Abstract
Alzheimer’s disease (AD) is a complex human neurodegenerative disease. Currently the therapeutics for AD only treat the symptoms. While numbers of excellent studies have used mammalian models to discover new compounds, the time and effort involved with screening large numbers of candidates is prohibitive. Cultured mammalian neurons are of often used to perform high through-put screens (HTS) however, cell culture lacks the organismal complexity involved in AD. To address these issues a number of researchers are turning to the round worm, C. elegans. C. elegans has numerous models of both Tau and Aβ induced toxicity; the two prime components observed to correlate with AD pathology. These models have lead to the discovery of numerous AD modulating candidates. Further, the ease of performing RNAi for any gene in the C. elegans genome allows for identification of proteins involved in the mechanism of drug action. These attributes make C. elegans well positioned to aid in the discovery of new AD therapies.
Introduction
Alzheimer’s disease (AD) is a chronic, neurodegenerative disorder that is the number one cause of senile dementia. Brains of AD patients contain neurofibrillary tangles of tau (a microtubule protein) and hallmark plaques, constituted primarily of insoluble Aβ [1]. According to the amyloid cascade theory, amyloid precursor protein (APP) is processed to form Aβ, which self-dimerizes into increasingly large oligomers, and is responsible for both sporadic and familial AD. Aβ is predominantly produced as a 40 residue peptide (Aβ1–40) and less commonly as a longer 42 residue peptide (Aβ1–42) [2]. A large body of evidence suggests that Aβ1–42 is more prone to forming oligomers and therefore the more toxic species. The toxic nature of Aβ1–42 makes it a marker of AD progression and a target of many screens for new therapeutic treatments [3, 4].
Currently there is a dearth of treatments for AD and the treatments that are available, acetyl cholinesterase inhibitors and NMDA receptor antagonists only treat the symptoms. Much of the research to determine the cause of AD as well as design therapies to treat it has utilized mammalian models. This approach is logical in that AD is a complex neurodegenerative disease, requiring the advanced nature of the rodent brain to see a plausible correlation to a human neurodegenerative disease. Unfortunately, due to the time and the number of animals required, mammals are not amenable to high-throughput screening (HTS). While mammalian neuronal cell culture is often used for (HTS) it lacks the complexity found in a living organism or even a whole organ, making this system a somewhat crude predictor of safety and efficacy. To fill the gap between cell culture and rodents, many have turned to a seemingly unlikely model, the round worm, C. elegans. An invertebrate with only 302 and neurons, C. elegans offers a number of advantages to identify candidate drugs for AD.
Drug Screening in C. elegans
Administration of drugs to C. elegans is conceptually simple; add varying concentrations of candidate drugs to the medium and add worms. Performing the screen with several concentrations allows for the creation of a classic dose response. The dose response can show the ideal dose, minimally effective dose, and, at higher concentrations, toxicity [5]. Although relatively simple, there is no easy way to know how much of a given drug is being ingested and/or absorbed [6]. Surrounding C. elegans is a weakly-permeable membrane known as the cuticle. The cuticle functions as an exoskeleton and as a barrier to environment. Therefore, while potentially functional, a small molecule may fail to produce a phenotype due to reduced bioavailability. Further, the bacteria that serve as food for C. elegans could also be metabolizing any drugs administered to the media. To address this issue it is now possible to use a structure based bioaccumulation model (SAM) to identify the candidate molecules most likely to be absorbed while remaining active [6]. While the cuticle may confound administration and dosage of candidates it is not prohibitive. Some drugs are still absorbed through the cuticle while other drugs are likely being ingested with the consumption of bacteria.
The screening of a drug library for modifiers of AD can employ a broad based approach where many molecules are screened. In a screen for enhancers of lifespan, the antidepressant, mianserin was isolated from a library of 88,000 molecules [7]. Mianserin functions as a serotonin receptor antagonist. The large collection of available genetic mutations in C. elegans allowed these researchers to identify the specific receptors required for lifespan extension by mianserin (SER-3 and SER-4), and to demonstrate that mianserin is likely to function as a calorie restriction mimetic. This study demonstrates that C. elegans is amenable to high-throughput screening and the genetic tools available in this model can be used to determine the mode of action of active compounds identified in an essentially unbiased chemical screen. To date, there have been no large scale screens described using C. elegans to identify compound leads for AD drugs. Below we characterize the available AD-relevant C. elegans models and suggest how studies in these models might be extended to enable high throughput screening.
Alzheimer’s disease modeled in C. elegans
The nematode, C. elegans, is a completely transparent, approximately 1mm long roundworm. As a hermaphrodite C. elegans is self-fertilizing, allowing for rapid production of numerous offspring. With a relatively short lifespan of 23 days, C. elegans is ideally suited to study aging and age-related diseases [8]. Although it is unlikely invertebrate models can really capture Alzheimer’s disease pathology in total, multiple transgenic C. elegans strains have been constructed to model aspects of AD, specifically β-amyloid peptide (Aβ) toxicity (Table 1). The first described model expressed the human Aβ 1–42 peptide under the control of a constitutive muscle-specific promoter derived from a myosin gene expressed in body wall muscle (unc-54) [9]. This transgenic construct employed an N-terminal artificial signal peptide to route the Aβ peptide to the secretory pathway. Mass spectrometry studies have demonstrated the signal peptide is efficiently removed, although this signal peptide cleavage apparently results in the production of Aβ 3–42 rather than Aβ 1–42 [10]. Constitutive muscle-specific expression of Aβ leads to the accumulation of intramuscular Aβ deposits (Figure 1, top panel) and the formation of fibrillar amyloid detectable with amyloid-specific dyes [11]. This pathology is reminiscent of that observed in Inclusion Body Myositis (IBM), and this transgenic model may be more directly relevant to this disease than AD. The best-characterized strain containing the Punc-54::Aβ 1–42 transgene is CL2006, which shows a progressive, adult onset paralysis.
Table 1.
C. elegans transgenic strains expressing human Aβ
| Strain | Aβ transgene | Aβ Expression | Phenotype |
|---|---|---|---|
| CL2006 | pCL12 (Punc-54:: SP::Aβ 1–42) | constitutive muscle | adult onset paralysis, β-amyloid formation[9] |
| CL2122 | pCL12 (Punc-54:: SP::Aβ 1–42) | constitutive muscle | slow adult movement, β-amyloid formation[11] |
| CL4176 | pAF29 (Pmyo-3::SP::Aβ1–42::long 3′ UTR) | inducible muscle | inducible larval paralysis[35] |
| CL2159 | pAF29 (Pmyo-3::SP::Aβ1–42::long 3′ UTR) | inducible muscle | inducible larval paralysis[19] |
| CL2355 | pCL45 (Psnb-1::SP::Aβ1–42::long 3′ UTR) | pan-neuronal | memory deficits, abnormal thrashing in liquid, partial sterility[23] |
| UA166 | Peat-4::SP::Aβ 1–42 | glutamatergic neurons | age-dependent loss of glutamatergic neurons[25] |
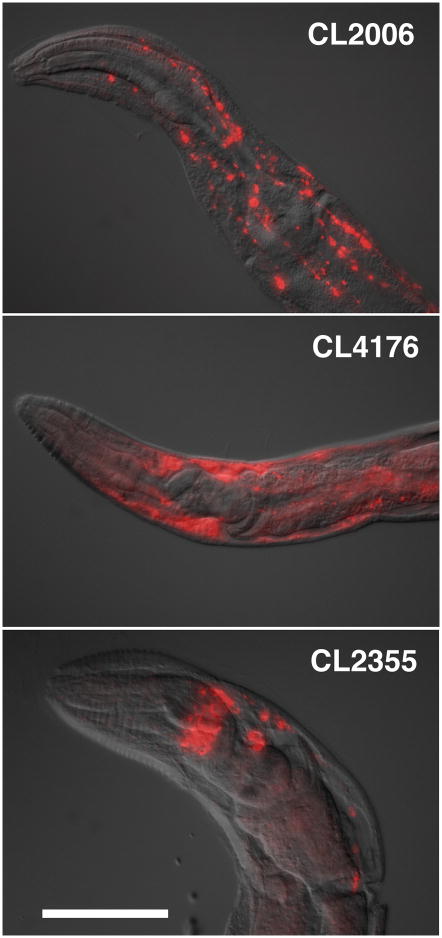
Figure 1.
Anterior regions of transgenic C. elegans worms fixed and probed for Aβ. Top panel, CL2006 adult probed with anti-Aβ mAb 4G8. Middle panel, CL4176 3rd stage larvae probed with anti-Aβ mAb 6E10. Bottom panel, CL2355 adult probed with anti-Aβ mAb 6E10. Images are overlays of DIC brightfield image and digitally deconvoluted epifluorescence images. Size bar = 50μM.
Strain CL2006 has been used to evaluate the effects of selected compounds on Aβ toxicity and fibrillar amyloid formation. These include natural compounds such gingko biloba extract [12], the soy isoflavone glycitein [13], and epigallocatechin gallate [14]. Drug-related compounds including reserpine [15], fluoxetine [16], and thioflavin T [17], have also been shown to modulate toxicity in this model. Only one study has examined the effects of more than a handful of compounds in the CL2006 model [5]. Thirty drugs that protect against glucose induced toxicity were identified using mammalian primary neurons to screen a library of FDA-approved drugs. These 30 candidates were subsequently screened for both amyloid induced toxicity and lifespan extension in C. elegans. From this two-species screen, three compounds, caffeine, tannic acid, and bacitracin, offered protection from Aβ-induced toxicity in the C. elegans CL2006 model. These studies indicate that a range of exogenous compounds can modulate Aβ-dependent phenotypes in this model. These compounds could be used as positive controls for higher-throughput assays.
A limitation of the CL2006 constitutive Aβ-expression model for more extensive compound screening may be the age dependence of the paralysis phenotype: it may be difficult to disentangle whether protective compounds are modulating Aβ toxicity or aging physiology (or both). Indeed, all the compounds identified by Lublin et al as protective in CL2006 also increased the lifespan in wild type worms, and genetic backgrounds that lead to increased lifespan also reduce paralysis rates in CL2006 [18]. In addition, the paralysis rate in CL2006 can be variable from experiment to experiment, and some worms never appear to become paralyzed. To circumvent these limitations, transgenic worms have been constructed in which body wall muscle expression of the signal peptide::Aβ 1–42 minigene can be up-regulated in larval animals by temperature up-shift [19]. (See Figure 1, middle panel). In the best-characterized transgenic strain of this type (CL4176), worms have wild type movement throughout their lifespan when raised at the permissive temperature (16°C). However, up-shift of larval worms to higher temperature (25°C) leads to rapid, reproducible, and 100% penetrant paralysis [20]. CL4176 has been used to demonstrate that coffee extracts [21] and tetracycline and related analogs [22] can be protective against Aβ toxicity.
An obvious limitation of the models described above is that Aβ expression is limited to muscle cells. Transgenic worms with pan-neuronal expression of the signal peptide::Aβ 1–42 minigene have also been constructed and characterized [23] (see Figure 1, bottom panel). While these worms have more subtle phenotypes than the paralysis that occurs in muscle expression strains, they do show chemotaxis defects and altered movement in liquid. Although suboptimal for high-throughput screens, perhaps the most interesting defect in these transgenic worms is a failure to retain associative memory [24]. A transgenic C. elegans model has been recently developed in which the signal peptide::Aβ 1–42 minigene is expressed solely in 5 glutamatergic neurons, using the promoter from the eat-4 gene [25]. While this transgenic strain was not reported to have any gross visible phenotypes, visualization of the glutamatergic neurons with a Peat-4::GFP construct was used to demonstrate that Aβ expression led to an age-dependent loss of glutamatergic cells. The transparency of C. elegans may allow GFP fluorescence-based assays to form the basis of high throughput screens.
Alzheimer’s disease is also associated with the deposition of neurofibrillary tangles composed of hyperphosphorylated tau protein. Three transgenic C. elegans tauopathy models have been described, two of which expressed human tau using pan-neuronal promoters [26]; [27], while one expressed tau only in touch cell neurons [28]. The strains with pan-neuronal tau expression displayed uncoordinated movement and age-dependent neurodegeneration, while tau expression in the touch neurons led to mechanosensory defects. These transgenic models have not yet been used for compound testing. Automated methods have been developed for assaying C. elegans movement [29], so the phenotypes of the pan-neuronal expression models may amenable to large scale compound screening.
A major advantage of C. elegans models lies in the sensitivity of C. elegans to RNA interference (RNAi), which can be very beneficial in identifying molecular mechanisms underlying the action of protective compounds. For example, the protective effects of coffee extracts are completely blocked by RNAi knockdown of skn-1, the worm ortholog of the master phase 2 detoxicification transcription factor Nrf2. Similarly, the lifespan extension created by treatment with either caffeine or tannic acid was completely blocked by RNAi of the FOXO transcription factor daf-16, suggesting that caffeine and tannic acid require DAF-16 to extend lifespan [5].
Conclusions
In the design of a screen, the short time required to create strains of C. elegans offers a clear advantage compared to the time involved in producing murine strains. This advantage is amplified by the ease of acquiring and maintaining previously created strains available from the C. elegans Genetics Center (CGC) (http://www.cbs.umn.edu/CGC/strains/), including most of the strains described in Table 1. While the strains with muscle-specific expression of Aβ do not directly capture the neurodegeneration seen in AD, they do provide an easy means to study Aβ-induced toxicity and protein aggregation [11, 19]. Furthermore, these models can be used to study inclusion body myositis, an aging-related disease were Aβ forms aggregates in muscle tissue [30–32]. More recently created strains have expressed Aβ1–42 in the neurons of C. elegans. These strains produce a less pronounced phenotype then the muscle expressing strains, making them potentially problematic for higher through-put screens, however, they may more accurately represent the amyloid induced toxicity seen in AD [23, 25].
Screening drugs, small molecules, or complex extracts in C. elegans is strait forward in design but difficulties in bio-accumulation can create obstacles in identification of promising candidates. To circumvent these issues there are several approaches. Using a large scale HTS reduces the difficulties by screening a large enough number of molecules that one or more candidates will produce a significant effect, as was the case with mianserin [7]. Another approach is to perform a more focused screen on candidates that have an increased probability of functioning in a specific model. The candidates for these screens come from many sources, including specific studies in higher organisms or from a HTS in tissue culture. This approach has lead to the discovery that, in addition to increasing lifespan, caffeine, bacitracin, and tannic acid can reduce the observed proteotoxicity seen in an Aβ expressing C. elegans strain (CL2006) [5].
In C. elegans, HTS offer the advantage of identifying promising novel, and otherwise unlikely drugs with the caveat that some interesting candidates are likely to be missed. With a more focused screen some of issues with drug administration can be surmounted using a wide range of doses. Furthermore, by using different concentrations a dose response curve can be produced, enabling the identification of the most efficacious dose as well as any doses that produce toxicity.
Once identified, candidates from the screen can be functionally analyzed by utilizing RNAi. Performing RNAi on genes potentially needed for drug activity allows for identification of specific proteins and protein pathways involved in the mechanism of its action. Knowing the mechanism responsible for drug action it is possible to investigate other drugs that might more specifically target the same mechanism. Further, combination therapies, similar to those used for the treatment of HIV [33], can be designed in C. elegans by utilizing additional drugs or other modulators of the target molecular mechanism.
In closing, with the number of people diagnosed with AD expecting to grow as average age of population increases it is paramount to find new and better ways to treat this growing pandemic [34]. The mouse models will ultimately be needed to demonstrate the efficacy of any therapy prior to clinical trials. However, the spate of candidate AD therapies combined with a large number of failed trails creates a need for a filter to isolate the most promising leads. As C. elegans is well suited to screening it serves as a scout, to rapidly identify, efficacy, toxicity, and the molecular mechanisms responsible for any observed benefit. Moving forward, the information gained from research in worms can be applied to the design of mammalian studies. This streamlined approach to the process of drug discovery, may ultimately aid in the production of a valid therapy for AD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lublin AL, Gandy S. Amyloid-β Oligomers: Possible Roles as Key Neurotoxins in Alzheimer’s Disease. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2010;77(1):43–49. doi: 10.1002/msj.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 3.Naslund J, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283(12):1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 4.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •5.Lublin A, et al. FDA-Approved Drugs that Protect Mammalian Neurons from Glucose Toxicity Slow Aging Dependent on Cbp and Protect Against Proteotoxicity. PLoS One. 2011;6(11):e27762. doi: 10.1371/journal.pone.0027762. The authors use a combination of mammalian cell culture and C. elegans to perform a HTS screen identifing 6 FDA approved drugs that protect against glucose toxicity, aging, and Aβ-induced toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns AR, et al. A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat Chem Biol. 2010;6(7):549–557. doi: 10.1038/nchembio.380. [DOI] [PubMed] [Google Scholar]
- 7.Petrascheck M, Ye X, Buck LB. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature. 2007;450(7169):553–556. doi: 10.1038/nature05991. [DOI] [PubMed] [Google Scholar]
- 8.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995;92(20):9368–72. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McColl G, et al. The Caenorhabditis elegans Aβ1–42 Model of Alzheimer Disease Predominantly Expresses Aβ3–42. Journal of Biological Chemistry. 2009;284(34):22697–22702. doi: 10.1074/jbc.C109.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fay DS, et al. In Vivo Aggregation of β-Amyloid Peptide Variants. Journal of Neurochemistry. 1998;71(4):1616–1625. doi: 10.1046/j.1471-4159.1998.71041616.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith JV, Luo Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J Alzheimers Dis. 2003;5(4):287–300. doi: 10.3233/jad-2003-5404. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Zepeda A, et al. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci. 2005;6:54. doi: 10.1186/1471-2202-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbas S, Wink M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine. 2010;17(11):902–9. doi: 10.1016/j.phymed.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Arya U, Dwivedi H, Subramaniam JR. Reserpine ameliorates Abeta toxicity in the Alzheimer’s disease model in Caenorhabditis elegans. Exp Gerontol. 2009;44(6–7):462–6. doi: 10.1016/j.exger.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Keowkase R, Aboukhatwa M, Luo Y. Fluoxetine protects against amyloid-beta toxicity, in part via daf-16 mediated cell signaling pathway, in Caenorhabditis elegans. Neuropharmacology. 2010;59(4–5):358–65. doi: 10.1016/j.neuropharm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alavez S, et al. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472(7342):226–9. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen E, et al. Opposing Activities Protect Against Age-Onset Proteotoxicity. Science. 2006;313(5793):1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 19.Link CD, et al. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiology of aging. 2003;24(3):397–413. doi: 10.1016/s0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- •20.Dostal V, Link CD. Assaying beta-amyloid toxicity using a transgenic C. elegans model. J Vis Exp. 2010;44 doi: 10.3791/2252. The authors give a comprehensive discription of the techniques and analysis (including video demonstrations) utilized to study Aβ-induced toxicity in the inducible Aβ C. elegans strain, CL4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dostal V, Roberts CM, Link CD. Genetic mechanisms of coffee extract protection in a Caenorhabditis elegans model of beta-amyloid peptide toxicity. Genetics. 2010;186(3):857–66. doi: 10.1534/genetics.110.120436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diomede L, et al. Tetracycline and its analogues protect Caenorhabditis elegans from beta amyloid-induced toxicity by targeting oligomers. Neurobiol Dis. 2010;40(2):424–31. doi: 10.1016/j.nbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, et al. Amyloid-β-Induced Pathological Behaviors Are Suppressed by Ginkgo biloba Extract EGb 761 and Ginkgolides in Transgenic Caenorhabditis elegans. The Journal of Neuroscience. 2006;26(50):13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •24.Dosanjh LE, et al. Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-beta. J Alzheimers Dis. 2010;19(2):681–90. doi: 10.3233/JAD-2010-1267. This study features behavioral defects, including the first description of a cognative impairment, seen in a neuronal Aβ expressing strain of C. elegans. [DOI] [PubMed] [Google Scholar]
- ••25.Treusch S, et al. Functional Links Between Aβ Toxicity, Endocytic Trafficking, and Alzheimer’s Disease Risk Factors in Yeast. Science. 2011 doi: 10.1126/science.1213210. The authors report the creation of a new C. elegans model that expresses Aβ in glutamatergic neurons. This is the first study to use both yeast and C. elegans to study Aβ-induced toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraemer BC, et al. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci U S A. 2003;100(17):9980–5. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandt R, et al. A Caenorhabditis elegans model of tau hyperphosphorylation: induction of developmental defects by transgenic overexpression of Alzheimer’s disease-like modified tau. Neurobiol Aging. 2009;30(1):22–33. doi: 10.1016/j.neurobiolaging.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Miyasaka T, et al. Progressive neurodegeneration in C. elegans model of tauopathy. Neurobiol Dis. 2005;20(2):372–83. doi: 10.1016/j.nbd.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Buckingham SD, Sattelle DB. Strategies for automated analysis of C. elegans locomotion. Invert Neurosci. 2008;8(3):121–31. doi: 10.1007/s10158-008-0077-3. [DOI] [PubMed] [Google Scholar]
- 30.Rebolledo DL, et al. Copper Reduces Aβ Oligomeric Species and Ameliorates Neuromuscular Synaptic Defects in a C. elegans Model of Inclusion Body Myositis. The Journal of Neuroscience. 2011;31(28):10149–10158. doi: 10.1523/JNEUROSCI.0336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewald CY, Li C. Caenorhabditis elegans as a model organism to study APP function. Exp Brain Res. 2011 doi: 10.1007/s00221-011-2905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vattemi G, et al. Amyloid-β42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis. Acta Neuropathologica. 2009;117(5):569–574. doi: 10.1007/s00401-009-0511-6. [DOI] [PubMed] [Google Scholar]
- 33.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. The Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebert LE, et al. Alzheimer Disease in the US Population: Prevalence Estimates Using the 2000 Census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 35.Fonte V, et al. Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc Natl Acad Sci U S A. 2002;99(14):9439–44. doi: 10.1073/pnas.152313999. [DOI] [PMC free article] [PubMed] [Google Scholar]