Abstract
Allergic diseases rob corneal allografts of immune privilege and increase immune rejection. Corneal allograft rejection in BALB/c allergic hosts was analyzed using a short ragweed (SWR) pollen model of allergic conjunctivitis. Allergic conjunctivitis did not induce exaggerated T cell responses to donor C57BL/6 (B6) alloantigens or stimulate cytotoxic T lymphocyte (CTL) responses. Allergic conjunctivitis did affect T regulatory cells (Tregs) that support graft survival. Exogenous IL-4, but not IL-5 or IL-13, prevented Treg suppression of CD4+ effector T cells isolated from naïve mice. However, mice with allergic conjunctivitis developed Tregs that suppressed CD4+ effector T cell proliferation. In addition, IL-4 did not inhibit Treg suppression of IL-4Rα−/− CD4+ T cell responses, suggesting that IL-4 rendered effector T cells resistant to Tregs. SRW-sensitized IL-4Rα−/− mice displayed the same 50% graft survival as non-allergic WT mice, that was significantly less than the 100% rejection that occurred in allergic WT hosts, supporting the role of IL-4 in the abrogation of immune privilege. Moreover, exacerbation of corneal allograft rejection in allergic mice was reversed by administering anti-IL-4 antibody. Thus, allergy-induced exacerbation of corneal graft rejection is due to the production of IL-4, which renders effector T cells resistant to Treg suppression of alloimmune responses.
Keywords: Eye, Cornea, Allergy, T regulatory cells, Tolerance
Introduction
Corneal transplants are routinely performed without HLA-matching or the use of systemically administered immunosuppressive drugs, yet they enjoy an immune privilege that is unparalleled by other forms of solid organ transplantation. The leading indicator for corneal transplantation is keratoconus, which is a developmental anomaly in which the corneal epithelium thins and takes on a “cone” shape that disturbs the refractive properties of the cornea (2009 Eye Banking Statistical Report; www.restoressight.org).
Animal studies have shown that hosts with allergic conjunctivitis, airway hyperreactivity (AHR) (a form of allergic asthma), or corneal allografts engineered to over-express Th2 cytokines have a significant increase in the incidence and tempo of corneal allograft rejection (1–4). Moreover, there is evidence that atopic diseases increase the risk for corneal transplant rejection (5–10). This risk is not a problem once the underlying inflammation resolves. However, inflammation associated with viral keratitis does pose a risk factor for corneal allograft survival (5).
It has been suggested that deviating the immune response to a Th2 pathway would increase corneal allograft survival. However, IFN-γ−/− mice and WT mice treated with anti-IFN-γ antibody display strong Th2 alloimmune responses and experience a dramatic exacerbation of corneal allograft rejection (11, 12). Moreover, induction of allergic conjunctivitis in BALB/c mice elicits a robust Th2-dominated alloimmune response and a sharp increase in both the tempo and incidence of corneal allograft rejection (1, 13). Interestingly, corneal allografts placed in the “quiet” contralateral eyes in BALB/c mice that express allergic conjunctivitis only in the opposite eyes undergo the same elevated incidence and tempo of graft rejection that occurs in mice with allergic conjunctivitis in the grafted eye (1). This indicates that allergic conjunctivitis produces a systemic, not local, effect that abolishes the immune privilege of corneal allografts. Allergic diseases in other mucosal tissues also exacerbate corneal allograft rejection. As previously mentioned, mice with allergic AHR, an allergic inflammation of the lungs, experience exacerbated corneal allograft rejection (14). The exacerbation of corneal allograft rejection in hosts with allergic conjunctivitis is not simply due to underlying inflammation in one eye, as inserting sutures in one eye exacerbates the rejection of corneal allografts placed into the previously sutured eye while corneal allografts placed into the contralateral eye do not display an increased incidence or tempo of rejection (4). Interestingly, Larkin and colleagues showed that topical treatment with anti-histamines mitigated allergic conjunctivitis, yet did not prevent the increased corneal allograft rejection associated with allergic conjunctivitis, which further supports the notion that the exacerbation of corneal allograft rejection in allergic hosts is not due to local effects of a “hot” eye (15). Together, these studies suggest that the increased corneal allograft rejection that occurs in mice with allergic diseases of mucosal tissues or mice with a Th2-biased immune response (i.e., IFN-γ−/− mice) is due to a systemic effect and is not simply a result of the local effects of an inflamed eye.
A growing body of evidence indicates that corneal allograft survival is intimately correlated with the generation of CD4+CD25+ T regulatory cells (Tregs) (16, 17). It has also been shown that Tregs from mice that have accepted corneal allografts (graft acceptors) suppress the proliferation and activation of CD4+ effector T cells (11, 16). However, the effects of allergic inflammation, and more specifically the secretion of Th2 cytokines, on Tregs that support corneal allograft survival have not been studied.
In addition to allergic conjunctivitis and allergic AHR, other conditions are associated with increased corneal allograft rejection. The corneal graft bed is normally devoid of blood and lymph vessels, a condition that is believed to contribute to the immune privilege of the ocular surface. However, blood and lymph vessels can be induced to penetrate the prospective corneal graft bed by inserting sutures into the central cornea. Corneal allografts placed into such prevascularized graft beds induce robust allospecific cytotoxic T lymphocyte (CTL) responses and invariably undergo rejection (18). Thus, the generation of donor-specific CTL responses coincides with the termination of immune privilege.
In this study, we explored the underlying mechanisms whereby allergic conjunctivitis abolishes immune privilege and exacerbates corneal allograft rejection. In particular, we tested whether allergic conjunctivitis promotes the generation of allospecific CTL responses, which might account for the exaggerated graft rejection that occurs in allergic mice. Since CD4+CD25+ Tregs are required for corneal allograft survival, we entertained a second hypothesis that allergic conjunctivitis prevents the generation and function of Tregs.
Materials and Methods
Animals
Eight- to ten-week old female WT BALB/c (H-2d) mice, IL-4Rα−/− BALB/c, and B6 (H-2b) were purchased from Taconic Farms (Germantown, NY). Animals were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology statement about the Use of Animals in Ophthalmic and Vision Research.
Induction of allergic conjunctivitis
Allergic conjunctivitis was induced in IL-4Rα−/− and WT BALB/c mice as previously described (19). Mice were immunized intraperitoneally (i.p.) with 50 μg of SRW pollen (International Biologicals, Piedmont, OK) in 5 mg of alum (Thermo Fisher Scientific Pierce, Rockford, IL) on day 0. Allergic conjunctivitis was evoked by repeated topical challenges in which immunized mice were given 1.5 mg of short ragweed pollen in 10 μl PBS in the right eye from days 10 to 16. For grafted mice, SRW pollen was applied three times a week after application of corneal allografts. IL-4Rα−/− mice and WT mice were examined clinically for signs of immediate hypersensitivity responses 20 minutes after each topical challenge with SRW pollen or PBS (20).
Induction of corneal neovascularization
Vascularized, high-risk corneal graft beds were produced by placing three interrupted sutures (11-0 nylon, 50 μm diameter needle; Sharpoint, Vanguard, Houston, TX) into the central corneas of BALB/c mice two weeks prior to applying orthotopic corneal allografts (18).
Orthotopic corneal transplantation
Orthotopic corneal allografts were transplanted to the right eyes of allergic and naïve WT BALB/c and IL-4Rα−/− mice as previously described (1). All of the animals had non-vascularized corneas. Grafts were transplanted to allergic mice 17 days after the initial SRW pollen immunization. Grafts were scored based on corneal opacity as previously described (1).
CTL assay
A standard 4 hr. 51Cr-release assay was used as previously described (21). Briefly, single-cell suspensions of lymph node cells were prepared from BALB/c mice 1–7 days after they rejected their B6 corneal allografts. Lymph node cells from low-risk rejectors and allergic rejectors were stimulated in vitro in 96 well plates (Corning Inc., Corning, NY) along with 2×104 51Cr-labeled B6 endothelial cells or B6 Con A blasts in a total volume of 200 μl/well for 4h. Assays were performed in triplicate using effector to target cell ratio 50:1. Plates were centrifuged at 110 × G for 5 min before harvesting 100 μl of the supernatant from each well and counting in a gamma counter (Packard BioScience, Meriden, CT). Cytotoxicity was determined by the amount of 51Cr released by the target cells as previously described (21).
Mixed Lymphocyte Reaction
CD4+ T cells from acceptors, low-risk rejectors and allergic rejectors were isolated using the mouse CD4 isolation kit (Miltenyi Biotec). Purified CD4+ T cells were harvested 4–7 days after rejection and incubated at 1 X 105 per well with respective APC at a 1:1 ratio for 76 hours and then pulsed with 3H-thymidine and incubated for an additional 18 hours. Incorporation of 3H-thymidine was measured using a liquid scintillation counter.
Cytokine ELISA
WT BALB/c and IL-4Rα−/− BALB/c mice were killed 17 days post challenge and their spleens removed. Single-cell suspensions of splenocytes were prepared by gently processing between the ends of two sterile frosted slides. 1 x 107 cells/ml were incubated with 25 μg/ml of soluble SRW pollen extract (Greer Labs, Lenoir, NC, USA) for 48 h in 2 ml medium. Six hours before harvest, 1 μg/ml ionomycin (Sigma-Aldrich) and 25 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) were added to stimulate cytokine release. For MLR, CD4+ T cells were harvested 4–7 days after rejection and incubated at 1 X 105 per well with B6 APC at a 1:1 ratio for 96 hours. ELISAs for IL-4, IL-5, IL-13, and IFN-γ were performed on culture supernatants according to the manufacturer’s instructions (R&D Systems).
In vitro suppression assay
CD4+CD25+ Tregs were collected from spleens of cornea grafted mice 3 wk post-transplantation using Treg isolation kits (Miltenyi Biotec, Auburn, CA). A total of 5x104 CD4+CD25+ Tregs isolated from corneal allograft acceptors or allergic rejectors were incubated in round bottom, 96-well plates with 1x105 CD4+ T effector cells from naïve WT mice or IL-4Rα−/− mice in the presence or absence of 20 ng/ml of recombinant murine IL-4, IL-5, or IL-13. The cells were stimulated in vitro with 2 mg/ml anti-CD3ε Ab (BD Biosciences) for 76 hours and then pulsed with 3H-thymidine and incubated for an additional 18 hours. Incorporation of 3H-thymidine was measured using a liquid scintillation counter. % suppression = [(Teff cpm) − (Teff + Tregs cpm) / (Teff cpm)] x 100.
LAT assay
CD4+CD25+ Tregs from corneal allograft acceptors, low-risk rejectors, or allergic rejectors were mixed with BALB/c APC pulsed with C57BL/6 splenocytes and effector CD4+ T cells from BALB/c corneal allograft rejectors in a 1:1:1 ratio. Left and right ear pinnae of naïve BALB/c mice were injected with 20 μl (1 x 106) of the mixed-cell population in the presence or absence of 20 ng/ml of recombinant murine IL-4, IL-5, or IL-13. The opposite ear was injected with HBSS as a negative control. Ear swelling was measured 24 hours later to assess DTH.
Antibody treatment
Mice were treated with i.p. injections of 1mg rat anti-mouse IL-4 mAb (hybridoma HB188; American Type Culture Collection, Manassas, VA, USA) and rat-IgG isotype control (Sigma-Aldrich, St. Louis, MO, USA) beginning the day they received a corneal transplant and 3X/week thereafter.
Histology
Eyes from mice were removed 17 days after SRW pollen challenge and fixed in 10% formalin for histology. Paraffin-embedded tissue sections were stained with Congo Red. Differential cell counts were performed counting all inflammatory cells in the forniceal conjunctiva in the histologic section of each mouse. Inflammatory cells were counted in masked fashion by two investigators and were recorded as either eosinophils, neutrophils, or mononuclear.
Statistics
The log-rank test was used for statistical analysis of the differences in the tempo of corneal graft rejection from the Kaplan–Meier survival curves. Unless otherwise specified, all results are represented as mean ± SE. Comparison between the WT immunized and knockout immunized mice were made using Student’s t-test. Significance of the histological data was tested by Student’s t-test. P values less than 0.05 were considered significant.
Results
Allergic conjunctivitis does not lead to the generation of donor-specific CTL
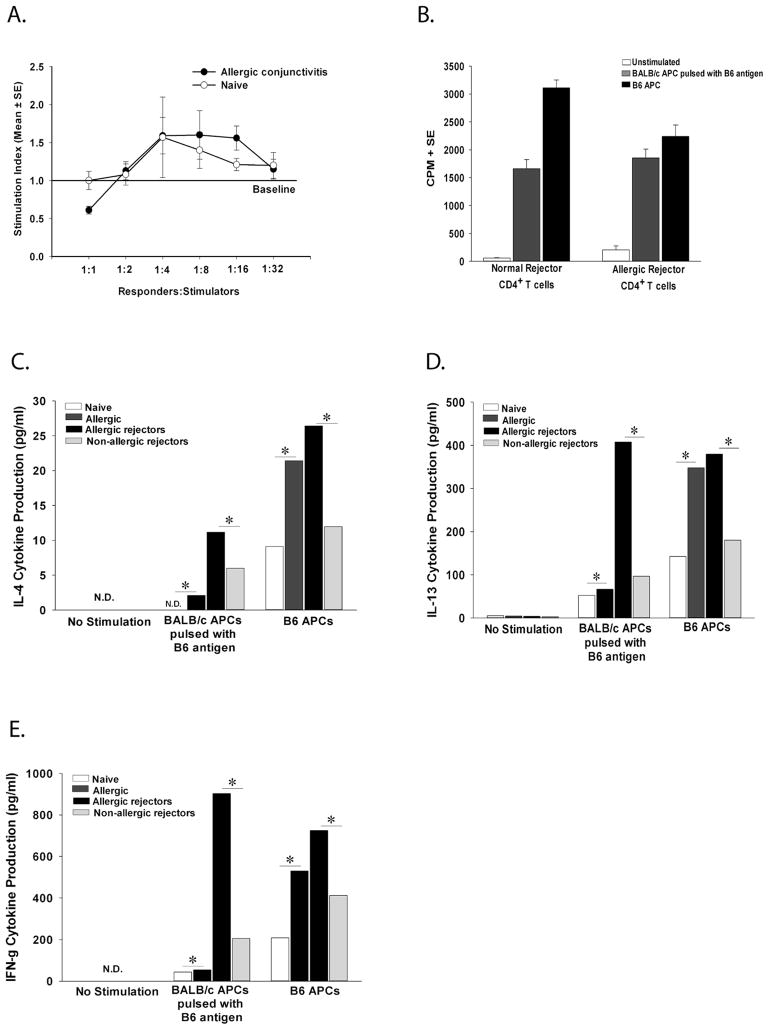
The presence of blood and in particular, lymph vessels in the corneal graft bed virtually ensures that immune rejection will occur (22–25). This increase in corneal allograft rejection is correlated with the development of donor-specific CD8+ CTLs that are not found in low-risk corneal allograft rejectors (18). Accordingly, we hypothesized that like other high-risk hosts, mice with allergic conjunctivitis generate donor-specific CTLs that are responsible for increased allograft rejection. To assess this, lymph node cells were isolated from BALB/c mice with or without allergic conjunctivitis one to seven days after they had rejected B6 corneal allografts (rejectors). Spleen cells were stimulated in vitro with B6 antigen presenting cells (APC). Lymph node cells were assessed for donor-specific CTL responses after a five-day in vitro boost. The results did not reveal elevated donor-specific CTLs in either the allergic conjunctivitis graft rejector group or the low-risk graft rejector group (Figure 1).
Figure 1.
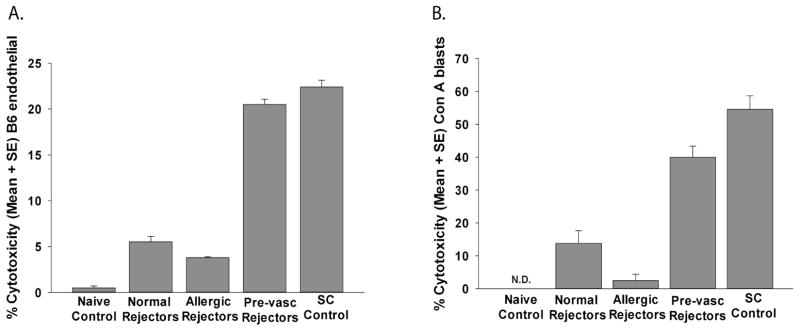
CTL responses directed against donor B6 alloantigens in grafted mice with allergic conjunctivitis. BALB/c mice with allergic conjunctivitis were grafted with B6 corneas 17 days after initial sensitization with SRW pollen. Vascularized graft beds were induced in other BALB/c mice by inserting 11-0 sutures into the central cornea 2 weeks prior to corneal transplantation. Lymph node cells were collected from allergic, low-risk, or mice with pre-vascularized graft beds 1–7 days after corneal allograft rejection. Lymph node cells were stimulated in vitro directly with mitomycin-C-treated B6 APCs for 96 hr. Responder cells were used in a CTL assay using (A) B6 corneal endothelial cells or (B) B6 Con A blasts target cells. Lymph node cells from BALB/c mice that had been immunized subcutaneously with B6 spleen cells served as positive controls. These results are representative of 2 independent experiments (N=4/group/experiment).
Mice with allergic conjunctivitis that receive corneal allografts do not generate enhanced T cell lymphoproliferative responses to donor alloantigens compared to non-allergic mice that reject corneal allografts
The presence of an ongoing inflammatory disease (i.e., allergic conjunctivitis) might result in the production of multiple cytokines and inflammatory molecules that act as adjuvants that stimulate heightened alloimmune responses in hosts receiving corneal allografts. This was tested by comparing primary lymphoproliferative responses in allergic and non-allergic mice. CD4+ T cells were collected immediately after the seventh topical challenge with SRW pollen and were cultured with BALB/c APC that were pulsed with B6 alloantigens to determine if allergic hosts mounted exaggerated primary lymphoproliferative responses. The results indicated that CD4+ T cells from mice with allergic conjunctivitis did not develop lymphoproliferative responses that were any greater than those found in naïve mice without allergic conjunctivitis (Figure 2A). We then assessed the lymphoproliferative responses of CD4+ T cells from allergic and low-risk mice that rejected B6 corneal allografts. The results indicated that CD4+ T cells from allergic rejectors and low-risk rejectors also had comparable secondary lymphoproliferative responses to B6 alloantigens (Figure 2B). It has been shown that lymph node cells from allergic rejectors produce more IL-4, IL-5, and IFN-γ compared to naïve mice and mice with only allergic conjunctivitis (1). Therefore, the levels of Th2 and Th1 cytokines were assessed in the CD4+ T cell population of allergic and low-risk mice following in vitro stimulation. CD4+ T cells from mice with allergic conjunctivitis and allergic rejectors produced significantly more IL-4, IL-13, and IFN-γ compared to naïve and low-risk rejector mice (Figure 2C–E). IL-5 was not detected in any of the groups.
Figure 2.
Proliferative responses of CD4+ T cells from naïve mice and mice with allergic conjunctivitis stimulated in vitro with B6 alloantigens. (A) Allergic conjunctivitis was induced with SRW pollen. CD4+ T cells from allergic (or naïve) mice were stimulated indirectly with BALB/c APC pulsed with B6 alloantigens. Lymphocyte proliferation was determined by 3H-thymidine incorporation 72 h later. This graph is representative of 3 independent experiments (N=5/group/experiment). (B) Mice with (or without) allergic conjunctivitis were grafted with B6 corneal allografts. CD4+ T cells were isolated 1–7 days after rejection and were stimulated indirectly with APC pulsed with B6 alloantigens or directly using B6 APC. Lymphocyte proliferation was determined by 3H-thymidine incorporation 96 h later. (C) CD4+ T cells from naïve, allergic, allergic rejectors, or non-allergic rejectors were isolated and stimulated with B6 APC for 96 h and assessed for IL-4, (D) IL-13, and (E) IFN-γ. These results are representative of two independent experiments (N=5/group/experiment). * p< 0.05.
IL-4 inhibits Treg suppression of effector T cells
We next turned our attention to the CD4+CD25+ Treg population and considered the hypothesis that allergic conjunctivitis impaired the induction or function of Tregs. This was examined by determining if Th2 cytokines, IL-4, IL-5, or IL-13, affected the suppressive function of Tregs induced by corneal transplantation. An in vitro suppression assay was employed in which CD4+CD25+ Tregs were isolated from the spleen of corneal allograft acceptors (day 21) and were co-cultured with anti-CD3-stimulated CD4+ effector T cells isolated from naïve mice. In Balb/c mice, Tregs represent about 3–5% of the total T cell population, therefore the spleen was used to obtain the desired number of Tregs needed for the suppression assay (26). Furthermore, Tregs isolated from the spleen of corneal transplant acceptors have been shown by other investigators to have suppressive function over effector T cells (16). As previously reported, we also found that Tregs from corneal graft acceptors suppressed the proliferation of anti-CD3-stimulated CD4+ effector T cells (Figure 3A) (11, 16). Interestingly, this suppression was abolished (Figure 3A) when IL-4 was added to the cultures. The suppression of CD4+ T cells by Tregs was unaffected by the presence of IL-5 or IL-13.
Figure 3.
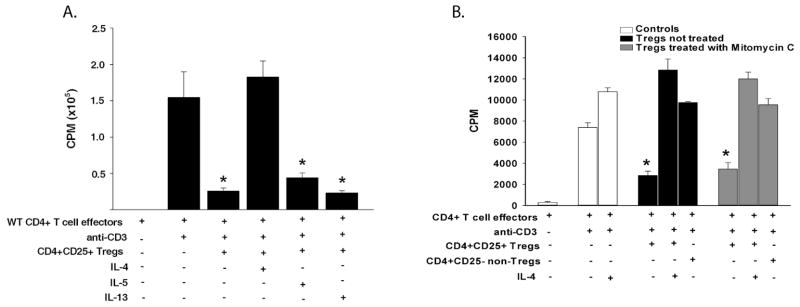
Suppressive activity of corneal allograft-induced Tregs in the presence of Th2 cytokines. (A) CD4+CD25+ Tregs were isolated from corneal allograft acceptors (day 21) and co-cultured in vitro with naïve anti-CD3-stimulated CD4+ T cells in the presence or absence of 25 ng/ml of Th2 cytokines (IL-4, IL-5, or IL-13). Proliferation was determined by 3H-thymidine incorporation 96 hr later. The results shown here are representative of 4 independent experiments (N=3/group/experiment). (B) In a separate experiment, CD4+CD25+ Tregs were isolated from corneal allograft acceptors (day 21) and treated with mitomycin-C for 30 min before co-culturing with naïve anti-CD3-stimulated CD4+ T cells in the presence or absence of 25 ng/ml of IL-4. Proliferation was determined by 3H-thymidine incorporation 96 h later. This graph is representative of 3 independent experiments (N=4/group/experiment). * p< 0.05.
Experiments were performed to confirm that the proliferation seen in these suppression assays was by effector T cells and not from Treg proliferation. Therefore, mitomycin-C-treated Tregs were tested in the presence or absence of IL-4. The results show that even when Tregs were unable to proliferate, they were still capable of suppressing effector T cell proliferation (Figure 3B). As seen in the previous experiments, Treg suppression of effector T cells was lost in the presence of IL-4 (Figure 3B).
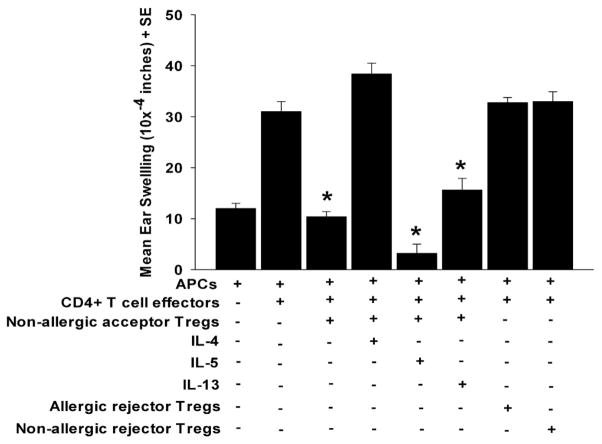
To confirm that IL-4 affects Treg suppressive activity in vivo, we performed a local adoptive transfer (LAT) assay (11). CD4+CD25+ Tregs were mixed with effector T cells, which were collected from corneal allograft rejector mice, and with APC, which had been pulsed in vitro with B6 alloantigens. The mixture of the three cell populations was injected into the left ears of naïve BALB/c mice in the presence or absence of IL-4, IL-5, or IL-13. Twenty-four hrs later, DTH was assessed by measuring ear swelling. The presence of CD4+CD25+ Tregs resulted in a significant reduction in ear swelling compared to mice that only received effector T cells and APC, indicating that co-injected Tregs suppressed DTH responses (Figure 4). However, the suppression of ear swelling was ablated when IL-4 was added to the co-injected cells. By contrast, suppression persisted when either IL-5 or IL-13 was co-injected with the three cell populations in the LAT assay. As expected no suppression was seen in the groups with CD4+CD25+ putative Tregs from allergic and low-risk rejectors (Figure 4). Together, these results suggest that IL-4, but not IL-5 or IL-13, is responsible for blocking Treg inhibition of effector T cell activity, either in vitro or in vivo.
Figure 4.
In vivo suppressive activity of CD4+CD25+ Tregs isolated from corneal allograft acceptors. CD4+CD25+ Tregs were isolated from corneal allograft acceptors on day 21 and were co-injected into the ears of naïve BALB/c mice along with CD4+ T cells from corneal allograft rejectors and APC pulsed with B6 alloantigens in the presence or absence of 25 ng/ml of IL-4, IL-5, or IL-13 in a LAT assay for measuring DTH ear swelling responses. This graph is representative of two independent experiments (N=5/group/experiment). * p< 0.05.
IL-4 renders effector T cells resistant to Treg suppression
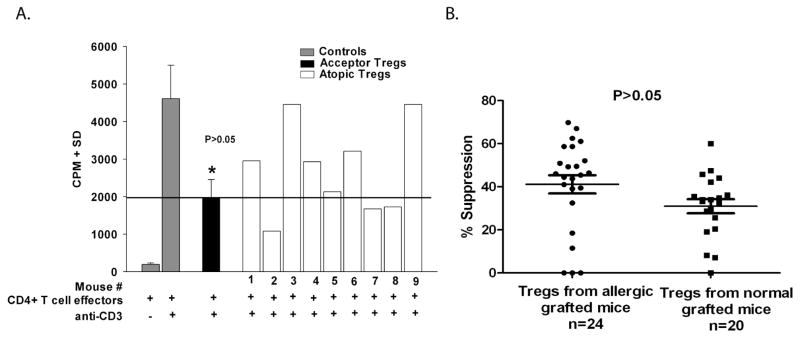
To determine if IL-4 directly affected Treg function, we assessed the suppressive ability of Tregs isolated from mice with allergic conjunctivitis that had also received corneal allografts. We have previously demonstrated that Tregs developed in low-risk BALB/c mice with avascular graft beds and that did not reject B6 corneal allografts (11). Since 50% of the low-risk BALB/c mice reject B6 corneal allografts, and do not display Tregs activity, we anticipated that none of the allergic mice would develop Tregs as 100% of such hosts reject their B6 corneal allografts. These experiments, like all of the experiments involving allergic conjunctivitis, were performed on hosts that had avascular graft beds and are normally deemed low-risk in the absence of allergic diseases. We isolated CD4+CD25+ Tregs from individual allergic mice that had received B6 corneal allografts and tested their suppressive activity against CD4+ effector cells from non-allergic mice in an in vitro suppression assay. As a control, a similar experiment evaluated Tregs isolated from individual non-allergic mice that had received B6 corneal allografts. The results show that approximately 50% (4/9) of the mice with allergic conjunctivitis displayed Treg activity equal to that found in non-allergic mice even though the allergic mice reject 100% of their corneal allografts (Figure 5A). In a follow up study, the suppressive profile of Tregs in each group was the same (p>0.05) (Figure 5B). These results suggest that Tregs develop in mice with allergic conjunctivitis and these Tregs can suppress CD4+ effector T cells that have not been exposed to an IL-4 rich environment.
Figure 5.
Suppressive profile of Tregs isolated from individual allergic and non-allergic grafted mice. (A) CD4+CD25+ Tregs were isolated from BALB/c mice without allergic conjunctivitis and bearing clear, accepted B6 corneal allografts on day 21 and were pooled prior for use in the in vitro suppression assay. CD4+CD25+ Tregs were isolated from individual BALB/c mice with allergic conjunctivitis that were grafted 21 days earlier. CD4+CD25+ Tregs from both groups were and were co-cultured with anti-CD3-stimulated CD4+ T cells from naïve WT BALB/c mice. Proliferation was determined by 3H-thymidine incorporation 96 h later. This experiment used pooled CD4+CD25+ Tregs from non-allergic mice and non-pooled CD4+CD25+ Tregs from nine individual mice with allergic conjunctivitis. p>0.05 compared to mice #2, 5, 7, and 9. (B) Similar experiments were performed using individual allergic and non-allergic mice that received B6 corneal allografts 21 days earlier. This graph shows data from 3 independent experiments with allergic mice (N=25 total) and 2 independent experiments with non-allergic mice (N=20 total). p>0.05.
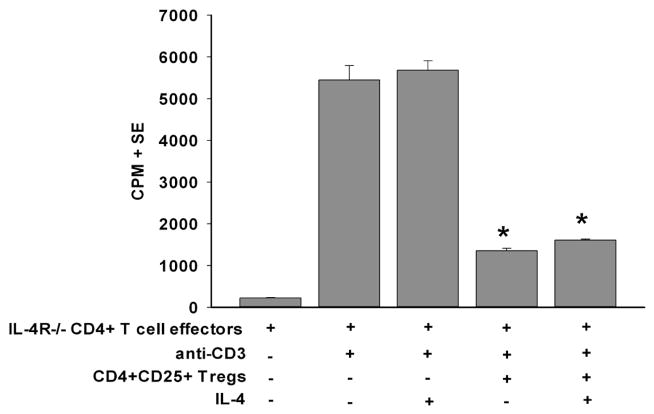
We hypothesized that in the in vivo setting IL-4 was produced in abundance and rendered effector T cells resistant to Treg suppression. To test this, we utilized effector T cells from IL-4Rα−/− mice, as these T cells cannot respond to IL-4. Accordingly, CD4+CD25+ Tregs were isolated from low-risk mice that had accepted their corneal allografts and were cultured with naïve anti-CD3-stimulated CD4+ IL-4Rα−/− effector T cells in the presence or absence of IL-4. CD4+CD25+ Tregs suppressed the proliferation of anti-CD3-stimulated CD4+ IL-4Rα−/− effector T cells in the absence of IL-4 (Figure 6). Importantly, suppression of IL-4Rα−/− effector T cells was unaffected when IL-4 was present in the cultures, demonstrating that Treg function is not affected in an IL-4-rich environment. Together, these results indicate that IL-4 enforces its effect on CD4+ effector T cells, and renders them resistant to suppression mediated by CD4+CD25+ Tregs.
Figure 6.
IL-4 renders CD4+ T cells resistant to suppression through IL-4R-mediated signaling. CD4+CD25+ Tregs were isolated from non-allergic BALB/c mice that harbored clear, surviving B6 corneal allografts on day 21 and were co-cultured with anti-CD3-stimulated CD4+ T cells from naïve IL-4Rα−/− BALB/c mice in the presence of absence of recombinant murine IL-4 (25 ng/ml). Proliferation was determined by 3H-thymidine incorporation 96 h later. This graph is representative of 3 independent experiments (N=4/group/experiment). * p<0.05 compared to effector cell groups stimulated with anti-CD3.
BALB/c IL-4Rα−/− mice are resistant to the exacerbation of corneal allograft rejection that is associated with allergic conjunctivitis
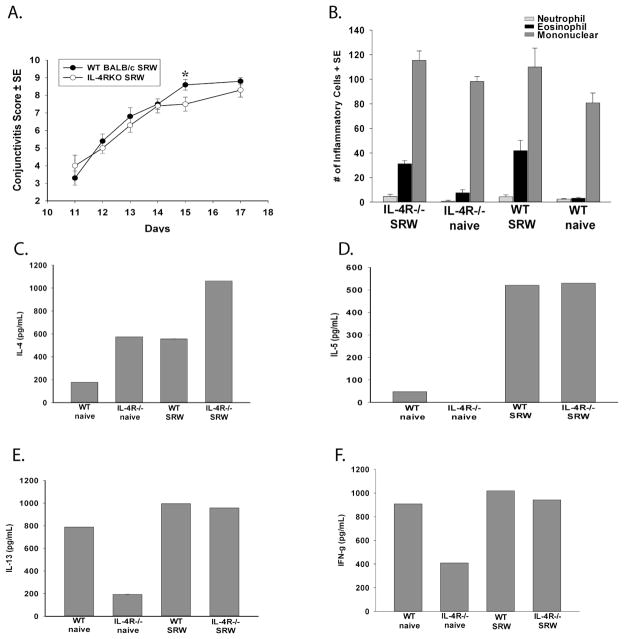
The results from the in vitro suppression assay indicated that IL-4 rendered CD4+ T cells resistant to suppression by CD4+CD25+ Tregs. If the same principle applies to the in vivo setting, IL-4Rα−/− mice should be resistant to the untoward effects of IL-4 and as a result, corneal allograft survival in these mice should mimic that in WT non-allergic mice (i.e., 50% corneal allograft acceptance). To assess this, it was important to determine if, and to what degree, IL-4Rα−/− mice developed allergic conjunctivitis. Allergic conjunctivitis was induced as previously described (27) and the early phase of allergic conjunctivitis was assessed clinically within 20 min of each daily topical challenge with SRW pollen (i.e. days 10–16) and the late phase was assessed at the end of the 7 day challenge (28). IL-4Rα−/− mice developed the clinical phenotype of allergic conjunctivitis that was comparable to WT mice (Figure 7A). There were also no differences in the late phase responses in IL-4Rα−/− mice compared to WT mice (Figure 7B). We also determined the cytokine profile of splenocytes from IL-4Rα−/− and WT mice and found that splenocytes from IL-4Rα−/− mice secreted Th2 cytokines, IL-4, IL-5, and IL-13, and the Th1 cytokine, IFN-γ, to the same degree as WT mice when stimulated with SRW allergens (Figure 7C–F).
Figure 7.
IL-4Rα−/− mice express clinical and histological features of allergic conjunctivitis that are similar to WT mice. (A) Clinical allergic conjunctivitis scores in WT BALB/c and IL-4Rα−/− mice sensitized and challenged with SRW pollen. The graph shows the average of 2 independent experiments (N=10 total). * p<0.05 for day 15; all other time points p>0.05. (B) Inflammatory infiltrates into the conjunctivae of SRW pollen-challenged mice. This graph is representative of 2 independent experiments (N=5/group/experiment). p>0.05 for the mononuclear cell counts between any of the groups. (C) IL-4, (D) IL-5, (E) IL-13, and (F) IFN-γ production by bulk spleen cell cultures from WT and IL-4Rα−/− mice with allergic conjunctivitis. Spleen cells were cultured in the presence of SRW extract. Supernatants were collected 72 h after in vitro culture and analyzed by ELISA. This graph is representative of 2 independent experiments (N=5/group/experiment).
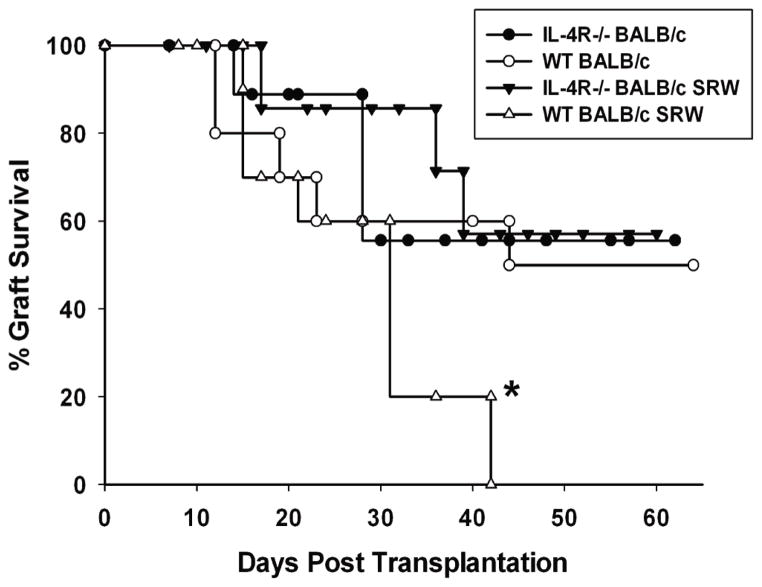
We next turned our attention to corneal graft survival in allergic IL-4Rα−/− mice and WT mice. Non-allergic IL-4Rα−/− mice had the same 50% corneal allograft survival as non-allergic WT that received corneal allografts (Figure 8). IL-4Rα−/− mice immunized with SRW pollen but challenged with the vehicle control also had 50% corneal allograft survival (data not shown; N=10). As has been previously reported (1), allergic BALB/c mice grafted with syngeneic BALB/c corneas in the present study had 100% graft survival (data not shown; N=10). Similarly, allergic IL-4Rα−/− mice grafted with IL-4Rα−/− corneas had 100% graft survival (data not shown; N=10). Interestingly, IL-4Rα−/− mice with allergic conjunctivitis also had a 50% incidence of corneal allograft survival, which was not significantly different from non-allergic WT controls (P>0.05). These results indicate that if CD4+ effector T cells are unresponsive to IL-4, they are amenable to Treg suppression.
Figure 8.
Survival curve for B6 corneal allografts transplanted orthotopically to IL-4Rα−/−BALB/c mice with allergic conjunctivitis. Allergic conjunctivitis was induced in IL-4Rα−/−(closed triangles) or WT BALB/c (open triangles) mice prior to receiving a B6 corneal allograft. As controls, naïve IL-4Rα−/− (closed circles) or WT BALB/c (open circles) mice were grafted with B6 corneal allografts. This graph is representative of 2 independent experiments (N=10/group/experiment). * p< 0.05 compared to the other 3 groups.
Neutralizing IL-4 restores immune privilege of corneal allografts in mice with allergic conjunctivitis
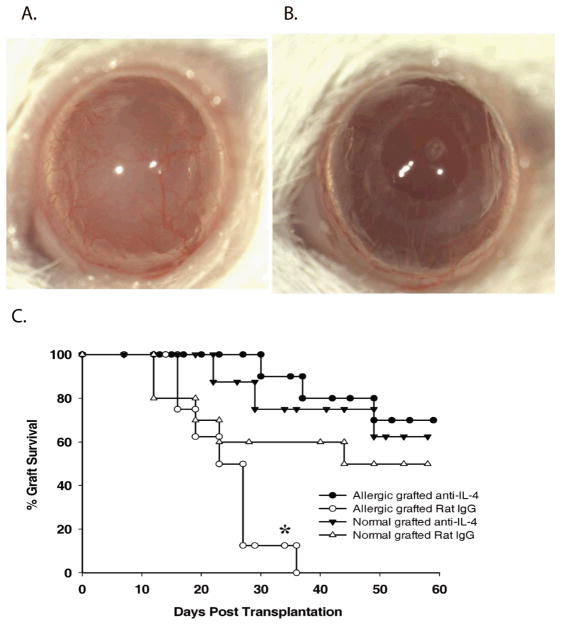
The results with IL-4Rα−/− mice indicated that circumventing the interaction between IL-4 and CD4+ T cells abrogated the exacerbation of corneal allograft rejection and restored immune privilege in mice with allergic conjunctivitis. Additional experiments were performed to determine if this principle could be applied in a clinically relevant setting. Accordingly, allergic conjunctivitis was induced in WT mice using SRW pollen and mice were challenged topically with SRW pollen as before. Treatment with either anti-IL-4 or an isotype control antibody was initiated on the same day that corneal allografts were applied (i.e., day 17 post i.p. immunization with SRW pollen). An additional group of mice that was not sensitized or challenged with SRW pollen was treated with anti-IL-4 antibody to determine if neutralizing this cytokine alone would affect corneal allograft survival. The results of three separate experiments show that anti-IL-4 treatment alone did not increase corneal allograft survival in low-risk mice (p>0.05) (Figure 9). However, anti-IL-4 treatment of mice with allergic conjunctivitis resulted in 70% graft survival (p<0.05) (Figure 9). These results indicate that neutralizing IL-4 in vivo restores immune privilege in mice with allergic conjunctivitis, presumably by rendering effector T cells amenable to Treg suppression even in the presence of IL-4 generated through the continuous exposure of the recipients to SRW allergens.
Figure 9.
Corneal allograft survival in BALB/c mice with allergic conjunctivitis and treated with anti-IL-4. Allergic conjunctivitis was induced in WT BALB/c mice. Anti-IL-4 (closed circles) or isotype control (open circles) antibody was administered on the day of transplantation and three times/week thereafter. Low-risk mice were also treated with anti-IL-4 (closed triangles) or isotype control (open triangles) antibody. (A) Clinical photograph of corneal allograft on a mouse with allergic conjunctivitis and treated with isotype control antibody (day 21). (B) Clinical photograph of corneal allograft on a mouse with allergic conjunctivitis and treated with anti-IL-4 antibody (day 21). (C) Graft survival curves for mice with or without allergic conjunctivitis and treated with either anti-IL-4 or an isotype control antibody. This graph is representative of two independent experiments (N = 10 mice/group/experiment). * p< 0.05 for allergic mice treated with normal rat IgG compared to anti-IL-4-treated allergic mice.
Discussion
Animal studies have shown that corneal allograft rejection is dependent on CD4+ T cells, as depletion of these cells by antibody treatment or by gene deletion results in increased graft survival (20, 29–31). Thus, a simple explanation for the exacerbation of corneal allograft rejection in hosts with allergic conjunctivitis is that the host’s CD4+ T cell-mediated immune responses are exaggerated as a result of the Th2 cytokine milieu. However, the present findings indicate that neither the primary nor the secondary CD4+ T cell lymphoproliferative responses to donor alloantigens were any greater in mice with allergic conjunctivitis than they were in non-allergic mice.
Although CD4+ T cells are required for the immune rejection of corneal allografts, CD8+ T cells can mediate rejection in the absence of CD4+ T cells (32). Mice with prevascularized graft beds develop robust CTL responses to donor alloantigens and display a remarkable increase in the immune rejection of corneal allografts (18). However, the present findings indicate that the exacerbation of corneal allograft rejection that occurs in mice with allergic conjunctivitis is not related to up regulation of CD8+ T cell activity, as CTL responses were undetectable in either allergic mice or the non-allergic mice that had rejected their corneal allografts.
Corneal allograft survival in mice correlates with development of Foxp3+ CD4+CD25+ Tregs and adoptive transfer of Tregs into graft recipients prevents corneal allograft rejection (16). Our results indicate that allergic conjunctivitis does not affect the generation of CD4+CD25+ Tregs, but renders CD4+ effector cells resistant to Treg-mediated suppression. The alloimmune response in mice with allergic conjunctivitis is heavily tilted toward a Th2 phenotype (1). The present findings indicate that IL-4, but not IL-5 or IL-13, prevents Treg suppression of alloimmune responses both in vitro and in vivo. Further analysis indicated that IL-4 imposed its influence on CD4+ effector T cells, rendering them unresponsive to the suppressive effects of CD4+CD25+ Tregs. Studies assessing the effect of IL-4 on CD4+CD25+ Treg function have yielded variable results. IL-4 has been shown to prevent spontaneous apoptosis of Tregs and the down regulation of Foxp3 mRNA in vitro, as well as enhancing CD25 expression on Tregs (33). IL-4 and IL-13 have also been shown to induce the generation of Foxp3+CD25+ Tregs from CD25− precursor T cells (34). IL-4 also enhances the ability of Tregs to inhibit proliferation of effector T cells, as well as production of IFN-γ (33, 34). By contrast, one study found that TGF-β-induced Foxp3 levels were maintained by blocking IL-4 or deleting the STAT6 gene (35). Furthermore, the presence of IL-4 at the time of T cell priming inhibited Foxp3 expression, while blocking IL-4 promoted Foxp3+ Treg differentiation (36, 37). Inhibition of Foxp3+ Treg induction requires full activation of the central signaling pathways for Th1/Th2 lineage differentiation (T-bet, STAT1, and STAT6) (36). Interestingly, one study found that while IL-4 was required for maintaining Foxp3 expression in Tregs and promoting their proliferation, it compromised Treg-mediated suppression (38).
Although we have no direct proof, we favor the hypothesis that Tregs act at the graft/host interface during the effector phase of graft rejection rather than in the regional lymph node at the initial alloantigen priming step. This proposition is based on the observation that Tregs from corneal allograft survivors suppress previously sensitized allospecific T cells in LAT assays (11). In the allergic host, CD4+ T cells are polarized to a Th2 phenotype and produce IL-4 when confronted with B6 alloantigens in vitro (1). Thus, in the allergic host, CD4+ T cells that enter the graft would be exposed to B6 alloantigens and as a result would generate IL-4, as they do in vitro, which would have an autocrine effect of CD4+ effector cells thereby rendering them resistant to Treg suppression.
The notion that IL-4 exerts its influence on CD4+ effector T cells was further demonstrated in experiments in which we found that IL-4Rα−/− mice 4Rα−/− mice, which are unable to respond to IL-4. CD4+ T cells from IL-4Rα−/− mice were amenable to Treg suppression even in the presence of IL-4. Moreover, IL-4Rα−/− mice with allergic conjunctivitis displayed the same incidence and tempo of corneal allograft rejection as non-allergic WT mice, indicating that the exacerbation of corneal allograft rejection in mice with allergic conjunctivitis occurs through an IL-4 signaling pathway. Although signaling through the IL-4 receptor is not an option in IL-4−/− mice, they nonetheless develop allergic conjunctivitis to the same severity as WT controls (39–41). On the surface this seems counterintuitive, however others have shown that signaling through the IL-4R is not essential for development of the Th2 phenotype. In allergic AHR, IL-13 can signal independently of the IL-4Rα chain to induce characteristic allergic disease (42).
The mechanisms whereby IL-4 renders CD4+ effector T cells resistant to Treg suppression remains to be elucidated. Effector T cells from patients with allergic asthma have a higher proliferative response when stimulated through their T cell receptor compared to healthy controls and are resistant to suppression by TGF-β and IL-10 (43). The resistance in suppression correlates with increased expression of MEK1, the upstream activator of ERK1/2. Inhibition of MEK1 expression rendered TGF-β treatment far more effective and inhibited T cell proliferation in asthmatic patients at a level that was comparable to the T cell proliferation observed in healthy subjects (43).
There is the possibility that the up-regulation of MEK1 renders effector T cells resistant by affecting the surface expression of several molecules needed for Tregs to mediate their suppressive function. Tregs constitutively express Foxp3 and GITR mRNA, as well as CTLA-4 (44, 45). As previously mentioned, in corneal transplantation Treg suppression of effector T cells is dependent on CTLA-4, GITR, and membrane-bound TGF-β (11). Other groups have confirmed that Treg suppressive function is related to the expression of both CTLA-4 and TGF-β that inhibits the expression of IL-2 receptor on effector T cells (46, 47). If MEK1 is up-regulated in effector T cells in hosts with allergic conjunctivitis, this could be enhancing the expression of IL-2 receptor. This over-expression of IL-2 receptor may counter-act the suppression mediated by Tregs. Treg inhibition of CD4+CD25− T cell proliferation has also been shown to be abrogated by anti-CTLA-4 mAb or by its Fab fragment (48). CTLA-4 functions by interacting with CD28 on an effector T cell resulting in inhibition of proliferation. The resistance of effector T cells under allergic conditions could be due to an altered expression in CD28 compared to the expression seen on effector T cells under non-allergic conditions. Interestingly, CTLA-4-deficient Tregs could still suppress through TGF-β and IL-10 in vitro and in vivo (44, 45). It is possible the TGF-β receptor or IL-10 receptor expression on an effector T cell is down-regulated under allergic conditions. This down-regulation could affect their ability to be suppressed. Further studies need to be conducted to determine the expression of these molecules on effector T cells under allergic and non-allergic conditions. It is interesting to note that although MEK1 is upregulated in asthmatic patients, this upregulation is not seen in patients with allergic rhinitis, suggesting that a Th2 phenotype alone, does not lead to increased MEK1 expression (43).
Our findings confirm and extend previous reports indicating that allergic diseases represent a risk factor for corneal allograft survival in both animals and atopic patients and indicate that IL-4 is a key player in disabling Treg-mediated suppression of immune effector cells. However, the risk for corneal allograft rejection in allergic hosts can be reversed through the administration of anti-IL-4 antibody. The prospect of simultaneously blocking IL-4 with antibody and inhibiting MEK1 expression with pharmacological agents might lead to the restoration of immune privilege and improved survival of corneal transplants in patients with seasonal allergic conjunctivitis.
Acknowledgments
This work supported by NIH grants EY0007641 and EY020799, and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Disclosure
This manuscript was not prepared or funded by a commercial organization. The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005;174(11):6577–6581. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- 2.Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest Ophthalmol Vis Sci. 2007;48(9):4044–4049. doi: 10.1167/iovs.06-0973. [DOI] [PubMed] [Google Scholar]
- 3.Klebe S, Sykes PJ, Coster DJ, Krishnan R, Williams KA. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation. 2001;71(9):1214–1220. doi: 10.1097/00007890-200105150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(5):1017–1026. doi: 10.1111/j.1600-6143.2009.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easty D, Entwistle C, Funk A, Witcher J. Herpes simplex keratitis and keratoconus in the atopic patient. A clinical and immunological study. Trans Ophthalmol Soc U K. 1975;95(2):267–276. [PubMed] [Google Scholar]
- 6.Hargrave S, Chu Y, Mendelblatt D, Mayhew E, Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. Am J Ophthalmol. 2003;135(4):452–460. doi: 10.1016/s0002-9394(02)02055-x. [DOI] [PubMed] [Google Scholar]
- 7.Kuchle M, Cursiefen C, Nguyen NX, Langenbucher A, Seitz B, Wenkel H, et al. Risk factors for corneal allograft rejection: intermediate results of a prospective normal-risk keratoplasty study. Graefes Arch Clin Exp Ophthalmol. 2002;240(7):580–584. doi: 10.1007/s00417-002-0496-5. [DOI] [PubMed] [Google Scholar]
- 8.Lyons CJ, Dart JK, Aclimandos WA, Lightman S, Buckley RJ. Sclerokeratitis after keratoplasty in atopy. Ophthalmology. 1990;97(6):729–733. doi: 10.1016/s0161-6420(90)32523-x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NX, Martus P, Seitz B, Cursiefen C. Atopic dermatitis as a risk factor for graft rejection following normal-risk keratoplasty. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2009;247(4):573–574. doi: 10.1007/s00417-008-0959-4. [DOI] [PubMed] [Google Scholar]
- 10.Reinhard T, Moller M, Sundmacher R. Penetrating keratoplasty in patients with atopic dermatitis with and without systemic cyclosporin A. Cornea. 1999;18(6):645–651. doi: 10.1097/00003226-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Cunnusamy K, Chen PW, Niederkorn JY. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186(12):6737–6745. doi: 10.4049/jimmunol.1100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargrave SL, Hay C, Mellon J, Mayhew E, Niederkorn JY. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004;45:1188–1193. doi: 10.1167/iovs.03-0515. [DOI] [PubMed] [Google Scholar]
- 13.Flynn TH, Ohbayashi M, Dawson M, Larkin DF, Ono SJ. The effect of perioperative allergic conjunctivitis on corneal lymphangiogenesis after corneal transplantation. Br J Ophthalmol. 2011;95(10):1451–1456. doi: 10.1136/bjo.2010.201939. [DOI] [PubMed] [Google Scholar]
- 14.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. Am J Transplant. 2009;9(5):1017–1026. doi: 10.1111/j.1600-6143.2009.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin DF, Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ. Early treatment of perioperative allergic conjunctivitis prolongs corneal allograft survival. ARVO abstract #1302. 2006;2006 (page 61 of abstract book) [Google Scholar]
- 16.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunnusamy K, Paunicka K, Reyes N, Yang W, Chen PW, Niederkorn JY. Two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010;51(12):6566–6574. doi: 10.1167/iovs.10-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ksander BR, Sano Y, Streilein JW. Role of donor-specific cytotoxic T cells in rejection of corneal allografts in normal and high-risk eyes. Transpl Immunol. 1996;4(1):49–52. doi: 10.1016/s0966-3274(96)80034-7. [DOI] [PubMed] [Google Scholar]
- 19.Reyes NJ, Mayhew E, Chen PW, Niederkorn JY. NKT cells are necessary for maximal expression of allergic conjunctivitis. Int Immunol. 2010;22(8):627–636. doi: 10.1093/intimm/dxq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He YG, Ross J, Niederkorn JY. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest Ophthalmol Vis Sci. 1991;32(10):2723–2728. [PubMed] [Google Scholar]
- 21.Hegde S, Niederkorn JY. The role of cytotoxic T lymphocytes in corneal allograft rejection. Invest Ophthalmol Vis Sci. 2000;41(11):3341–3347. [PubMed] [Google Scholar]
- 22.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22(3):273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Cursiefen C, Ikeda S, Nishina PM, Smith RS, Ikeda A, Jackson D, et al. Spontaneous corneal hem- and lymphangiogenesis in mice with destrin-mutation depend on VEGFR3 signaling. Am J Pathol. 2005;166(5):1367–1377. doi: 10.1016/S0002-9440(10)62355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25(4):443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 25.Cursiefen C, Rummelt C, Junemann A, Vorwerk C, Neuhuber W, Kruse FE, et al. Absence of blood and lymphatic vessels in the developing human cornea. Cornea. 2006;25(6):722–726. doi: 10.1097/01.ico.0000214230.21238.3d. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Oppenheim JJ, Howard OM. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25− responder T cells than C57BL/6 mice. J Leukoc Biol. 2005;78(1):114–121. doi: 10.1189/jlb.0604341. [DOI] [PubMed] [Google Scholar]
- 27.Reyes NJ, Mayhew E, Chen PW, Niederkorn JY. NKT cells are necessary for maximal expression of allergic conjunctivitis. Int Immunol. 2010;22(8):627–636. doi: 10.1093/intimm/dxq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laitinen A, Laitinen LA. Pathology of asthma. Allergy Proc. 1994;15(6):323–328. doi: 10.2500/108854194778816436. [DOI] [PubMed] [Google Scholar]
- 29.Ayliffe W, Alam Y, Bell EB, McLeod D, Hutchinson IV. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br J Ophthalmol. 1992;76(10):602–606. doi: 10.1136/bjo.76.10.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation. 2005;79(1):23–31. doi: 10.1097/01.tp.0000147196.79546.69. [DOI] [PubMed] [Google Scholar]
- 31.Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999;40(11):2614–2621. [PubMed] [Google Scholar]
- 32.Niederkorn JY, Stevens C, Mellon J, Mayhew E. CD4+ T-Cell-Independent Rejection of Corneal Allografts. Transplantation. 2006;81(8):1171–1178. doi: 10.1097/01.tp.0000203140.70742.cb. [DOI] [PubMed] [Google Scholar]
- 33.Maerten P, Shen C, Bullens DM, Van Assche G, Van Gool S, Geboes K, et al. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J Autoimmun. 2005;25(2):112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175(9):6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 35.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, et al. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283(22):14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104(46):18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS biology. 2007;5(12):e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol. 2009;183(1):155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balic A, Harcus YM, Taylor MD, Brombacher F, Maizels RM. IL-4R signaling is required to induce IL-10 for the establishment of T(h)2 dominance. Int Immunol. 2006;18(10):1421–1431. doi: 10.1093/intimm/dxl075. [DOI] [PubMed] [Google Scholar]
- 40.Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul WE. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Found Symp. 1997;204:208–216. doi: 10.1002/9780470515280.ch14. discussion 216–209. [DOI] [PubMed] [Google Scholar]
- 42.Mattes J, Yang M, Siqueira A, Clark K, MacKenzie J, McKenzie AN, et al. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J Immunol. 2001;167(3):1683–1692. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 43.Liang Q, Guo L, Gogate S, Karim Z, Hanifi A, Leung DY, et al. IL-2 and IL-4 stimulate MEK1 expression and contribute to T cell resistance against suppression by TGF-beta and IL-10 in asthma. J Immunol. 2010;185(10):5704–5713. doi: 10.4049/jimmunol.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177(7):4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9(3):239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. The Journal of experimental medicine. 2002;196(3):379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosmi L, Liotta F, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103(8):3117–3121. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]