Abstract
OBJECTIVES
Clinical outcomes in older adults with metastatic renal cell carcinoma (mRCC) are poorly understood, particularly in the era of targeted therapies. We characterize survival and relevant treatment-related variables in a modern series.
MATERIALS AND METHODS
From an institutional database including 562 patients with RCC, a total of 219 patients with metastatic disease were identified for the current analysis. Survival was assessed in four age-based cohorts: (1) age < 55, (2) age 55–64, (3) age 65–74, and (4) age ≥ 75. The number of lines of therapy rendered was collected for each patient, and the reason for treatment discontinuation was characterized.
RESULTS
Of the 219 patients assessed, median age was 58 (range, 26–87), and most patients had clear cell histology (82%) and prior nephrectomy (70.9%). The majority of patients were characterized as intermediate-risk (53%) by MSKCC criteria. Median survival in patients age ≥ 75 was 12.5 months, as compared to 26.4 months for patients age < 75 (P=0.003). Patients age ≥ 75 received fewer lines of systemic therapy as compared to other age-based subsets, and more frequently discontinued therapies due to toxicity.
CONCLUSIONS
Older adults represent a unique subpopulation of patients with mRCC, with distinct clinical outcomes. Further research is warranted to better understand the safety and tolerability of current therapies for mRCC in this group.
Keywords: Older adults, elderly, survival, renal cell carcinoma, targeted therapy
Introduction
The past decade has been marked by a dramatic paradigm shift in the treatment of metastatic renal cell carcinoma (mRCC) – specifically, a total of 7 targeted agents have been approved by the United States Food and Drug Administration.(1) These targeted agents antagonize the vascular endothelial growth factor (VEGR) receptor signaling pathway through ligand binding (bevacizumab), tyrosine kinase inhibition (sunitinib, sorafenib, pazopanib and axitinib), or inhibition of a downstream moiety, the mammalian target of rapamycin (mTOR; temsirolimus and everolimus). These agents possess several advantages over traditional immunotherapeutic strategies, such as interferon-α (IFN-α) and interleukin-2 (IL-2).(2) Several of these agents (e.g., sunitinib and temsirolimus) have demonstrated a benefit in clinical outcome (i.e., progression-free survival, PFS, and overall survival, OS) relative to IFN-α.(3–4) Furthermore, although high-dose IL-2 therapy may elicit a durable response in a small proportion of patients with mRCC (estimated in the range of 5–7%), the treatment is generally rendered to younger patients with more limited co-morbidities.(5)
In contrast to IFN-α and IL-2, the targeted therapies appear to represent an efficacious option applicable to a broader patient population. Efforts have been made or are currently underway to understand the relative efficacy of both VEGF- and mTOR- directed therapies in a number of different clinical settings (i.e., mRCC in patients with non-clear cell histologies, brain metastases, etc.).(6–7) Older adults arguably represent a population warranting further study in this context. While subset analyses by age do accompany the majority of phase III clinical trials in mRCC, there is little consistency in the manner in which this data is reported. For instance, the pivotal studies of everolimus, pazopanib, and sunitinib report outcomes based on an age cut-off of 65, while a pivotal study of bevacizumab utilizes an age cut-off of 70.(4, 8–9) Regardless, the utility of these analyses is challenged by the fact that the median age at diagnosis of RCC is 64 – thus, these cut-offs may not accurately characterize an “older” population.(10) Furthermore, the distribution of patients above these thresholds vary across studies – in the majority, one-third or fewer patients lie above these age cut-offs.(11)
In the current study, we report a detailed examination of a modern series including 219 patients with mRCC. The vast majority of patients in this cohort were treated with targeted therapies. Acknowledging the median age of 65 for patients with RCC, we examined clinical outcomes in several age-based cohorts, with particular attention to patients over the age of 75. The number of lines of therapy rendered and reasons for treatment discontinuation in each age group were also the focus of study.
Methods
Patients
From an IRB-approved institutional database, a total of 562 patients were identified with a diagnosis of RCC. Of these patients, a total of 219 patients were noted to have metastatic disease. We collected extensive clinicopathologic data related to each patient, including age, gender, tumor histology (clear cell, papillary, chromophobe, or other), sites of metastasis (bone, brain, liver and lung), nephrectomy status, and the presence or absence of sarcomatoid features. Furthermore, sufficient data was available to characterize the Memorial Sloan-Kettering Cancer Center (MSKCC) Risk Score for each patient, a validated prognostic tool for patients with mRCC based on 5 characteristics: (1) recurrence < 12 months after nephrectomy, (2) serum calcium > 10 mg/dL, (3) hemoglobin < lower limit of normal, (4) lactate dehydrogenase > 1.5x upper limit of normal, and (5) Karnofsky performance status < 80%.(2) Patients were characterized as good-, intermediate- or poor-risk based on the presence of 0, 1–2, or ≥ 3 features, respectively.
Collection of treatment-related data and clinical outcome
Survival was characterized as the time elapsed between the time of diagnosis with metastatic disease and the time of death. Date of death was ascertained through use of an institutional cancer registry, which regularly updates this data point using information from the Social Security Death Index or information derived from patient medical records. The time of diagnosis with metastatic disease was defined as the date of radiographic studies identifying metastases. Treatment-related data was captured separately for each line of therapy. These data included the specific type of treatment rendered, the date of treatment initiation, the date of discontinuation, and (when available) the reason for discontinuation. Of note, a “line of therapy” was regarded as a unique therapeutic agent used for mRCC (i.e., sunitinib, IL-2, etc.), as opposed to a therapeutic drug class (i.e., immunotherapy, VEGF-TKI, mTOR inhibitors, etc.). The reason for treatment discontinuation was delineated as follows: (1) discontinuation following progression, (2) discontinuation due to toxicity, or (3) discontinuation due to reasons other than progression or toxicity (i.e., patient preference).
Statistical Analysis
Patients were stratified into four subgroups based on age, as follows: (1) age < 55, (2) age 55–64, (3) age 65–74, and (4) age ≥ 75. Survival was characterized using the Kaplan-Meier method, and the log-rank test was used to compare survival amongst (1) all four age-based cohorts, and (2) age < 75 versus age ≥ 75. The average number of therapies rendered was determined for each subgroup and compared using 1-way analysis of variance (ANOVA). Descriptive statistics were used to express the reasons for treatment discontinuation, represented in a cumulative fashion across age groups. The data analyses were performed using SAS version 9.1.
Results
Patient Characteristics
Of 219 patients with documented mRCC, the majority of patients were male (72%) and the median age of the cohort was 58 (range, 26–87). Seventy-nine patients (36.1%) were age < 55, 77 patients (35.2%) were age 55–64, 43 patients (19.6%) were age 65–74, and 20 patients (9.1%) were age ≥ 75. Clear cell histology was noted in 82% of patients, and sarcomatoid features were present in approximately 14% of patients (a breakdown of histology by age is provided in Table 1). Most patients in the overall cohort had received prior nephrectomy (70.9%) and were characterized as having intermediate-risk disease (53%) by MSKCC criteria. In contrast, relatively few patients (6.8%) were noted to be poor risk.
Table 1.
Clinicopathologic characteristics of patients with mRCC categorized by age.
| Age < 55 | Age 55–64 | Age 65–74 | Age ≥ 75 | |
|---|---|---|---|---|
| N | 79 | 77 | 43 | 20 |
| Gender | ||||
| Male | 66 (84%) | 55 (71%) | 27 (63%) | 12 (60%) |
| Female | 13 (16%) | 22 (29%) | 16 (37%) | 8 (40%) |
| Histology* | ||||
| Clear cell | 63 (80%) | 67 (87%) | 37 (86%) | 15 (75%) |
| Papillary | 10 (13%) | 7 (9%) | 4 (9%) | 4 (20%) |
| Chromophobe | 3 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Sites of disease | ||||
| Bone | 33 (42%) | 34 (44%) | 25 (58%) | 8 (40%) |
| Brain | 6 (8%) | 16 (21%) | 7 (16%) | 2 (10%) |
| Liver | 22 (28%) | 22 (29%) | 8 (19%) | 4 (20%) |
| Lung | 56 (71%) | 54 (70%) | 35 (81%) | 12 (60%) |
| Prior nephrectomy | 55 (70%) | 57 (74%) | 29 (67%) | 15 (75%) |
| MSKCC risk status | ||||
| Good | 31 (39%) | 33 (43%) | 18 (42%) | 5 (25%) |
| Intermediate | 39 (49%) | 41 (53%) | 25 (58%) | 12(60%) |
| Poor | 9 (11%) | 3 (4%) | 0 (0%) | 3 (15%) |
| Sarcomatoid features | ||||
| Present | 15 (19%) | 8 (10%) | 5 (12%) | 3 (15%) |
| Absent | 64 (81%) | 69 (90%) | 38 (88%) | 17 (85%) |
| Receipt of systemic therapy | ||||
| Yes | 62 (78%) | 57 (74%) | 30 (70%) | 6 (30%) |
| No | 17 (22%) | 20 (26%) | 13 (30%) | 14 (70%) |
Histologic data was either unavailable or recorded as “other” for a total of 9 patients.
Clinical Outcome
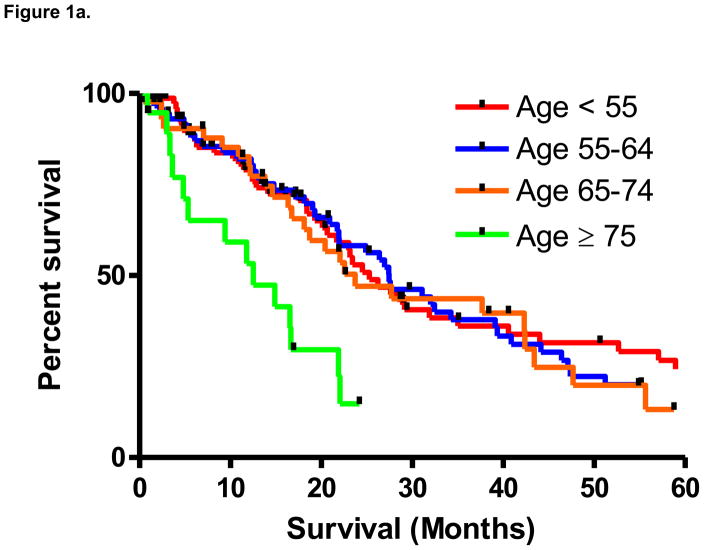
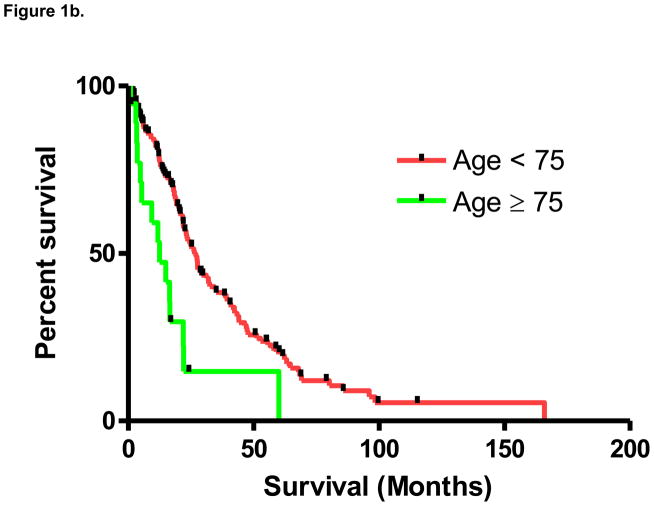
From the time of diagnosis with metastatic disease, median survival in the overall cohort was 23.7 months. As noted in Figure 1a, based on the pre-specified distribution of age groups, a significant difference in median survival was noted between groups (P=0.005 by the log-rank test). As compared to the remainder of the cohort, median survival in patients age ≥ 75 was significantly lower (12.5 months v 26.4 months; P=0.003 by the log-rank test; Figure 1b). Median survival was 25.2 months in patients age < 55, 27.5 months in patients age 55–64, and 23.7 in patients age 65–74.
Figure 1.
Figure 1a. Survival in patients with mRCC stratified into four age-based cohorts (P=0.005).
Figure 1b. Survival in patients with mRCC stratified above and below age 75 (P=0.003).
Treatment-Related Data
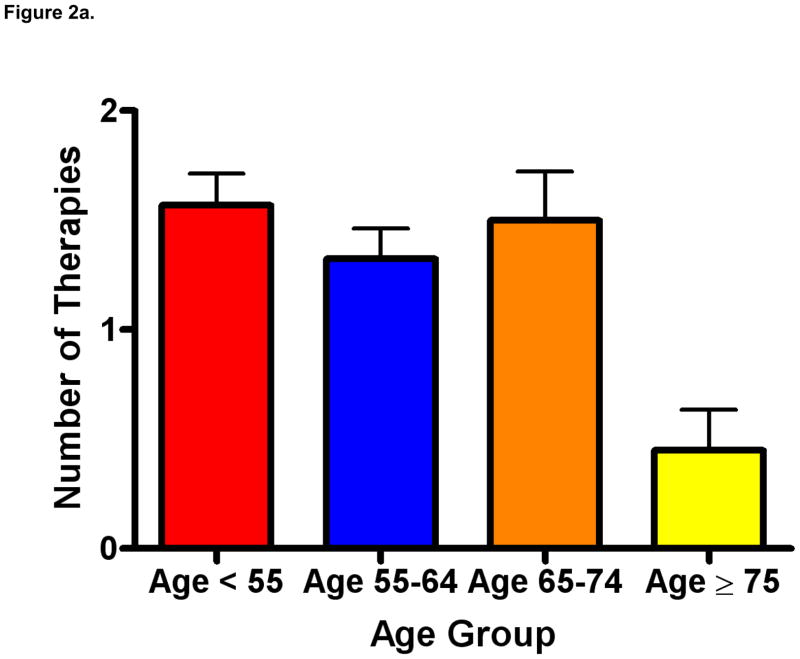
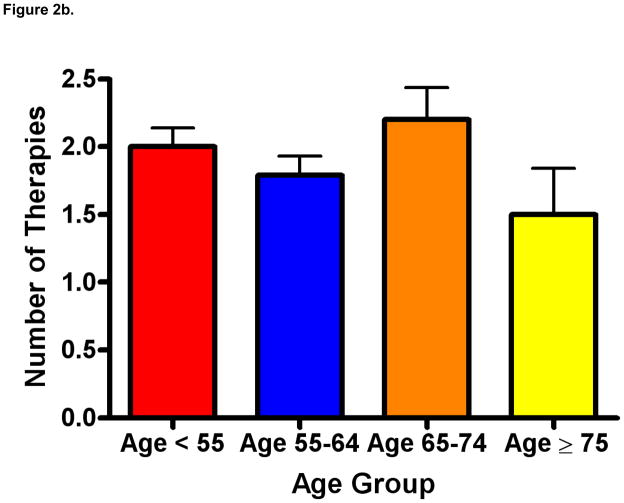
As depicted in Figure 2a, patients age ≥ 75 received a mean of 0.45 lines of therapy (standard error of the mean [SEM] 0.18) as compared to 1.57 therapies (SEM 0.14) in patients under the age of 55, 1.32 therapies (SEM 0.14) in patients 55–64, and 1.5 therapies (SEM 0.22) in patients 65–74 (P=0.001). In a separate analysis (depicted in Figure 2b), we censored patients who had received no prior therapy. In this analysis, the mean number of therapies rendered was not significantly different across age groups (P=0.24), but was numerically lower in patients age ≥ 75 (mean 1.5, SEM 0.34) as compared to patients age < 55 (mean 2.0, SEM 0.14), age 55–64 (mean 1.8, SEM 0.14), and age 65–74 (mean 2.2, SEM 0.24). As these data imply, with increasing age, an increasing proportion of patients received no therapy. Specifically, 21% of patients under the age of 55, 26% of patients 55–64, 32% of patients 65–74, and 70% of patients over age 75 had no systemic therapies recorded.
Figure 2.
Figure 2a. Lines of therapy rendered by age (mean ± SEM), including patients who have received no prior therapy (P=0.001).
Figure 2b. Lines of therapy rendered by age (mean ± SEM), censored for patients who have received no prior therapy (P=NS).
Data pertaining to reason for treatment discontinuation was recorded for a total of 271 distinct therapies rendered across all age groups. Less data was available for subjects in increasing age cohorts, possibly reflecting (a) a smaller sample size in older age-based cohorts and (b) fewer therapies rendered in older age-based cohorts, as noted previously. As noted in Table 2, in comparison to younger patients, fewer patients age ≥ 75 discontinued therapy as a consequence of progression and more patients discontinued therapy secondary to toxicity.
Table 2.
Reasons for treatment discontinuation. Within each age group, the reason for treatment discontinuation (when available) was documented for each therapy rendered, and expressed as a percentage of the cumulative number of therapies rendered for each age group.
| Age <55 (N=79) | Age 55–64 (N=77) | Age 65–75 (N=43) | Age ≥ 75 (N=20) | |
|---|---|---|---|---|
| Number of lines of therapy rendered | 109 | 95 | 58 | 9 |
| Discontinuation of treatment due to progression or death | 73 (67%) | 61 (64%) | 28 (48%) | 3 (33%) |
| Discontinuation of treatment due to toxicities | 17 (16%) | 15 (16%) | 14 (24%) | 3 (33%) |
| Discontinuation of treatment for other reasons (i.e., patient preference) | 19 (17%) | 19 (20%) | 16 (28%) | 3 (33%) |
Discussion
The International Society of Geriatric Oncology (SIOG) have issued treatment recommendations for patients with mRCC.(12) Admittedly, these recommendations draw from limited pools of prospective evidence and rely heavily on subset analyses. Age is omitted from the standard prognostic schema used for patients with mRCC, such as the Motzer criteria (developed in the era of cytokine therapy) or the more recent Heng criteria (more applicable to patients receiving targeted therapies).(2, 13) In our series, median survival patients age ≥ 75 with mRCC was statistically inferior to patients age < 75. Although it is impossible to assign causality in a retrospective analysis such as this, our results implicate the possibility that older adults receive fewer lines of systemic therapy. Further, our data suggest that older adults may be more prone to discontinue therapy as a consequence of toxicity as opposed to disease progression. These data underscore the need to better understand both the safety and efficacy of targeted therapies in older patients.
Our effort to characterize clinical outcomes in older adults with mRCC is not the first. The International mRCC Database Consortium (utilizing pooled data from 14 academic centers) has previously reported survival data for 144 patients age ≥ 75, comparing these endpoints to 1,241 patients age < 75.(14) Median survival in patients age ≥ 75 was 16.8 months, as compared to 19.7 months in patients age < 75 (P=0.33). Although this difference was not statistically significant, the overall trend was akin to what we observed in our study. The study also reported a statistically insignificant decrease in response rate and length of treatment duration in older adults, representing data not collected in our study. However, individual patient-level data pertaining to lines of therapy and reasons for treatment discontinuation were not reported. Furthermore, the study only included patients who received 1st-line VEGF-directed therapy. As such, patients with a non-clear cell phenotype (who may receive 1st-line therapy with agents such as temsirolimus) would likely be poorly represented. Older adults may be more likely to have relative contraindications for VEGF-directed treatments (described subsequently). As evidenced by our dataset, these patients may be more likely to receive no treatment altogether – the International mRCC Database Consortium experience does not account for these caveats.
There are several reasons to suspect that older adults may garner a different extent of benefit from targeted agents. First, the biology of the disease is distinct – recent studies suggest that von Hippel Lindau (VHL) gene mutations may be more frequent in older adults.(15) Aberrations in VHL ultimately lead to dysregulation of VEGF-mediated signaling, driving tumor angiogenesis and growth.(16) Older adults may also more frequently possess clear cell histology – notably, studies of VEGF-directed therapies have been largely restricted to this subset of patients.(17–18) Beyond these biologic differences, physiologic differences may challenge the receipt of targeted agents. Older adults may have decreased liver mass and cytochrome P450 (CYP) enzyme content, challenging the administration of VEGF-tyrosine kinase inhibitors (VEGF-TKIs) and mTOR inhibitors, which are largely metabolized through CYP3A4.(19–20) The documented increase in co-morbidities amongst older adults could also be problematic. For instance, treatment with bevacizumab is thought to evoke a 5.28-fold increase in the rate of grade 3/4 hypertension.(21) Given the increased frequency of baseline hypertension in older adults, these drugs would have to be used with particular caution.(22)
In the pivotal phase III studies leading to the approval of the currently available VEGF- and mTOR-directed therapies for mRCC, subset analyses have been conducted to ascertain the relative benefit of these targeted agents. Several examples are provided in Table 3. However, a cumulative interpretation of these results can be seen through the use of different age-cutoffs in these subset analyses. For instance, the phase III TARGET study comparing sorafenib and placebo assesses progression-free survival (PFS) in patients above and below age 70, while the phase III AVOREN study comparing bevacizumab/IFN-α and placebo/IFN-α explores the same endpoint in subgroups <40, 40–64, and ≥ 65.(23–24) This harkens to the difficulty in characterizing the older adult – as opposed to a certain age threshold that might to delineate the “elderly”, an array of features (such as cognitive function, physical function, comorbidity, etc.) might better serve to characterize this group. Tools such as the Comprehensive Geriatric Assessment (CGA) incorporate these diverse features, and are being prospectively assessed in the setting of large cooperative group trials.(25)
Table 3.
Subset analyses of progression-free survival (PFS) benefit by age in several pivotal phase III studies including patients with mRCC.
| Experimental arm | Control arm | Age Group | HR* | |
|---|---|---|---|---|
| TARGET(24) | Sorafenib | Placebo | < 70 | 0.55 |
| ≥ 70 | 0.43 | |||
| VEGF105192(9) | Pazopanib | Placebo | < 65 | 0.41 |
| ≥ 65 | 0.52 | |||
| RECORD-1(8) | Everolimus | Placebo | < 65 | 0.33 |
| ≥ 65 | 0.19 |
The hazard ration (HR) expressed refers to the relative PFS benefit of the experimental arm relative to the control arm.
Several limitations to the current study should be acknowledged. First, the sample size was relatively small, particularly with respect to the subset of interest (i.e., those patients age ≥ 75). The results herein should be considered hypothesis generating, and provoke larger efforts to explore outcomes in this population. Second, the retrospective methods utilized herein pose a challenge in ascertaining complete data related to treatment. It is possible that patients noted to have metastatic disease in our database received systemic therapies at other institutions unbeknownst to the investigators. A surprisingly large proportion of patients received no therapy (especially considering the relatively small proportion of patients in our study with poor-risk disease); incomplete data could account for this observation. However, there is little reason to surmise that the extent of data collection would vary across age-based subgroups – thus, the trends observed (a lesser number of therapies rendered in older patients) are likely accurate. A third limitation of our work is an inability to fully account for confounders that may affect the utilization of treatments. We do not have data available relative to comorbidity; certainly, this could have played a similarly important role in defining mortality, and could have had a substantial effect on the number of lines of therapy rendered and reasons for treatment discontinuation.
Despite these limitations, the current report represents a unique analysis of clinical outcome in older adults with mRCC treated in the era of targeted therapy, distinguishing itself from similar reports by additionally including (1) untreated patients and (2) patients who may have received non-VEGF-directed treatments in the 1st-line setting. Our observation of decreased survival in patients age ≥ 75 should serve as the rationale for larger multi-institutional efforts exploring this phenomenon. Novel strategies of characterizing older adults (such as the aforementioned CGA) may lead to a better understanding of patterns of care in this population. Numerous recent studies have focused on differences in outcome amongst older adults with non-metastatic RCC, highlighting the differential benefit of cytoreductive nephrectomy and nephron-sparing strategies in this setting.(26) Undoubtedly, the treatment of metastatic disease in older adults warrants similar attention.
Acknowledgments
Dr. Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP) and NIH K12 2K12CA001727-16A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pal SK, Figlin RA. Targeted therapies for renal cell carcinoma: understanding their impact on survival. Target Oncol. 2010 Jun;5(2):131–8. doi: 10.1007/s11523-010-0145-6. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: memorial sloan-kettering cancer center experience. Clin Cancer Res. 2004 Sep 15;10(18 Pt 2):6302S–3S. doi: 10.1158/1078-0432.CCR-040031. [DOI] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N Engl J Med. 2007 May 31;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009 Aug 1;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995 Mar;13(3):688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 6.Plimack ER, Jonasch E, Bekele BN, Qiao W, Ng CS, Tannir NM. Sunitinib in papillary renal cell carcinoma (pRCC): Results from a single-arm phase II study. J Clin Oncol (Meeting Abstracts) 2010 May 20;28(15_suppl):4604. [Google Scholar]
- 7.Verma J, Jonasch E, Allen P, Tannir NM, Mahajan A. Tyrosine kinase inhibitors and development of brain metastasis in metastatic renal cell carcinoma: A retrospective review. ASCO Meeting Abstracts. 2011;29(7_suppl):340. [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 9;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. 2010 Feb 20;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 10. [last accessed July 15, 2012];Surveillance, Epidemiology and End Results (SEER) Fast Facts Sheets: Kidney and Renal Pelvis. Available on-line at http://seer.cancer.gov/statfacts/html/kidrp.html.
- 11.Pal SK, Vanderwalde A, Hurria A, Figlin RA. Systemic therapies for metastatic renal cell carcinoma in older adults. Drugs Aging. 2011 Aug 1;28(8):635–49. doi: 10.2165/11592880-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellmunt J, Negrier S, Escudier B, Awada A, Aapro M. The medical treatment of metastatic renal cell cancer in the elderly: position paper of a SIOG Taskforce. Crit Rev Oncol Hematol. 2009 Jan;69(1):64–72. doi: 10.1016/j.critrevonc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009 Dec 1;27(34):5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 14.Khambati H, Choueiri TK, Kollmannsberger CK, North S, Bjarnason GA, Vaishampayan UN, et al. Efficacy of targeted drug therapy for metastatic renal cell carcinoma in the elderly patient population. Presented at the 2011 Genitourinary Cancers Symposium [Abstr 318]; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1(3):237–46. doi: 10.1016/S1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim WY, Kaelin WG. Role of VHL Gene Mutation in Human Cancer. J Clin Oncol. 2004 Dec 15;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez A, Patard JJ, Lobel B. Renal cell carcinoma in young adults: incidence, disease outcome and review of the literature. Arch Esp Urol. 2002 Oct;55(8):969–75. [PubMed] [Google Scholar]
- 18.Gillett MD, Cheville JC, Karnes RJ, Lohse CM, Kwon ED, Leibovich BC, et al. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J Urol. 2005 Jun;173(6):1893–6. doi: 10.1097/01.ju.0000158157.57981.80. [DOI] [PubMed] [Google Scholar]
- 19.Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res. 2004 Dec 15;10(24):8341–50. doi: 10.1158/1078-0432.CCR-04-1371. [DOI] [PubMed] [Google Scholar]
- 20.Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997 Mar;61(3):331–9. doi: 10.1016/S0009-9236(97)90166-1. [DOI] [PubMed] [Google Scholar]
- 21.Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010 May;23(5):460–8. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 22.Cohen L, Curhan GC, Forman JP. Influence of age on the association between lifestyle factors and risk of hypertension. J Am Soc Hypertens. 2012 Jul;6(4):284–90. doi: 10.1016/j.jash.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III Trial of Bevacizumab Plus Interferon Alfa-2a in Patients With Metastatic Renal Cell Carcinoma (AVOREN): Final Analysis of Overall Survival. J Clin Oncol. 2010 May 1;28(13):2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Cirrincione CT, Muss HB, Kornblith AB, Barry W, Artz AS, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011 Apr 1;29(10):1290–6. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kader AK, Tamboli P, Luongo T, Matin SF, Bell K, Jonasch E, et al. Cytoreductive nephrectomy in the elderly patient: the M. D. Anderson Cancer Center experience. J Urol. 2007 Mar;177(3):855–60. doi: 10.1016/j.juro.2006.10.058. discussion 60–1. [DOI] [PubMed] [Google Scholar]