Abstract
Purpose
To investigate possible association between mental stress and psychosomatic symptoms, socioeconomic status, lifestyle, as well as incident mortality in a middle-aged female population followed over 37 years.
Methods
A prospective observational study initiated in 1968–1969, including 1462 women aged 60, 54, 50, 46, and 38 years, with follow-ups in 1974–1975, 1980–1981, and 2000–2001, was performed. Measures included self-reported mental stress as well as psychosomatic symptoms and smoking, physical activity, total cholesterol, S-triglycerides, body mass index, waist–hip ratio, blood pressure, socioeconomic status and mortality.
Results
Smoking, not being single, and not working outside home were strongly associated with reported mental stress at baseline. Women who reported high mental stress in 1968–1969 were more likely to report presence of abdominal symptoms (odds ratio [OR] = 1.85, 95% confidence interval [CI]: 1.39–2.46), headache/migraine (OR = 2.04, 95% CI: 1.53–2.72), frequent infections (OR = 1.75, 95% CI: 1.14–2.70), and musculoskeletal symptoms (OR = 1.70, 95% CI: 1.30–2.23) than women who did not report mental stress. Women without these symptoms at baseline 1968–1969, but with perceived mental stress were more likely to subsequently report incident abdominal symptoms (OR = 2.15, 95% CI: 1.39–3.34), headache/migraine (OR = 2.27, 95% CI: 1.48–3.48) and frequent infections (OR = 2.21, 95% CI: 1.12–4.36) in 1974–1975 than women without mental stress in 1968–1969. There was no association between perceived mental stress at baseline and mortality over 37 years of follow-up.
Conclusion
Women reporting mental stress had a higher frequency of psychosomatic symptoms than women who did not report these symptoms. Not working outside home and smoking rather than low socioeconomic status per se was associated with higher stress levels. Perception of high mental stress was not associated with increased mortality.
Keywords: cardiovascular disease, mental stress, mortality, psychosomatic symptoms, population study, women
Introduction
Mental stress and its association with morbidity and mortality has long been studied, with contradictory results, especially concerning women.1 One reason for these conflicting results is that the concept of mental stress has been defined in several different ways. Hinton and Burton’s definition of mental stress describes “a given individual in a given life situation with perceived lack of control over the situation, reacts with specific emotions, reduced efficiency of performance, and psychosomatic symptoms”.2 The American Institute of Stress defines mental stress as “physical, mental, or emotional strain or tension as well as a condition or feeling experienced when a person perceives that demands exceed the personal and social resources the individual is able to mobilize”,3 indicating that perception of mental stress widely varies between individuals. Perception of mental stress in populations also varies with time. Mental stress can lead to a long-term deregulation of allostasis and may result in somatic symptoms through a series of neural and endocrine mechanism.4 One possible mechanism through which psychosocial stress may influence the risk of coronary heart disease is progression of artery calcification as a reaction of high levels of cortisol.5
Mental stress has been observed to be associated with a number of symptoms and diseases, such as cardiovascular diseases (CVD) including stroke6–9 and myocardial infarction,10 where the patients with myocardial infarction reported higher prevalence of working stress during the previous year than did individuals without myocardial infarction. A Swedish study of women who had suffered from myocardial infarction indicated that mental stress from family or work life may accelerate coronary disease processes over 3 years, whereas relative protection may be obtained from a satisfactory job and a happy marriage.11
Mental stress has also been associated with psychosomatic symptoms such as sleep problems, gastrointestinal symptoms, and joint, back, or muscular pain. In women working within the health care system, sleep problems were connected with decreased resilience to stress,12 and mental stress predicted multiple-site musculoskeletal pain among female kitchen workers and musculoskeletal pain predicted mental stress in a cumulative process.13 Association between mental stress and asthma as well as an increased risk of acute infectious respiratory illness has been reported.6,14,15 Mental stress has been shown to be a central contributor to primary headache.16 Persons who were older, female, black, previously married, and of lower socioeconomic status (SES) were more likely than others to develop psychosomatic symptoms and conditions over 3 years, but the risk was significantly higher only in those of lower SES.17
There has been a long-standing discussion about associations between personality, depression, stress, and stressful life events, respectively, and the development of malignant neoplasms, but the results are inconclusive. For example in a Scottish study, the authors found that men with reported high stress levels were protected against mortality from smoking-related cancers, but they interpreted the results as a product of confounding factors and of no causal significance.18 The association between stress and breast cancer has been studied, mostly using case-control designs. Women reporting experience of stress during the 5 years preceding the first examination displayed a twofold rate of breast cancer compared with women reporting no stress during 24 years of follow up.19,20 The presence or absence of physical and mental/psychological risk factors for mammary cancer were studied to predict mortality, and it was found that the physical factors were more predictive than mental, but both interacted to predict mortality in 15 years.21
A recent report from the prospective Population Study of Women in Gothenburg, including 1270 women who represented the population, showed that perceived mental stress levels at a group level increased over 36 years in two generations of 38- and 50-year-old Swedish women.22 In that report as well as in the present study, the women’s own perception of mental stress was used.
Most of the related studies have follow-up times not exceeding 10 years. In the Population Study of Women in Gothenburg, the follow-up time now exceeds 30 years, and makes possible both the short- and long-time assessment of the effects of perception of high mental stress on morbidity and mortality in middle-aged women.
The aims of this study were to investigate the association between perceived mental stress and psychosomatic symptoms, and the association between perceived mental stress and mortality. Secondary objectives were to examine the relation between SES and selected lifestyle factors, on one hand, and symptoms on the other. Our hypothesis was that women with perceived mental stress had a higher frequency of psychosomatic symptoms as well as higher risk of increased mortality in a long-time perspective.
Participants and methods
Study of Women in Gothenburg 1968–1969 until 2000–2001
In 1968–1969, 1462 women in Gothenburg, Sweden, aged 38, 46, 50, 54, and 60 years, participated in the Prospective Population Study of Women in Gothenburg.23 The sampling method was based on date of birth, which, given the high participation rate (90.1%), ensured that the participants were representative of women from the community in the age groups studied.23
All women examined in 1968–1969 were offered a second examination in 1974–1975,23,24 which 1302 women attended (89.1% of the original group). In 1980–1981, a third examination was conducted in 1154 participants (78.9% of the original group).25 In addition to data collected during the health examinations, the National Swedish Death Registry was used to identify the date of death until 2005.
Mental stress in the women at the different examinations
The prevalence of mental stress was based on a question describing the extent to which the women had experienced mental stress previously or presently (Table 1). The mental stress question was asked in exactly the same way in 1968–1969, 1974–1975, and 1980–1981.
Table 1.
Mental stress question: the women were asked by a physician whether they had had a feeling of mental stress for a month or longer, including tension, fear, anxiety or sleep disturbances connected with conflict in the family, at work etc
| Period of mental stress | |
| Have you experienced any period of mental stress (1 month or more), and with mental stress we mean that you have been | |
| Irritable | |
| Tense | |
| Nervous | |
| Anxious | |
| Afraid | |
| Anguished | |
| Sleepless | |
| Connected with concern for | |
| Your work | |
| Your health | |
| Your family | |
| Conflict with the people around you (at home, at work) | |
| Check one | |
| 0 = Never experienced mental stress | |
| 1 = Experienced mental stress, but not during the last 5 years | |
| 2 = Occasionally experienced mental stress during the last 5 years | |
| 3 = Experienced mental stress several times during the last 5 years | |
| 4 = Experienced mental stress constantly during the last year | |
| 5 = Experienced mental stress constantly during the last 5 years | |
To describe the prevalence of mental stress at baseline and its relation to anthropometric, lifestyle and sociodemographic variables (Table 2) or to psychosomatic symptoms (Table 3), we used a model with three levels of mental stress:
Table 2.
Mental stress and association to socio-demographic, anthropometric and lifestyle variables, cross-sectionally in 1968–1969
| Periods of mental stress | Mean (SD) | ||
|---|---|---|---|
|
|
|||
| Level 0–1 | Level 2 | Level 3–5 | |
|
|
|
|
|
| No period of mental stress during the last 5 years (n = 934) | Some periods of stress during the last 5 years (n = 204) | Several periods of stress or permanent stress (n = 277) | |
| Age (years) | 46.9 (6.2) | 46.7 (6.3) | 47.1 (6.2) |
| SBP (mmHg) | 134.5 (22.0) | 131.9 (21.1) | 131.5 (22.6)* |
| DBP (mmHg) | 86.1 (10.9) | 84.7 (9.8) | 85.0 (11.5) |
| BMI (kg/m2) | 24.2 (3.8) | 23.8 (3.6) | 23.9 (3.8) |
| WHR (cm/cm) | 0.74 (0.05) | 0.74 (0.05) | 0.74 (0.06) |
| S-triglycerides (mmol/L) | 1.22 (0.62) | 1.19 (0.48) | 1.31 (0.60)* |
| Total cholesterol (mmol/L) | 6.85 (1.24) | 6.83 (1.04) | 6.93 (1.22) |
| Blood glucose (mmol/L) | 4.17 (0.97) | 4.05 (0.72) | 4.05 (0.68)* |
| Total income in the household (SEK) | 39,700 (21,100) | 38,000 (18,600) | 34,100 (23,800)*** |
| n (%) | |||
|
|
|||
| Higher education | 266 (29) | 74 (37) | 86 (31) |
| Grown up in town | 649 (70) | 159 (78) | 197 (71) |
| Married | 757 (81) | 154 (76) | 187 (68)*** |
| Children (yes/no) | 750 (81) | 160 (80) | 224 (81) |
| Living in a house | 216 (23) | 35 (17) | 41 (15)** |
| Working outside home | 596 (64) | 132 (65) | 153 (56)* |
| Higher social class | 591 (63) | 136 (67) | 171 (62) |
| High LTPA# | 771 (83) | 168 (82) | 215 (78) |
| High OPA## | 263 (28) | 69 (34) | 86 (31) |
| Ever smoking | 389 (42) | 99 (49) | 156 (56)*** |
| Current smoking | 345 (37) | 92 (45) | 137 (49)*** |
Notes:
P < 0.05;
P < 0.01;
P < 0.001 significance level for test of trend across mental stress categories, linear regression for logarithms of continuous variables, Cochran–Armitage test of trend for binary variables;
leisure time physical activity;
occupational physical activity.
Abbreviations: LTPA, leisure time physical activity; OPA, occupational physical activity; SEK, Swedish Crones; WHR, waist hip ratio; BMI, body mass index; SBP, systolic blood pressure; DMP, diastolic blood pressure; SD, standard deviation.
Table 3.
Perception of mental stress at baseline, prevalence of symptoms in 1968 and incidence of symptoms in 1974
| 1968 (cross-sectional data) | 1974 (prospective data) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n68 | 2 vs none | 3–5 vs none | n74 | 2 vs none | 3–5 vs none | |
|
|
|
|
|
|||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Abdominal symptoms | 401 | 1.29 (0.92–1.80) | 1.94 (1.46–2.57)* | 139 | 1.02 (0.59–1.77) | 2.19 (1.42–3.39)* |
| 1.28 (0.92–1.80) | 1.85 (1.39–2.46) | 1.01 (0.58–1.75) | 2.15 (1.39–3.34)* | |||
| Obstructive symptoms | 308 | 1.30 (0.91–1.85) | 1.22 (0.89–1.68) | 109 | 1.97 (1.18–3.29) | 1.38 (0.83–2.29) |
| 1.23 (0.86–1.78) | 1.10 (0.79–1.53) | 1.93 (1.15–3.23) | 1.28 (0.77–2.15) | |||
| Headache/migraine | 402 | 1.25 (0.89–1.75) | 2.00 (1.48–2.61)* | 148 | 0.92 (0.53–1.59) | 2.27 (1.48–3.48)** |
| 1.29 (0.92–1.81) | 2.04 (1.53–2.72)* | 0.92 (0.53–1.59)¶ | 2.27 (1.48–3.48)**¶ | |||
| Frequent infections | 130 | 1.68 (1.03–2.75) | 1.93 (1.26–2.95) | 47 | 1.84 (0.84–4.03) | 2.21 (1.12–4.36) |
| 1.63 (0.99–2.68) | 1.75 (1.14–2.70) | 1.84 (0.84–4.03)¶ | 2.21 (1.12–4.36)¶ | |||
| Musculoskeletal symptoms | 564 | 1.16 (0.85–1.58) | 1.70 (1.30–2.23)* | 209 | 1.31 (0.84–2.04) | 1.21 (0.78–1.86) |
| 1.16 (0.85–1.58)¶ | 1.70 (1.30–2.23)*¶ | 1.29 (0.83–2.01) | 1.14 (0.74–1.77) | |||
| Hypertension | 290 | 0.72 (0.48–1.10) | 0.86 (0.61–1.23) | 148 | 1.16 (0.72–1.87) | 0.74 (0.45–1.20) |
| 0.78 (0.50–1.19) | 0.89 (0.62–1.28) | 1.20 (0.74–1.96) | 0.77 (0.47–1.27) | |||
Notes: Intermediate (level 2) and high level of mental stress (level 3–5) was compared with no experience of mental stress during the last 5 years (level 0–1). The upper line gives the result for mental stress in a model adjusted for age only, the lower line for the adjusted model, where covariates where selected from smoking (former, current, ever), OPA, LTPA, WHR, BMI by stepwise selection. The number of individuals with symptoms at baseline 1968 is denoted by n68; n74 gives the number of women with symptoms in 1974 among all women without symptoms at baseline.
P < 0.05;
P < 0.01 significance level for test of mental stress level 3–5 vs level 2;
no covariates were selected in addition to age and stress level.
Abbreviations: LTPA, leisure time physical activity; OPA, occupational physical activity; WHR, waist hip ratio; BMI, body mass index; OR, odds ratio; CI, confidence interval.
Mental stress level 0–1: No mental stress or no mental stress during the last 5 years
Mental stress level 2: occasionally experienced mental stress during the last 5 years
Mental stress level 3–5: experienced constant or several periods of mental stress during the last 5 years.
When comparing the prevalence of mental stress at different examinations (Figure 1A and B), we created a dichotomous variable, reflecting no experience of mental stress during the last 5 years (mental stress level 0–1) versus any mental stress experience during the last 5 years (mental stress level 2–5).
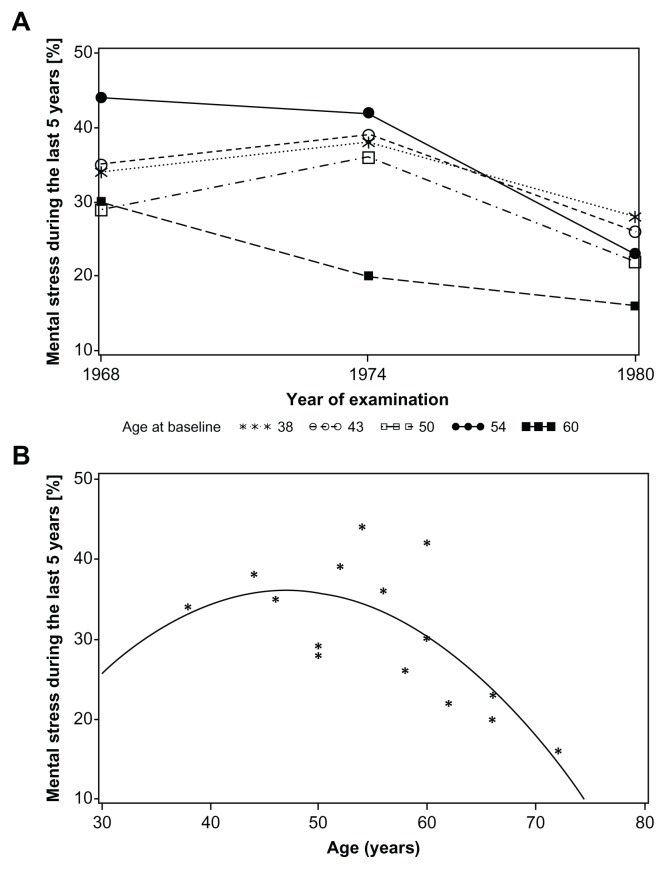
Figure 1.
Prevalence of mental stress during the last 5 years (level 2–5) versus year of examination longitudinally for each of the different birth cohorts (A) and versus age at which mental stress was reported (B).
The mental stress question has been used in different epidemiological studies20,26,27 and has been evaluated in studies of women with ischemic heart disease.28 It has been found to measure mental stress adequately compared to other psychometric instruments.
Psychosomatic symptoms
The prevalence of psychosomatic symptoms was based on a self-administered questionnaire and was defined as answering yes to any of the questions: “Have you had any of the following illnesses/symptoms: abdominal symptoms (constipation, diarrhea, or other abdominal symptoms); asthma/obstructive symptoms; headache/migraine, hypertension, infections (pneumonia or frequent colds as well as otitis, sinusitis or tonsillitis); musculoskeletal symptoms (joint or back problems or sciatica)?”. The subjects reported if they had experienced any of these symptoms (the time frame was not specified) and if they had consulted a doctor and/or been hospitalized because of symptoms, and if they still had these problems. The women were defined as having the symptoms if they had consulted a doctor, been hospitalized, and/or still had the symptoms. Women confirming having asthma or wheezing or using medication for asthma/obstructive symptoms or whose physical examination revealed wheezing were defined as having asthma/obstructive symptoms.
Hypertension/high blood pressure was defined as using antihypertensive medication and/or a systolic pressure > 160 mmHg and/or diastolic blood pressure > 95 mmHg at the time of examination.
In 1974–1975, participants were asked, with the same self-administered questionnaire, whether they had experienced psychosomatic symptoms since their first examination in 1968–1969.
Information on mortality from 1968–2005 was obtained from death certificates via the National Swedish Death Registry. Diabetes, used as a covariate in some analyses, was determined from health questionnaires, medical examinations, and/or the Swedish Hospital Discharge Register.
Measured CVD risk factors at baseline
Physical examinations were conducted according to the same protocol at all examinations with respect to body weight and height, body mass index (BMI), waist circumference, waist–hip ratio (WHR), systolic and diastolic blood pressure measurements (sitting position after 5 minutes rest).23 Blood samples were drawn after an overnight fast. Serum levels of total total cholesterol, S-triglycerides, and blood glucose were analyzed according to the standard methods of the laboratory of the Sahlgrenska University Hospital, Gothenburg.
Social and lifestyle-related variables
Several binary variables were created to describe lifestyle and education, ie, whether a woman had grown up in a city (1) or in the countryside (0), whether she lived in a private house (1) or not (0), whether she was married (1) or not (0) (note that marriage was the common form of partnership in Sweden 1968–1969 and was used as a proxy for ‘living together’), and whether she had children (1) or not (0). Women with more than compulsory education (ie, more than 6–7 years depending on age cohort) were classified as highly educated (1) vs those who only had attended elementary school (0). They were asked if they were working outside home (1) or not (0), and reported their own occupation and income and, if they were married, their husband’s occupation and income. For couples, the total income available in the household was calculated. The occupational information was transformed according to Carlsson’s standard occupations grouping system.29,30 We combined group one (large-scale employers and officials of high or intermediate rank) and groups two and three (small-scale employers, lower-ranked officials, and supervisors) into a “high and medium social class group”. Groups four and five (skilled and unskilled workers) were classified as a “lower social class group”. Couples were classified according to the higher social class index if both partners worked.
Women were classified as being physically active during leisure time (LTPA) if they reported, spending at least 4 hours a week during the last year gardening, running, dancing, and playing golf, tennis, or similar activities. Occupational physical activity (OPA) compared a medium-to-vigorous level of walking, moving, or lifting (eg, nurse, cleaner, etc, and housewife with two or more children) to a low level of physical work. Smoking status was categorized as current, former, or never smoking.
Statistical methods
Cochran–Armitage tests of trend and linear regression were used for categorical and continuous variables, respectively, to analyze changes of background variables with increasing level of mental stress in 1968–1969. The logarithm of continuous variables was used for those with a right-skewed distribution and the normal distribution of residuals was checked. Associations between psychosomatic symptoms in 1968–1969 and 1974–1975 (yes/no) and mental stress levels at baseline were tested in a binary logistic regression model, which always included age. The dose-response relationship between mental stress and outcome was tested by comparing the effect of the three highest mental stress categories combined (several periods or permanent mental stress over the last 5 years) with an intermediate mental stress level (some periods of mental stress during the last 5 years), vs no or former mental stress (mental stress levels 2 and 3–5 vs 0–1). For the association between symptoms 1974–1975 and mental stress at baseline, only women without symptoms at baseline were included. All associations were tested in a larger model where explanatory variables, in addition to mental stress and age, were chosen by stepwise selection from smoking, BMI, WHR, LTPA, and OPA. As a result, we define the odds ratio (OR) for being in a certain category relative to a reference category (categorical variables) or for increase by one standard deviation (continuous variables), together with a 95% confidence interval (CI).
To determine which socioeconomic variables (total income, marital status, education, etc) were associated with mental stress at baseline, we used ordinal logistic regression for mental stress (levels 0–1, 2, and 3–5) and stepwise selection of variables, using the score test to check the proportional odds assumption. Similarly, we applied ordinal logistic regression to model the sum of psychosomatic symptoms as a function of mental stress and covariates.
We used the Cox proportional hazard model to assess the association between mental stress, age, and covariates at baseline and mortality. The proportional hazard assumption for categorized variables was checked using the log-cumulative hazard plot. As a result, we give the hazard ratio (HR) and its 95% CI for being in a high (level 3–5) or intermediate (level 2) mental stress category, relative to no or former mental stress (level 0–1), adjusted for age and other covariates selected by stepwise selection.
Significance level was set at 0.05 (two-sided tests). SAS software (v. 9.2; SAS Institute, Cary, NC, USA) was used for the statistical analyses.
Results
Prevalence of mental stress and its association with sociodemographic, anthropometric, and lifestyle variables – 1968–1969
Out of 1415 women reporting on mental stress at baseline, 934 (66%) experienced no mental stress during the last 5 years, 204 (14%) reported some periods of mental stress, and 277 (20%) reported several periods or permanent mental stress.
Women who reported a higher level of mental stress were more often smokers and had lower mean systolic blood pressure, higher S-triglyceride levels, lower blood glucose levels, and less often diabetes at baseline. They were less likely to live in a house, to work outside home and were more often single than women with a lower level of mental stress. Their total income in the household was lower than for women who reported less mental stress (Table 2). Ordinal logistic regression of the three stress levels on all variables listed in Table 2 showed that smoking was associated with higher level of mental stress (ever vs never smoking: OR = 1.56, 95% CI: 1.24–1.96), while working outside home (OR = 0.60, 95% CI: 0.47–0.77) and being married (OR = 0.60, 95% CI: 0.44–0.81) were associated with a lower level of stress. Total income and SES were not associated with mental stress when adjusted for marital and working status.
Perception of mental stress and its association with psychosomatic symptoms: cross-sectional associations at baseline
The most commonly reported psychosomatic symptoms in 1968–1969 were musculoskeletal symptoms (40%), headache/migraine (28%), and abdominal symptoms (28%). There was a dose-response relationship for these three symptoms with increasing mental stress level, which persisted when adjusted for background variables such as smoking, BMI, WHR, LTPA and OPA (Table 3). High or intermediate mental stress was equally associated with frequent infections while stress was not related with obstructive symptoms or hypertension. Psychosomatic symptoms were also converted into a sum score. Collapsing the three highest categories (≥ 4 symptoms present, n = 74) and using ordinal logistic regression we found a dose response with respect to stress (level 2 vs 0–1: OR = 1.31, 95% CI: 1.00–1.72; levels 3–5 vs 0–1: OR = 2.03, 95% CI: 1.59–2.59; levels 3–5 vs 2: OR = 1.55, 95% CI: 1.12–2.14). Selected covariates were BMI (OR = 1.04, 95% CI: 1.01–1.08 for increase by 1 kg/m2), WHR (OR = 1.26, 95% CI: 1.02–1.55 for increase by 0.1) and smoking (former vs never: OR = 1.96, 95% CI: 1.35–2.83; current vs never: OR = 1.27, 95% CI: 1.04–1.56).
Perception of mental stress in 1968–1969 and incidence of psychosomatic symptoms at follow-up in 1974–1975
The prevalence of psychosomatic symptoms at follow-up was similar to their prevalence at baseline; most common were musculoskeletal symptoms (43%), headache/migraine (29%), and abdominal symptoms (26%). Among those participants who did not report a particular symptom at baseline, most new cases were reported for musculoskeletal symptoms (28%), headache/migraine (17%), and abdominal symptoms and hypertension (both 15%).
We found that a higher level of mental stress at baseline was not only associated with higher prevalence of abdominal symptoms, headache/migraine, and frequent infections at baseline, but also predicted the incidence of these symptoms 6 years later (Table 3, right columns). We used the 6-year prospective design to study stress at baseline as a risk factor for incident psychosomatic symptoms, ie, that were not present at baseline, in order to separate out chronology of possible cause and effect. Due to the reduced number of women that could be included in this analysis, the group of women with symptoms was too modest for longer follow-up conclusions.
The influence of other risk factors measured at baseline was reduced. Mental stress at baseline was not found to be associated with musculoskeletal symptoms, and did not predict incident hypertension. An analysis of a sum score of incident symptoms was not carried out since the data set was reduced to less than 400 women who did not experience any symptom at baseline.
Longitudinal observations of experience of mental stress
Figure 1A shows the percentage of women who experienced several periods of mental stress or more during the last 5 years (levels 2–5) compared to former or no periods of mental stress (levels 0–1) as reported at baseline (when the women were between 38 and 60 years old), 1974–1975 as well as 1980–1981 (when the women were between 50 and 72 years old).
The percentage of mental stress perception in the oldest cohorts, aged 54 and 60 years in 1968–1969 decreased after the first examination while it increased in women aged 38, 44, and 50 years to a maximum in 1974–1975, with a decrease thereafter again in the follow-up examination in 1980–1981. Prevalence of mental stress among women born in 1908 (60 years old at baseline) was lowest and declined to 16% in 1980–1981. The cohort of women born in 1914 (54 years old at baseline) showed the highest prevalence of mental stress, >40%, which is above the value for women aged 60 years in 1968 or 52–56 years in 1974. The prevalence of mental stress reported in 2000 was similar to its value in 1980 among the four younger cohorts (data not shown).
Figure 1B shows the prevalence of mental stress versus age including a quadratic polynomial fit. There was a broad maximum of prevalence of mental stress among women aged 46 to 54 years and a decline of mental stress after the age of 60 years.
Mental stress and associations with mortality: 37-year longitudinal follow-up
We investigated whether mental stress at baseline could predict total mortality using the Cox proportional hazard model. Among the 1415 who answered the mental stress question at baseline, 699 died before the end of 2005. There was a weak risk increase of mortality associated with high mental stress level (several periods of mental stress during the last 5 years or more [HR = 1.21, 95% CI: 1.01–1.45]). However, this association was reduced to nonsignificance when adjusted for other mortality risk factors of which the most important were current smoking (OR = 1.86, 95% CI: 1.59–2.18), triglyceride level (HR = 1.21, 95% CI: 1.10–1.33 for increase by 1 mmol/L) and diabetes (OR = 3.09, 95% CI: 1.76–5.42).
Discussion
We followed a cohort of middle-aged women and collected information about their experience of mental stress, psychosomatic symptoms, and a large set of anthropometric, sociodemographic, and lifestyle variables. Earlier studies have shown that stressful life events are predictive factors for long-term sick leave and disability pension among primary care patients31 and an important question today and for the future is the influence of mental stress on morbidity and mortality.
It was earlier observed that low income is associated with mental stress. Rates of reported psychological distress were much higher in low-income populations.32 In our study, lower total income was associated with higher mental stress at baseline in a univariate analysis, but the association was no longer observed when adjusted for being married and working outside home. Married women and women who worked outside home reported less mental stress than single and housewives, a finding also confirmed by other studies.32 These findings illustrate that single women and those not working outside home might have an increased need of preventive actions in the community aimed at reducing mental stress.
Among the physiological and lifestyle variables associated with mental stress, smoking was the most important. Smoking was also strongly associated with symptoms of psychological distress in the Australian community in a recently published study.33 The biological evidence presented earlier concerning neural and hormonal processes associated with smoking and stress has enhanced the understanding of administration of nicotine as well as other drugs of abuse and their neuroregulatory mechanisms. It is still unclear whether nicotine reduces stress among smokers or not, and smoking appears to have no effect on stress among relapsed smokers.34
We have previously shown that perception of mental stress in middle-aged women has more than doubled in frequency from 1968–1969 to 2004–2005 in a cohort comparison.22 In the present study, we longitudinally follow the five age cohorts from baseline through follow-up examinations over 37 years, which shows that the prevalence of mental stress (at least some periods of mental stress during the last 5 years or more) was highest in the age interval of 40–60 years and decreased below 30% in higher ages.
Some psychosomatic symptoms were found to be associated with mental stress in a dose-responsive way at baseline as well as in 1974, particularly abdominal, headache/migraine, and musculoskeletal symptoms. Experience of mental stress activates the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system, resulting in a series of neural and endocrine adaptations with physiological and metabolic changes.35 It could be hypothesized that these changes require long duration to exert their full effects and that many social and lifestyle factors, eg, marriage, counteract against these effects. It seems reasonable to study symptoms in 1974 as a possible consequence of mental stress 6 years earlier, but we assume that longer time associations would not be adequate to investigate. Other studies have investigated mental stress in 3 years to 1 year before baseline examinations.11,36
Patients with irritable bowel syndrome (IBS) have negatively impacted health-related quality of life37 and recently a meta-analysis confirmed that some somatic syndromes, such as IBS, are related to depression and anxiety.38
There was no association between mental stress and hypertension whether in 1968–1969, or in 1974–1975. It is often assumed that hypertension is a long-time consequence of mental stress. A British study showed that the hypertension risk category had no overall effect upon short-term cardiovascular reactions to mental stress.39 Another study investigating the mental stress responsively related to age and gender in CVD risk showed that systolic blood pressure stress responsively increased with age in women, but not in men,40 which indicates that a direct association between hypertension and stress could be questioned.
While at least some of the psychosomatic symptoms could be viewed as short-term effects of mental stress with a dose-response relationship, similarly to hypertension, we found no clear association between mental stress and total mortality. However, other studies have estimated that between 20% to 40% of sudden cardiac deaths are precipitated by acute emotional stressors.41
Among the advantages of this study are the cross-sectional and longitudinal designs, the long follow-up from 1968–1969 to 2000–2001 with exactly the same examination protocol across the years.
There are also some limitations to this study. We used the women’s own perception of experience of mental stress rather than a more sophisticated medical instrument. However, this method of documentation of mental stress was also used in other Swedish studies.20,28
The mental stress question (Table 1) included general experiences of mental stress. Even if we used exactly the same question in 1968–1969 as in later examinations, we cannot assume that mental stress had exactly the same meaning in 1968–1969 as today. Maybe mental stress as a conception is more common today than in the late 1960s as well as it can be regarded as more acceptable by society to acknowledge and perceive mental stress.
The lifestyle of women has changed from 1968 to 2004 in many ways such as more gainful employment.42 Changes of lifestyle could probably influence perceived mental stress. A final limitation is the fact that the mental stress variable, in its wording, may have included some aspects of the symptoms that it is predicting.
In conclusion, bodily symptoms, often assumed to be of psychosomatic origin, eg, headache/migraine, abdominal, and musculoskeletal symptoms, are more common in women who have experienced mental stress. Our findings support the significance of perceived stress as a cause of psychosomatic symptoms in women, but we found no association between high mental stress level and mortality even after more than 3 decades.
Our findings illustrate the importance of intensifying preventive actions against factors that give rise to continuous mental stress in women’s lives.
Acknowledgments
This study was supported by grants from Swedish Medical Research Council, Swedish Research Council (11267, 825-2007-7462), and the Swedish Council for Life and Social Research (no 2001-2835, 2001-2646, 2003-0234, 2004-0150, 2006-0020, 2008-1229, 2004-0145, 2006-0596, 2008-1111) (EpiLife, FAS, and FAS WISH). The Alzheimer’s Association Zenith Award (ZEN-01-3151), the Alzheimer’s Association Stephanie B. Overstreet Scholars (IIRG-00-2159) and the Bank of Sweden Tercentenary Foundation.
Footnotes
Disclosure
The Ethics Committee of University of Gothenburg approved the study. All subjects gave their informed consent to participate in accordance with the provisions of the Declaration of Helsinki. The authors declare no conflicts of interest in this work.
References
- 1.Aro S, Hasan J. Occupational class, psychosocial stress and morbidity. Ann Clin Res. 1987;19(2):62–68. [PubMed] [Google Scholar]
- 2.Hinton JW, Burton RF. Clarification of the concept of psychological stress (“psystress”) Int J Psychosom. 1992;39(1–4):42–43. [PubMed] [Google Scholar]
- 3.American Institute of Stress [homepage on the Internet] Fort Worth, TX: [Accessed March 26, 2013]. [cited Dec 2012]. Available from http://www.stress.org/ [Google Scholar]
- 4.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamer M, Endrighi R, Venuraju SM, Lahiri A, Steptoe A. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One. 2012;7(2):e31356. doi: 10.1371/journal.pone.0031356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A. Psychological stress increases the risk of cardiovascular disease. Lakartidningen. 2010;107(36):2096–2099. [PubMed] [Google Scholar]
- 8.Jood K, Redfors P, Rosengren A, Blomstrand C, Jern C. Self-perceived psychological stress and ischemic stroke: a case-control study. BMC Med. 2009;7:53. doi: 10.1186/1741-7015-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surtees PG, Wainwright NW, Luben RN, Wareham NJ, Bingham SA, Khaw KT. Psychological distress, major depressive disorder, and risk of stroke. Neurology. 2008;70(10):788–794. doi: 10.1212/01.wnl.0000304109.18563.81. [DOI] [PubMed] [Google Scholar]
- 10.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang HX, Leineweber C, Kirkeeide R, et al. Psychosocial stress and atherosclerosis: family and work stress accelerate progression of coronary disease in women. The Stockholm Female Coronary Angiography Study. J Intern Med. 2007;261(3):245–254. doi: 10.1111/j.1365-2796.2006.01759.x. [DOI] [PubMed] [Google Scholar]
- 12.Edell-Gustafsson UM, Kritz EI, Bogren IK. Self-reported sleep quality, strain and health in relation to perceived working conditions in females. Scand J Caring Sci. 2002;16(2):179–187. doi: 10.1046/j.1471-6712.2002.00078.x. [DOI] [PubMed] [Google Scholar]
- 13.Haukka E, Leino-Arjas P, Ojajarvi A, Takala EP, Viikari-Juntura E, Riihimaki H. Mental stress and psychosocial factors at work in relation to multiple-site musculoskeletal pain: A longitudinal study of kitchen workers. Eur J Pain. 2011;15(4):432–438. doi: 10.1016/j.ejpain.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Oh YM, Kim YS, Yoo SH, Kim SK, Kim DS. Association between stress and asthma symptoms: a population-based study. Respirology. 2004;9(3):363–368. doi: 10.1111/j.1440-1843.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 16.Nash JM, Thebarge RW. Understanding psychological stress, its biological processes, and impact on primary headache. Headache. 2006;46(9):1377–1386. doi: 10.1111/j.1526-4610.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwab JJ, Traven ND. Factors related to the incidence of psychosomatic illness. Psychosomatics. 1979;20(5):307–311. 315. doi: 10.1016/S0033-3182(79)70827-9. [DOI] [PubMed] [Google Scholar]
- 18.Macleod J, Smith GD, Heslop P, Metcalfe C, Carroll D, Hart C. Are the effects of psychosocial exposures attributable to confounding? Evidence from a prospective observational study on psychological stress and mortality. J Epidemiol Community Health. 2001;55(12):878–884. doi: 10.1136/jech.55.12.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz S, Messerschmidt H, Dören M. Psychosocial risk factors for cancer development. Med Klin (Munich) 2007;102(12):967–979. doi: 10.1007/s00063-007-1128-y. [DOI] [PubMed] [Google Scholar]
- 20.Helgesson Ö, Cabrera C, Lapidus L, Bengtsson C, Lissner L. Self-reported stress levels predict subsequent breast cancer in a cohort of Swedish women. Eur J Cancer Prev. 2003;12(5):377–381. doi: 10.1097/00008469-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Grossarth-Maticek R, Eysenck HJ, Boyle GJ, Heep J, Costa SD, Diel IJ. Interaction of psychosocial and physical risk factors in the causation of mammary cancer, and its prevention through psychological methods of treatment. J Clin Psychol. 2000;56(1):33–50. doi: 10.1002/(sici)1097-4679(200001)56:1<33::aid-jclp4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Lissner L, Sjöberg A, Schütze M, Lapidus L, Hulthén L, Björkelund C. Diet, obesity and obesogenic trends in two generations of Swedish women. Eur J Nutr. 2008;47(8):424–431. doi: 10.1007/s00394-008-0744-5. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson C, Blohmé G, Hallberg L, et al. The study of women in Gothenburg 1968–1969 – a population study. General design, purpose and sampling results. Acta Med Scand. 1973;193(4):311–318. doi: 10.1111/j.0954-6820.1973.tb10583.x. [DOI] [PubMed] [Google Scholar]
- 24.Bengtsson C, Hallberg L, Hällström T, et al. The population study of women in Göteborg 1974–1975-the second phase of a longitudinal study. General design, purpose and sampling results. Scand J Soc Med. 1978;6(2):49–54. doi: 10.1177/140349487800600201. [DOI] [PubMed] [Google Scholar]
- 25.Bengtsson C, Gredmark T, Hallberg L, et al. The population study of women in Gothenburg 1980–1981 – the third phase of a longitudinal study. Comparison between participants and non-participants. Scand J Soc Med. 1989;17(2):141–145. doi: 10.1177/140349488901700203. [DOI] [PubMed] [Google Scholar]
- 26.Cabrera C, Helgesson Ö, Wedel H, Björkelund C, Bengtsson C, Lissner L. Socioeconomic status and mortality in Swedish women: opposing trends for cardiovascular disease and cancer. Epidemiology. 2001;12(5):532–536. doi: 10.1097/00001648-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Rosengren A, Wedel H, Wilhelmsen L. Coronary heart disease and mortality in middle aged men from different occupational classes in Sweden. BMJ. 1988;297(6662):1497–1500. doi: 10.1136/bmj.297.6662.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bengtsson C, Blohmé G, Hallberg T, Tibblin G. Social factors, stress experience, and personality traits in women with iscaemic heart disease, compared to a population sample of women. Acta Med Scand. 1973;(Suppl 549):82–92. [Google Scholar]
- 29.Carlsson G. Socialgruppering: Social Mobility and Class Structure. Lund, Sweden: University of Lund, GWK Gleerup; 1958. [Google Scholar]
- 30.Statistiska centralbyrån, Avdelningen för planering och samordning. Sweden. ISSN 0082-0229 Stockholm: SCB; 1983. Socioekonomisk indelning: SEI = Swedish socioeconomic classification. 1982:4. [Google Scholar]
- 31.Bergh H, Baigi A, Mansson J, Mattsson B, Marklund B. Predictive factors for long-term sick leave and disability pension among frequent and normal attenders in primary health care over 5 years. Public Health. 2007;121(1):25–33. doi: 10.1016/j.puhe.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Caron J, Liu A. A descriptive study of the prevalence of psychological distress and mental disorders in the Canadian population: comparison between low-income and non-low-income populations. Chronic Dis Can. 2010;30(3):84–94. [PubMed] [Google Scholar]
- 33.Leung J, Gartner C, Dobson A, Lucke J, Hall W. Psychological distress is associated with tobacco smoking and quitting behaviour in the Australian population: evidence from national cross-sectional surveys. Aust N Z J Psychiatry. 2011;45(2):170–178. doi: 10.3109/00048674.2010.534070. [DOI] [PubMed] [Google Scholar]
- 34.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 35.Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51(6 Suppl 1):5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- 36.Rosengren A, Orth-Gomér K, Wedel H, Wilhelmsen L. Stressful life events, social support, and mortality in men born in 1933. BMJ. 1993;307(6912):1102–1105. doi: 10.1136/bmj.307.6912.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naliboff BD, Kim SE, Bolus R, Bernstein CN, Mayer EA, Chang L. Gastrointestinal and psychological mediators of health-related quality of life in IBS and IBD: A structural equation modeling analysis. Am J Gastroenterol. 2011;107:451–459. doi: 10.1038/ajg.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65(4):528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 39.Vögele C, Steptoe A. Emotional coping and tonic blood pressure as determinants of cardiovascular responses to mental stress. J Hypertens. 1992;10(9):1079–1087. [PubMed] [Google Scholar]
- 40.Steptoe A, Fieldman G, Evans O, Perry L. Cardiovascular risk and responsivity to mental stress: the influence of age, gender and risk factors. J Cardiovasc Risk. 1996;3(1):83–93. [PubMed] [Google Scholar]
- 41.Vlastelica M. Emotional stress as a trigger in sudden cardiac death. Psychiatr Danub. 2008;20(3):411–414. [PubMed] [Google Scholar]
- 42.Björkelund C, Andersson-Hange D, Andersson K, et al. Secular trends in cardiovascular risk factors with a 36-year perspective: Observations from 38- and 50-year-olds in the Population Study of Women in Gothenburg. Scand J Prim Health Care. 2008;26(3):140–146. doi: 10.1080/02813430802088403. [DOI] [PMC free article] [PubMed] [Google Scholar]