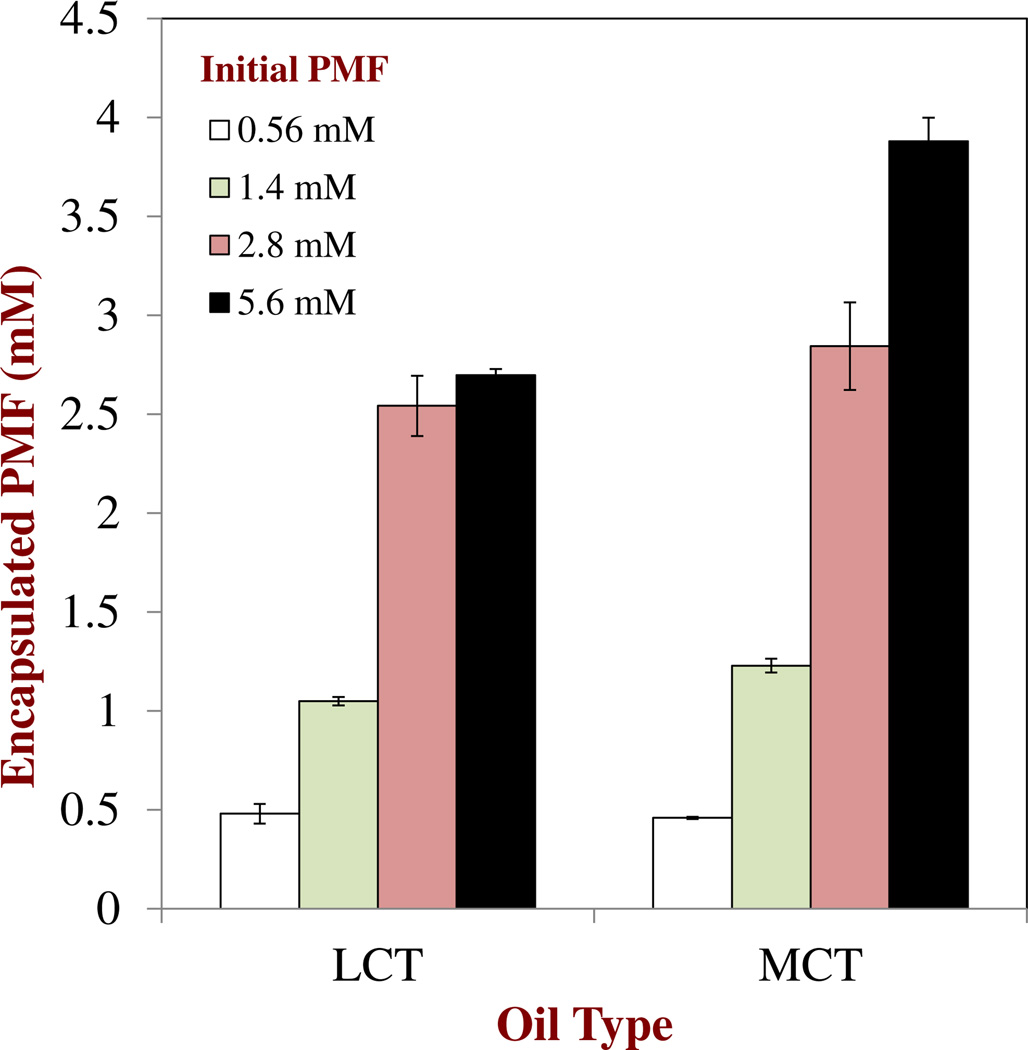
In another experiment, we kept the total oil concentration in the nanoemulsions constant at 20 wt%, but varied the oil type (LCT versus MCT) and the initial amount of PMF present (0.56, 1.4, 2.8 or 5.6 mM). The amount of soluble PMF was then measured after they were stored at ambient temperature for 72 h (Figure 5). In this case the saturation concentration of PMF in the 20 wt% oil-in-water nanoemulsions calculated using Eq. 1 was 1.22 and 0.84 mM for MCT and LCT, respectively. The experimental results indicated that the amount of soluble PMF present in the nanoemulsions was lower than the initial amount added, but higher than the value predicted by Eq. 1 assuming saturation. These results suggest that some of the PMF had crystallized and sedimented to the bottom of the nanoemulsions, but that the remainder was either solubilized (due to supersaturation) or dispersed as crystals (due to inhibition of crystal sedimentation) within the nanoemulsions. Slightly more PMF could be incorporated into the MCT nanoemulsions than into the LCT nanoemulsions (Figure 5), which can be attributed to the higher saturation concentration of PMF in MCT than in LCT (Figure 1b). The encapsulation efficiency of the nanoemulsions was calculated as the percentage of soluble PMF measured over the initial PMF added. For the nanoemulsions containing relatively low initial amounts of PMF (0.56, 1.4, and 2.8 mM) the encapsulation efficiency was relatively high (75 to 100 %), which can be attributed to the fact that most of the PMF remained soluble. On the other hand, for the nanoemulsions containing the highest initial PMF content (5.6 mM) the encapsulation efficiencies were around 48 % and 69 % for the LCT and MCT oils, respectively. This suggests that a greater fraction of the PMF added to these systems had formed crystals that sedimented to the bottom of the samples during storage. This can be attributed to the fact that the degree of PMF supersaturation in the nanoemulsions increased as the total amount of PMF added was increased or the total amount of oil present decreased.
Fig. 5.
Influence of oil type and initial PMF concentration on the amount of encapsulated PMF remaining in oil-in-water nanoemulsions after 72 h storage. The initial PMF-to-fat content ratio was kept constant (1:100) in all systems
We also used Eq. 1 to predict the influence of a water-soluble polymer (0.5 % HPMC) on the amount of PMF solubilized in MCT nanoemulsions. This polymer was selected because it was found to be the most effective at increasing the solubility of PMF in aqueous solutions (previous section). We found that increasing the solubility of PMF in the water phase using HPMC had little influence on the predicted values of the PMF saturation concentration in the nanoemulsions (data not shown), which can be attributed to the fact that the oil-solubility is so much higher than the water-solubility even in the presence of polymer. For example, the equilibrium solubility of PMF in MCT is around 6 mM (Figure 1), but its value in a 0.5 % HPMC aqueous solution is less than 0.01 mM (Figure 2).
Overall, the theoretical predictions and experimental results indicate that the amount of PMF that can be incorporated into a nanoemulsion increases as the oil content increases, and is higher for MCT than LCT. To obtain relatively high encapsulation efficiencies it is important to ensure that the initial concentration of the bioactive does not exceed the saturation level for the system by too much. Addition of water-soluble polymers to the aqueous phase of the nanoemulsions would be expected to have little influence on the overall amount of PMF solubilized in the system. This means that polymer addition may not be a useful method of inhibiting crystal formation during the long-term storage of nanoemulsions. However, polymer addition may still prove useful for inhibiting PMF precipitation during the relatively short time (a few hours) that a nanoemulsion remains within the upper gastrointestinal tract, which will be investigated in the following section.
Solubility of PMF in Simulated Small Intestinal Fluids
An effective nanoemulsion-based delivery system should retain an encapsulated bioactive component during storage, but then release it at an appropriate site within the GIT after ingestion.43 The bioavailability of hydrophobic bioactive components within the GIT tends to be higher when they are in a soluble form, than when they are in an insoluble (crystalline) form.8 It is therefore important to understand the influence of GIT conditions on the potential solubilization and crystallization of bioactive components once they are released from a delivery system into the aqueous environment of the intestinal juices. For this reason we examined the influence of simulated small intestinal conditions on the solubility behavior of PMF in aqueous solutions. In particular, we examined whether addition of water-soluble polymers (HPMC or PVA) could be used to improve the solubility of PMF in GIT conditions.
Initially, we carried out an experiment to determine the influence of simulated small intestinal fluids (SSIF) on PMF solubility. Both a fed-state SSIF and a fasted-state SSIF were tested to establish the potential influence of media composition on PMF solubility. These fluids differ primarily in the amounts of lipase and bile salts they contain (Table 1), with the fed-state SSIF having higher values than the fasted-state SSIF. PMF was dissolved in DMSO and the resulting solution was then mixed with SSIF media or buffer solution. In some experiments, we included water-soluble polymer in the SSIF (0.05 % PVA or HPMC). The samples were then stored for 72 h at 37 °C with constant stirring, and the concentration of soluble PMF was measured after centrifugation as described earlier.
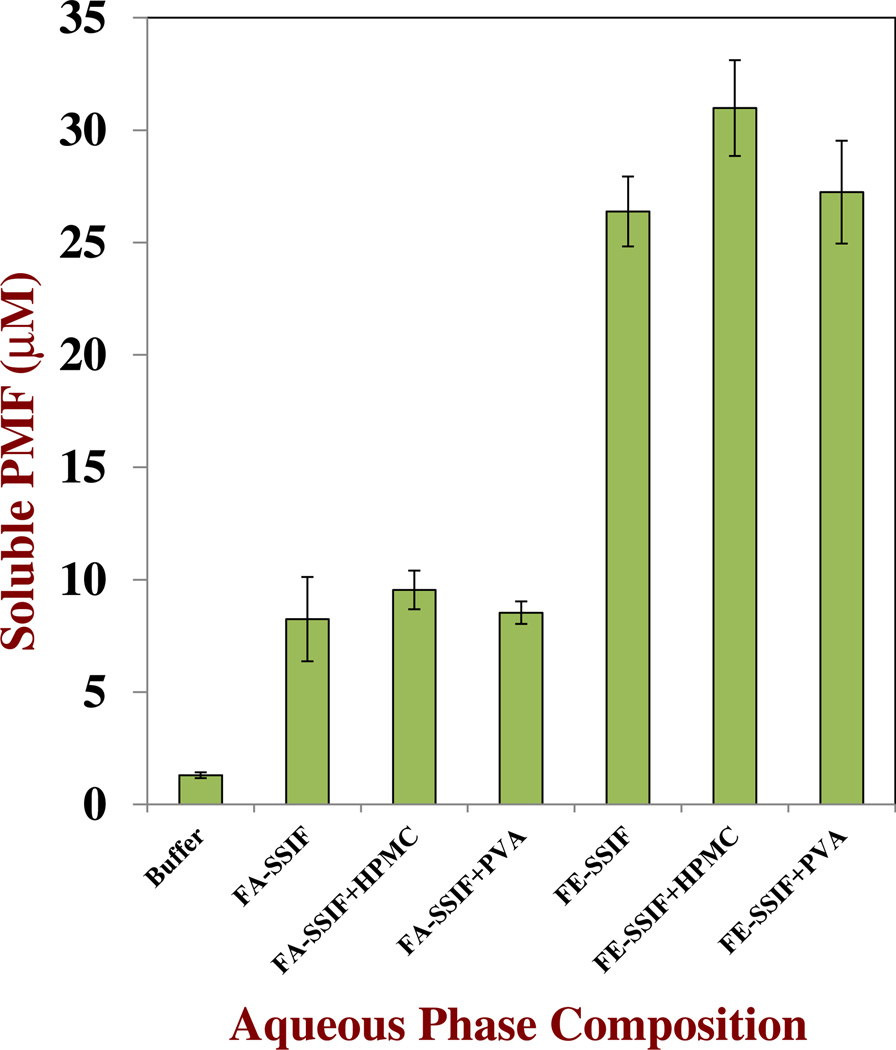
The soluble PMF concentration was considerably higher in both SSIFs than in pure buffer solution, which can be attributed to the influence of bile salts on the solubilization of hydrophobic components in aqueous media (Figure 6). Bile salts form micelle-like structures with hydrophobic interiors capable of incorporating non-polar substances.44 As expected, the amount of soluble PMF in the fed-state SSIF was appreciably higher (26 µM) than in the fasted-state SSIF (8 µM) since the former had a higher concentration of bile salts capable of solubilizing PMF. Interestingly, the addition of water-soluble polymers had little influence on the solubility characteristics of the PMF in the SSIFs, which may have been because some of the components within them prevented the polymer from interacting with the PMF. The incorporation of water-soluble polymers into a nanoemulsion would therefore seem to be ineffective at improving the encapsulation of PMF during storage or within the GIT. For this reason, we did not use water-soluble polymers in the remainder of the study.
Fig. 6.
Influence of composition on the amount of soluble PMF remaining in aqueous solutions after 72 h storage at 37 °C with stirring. The aqueous phase compositions consisted of buffer, fasted-SSIF and fed-SSIF in the presence and absence of water-soluble polymers (0.05 % PVA or HPMC): Fe-SSIF015 mg/mL bile, 2.4 mg/mL lipase, 10 mM CaCl2, 150 mM NaCl; Fa-SSIF05 mg/mL bile, 0.6 mg/mL lipase, 5 mM CaCl2, 100 mM NaCl
Behavior of PMF Nanoemulsions Under Simulated Digestion Conditions
Finally, we examined the behavior of PMF encapsulated within nanoemulsion-based delivery systems under simulated digestion conditions. An oil-in-water nanoemulsion (20 wt% MCT, 2 wt% Tween 20, 0.1 % (2.8 mM) PMF, d=210±30 nm) was freshly prepared, and then added to a SSIF containing lipase.We would have expected the PMF in this system to initially be fully soluble based on our preliminary studies of the amount of encapsulated PMF remaining in 20 % MCT nanoemulsions containing 2.8 mM PMF after prolonged storage (Figure 5). The enzymatic digestion of the triacylglycerols in the nanoemulsion was monitored using a pH-stat method that involves measuring the concentration of base (NaOH) required to neutralize the free fatty acids produced.45 It was assumed that each triacylglycerol (TAG) molecule was hydrolyzed into two free fatty acids (FFA) and one monoacylglycerol (MAG) molecule due to the action of pancreatic lipase.46 After the digestion step was completed, the samples were centrifuged and the supernatant and pellet were collected to determine the PMF concentration in them. After centrifugation we did not observe a layer of oil on top of the samples, which suggested that all of the triacylglycerols had been digested. Bioactive components solubilized within the micelle phase are expected to be bioavailable, whereas those remaining as crystals in the pellet are not.16 In this study, “bioavailability” was defined as the percentage of PMF in the micelle phase divided by the total amount of PMF present in the nanoemulsion. In practice, it should be recognized that the in vitro digestion model can only give a rough estimate of the true bioavailability of a substance within the complex environment of the GIT, and that in vitro – in vivo correlations are required to ensure that the value measured in the digestion model is reliable.
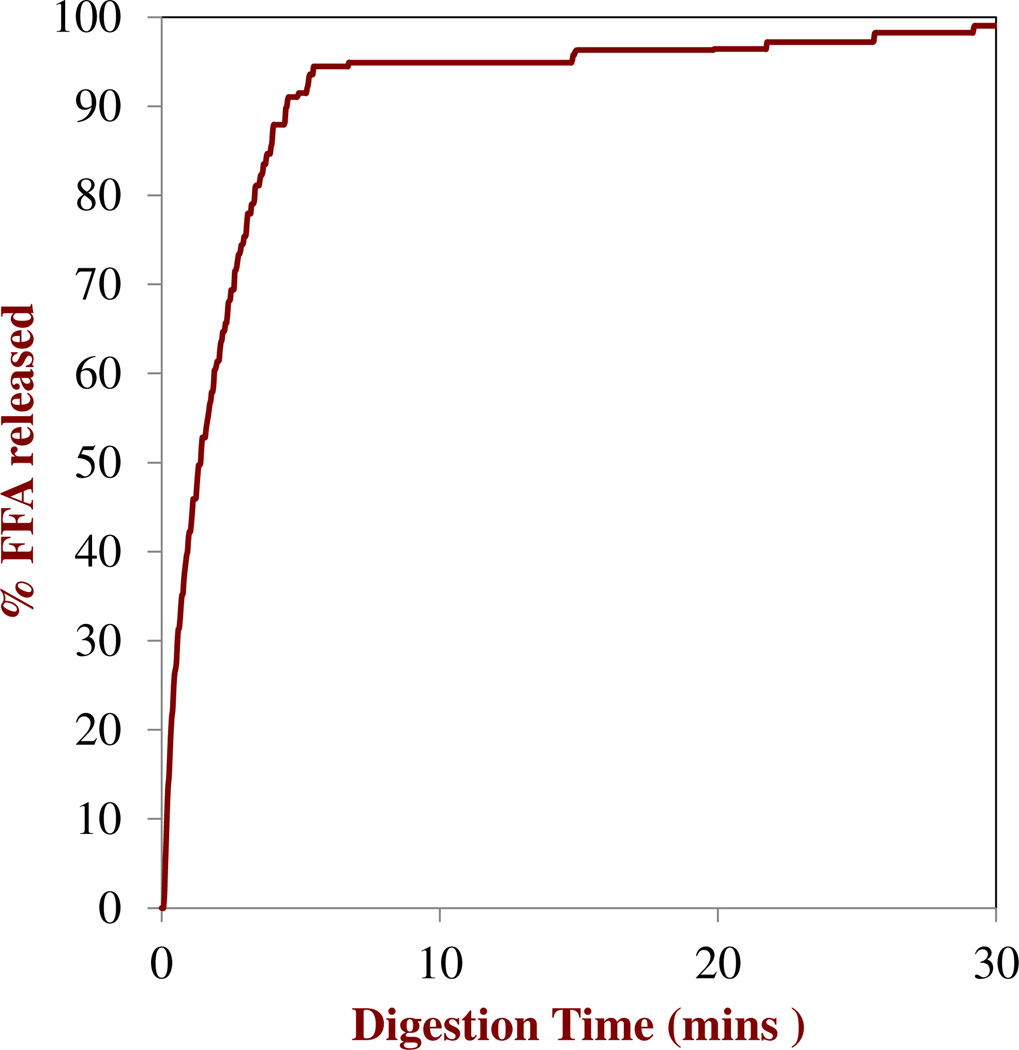
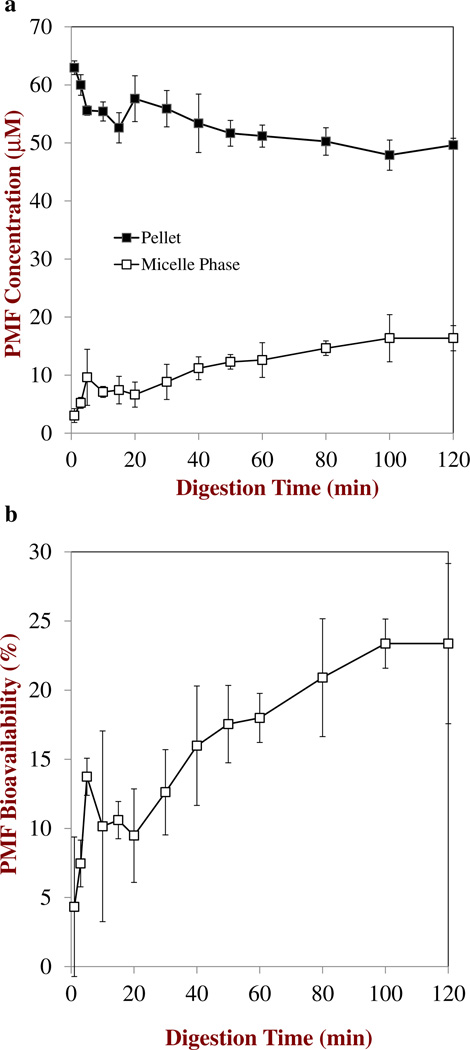
The digestion profiles of the nanoemulsions determined by the pH stat method are shown in Figure 7. The amount of FFA released increased rapidly during the first few minutes and then reached a relatively constant level close to 100 % at longer times, suggesting that most of the triacylglycerols were rapidly and fully digested. The concentration of PMF in the micelle phase increased from around 3 µM after 3 min to around 16 µM after 120 min (Figure 8a),which resulted in a corresponding increase in the PMF bioavailability during digestion (Figure 8b). Interestingly, the amount of soluble PMF in the micelle phase after digestion (≈ 16 µM) was appreciably higher than the amount of soluble PMF in the Fa-SSIF (≈ 8 µM) measured in the absence of oil (Figure 6). Previous studies have shown that MAGs and FFAs produced by TAG digestion process combine with bile salts and phospholipids to form mixed micelles and other colloidal structures, which leads to an increase in the solubilization capacity of the intestinal lumen for hydrophobic compounds.47, 48 The increase in bioavailability during digestion may therefore have been due to an increase in the number of mixed micelles available to solubilize the PMF released from the nanoemulsions. Nevertheless, the majority of PMF was still present in the pellet phase at the end of 2 h digestion (Fig. 8a), suggesting that it was in a crystalline state that would be unavailable for absorption. Indeed, optical microscopy images of the digesta collected at the end of the digestion process showed that they contained PMF crystals (Fig. 9). These results suggest that the bioavailability of PMF may be limited due to precipitation within the aqueous fluids in the simulated GIT. Even though the PMF may be fully dissolved within the initial oil-in-water nanoemulsion used as a delivery system, a large proportion of it precipitates after the digestion process. It may be possible to further increase the oral bioavailability of PMF by carefully designing the delivery system used, e.g., by increasing the type or amount of carrier oil present. Previous studies have shown that long chain triacylglycerols (LCT)may have higher solubilization capacities than medium chain triacylglycerols (MCT). In addition, studies have shown that increasing the amount of fat consumed with a hydrophobic component increases its oral bioavailability since this increases the amount of mixed micelles formed during digestion.49, 50
Fig. 7.
Measured percentage of free fatty acids released (assuming 2 FFAs produced per TAG) from nanoemulsions in a simulated small intestinal fluid: Fa-SSIF05 mg/mL bile, 0.8 mg/mL lipase, 5 mM CaCl2, 100 mM NaCl.
Fig. 8.
a. Change in PMF concentration in the micelle phase and pellet collected after centrifuging MCT nanoemulsions exposed to simulated small intestinal conditions (Fa-SSIF). b. Bioavailability of PMF from MCT nanoemulsions after exposure to simulated small intestinal conditions (Fa-SSIF). The bioavailability is defined as the amount of PMF present in the micelle phase divided by the total amount in the system×100
Fig. 9.
Optical microscopy images of material collected after digestion of MCT nanoemulsions initially containing 5.6 mM PMF. Images were taken with crossed-polarizers to highlight crystal formation within the whole sample after mild shaking. Photomicrograph images have dimensions of 553 by 418 µm
It should be noted that water-soluble polymers will also alter the rheological properties of the aqueous fluids within the GIT, which would be expected to influence various mass transfer processes that may impact the rate and extent of absorption of lipophilic components. For example, a change in the viscosity of the intestinal fluids would alter the diffusion of digestive enzymes, as well as the movement of digestion products from the oil droplets, into mixed micelles, and then into epithelium cells. This phenomenon was not studied in the current work, but would be interesting to investigate in future studies.
Potential Use of PMF in Delivery Systems
It is important that any food-grade delivery system is capable of incorporating sufficient levels of a bioactive component to have a biological effect. Animal (rat) feeding studies have shown that consumption of from 2 to 9 mg of a PMF (nobiletin) per day (in a 71 kcal per day diet) was sufficient to have a significant anti-carcinogenic effect.51 For humans, this value translates to about 50–250 mg PMF per day (based on a 2000 kcal per day diet). Typically, food manufacturers aim to fortify foods with about 10 % of the daily recommended amount of a bioactive compound. Thus, a functional food or beverage product should contain about 5–25 mg of PMF per serving. Our study suggests that about 1–2 mM of PMF could be incorporated into a 20 wt% oil-in-water nanoemulsion or emulsion without observing crystal formation, which corresponds to about 36–mg PMF per 100 g. These levels are therefore in the range expected to provide biological activity. In practice, many food products contain a lower fat content than 20 % and so the amount of PMF delivered would be somewhat less.
In contrast, if PMF was solubilized within an aqueous solution that contained no oil, then it would be difficult to reach the levels required for biological activity even in the presence of water-soluble polymers. For example, it was only possible to solubilize about 10 µM of PMF in an aqueous phase containing 0.5 % HPMC (Figure 2), which corresponds to about 0.36 mg per 100 g of solution. It would therefore be necessary to consume nearly 14 kg of a food or beverage to reach a biologically active level.
Conclusions
The purpose of this study was to elucidate the main factors influencing the dissolution of a crystalline hydrophobic bioactive component (PMF) in nanoemulsion-based delivery systems under storage and gastrointestinal conditions. A study of the solubility of PMF in oil phases showed that it had a higher saturation concentration when it was dispersed within MCT oil (6.1 mM) than in LCT (4.2 mM). PMF remained fully soluble in the oils below these levels, but progressively formed needle-like crystals that either sedimented (low crystal concentrations) or created a three-dimensional network (high crystal concentrations) above these levels. A study of PMF solubility in aqueous solutions showed that its saturation concentration increased almost 10-fold by adding certain water-soluble polymers. In particular, HPMC and PVA were able to increase the water-solubility of PMF appreciably, whereas CD and PEG were not. HPMC and PVA may have increased the water-solubility of PMF through a thermodynamic effect (e.g., solubilization) or a kinetic effect (e.g., inhibiting crystal nucleation and growth), but further studies are required to elucidate the physicochemical mechanism involved.
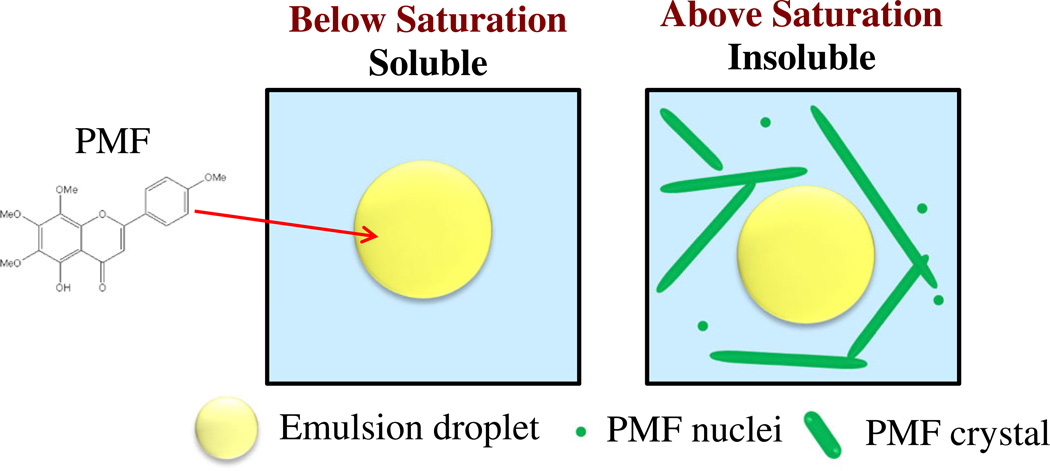
In nanoemulsions, PMF was soluble and mainly present within the oil phase below its saturation level, but formed crystals above at higher levels which were mainly distributed throughout the aqueous phase (Figure 10). The solubility of PMF in nanoemulsions increased with increasing oil content, which can be attributed to the fact that the equilibrium saturation concentration of PMF is much higher in oil than in water. PMF encapsulation in MCT nanoemulsions was slightly higher than in LCT nanoemulsions, due to the higher oil-water partition coefficient of PMF in MCT than in LCT. At greater PMF and oil levels the amount of encapsulated PMF was found to be higher than that expected from equilibrium saturation predictions, which may have been due to the formation of a crystal network that inhibited PMF sedimentation. The aqueous solubility of PMF was found to increase in the presence of simulated small intestinal fluids, which was attributed to solubilization within bile salt micelles or mixed micelles formed during triacylglycerol digestion. This information has important consequences for the design and fabrication of oral delivery systems for PMF, since soluble bioactive components are believed to be more bioavailable than insoluble ones.
Fig. 10.
Schematic representation of PMF distribution in nanoemulsions: soluble below saturation; insoluble above saturation.
Acknowledgments
This work was supported in part by an EPANSF-NIFA (AFRI) joint grant (2010-05266) program, NIH grant CA139174, a USDA-AFRI grant, a special call grant from Massachusetts Center for Agriculture, and a CVIP grant from the University of Massachusetts Amherst.
Footnotes
The authors have no declared conflict of interest.
References
- 1.Brouwers J, Brewster ME, Augustijns P. J. Pharm. Sci. 2009;98(8):2549–2572. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]
- 2.Bevernage J, Brouwers J, Clarysse S, et al. J. Pharm. Sci. 2010;99(11):4525–4534. doi: 10.1002/jps.22154. [DOI] [PubMed] [Google Scholar]
- 3.Kleberg K, Jacobsen J, Mullertz A. J. Pharm, Pharmacol. 2010;62(11):1656–1668. doi: 10.1111/j.2042-7158.2010.01023.x. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Zheng JK, Xiao H, McClements DJ. Food Hydrocolloids. 2012;27(2):517–528. doi: 10.1016/j.foodhyd.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Int. J. Pharm. 2011;420(1):1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Wang JM, Hou TJ. Comb. Chem. High. Throughput. Screen. 2011;14(5):328–338. doi: 10.2174/138620711795508331. [DOI] [PubMed] [Google Scholar]
- 7.Brewster ME, Loftsson T. Adv. Drug. Deliv. Rev. 2007;59(7):645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Warren DB, Benameur H, Porter CJH, Pouton CW. J. Drug. Target. 2010;18(10):704–731. doi: 10.3109/1061186X.2010.525652. [DOI] [PubMed] [Google Scholar]
- 9.Muller RH, Keck CM. J. Biotechnol. 2004;113(1–3):151–170. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Hauss DJ. Adv. Drug. Deliv. Rev. 2007;59(7):667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ziani K, Fang Y, McClements DJ. Food. Chem. 2012;134(2):1106–1112. doi: 10.1016/j.foodchem.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Miller JM, Dahan A. Int. J. Pharm. 2012;430(1–2):388–391. doi: 10.1016/j.ijpharm.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Bevernage J, Forier T, Brouwers J, Tack J, Annaert P, Augustijns P. Mol. Pharm. 2011;8(2):564–570. doi: 10.1021/mp100377m. [DOI] [PubMed] [Google Scholar]
- 14.Tian Y, Mao SR. Expert. Opin. Drug. Deliv. 2012;9(6):687–700. doi: 10.1517/17425247.2012.681299. [DOI] [PubMed] [Google Scholar]
- 15.Alhnan MA, Murdan S, Basit AW. Int. J. Pharm. 2011;416(1):55–60. doi: 10.1016/j.ijpharm.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 16.Dahan A, Hoffman A. Pharm. Res. 2006;23(9):2165–2174. doi: 10.1007/s11095-006-9054-x. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SA, Reynolds TD, Dasbach TP. Int. J. Pharm. 2003;250(1):3–11. doi: 10.1016/s0378-5173(02)00293-4. [DOI] [PubMed] [Google Scholar]
- 18.Tajarobi F, Larsson A, Matic H, Abrahmsén-Alami S. Eur. J. Pharm. Biopharm. 2011;78(1):125–133. doi: 10.1016/j.ejpb.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Alonzo DE, Raina S, Zhou D, Gao Y, Zhang GGZ, Taylor LS. Cryst. Growth. Des. 2012;12(3):1538–1547. [Google Scholar]
- 20.Li S, Lo C-Y, Ho C-T. J. Agric, Food Chem. 2006;54(12):4176–4185. doi: 10.1021/jf060234n. [DOI] [PubMed] [Google Scholar]
- 21.Hirata T, Fujii M, Akita K, et al. Bioorg. Med. Chem. 2009;17(1):25–28. doi: 10.1016/j.bmc.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Xiao H, Yang CS, Li S, Jin H, Ho C-T, Patel T. Mol. Nutr. Food. Res. 2009;53(3):398–406. doi: 10.1002/mnfr.200800057. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Pan M-H, Lo C-Y, et al. J. Funct. Foods. 2009;1(1):2–12. [Google Scholar]
- 24.Lai C-S, Li S, Chai C-Y, et al. Carcinogenesis. 2008;29(12):2415–2424. doi: 10.1093/carcin/bgn222. [DOI] [PubMed] [Google Scholar]
- 25.Lai C-S, Li S, Chai C-Y, et al. Carcinogenesis. 2007;28(12):2581–2588. doi: 10.1093/carcin/bgm231. [DOI] [PubMed] [Google Scholar]
- 26.Sergeev IN, Ho C-T, Li S, Colby J, Dushenkov S. Mol. Nutr. Food. Res. 2007;51(12):1478–1484. doi: 10.1002/mnfr.200700136. [DOI] [PubMed] [Google Scholar]
- 27.Sergeev IN, Li S, Colby J, Ho C-T, Dushenkov S. Life Sci. 2006;80(3):245–253. doi: 10.1016/j.lfs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita T, Firman K. Phytochemistry. 1996;42(4):1207–1210. [Google Scholar]
- 29.Dong P, Qiu P, Zhu Y, et al. J. Chromatogr. A. 2010;1217(5):642–647. doi: 10.1016/j.chroma.2009.11.097. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Hu M, McClements DJ. Food Chem. 2011;126(2):498–505. [Google Scholar]
- 31.Sassene PJ, Knopp MM, Hesselkilde JZ, et al. J. Pharm. Sci. 2010;99(12):4982–4991. doi: 10.1002/jps.22226. [DOI] [PubMed] [Google Scholar]
- 32.McClements DJ. Adv. Coll. Int. Sci. 2012;174:1–30. doi: 10.1016/j.cis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Gao P, Rush BD, Pfund WP, et al. J. Pharm. Sci. 2003;92(12):2386–2398. doi: 10.1002/jps.10511. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Pollock-Dove C, Dong LC, Chen J, Creasey AA. W.G. Dai, Mol. Pharm. 2012;9(5):1100–1108. doi: 10.1021/mp200352q. [DOI] [PubMed] [Google Scholar]
- 35.Tajarobi F, Larsson A, Matic H, Abrahmsen-Alami S. Eur. J. Pharm. Biopharm. 2011;78(1):125–133. doi: 10.1016/j.ejpb.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Yin YN, Chow PS, Tan RBH. Mol. Pharm. 2011;8(5):1910–1918. doi: 10.1021/mp200277u. [DOI] [PubMed] [Google Scholar]
- 37.Budke C, Koop T. ChemPhysChem. 2006;7(12):2601–2606. doi: 10.1002/cphc.200600533. [DOI] [PubMed] [Google Scholar]
- 38.Inada T, Modak PR. Chem. Eng. Sci. 2006;61(10):3149–3158. [Google Scholar]
- 39.McClements DJ. Ann. Rev. Food Sci. Tech. 2010;1(1):241–269. doi: 10.1146/annurev.food.080708.100722. [DOI] [PubMed] [Google Scholar]
- 40.McClements DJ. Soft Matter. 2011;7(6):2297–2316. [Google Scholar]
- 41.McClements DJ, Rao J. Crit. Rev. Food. Sci. Nutr. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 42.Pal R. Curr. Op. Colloid Int. Sci. 2011;16(1):41–60. [Google Scholar]
- 43.McClements DJ, Xiao H. Food Func. 2012;3(3):202–220. doi: 10.1039/c1fo10193e. [DOI] [PubMed] [Google Scholar]
- 44.Salentinig S, Sagalowicz L, Leser ME, Tedeschi C, Glatter O. Soft Matter. 2011;7(2):650–661. [Google Scholar]
- 45.Wiedmann TS, Kamel L. J. Pharm, Sci. 2002;91(8):1743–1764. doi: 10.1002/jps.10158. [DOI] [PubMed] [Google Scholar]
- 46.Porter CJH, Charman WN. Adv. Drug. Deliv. Rev. 2001;50:S127–S147. doi: 10.1016/s0169-409x(01)00182-x. [DOI] [PubMed] [Google Scholar]
- 47.Porter CJH, Trevaskis NL, Charman WN. Nat. Rev. Drug. Discov. 2007;6(3):231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 48.Porter CJH, Wasan KM. Adv. Drug. Deliver. Rev. 2008;60(6):615–616. doi: 10.1016/j.addr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Huo T, Ferruzzi MG, Schwartz SJ, Failla ML. J. Ag, Food Chem. 2007;55(22):8950–8957. doi: 10.1021/jf071687a. [DOI] [PubMed] [Google Scholar]
- 50.Thakkar SK, Maziya-Dixon B, Dixon AGO, Failla ML. J. Nutr. 2007;137(10):2229–2233. doi: 10.1093/jn/137.10.2229. [DOI] [PubMed] [Google Scholar]
- 51.Kohno H, Yoshitani S, Tsukio Y, et al. Life Sci. 2001;69(8):901–913. doi: 10.1016/s0024-3205(01)01169-9. [DOI] [PubMed] [Google Scholar]