Abstract
Weight loss induced by caloric restriction (CR) or aerobic exercise can reduce pericardial fat, and these reductions may help improve cardiovascular health.
Purpose
We examined whether combining CR with aerobic exercise enhances pericardial fat loss compared with a CR-only intervention designed to elicit equivalent reductions in body weight. We also examined the relationship between changes in pericardial fat and changes in maximal oxygen consumption (V̇O2max), a measure of cardiorespiratory fitness.
Methods
Thirty-two abdominally obese postmenopausal women (mean age = 58 yr; 78% Caucasian) were randomly assigned to one of three interventions of equal energy deficit (~2800 kcal·wk−1) for 20 wk: CR only (n = 8), CR + moderate-intensity exercise (n = 15), or CR + vigorous-intensity exercise (n = 9). The volume of pericardial fat around the coronary arteries was measured by computed tomography.
Results
Women in the CR, CR + moderate-intensity, and CR + vigorous-intensity groups had similar baseline characteristics. The mean ± SD value for pericardial fat before weight loss was 79.07 ± 32.90 cm3 (range = 34.04–152.74 cm3), with no difference among groups (P = 0.89). All three interventions significantly reduced body weight (15%), waist circumference (10%), and abdominal visceral fat (28%) to a similar degree. There was also a 17% reduction in pericardial fat (−12.75 ± 6.29 cm3, P < 0.0001), which did not differ among groups (P = 0.84). Changes in pericardial fat were inversely correlated with changes in V̇O2max (r = −0.37, P = 0.05), but not after adjusting for intervention group and change in body weight.
Conclusions
Weight loss interventions of equal energy deficit have similar effects on pericardial fat in postmenopausal women, regardless of whether the energy deficit is due to CR alone or CR plus aerobic exercise.
Keywords: OBESITY, CALORIC RESTRICTION, AEROBIC EXERCISE, ECTOPIC FAT, CARDIORESPIRATORY FITNESS
Obesity is an independent risk factor for cardiovascular disease and mortality and is strongly associated with the development of diabetes, hypertension, and dyslipidemia (1,13,26). Obesity is also recognized as a major determinant of structural and functional changes in the cardiovascular system (18). In this regard, the location of body fat is often more important than the overall amount of fat. Although the focus has largely been on abdominal obesity, excess fat accumulation around the heart and coronary arteries (i.e., pericardial fat) may be more detrimental for cardiovascular health given its anatomic location. Indeed, higher pericardial fat is associated with left ventricular (LV) hypertrophy, lower stroke volume and cardiac output, higher coronary calcium, increased carotid stiffness and intima-media thickness, autonomic dysfunction, poor cardiorespiratory fitness, and increased risk for coronary heart disease (4,6–8, 10,22–24). In addition, pericardial fat has been found to be a stronger predictor of LV mass, carotid stiffness, coronary calcium, and coronary heart disease than other indices of obesity, including body mass index (BMI), abdominal visceral fat, and waist circumference (4,6–8).
Weight loss is advocated for the improvement and prevention of many obesity-related cardiovascular disease risk factors, and the beneficial effects are partly related to reductions in pericardial fat. Bariatric surgery and a very low calorie diet (900 kcal·d−1) have been shown to significantly reduce pericardial fat in severely obese persons (mean BMI ≥ 45 kg·m−2) (9,25). Interestingly, improvements in LV size and diastolic function were better correlated with reductions in pericardial fat than reductions in BMI and waist circumference (9). Pericardial fat is also reduced after 12 wk of diet-or exercise-induced weight loss in men with a lesser degree of obesity (mean BMI = 31 kg·m−2) (11,12). We previously showed that greater improvements in maximal oxygen consumption (V̇O2max), a measure of cardiorespiratory fitness, are associated with greater reductions in visceral fat during weight loss (15,20). Although these studies examined visceral fat in the abdominal region, pericardial fat is likely to have a more localized effect on the heart and its ability to pump blood to peripheral tissues.
The current consensus is that a combination of caloric restriction (CR) and regular aerobic exercise is the most effective treatment of both total and abdominal obesity. We and others have shown that although combining CR with exercise leads to significant improvements in cardiovascular and metabolic fitness compared with CR alone, it does not generally lead to greater improvements in body composition and fat distribution when the total energy deficit is carefully matched between groups (14,20,21). However, it is not known if this is also true for cardiac obesity because changes in pericardial fat in response to weight loss is an area of research that has only recently begun to be explored. It is possible that the additional cardiovascular benefits beyond CR alone may be mediated by exercise-induced lipolysis in pericardial fat. In addition, although a greater volume of exercise results in a greater loss of fat, the ideal exercise intensity necessary to maximize these benefits for a given level of caloric expenditure is not known. Thus, while prior studies show that weight loss induced by either CR or aerobic exercise training can significantly reduce pericardial fat in men, we sought to clarify the effects of equivalent energy deficits induced by different combinations of CR and aerobic exercise on pericardial fat in overweight and obese postmenopausal women. As a secondary objective, we examined whether improvements in V̇O2max are correlated with changes in pericardial fat with weight loss.
METHODS
Participants
The present study is an ancillary study to the Diet, Exercise, and Metabolism for Older Women Study, a randomized clinical trial of the effects of aerobic exercise intensity on abdominal fat and cardiovascular disease risk factors under conditions of equal energy deficit in 95 women with abdominal obesity. This study was approved by the Wake Forest University Institutional Review Board. The primary study results were previously published (20). All women were recruited from the Piedmont Triad area of North Carolina and enrolled in the study on the basis of the following inclusion criteria: overweight or obese (BMI = 25–40 kg·m−2 and waist circumference > 88 cm), older (age = 50–70 yr), postmenopausal (at least 1 yr without menses), nonsmoking, not on hormone replacement therapy, sedentary (<15 min of exercise, two times per week) in the past 6 months, and weight stable (<5% weight change) for at least 6 months before enrollment. Women with evidence of untreated hypertension (blood pressure > 160/90 mm Hg), triglycerides > 400 mg·dL−1, insulin-dependent diabetes, active cancer, liver, renal or hematological disease, or other medical disorders were excluded. Women with an abnormal cardiovascular response to a graded exercise test were also excluded (2). All women provided informed consent to participate in the study. Because pericardial fat was only measured in a subset of the study participants, the present analysis is based on the 32 women who had preintervention and postintervention pericardial fat measurements available. These women were similar to those excluded from the analysis with respect to age (58.0 vs 58.4 yr, P = 0.72), race (22% vs 35% African American, P = 0.19), BMI (33.5 vs 33.0 kg·m−2, P = 0.60), waist circumference (97.7 vs 98.3 cm, P = 0.75), and V̇O2max (21.9 vs 20.8 mL·kg−1·min−1, P = 0.15).
Study design
Women were randomly assigned to either a CR alone (CR only), a CR plus moderate-intensity exercise (CR + moderate-intensity), or a CR plus vigorous-intensity exercise (CR + vigorous-intensity) intervention for 20 wk. Baseline measurements of body composition and V̇O2max were performed after at least 2 wk of weight stability. The calorie deficits of all women were adjusted to ~2800 kcal·wk−1. The deficits for the CR-only group resulted totally from a reduction in dietary intake, whereas deficits for the exercise groups resulted from reductions in dietary intake (~2400 kcal·wk−1) and increases in energy expenditure (~400 kcal·wk−1). The CR-only group was asked not to alter their physical activity habits during the study. As reported previously, compliance to the CR and exercise interventions was excellent and did not differ among intervention groups (20).
Dietary interventions
During the 20-wk interventions, all women were provided food for their lunch and dinner, which was prepared by the Wake Forest University General Clinical Research Center metabolic kitchen staff. These meals were prepared individually after women chose from a hypocaloric menu designed by a registered dietitian. Women purchased and prepared their breakfast meal in consultation with the General Clinical Research Center dietitian from this same menu. They were allowed two free days per month, during which they were given guidelines for diet intake and asked to report all intake. They were also allowed to consume as many noncaloric, noncaffeinated beverages as they liked. In addition, all women were provided with a daily calcium supplement (1000 mg·d−1).
Exercise interventions
Both exercise groups walked on a treadmill 3 d·wk−1 at a target heart rate calculated from the Karvonen equation (heart rate reserve × (intensity) + resting heart rate), where heart rate reserve (HRR) is calculated as the maximal HR minus the resting HR obtained from each subject’s V̇O2max test. The duration and intensity of the exercise progressed from 15 to 20 min at 45%–50% of HRR during the first week to 55 min at 45%–50% HRR for the low-intensity group and 30 min at 70%–75% HRR for the high-intensity group by the second month.
Pericardial fat
The volume of pericardial fat encasing the coronary arteries was measured by computed tomography as described previously (7). The superior extent of the left main coronary artery was identified in a cross-sectional scan, and slices within 15 mm above and 30 mm below this slice were included in the measurement. This region of the heart was selected because it includes the fat located around the proximal coronary arteries (left main coronary, left anterior descending, right coronary, and circumflex arteries). The anterior border of the volume was defined by the chest wall and the posterior border by the aorta and the bronchus. Volume Analysis software (GE Healthcare, Waukesha, WI) was used to discern fat from the remaining portions of the heart with a threshold of −190 to −30 Hounsfield units. The volume was the sum of all voxels containing pericardial fat. This measurement is highly reproducible, with intraclass correlation coefficients for intrareader and interreader reliability of 0.999 and 0.997, respectively (7).
Body composition and body fat distribution
BMI was calculated from measured height (cm) and weight (kg). Total fat mass, total lean mass, and percent body fat were measured by dual-energy x-ray absorptiometry (Hologic Delphi QDR, Bedford, MA). Waist (minimal circumference) and hip (maximal gluteal protuberance) were measured in triplicate, and waist-to-hip ratio was calculated. Abdominal subcutaneous and visceral fat volumes at the L4 and L5 level were measured by multidetector computed tomography (GE Medical Systems, Milwaukee, WI) using standard procedures (20).
Maximal aerobic capacity
V̇O2max was measured on a motor-driven treadmill (Medical Graphics Corporation, Minneapolis, MN) using a ramp protocol during a progressive exercise test to voluntary exhaustion (19). A valid V̇O2max was obtained when at least two of the following criteria were achieved: 1) <200 mL·min−1 change in V̇O2max with increasing work rate, 2) heart rate > 90% of age-predicted maximal heart rate, and 3) respiratory exchange ratio ≥ 1.10. If a valid V̇O2max test was not achieved, then the test was repeated.
Other laboratory and clinical measurements
Blood samples were drawn into ethylenediaminetetraacetic acid tubes by venipuncture in the morning after an overnight fast. Plasma cholesterol and triglyceride levels were measured using standardized procedures in samples collected on duplicate testing days both preintervention and postintervention. Plasma glucose was measured with the glucose hexokinase method (Bayer Diagnostics, Tarrytown, NY). Plasma insulin was measured using a chemiluminescent immunoassay on an IMMULITE® analyzer (Diagnostics Products Corporation, Los Angeles, CA). Blood pressure was measured in the right arm, using a conventional mercury sphygmomanometer and the appropriately sized cuff, with the participant in a seated position after having rested quietly for 10–15 min. The values reported are the average of two repeated measures.
Statistical analyses
Analyses were performed using SAS Version 9.1 (SAS Institute, Inc., Cary, NC). All data were treated as continuous variables (except race) and met parametric assumptions. Chi-square frequency tests and ANOVA were used to compare differences in categorical and continuous variables, respectively, among intervention groups. Changes in pericardial fat, body weight, body composition, body fat distribution, and V̇O2max with weight loss were examined using paired t-tests. Pearson correlation coefficients were used to examine relationships between changes in pericardial fat and changes in body composition, body fat distribution, and V̇O2max. Partial correlations were also used to examine these relationships after adjusting for potential confounders. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Overall, the women in this analysis were 58.0 ± 5.2 yr old and 14.5 ± 10.5 yr past menopause (mean ± SD). These women were also predominately Caucasian (78%) and obese (BMI = 33.5 ± 4.4 kg·m−2, total body fat = 43.5% ± 3.2%), with low fitness levels (V̇O2max = 21.9 ± 2.7 mL·kg−1·min−1). Baseline characteristics for the women by intervention group are shown in Table 1. Women in the CR only, CR + moderate-intensity, and CR + vigorous-intensity groups were similar at baseline with respect to age, race, total and LDL cholesterol, triglycerides, fasting glucose, and blood pressure. Baseline body weight, body composition, body fat distribution, and V̇O2max were also similar among intervention groups. On the other hand, HDL cholesterol levels were lower (P = 0.008) and fasting insulin levels were higher (P < 0.05) in the CR + vigorous-intensity group compared with the other groups.
TABLE 1.
Baseline characteristics of study participants randomized to weight loss interventions of equal energy deficit.
| CR Only (n = 8) | CR + Moderate Intensity (n = 15) | CR + Vigorous Intensity (n = 9) | Pa | |
|---|---|---|---|---|
| Age (yr) | 57.6 ± 4.8 | 57.3 ± 5.7 | 59.4 ± 4.9 | 0.63 |
| Race, n (%) | 0.10 | |||
| Caucasian | 6 (75) | 11 (73) | 8 (89) | |
| African American | 2 (25) | 4 (27) | 1 (11) | |
| Total cholesterol (mg·dL−1) | 202 ± 44 | 207 ± 37 | 221 ± 23 | 0.83 |
| HDL cholesterol (mg·dL−1) | 53 ± 5 | 64 ± 14 | 49 ± 10 | 0.008 |
| LDL cholesterol (mg·dL−1) | 124 ± 42 | 118 ± 24 | 137 ± 21 | 0.29 |
| Triglycerides (mg·dL−1) | 126 ± 43 | 125 ± 58 | 130 ± 45 | 0.97 |
| Fasting glucose (mg·dL−1) | 94.1 ± 13.0 | 92.9 ± 7.1 | 103.9 ± 21.3 | 0.16 |
| Fasting insulin (mg·dL−1) | 12.6 ± 5.2 | 9.3 ± 5.4 | 16.9 ± 9.9 | 0.05 |
| Systolic BP (mm Hg) | 122 ± 17 | 126 ± 18 | 133 ± 17 | 0.42 |
| Diastolic BP (mm Hg) | 80 ± 5 | 76 ± 8 | 80 ± 10 | 0.30 |
| Body weight (kg) | 88.9 ± 11.0 | 88.9 ± 10.4 | 94.9 ± 14.3 | 0.43 |
| BMI (kg·m−2) | 32.2 ± 4.0 | 33.6 ± 4.5 | 34.4 ± 4.7 | 0.58 |
| Total fat mass (kg) | 38.5 ± 7.8 | 39.5 ± 5.9 | 44.0 ± 7.8 | 0.21 |
| Total lean mass (kg) | 52.8 ± 4.0 | 51.2 ± 6.8 | 53.5 ± 7.1 | 0.68 |
| Total body fat (%) | 41.9 ± 3.6 | 43.5 ± 3.3 | 45.0 ± 2.0 | 0.13 |
| Abdominal visceral fat (cm3) | 2432 ± 823 | 2066 ± 784 | 2733 ± 791 | 0.16 |
| Abdominal subcutaneous fat (cm3) | 5443 ± 1835 | 6144 ± 1463 | 6047 ± 1811 | 0.62 |
| Visceral-to-subcutaneous fat ratio | 0.49 ± 0.21 | 0.37 ± 0.23 | 0.50 ± 0.22 | 0.30 |
| Waist circumference (cm) | 95.9 ± 9.7 | 96.1 ± 6.7 | 102.2 ± 8.1 | 0.16 |
| Hip circumference (cm) | 117.8 ± 11.5 | 120.2 ± 8.0 | 121.0 ± 11.8 | 0.78 |
| Waist-to-hip ratio | 0.82 ± 0.06 | 0.80 ± 0.05 | 0.85 ± 0.05 | 0.13 |
| V̇O2max (mL·kg−1·min−1) | 21.2 ± 1.8 | 22.3 ± 3.3 | 21.6 ± 2.3 | 0.63 |
Values are presented as mean ± SD.
On the basis of one-way ANOVA across intervention groups.
Pericardial fat
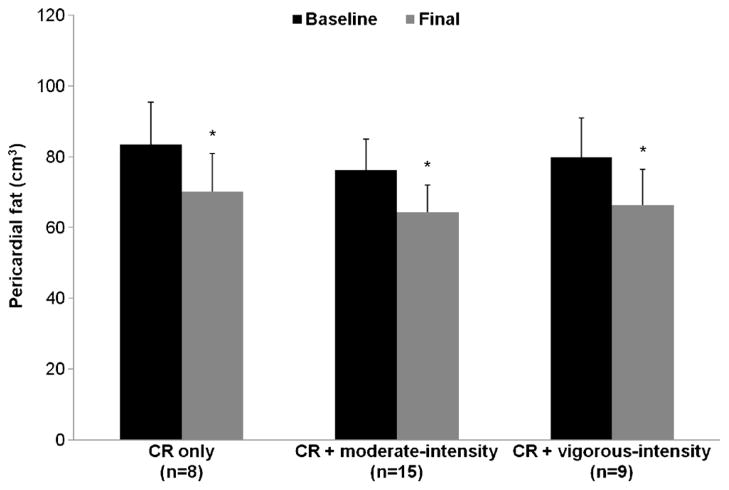
Pericardial fat at baseline ranged from 34.04 to 152.74 cm3 (overall mean = 79.07 ± 32.90 cm3). As shown in Figure 1, baseline pericardial fat was similar among intervention groups (P = 0.89): 83.44 ± 11.98 cm3 in the CR only group, 76.29 ± 8.75 cm3 in the CR + moderate-intensity group, and 79.81 ± 11.29 cm3 in the CR + vigorous-intensity group. Overall, there was a −12.75 ± 6.29 cm3 decrease in pericardial fat (P < 0.0001), with significant reductions in pericardial fat in all three groups (CR only group = −13.25 ± 2.29 cm3, CR + moderate-intensity group = −12.03 ± 1.67 cm3, CR + vigorous-intensity group = −13.50 ± 2.16 cm3; all P values < 0.0001; Fig. 1). These changes were similar among intervention groups (P = 0.84).
FIGURE 1.
Pericardial fat before and after weight loss by intervention group; *significantly different from baseline, P < 0.05.
Changes in body composition, fat distribution, and cardiorespiratory fitness
Table 2 shows the effect of the weight loss interventions on body composition, body fat distribution, and V̇O2max. The average weight loss for all women combined was −13.5 ± 4.6 kg (15% ± 4%, P < 0.0001), which was not significantly different among intervention groups (P = 0.12). There were also similar reductions in abdominal fat, visceral-to-subcutaneous fat ratio, waist and hip circumferences, and waist-to-hip ratio among groups. However, there was a slight difference in the change in total fat mass (P = 0.02) and percent body fat (P = 0.04) across groups. Among all the anthropometric, body composition, and body fat distribution measures, the percent change in abdominal visceral fat was the highest (28%), followed by total fat mass (22%), abdominal subcutaneous fat (19%), pericardial fat (17%), body weight (15%), BMI (14%), waist circumference (10%), hip circumference (9%), visceral-to-subcutaneous fat ratio (8%), and waist-to-hip ratio (1%). There were also significant improvements in V̇O2max in all three groups. These changes were not different among groups (P = 0.13). Changes in lipid levels, fasting glucose and insulin and levels, and blood pressure were also similar among intervention groups (data not shown).
TABLE 2.
Changes in body composition, body fat distribution, and V̇O2max in response to weight loss interventions of equal energy deficit.
| Characteristics | CR Only (n = 8) | CR + Moderate Intensity (n = 15) | CR + Vigorous Intensity (n = 9) | Pa |
|---|---|---|---|---|
| Δ Body weight (kg) | −11.4 ± 4.9* | −13.3 ± 4.2* | −15.8 ± 4.2* | 0.12 |
| Δ BMI (kg·m−2) | −3.7 ± 1.3* | −5.0 ± 3.1* | −5.2 ± 1.4* | 0.41 |
| Δ Total fat mass (kg) | −7.0 ± 3.0* | −8.5 ± 3.3* | −11.7 ± 3.5* | 0.02 |
| Δ Total lean mass (kg) | −3.7 ± 1.9* | −3.6 ± 2.2* | −3.9 ± 1.8* | 0.94 |
| Δ Total body fat (%) | −3.3 ± 2.2* | −4.1 ± 1.6* | −5.7 ± 2.7* | 0.04 |
| Δ Abdominal visceral fat (cm3) | −630 ± 349* | −576 ± 364* | −813 ± 143* | 0.22 |
| Δ Abdominal subcutaneous fat (cm3) | −1265 ± 1185* | −1384 ± 897* | −1211 ± 1698 | 0.94 |
| Δ Visceral-to-subcutaneous fat ratio | −0.04 ± 0.12 | −0.05 ± 0.14 | −0.10 ± 0.13* | 0.53 |
| Δ Waist circumference (cm) | −7.9 ± 4.5* | −10.2 ± 4.6* | −11.7 ± 2.6 | 0.18 |
| Δ Hip circumference (cm) | −8.9 ± 3.4* | −11.2 ± 4.3* | −11.2 ± 4.1* | 0.38 |
| Δ Waist-to-hip ratio | −0.01 ± 0.04 | −0.01 ± 0.03 | −0.02 ± 0.03* | 0.52 |
| Δ V̇O2max (mL·kg−1·min−1) | 3.7 ± 1.4* | 2.8 ± 3.1* | 5.4 ± 2.4* | 0.13 |
Values are presented as mean ± SE.
On the basis of one-way ANOVA across intervention groups.
Significant change with intervention, P ≤ 0.05.
Correlation analyses
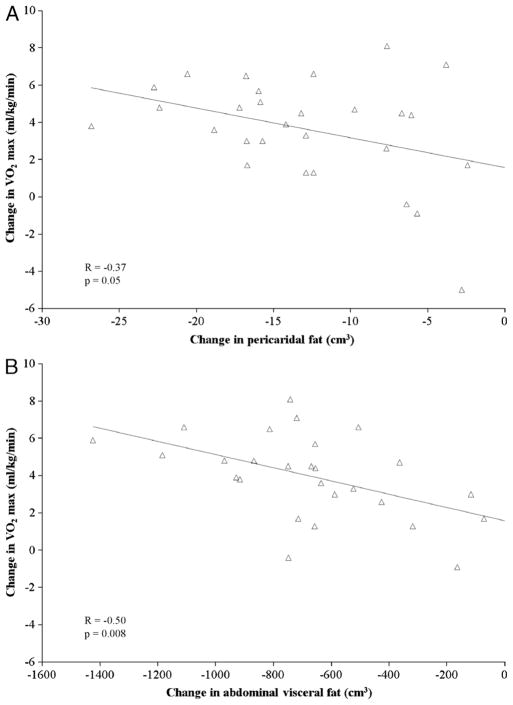
In all women combined, reductions in pericardial fat were positively correlated with reductions in abdominal visceral fat (r = 0.60, P = 0.0003), visceral-to-subcutaneous fat ratio (r = 0.56, P = 0.001), total lean mass (r = 0.51, P = 0.02), waist circumference (r = 0.45, P = 0.01), hip circumference (r = 0.42, P = 0.02), and BMI (r = 0.38, P = 0.03); negatively correlated with changes in abdominal subcutaneous fat (r = −0.43, P = 0.02); and unrelated to reductions in total fat mass, percent body fat, waist-to-hip ratio, and body weight. There were also significant correlations between increases in V̇O2max and reductions in pericardial fat (r = −0.37, P = 0.05; Fig. 2A), abdominal visceral fat (r = −0.50, P = 0.008; Fig. 2B), and total fat mass (r = −0.42, P = 0.03). After adjusting for intervention group and change in body weight, these correlations were no longer significant.
FIGURE 2.
Association of changes in V̇O2max with changes in pericardial fat (A) and abdominal visceral fat (B) in all women combined.
DISCUSSION
Accumulating evidence indicates that higher amounts of pericardial fat have adverse effects on the cardiovascular system, independent of total and abdominal obesity. As such, reductions in pericardial fat may contribute more to improvements in cardiovascular health. We compared the effects of three weight loss interventions of equal energy deficit on changes in pericardial fat in abdominally obese postmenopausal women. The main findings were as follows: 1) weight loss induced by CR alone, CR plus moderate-intensity aerobic exercise, and CR plus vigorous-intensity aerobic exercise leads to similar reductions in pericardial fat; 2) exercise intensity does not influence the magnitude of change in pericardial fat when performed in conjunction with CR; and 3) reductions in pericardial fat with weight loss are not independently associated with improvements in cardiorespiratory fitness.
Despite the emerging role of pericardial fat as an independent risk factor for obesity-related cardiovascular dysfunction, only a few studies have examined the effect of weight loss on this local fat depot, including two studies in severely obese persons (mean BMI ≥ 45 kg·m−2). Willens et al. (25) reported that a loss of 40 kg (26%) of body weight after bariatric surgery was accompanied by a 24% reduction in pericardial fat. Similarly, Iacobellis et al. (9) reported that after 6 months on a very low calorie diet program (900 kcal·d−1), subjects lost 25 kg (20%) of body weight and reduced their pericardial fat by 32%. In obese middle-aged men, a weight loss intervention involving 12 wk of moderate-intensity exercise with no dietary restrictions decreased body weight by 4% and pericardial fat by 8% (12), whereas a 12-wk diet-induced weight loss program (~1550 kcal·d−1, 30% caloric reduction) decreased body weight by 11% and pericardial fat by 17% (11). These data suggest that the magnitude of change in pericardial fat may be influenced by the type of weight loss intervention and/or the total amount of weight loss. However, in our study of overweight and obese postmenopausal women randomized to one of three weight loss interventions for 20 wk, there were similar reductions in body weight and pericardial fat in all three groups, and reductions in body weight were not correlated with reductions in pericardial fat. Moreover, in response to equal amounts of total weight loss, pericardial fat loss was not enhanced by adjusting the degree of CR to accommodate increases in energy expenditure due to moderate- or vigorous-intensity exercise. Taken together, our data suggest that the type of weight loss intervention (i.e., CR + exercise vs CR alone) does not appear to influence the magnitude of change in pericardial fat, at least in the context of interventions of equal caloric deficit. Given the small sample size, a larger study is warranted to confirm these findings.
Although the combination of CR and aerobic exercise did not result in greater reductions in pericardial fat, previous reports in this population demonstrate that compared with CR alone, performing moderate- or vigorous-intensity exercise in conjunction with CR leads to greater improvements in V̇O2max, significant reductions in subcutaneous abdominal adipocyte size, and greater preservation of lean mass (20,27). In addition, it is possible that CR + exercise could also lead to greater improvements in other risk factors that were not examined in the present study. Furthermore, there is some evidence that the addition of aerobic exercise to a CR intervention helps to maintain weight loss for a longer period of time (5,16).
Weight loss appears to have variable effects on the relative change in pericardial fat compared with changes in other obesity measures. In this regard, the results of this study are consistent with previous studies and extend the effects of weight loss on pericardial fat to women without severe obesity. In the previous diet- and exercise-induced weight loss studies, the percent change in pericardial fat was greater than that for the anthropometric measures but lower than the percent changes in body fat. We found similar results in our population. Previous studies also show that changes in pericardial fat with weight loss are highly correlated with changes in other measures of total and regional adiposity. Most notably, correlations of 0.53–0.80 have been reported for changes in abdominal visceral fat and pericardial fat (11,12). We found a similar correlation of 0.60 in our study as well as positive associations with changes in BMI and hip and waist circumferences. In addition, we found that changes in pericardial fat with weight loss were positively associated with changes in lean mass and inversely associated with changes in abdominal subcutaneous fat and visceral-to-subcutaneous fat ratio. These findings highlight the importance of body composition and body fat distribution as correlates of the change in pericardial fat with weight loss.
Our secondary objective was to examine the association between weight loss–induced changes in pericardial fat and V̇O2max. Lower cardiorespiratory fitness, as assessed by V̇O2max, has been reported in individuals with increased total and abdominal obesity (3,17). Kim et al. (10) recently demonstrated that higher pericardial fat is also associated with a lower V̇O2max in overweight and obese middle-aged men. This is supported by findings of an inverse association between pericardial fat and stroke volume and cardiac output in obese subjects (23). We previously showed that improvements in V̇O2max with weight loss are associated with reductions in abdominal visceral fat in postmenopausal women (15,20). In the present study, we hypothesized that reductions in pericardial fat may be more relevant given the location of this fat depot. Although changes in pericardial fat and V̇O2max with weight loss were significantly correlated in univariate analysis, after adjusting for intervention group and change in body weight, the association was no longer significant. In addition, a stronger correlation was found between changes in abdominal visceral fat and changes in V̇O2max in this population. These data indicate that the amount of fat in the abdomen is more relevant to V̇O2max than fat in a small localized area, which is consistent with the fact that V̇O2max is determined by both central (i.e., heart and lungs) and peripheral factors (i.e., skeletal muscle). Moreover, there is some evidence that the independent effect of pericardial fat depends on the outcome studied. In this regard, pericardial fat appears to have a greater effect on local cardiovascular health, whereas abdominal visceral fat has more systemic effects (22).
Our study has several strengths. First, we used a highly controlled dietary intervention and a well-standardized exercise program that allowed us to examine the independent effects of adding exercise to CR on pericardial fat, without the confounding effect of differences in the total amount of weight loss. Second, we are the first study to report the effects of weight loss on changes in the volume of pericardial fat (cm3), as opposed to the thickness (mm) of the fat pad that covers the free wall of the right ventricle. Our measurement includes not only the fat surrounding the right and left ventricles but also the fat around the coronary arteries, which together may provide a more relevant assessment of fat around the heart. However, the small sample size may have limited our ability detect differences between intervention groups. On the basis of our estimates, the smallest difference we could detect between intervention groups with eight participants per group, alpha ≤ 0.05, and power ≥ 0.80 is 9.72 cm3, an amount that is probably not clinically relevant on the basis of our previous studies looking at the association of pericardial fat with clinical and subclinical cardiovascular disease (6,7,24). In addition, because this study was designed to elicit similar reductions in body weight across intervention groups, our results are only applicable in this context. Nevertheless, we found that weight loss interventions of equal energy deficit lead to similar reductions in pericardial fat in abdominally obese postmenopausal women, regardless of whether the energy deficit is due to CR alone or CR plus aerobic exercise. These data contribute to the growing body of knowledge demonstrating the efficacy of weight loss interventions on pericardial fat, an important fat depot with adverse effects on cardiovascular health.
Acknowledgments
This study was supported by the National Institutes of Health (grants R01-AG/DK20583 and R01-HL093713), the Wake Forest University Claude D. Pepper Older Americans Independence Center (grant P30-AG21332), and the Wake Forest University General Clinical Research Center (grant M01-RR07122).
The authors are grateful to the study coordinators, dietitians, exercise physiologists, nurses, and other research staff of the Wake Forest University General Clinical Research Center and Geriatric Research Center for their assistance in the conduct of this study. They also thank all the women who volunteered to participate in this study.
Footnotes
The authors have no conflicts of interest.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–8. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 2.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Baltimore (MD): Lippincott Williams & Wilkins; 2000. pp. 189–90. [Google Scholar]
- 3.Bertoli A, Di Daniele N, Ceccobelli M, Ficara A, Girasoli C, De Lorenzo A. Lipid profile, BMI, body fat distribution, and aerobic fitness in men with metabolic syndrome. Acta Diabetol. 2003;40(Suppl 1):S130–3. doi: 10.1007/s00592-003-0045-7. [DOI] [PubMed] [Google Scholar]
- 4.Brinkley TE, Hsu FC, Carr JJ, et al. Pericardial fat is associated with carotid stiffness in the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2010 Feb 11; doi: 10.1016/j.numecd.2009.10.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29(10):1168–74. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90(3):499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Kritchevsky SB, Harris TB, et al. Multi-Ethnic Study of Atherosclerosis. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16(8):1914–9. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94(8):1084–7. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 9.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16(7):1693–7. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 10.Kim MK, Tanaka K, Kim MJ, et al. Epicardial fat tissue: relationship with cardiorespiratory fitness in men. Med Sci Sports Exerc. 2010;42(3):463–9. doi: 10.1249/MSS.0b013e3181b8b1f0. [DOI] [PubMed] [Google Scholar]
- 11.Kim MK, Tanaka K, Kim MJ, et al. Comparison of epicardial, abdominal and regional fat compartments in response to weight loss. Nutr Metab Cardiovasc Dis. 2009;19(11):760–6. doi: 10.1016/j.numecd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106(1):5–11. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]
- 13.Lamon-Fava S, Wilson PW, Schaefer EJ. Impact of body mass index on coronary heart disease risk factors in men and women. The Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 1996;16(12):1509–15. doi: 10.1161/01.atv.16.12.1509. [DOI] [PubMed] [Google Scholar]
- 14.Larson-Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E. Caloric restriction with or without exercise: the fitness versus fatness debate. Med Sci Sports Exerc. 2010;42(1):152–9. doi: 10.1249/MSS.0b013e3181ad7f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch NA, Nicklas BJ, Berman DM, Dennis KE, Goldberg AP. Reductions in visceral fat during weight loss and walking are associated with improvements in VO(2 max) J Appl Physiol. 2001;90(1):99–104. doi: 10.1152/jappl.2001.90.1.99. [DOI] [PubMed] [Google Scholar]
- 16.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord. 1997;21(10):941–7. doi: 10.1038/sj.ijo.0800499. [DOI] [PubMed] [Google Scholar]
- 17.Miyatake N, Takanami S, Kawasaki Y, Fujii M. Relationship between visceral fat accumulation and physical fitness in Japanese women. Diabetes Res Clin Pract. 2004;64(3):173–9. doi: 10.1016/j.diabres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28(Suppl4):S58–65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- 19.Myers J, Buchanan N, Smith D, et al. Individualized ramp treadmill. Observations on a new protocol. Chest. 1992;101(Suppl 5):236S–41S. [PubMed] [Google Scholar]
- 20.Nicklas BJ, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89(4):1043–52. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–13. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 23.Ruberg FL, Chen Z, Hua N, et al. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity (Silver Spring) 2009;18(6):1116–21. doi: 10.1038/oby.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soliman EZ, Ding J, Hsu FC, Carr JJ, Polak JF, Goff DC., Jr Association between carotid intima-media thickness and pericardial fat in the Multi-Ethnic Study of Atherosclerosis (MESA) J Stroke Cerebrovasc Dis. 2010;19(1):58–65. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99(9):1242–5. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 27.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes (Lond) 2006;30(8):1211–6. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]