Abstract
Methamphetamine-induced partial dopamine depletions are associated with impaired basal ganglia function, including decreased preprotachykinin mRNA expression and impaired transcriptional activation of activity-regulated, cytoskeleton-associated (Arc) gene in striatum. Recent work implicates deficits in phasic dopamine signaling as a potential mechanism linking methamphetamine-induced dopamine loss to impaired basal ganglia function. The present study thus sought to establish a causal link between phasic dopamine transmission and altered basal ganglia function by determining whether the deficits in striatal neuron gene expression could be restored by increasing phasic dopamine release. Three weeks after pretreatment with saline or a neurotoxic regimen of methamphetamine, rats underwent phasic- or tonic-like stimulation of ascending dopamine neurons. Striatal gene expression was examined using in situ hybridization histochemistry. Phasic-like, but not tonic-like, stimulation induced immediate-early genes Arc and zif268 in both groups, despite the partial striatal dopamine denervation in methamphetamine-pretreated rats, with the Arc expression occurring in presumed striatonigral efferent neurons. Phasic-like stimulation also restored preprotachykinin mRNA expression. These results suggest that disruption of phasic dopamine signaling likely underlies methamphetamine-induced impairments in basal ganglia function, and that restoring phasic dopamine signaling may be a viable approach to manage long-term consequences of methamphetamine-induced dopamine loss on basal ganglia functions.
Keywords: methamphetamine, striatum, dopamine, Arc, preprotachykinin, zif268
Introduction
Methamphetamine (METH) is an addictive psychostimulant that is neurotoxic to dopamine (DA) neurons. Markers of DA innervation in both the dorsal and ventral striatum are reduced in brains of chronic METH abusers (Wilson et al. 1996), and individuals with a history of hospitalization associated with METH use are more likely to develop Parkinsonism (Callaghan et al. 2012). Thus, it is likely that some proportion of individuals who abuse METH will experience a period of partial DA loss as a consequence of their METH abuse prior to the development of Parkinsonism. Prior studies have suggested a relation between METH-induced DA loss and cognitive impairment in individuals with a history of METH abuse, as reduction of dopamine transporter (DAT) binding in the caudate-putamen correlates with motor and cognitive impairments in METH users (Volkow et al. 2001). Furthermore, METH use is associated with cognitive decline, as executive functions, learning, and memory, which are dependent on intact striatal circuitry (Brown et al. 1997; Packard and Knowlton 2002), are impaired in METH users (Scott et al. 2007; Dean et al. 2013 , but see Hart et al. 2012). Similarly, in rodents, exposure to METH causes significant striatal DA denervation (Hotchkiss and Gibb 1980; Ricaurte et al. 1980; Ricaurte et al. 1982). Associated with this depletion are learning impairments, including impaired recognition of novel stimuli (Schroder et al. 2003; Marshall et al. 2007; Herring et al. 2008; O'Dell et al. 2011), impaired motor-sequence learning (Chapman et al. 2001; Daberkow et al. 2005), and altered reversal learning (Izquierdo et al. 2010; Pastuzyn et al. 2012). Despite the association between METH-induced DA denervation and learning impairments, the molecular mechanisms linking these phenomena are not fully understood.
Approximately 95% of striatal neurons are GABAergic medium spiny neurons (MSNs), which are found in two roughly equal subtypes. Striatonigral (“direct pathway”) MSNs express D1 DA receptors, as well as the neuropeptides substance P (and its preprotachykinin (ppt) precursor) and dynorphin (Gerfen et al. 1990; Surmeier et al. 1996). Striatopallidal neurons (“indirect pathway”) express D2 DA receptors, as well as the neuropeptide enkephalin (and its preproenkephalin (ppe) precursor) (Gerfen et al. 1990; Le Moine et al. 1990; Surmeier et al. 1996). Partial striatal DA loss induced by either METH or 6-hydroxydopamine (6-OHDA) results in a reduction in basal ppt expression in striatum, but no change in ppe expression (Nisenbaum et al. 1996; Chapman et al. 2001; Johnson-Davis et al. 2002). Furthermore, METH-induced neurotoxicity is associated with loss of Arc (activity-regulated, cytoskeleton-associated gene) transcription in response to behavioral activation (Daberkow et al. 2008; Barker-Haliski et al. 2012a), and only the impairment in the numbers of striatonigral neurons with Arc mRNA in the cytoplasm correlates significantly with the degree of METH-induced striatal DA loss (Barker-Haliski et al. 2012a). Taken together, these data suggest that partial DA loss, such as that induced by METH, affects striatal efferent neuron function and that this dysfunction may predominantly affect striatonigral neurons.
METH-induced neurotoxicity selectively impairs phasic-like DA signaling (Howard et al. 2011), which is thought to preferentially affect striatonigral neurons (Chergui et al. 1997; Gonon 1997; Onn et al. 2000). METH-induced partial DA depletion is associated with diminished amplitude of DA signals evoked using phasic-like stimulation of the DA neurons ascending through the medial forebrain bundle (MFB)(Howard et al. 2011), as well as the amplitude of endogenous, spontaneously occurring phasic DA transients (CD Howard, et al., submitted). On the other hand, tonic DA levels, which are thought to be sufficient to activate D2 receptors expressed by striatopallidal neurons (Richfield et al. 1989; Dreyer et al. 2010), are not disrupted following METH pretreatment, as assessed by microdialysis (Cass and Manning 1999). Interestingly, enhancing phasic-like, but not tonic-like, DA signaling via electrical stimulation of DA neurons results in increased zif268 expression in D1 DA receptor-containing striatonigral neurons (Chergui et al. 1997). Therefore, METH-induced dysfunction in direct pathway MSNs may be due to impairments in phasic, but not tonic, DA signaling.
If reduced phasic DA signaling is related to the gene expression deficits observed following METH-induced partial DA denervation, then augmenting phasic DA signaling should restore normal gene expression in the partially DA denervated striatum. We tested this hypothesis by pretreating rats with a neurotoxic regimen of METH and then, three weeks later, stimulating DA neurons ascending through the MFB in either a phasic- or tonic-like pattern. We then assessed striatal expression of ppt and ppe and the immediate-early genes (IEGs) Arc and zif268. We show that phasic-like stimulation increases IEG expression in both METH- and saline-pretreated rats and that the increase in Arc mRNA expression is preferentially in direct pathway neurons. Furthermore, the phasic-like stimulation restores ppt expression in METH-pretreated rats without altering ppe expression. These findings suggest that restoring phasic DA signaling may ameliorate basal ganglia dysfunction arising consequent to partial DA depletion, such as that induced by METH.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (n=30, 240–350 g at time of pretreatment) were purchased from Harlan (Indianapolis, IN, USA) and housed in a light- and temperature-controlled vivarium. Access to food and water was provided ad libitum. All procedures conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Illinois State University.
Drugs
(±)-Methamphetamine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA). METH doses were calculated as free base. All other chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA).
METH pretreatment
The “binge” neurotoxic METH regimen was conducted as previously described (Howard et al. 2011). Briefly, animals were housed in plastic tub cages (50 cm length × 40 cm width × 20 cm height: four rats/cage). METH was dissolved in 0.9% saline and all injections were made subcutaneously. Four injections of either METH (7.5 mg/kg) or saline were administered at 2-h intervals. Temperature was monitored rectally using a Thermalert TH-5 (Physitemp, Clifton, NJ, USA) prior to, immediately after the first injection, and every hour thereafter, continuing two hours after the final injection of METH. Health was assessed at least every hour, and if rats showed signs of overheating, they were placed in a separate tub on ice for ~10 min.
Electrical stimulation and in vivo voltammetry
Three weeks after METH or saline pretreatment, animals to be stimulated were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tajunga, CA, USA). Four holes were drilled into the skull to allow for lowering of the stimulating electrode, two carbon fiber microelectrodes (CFM), and a Ag/AgCl reference electrode. The twisted, bipolar stimulating electrode (Plastics One, Roanoke, VA, USA) was placed dorsal to the MFB (−4.6 AP; +1.4 ML; −7.0 DV; Paxinos and Watson 1986) and was incrementally lowered until a robust DA signal was recorded in the striatum at the CFMs placed in the dorsomedial (DM) and dorsolateral (DL) striatum (+1.2 AP; +1.0 and +4.0 ML, respectively; −4.5 DV; Paxinos and Watson 1986) at 6° angles to allow side by side ipsilateral placement. Changes in DA concentration were recorded using fast-scan cyclic voltammetry (FSCV), where a triangular waveform (−0.4 V to 1.3 V and back at 400 V/s) was applied to the tip of the CFM every 100 ms (Cahill et al. 1996) using an EI400 bipotentiostat (Ensman Instruments, Bloomington, IN, USA) that was computer-controlled using TH-1 software (ESA, Chelmsford, MA, USA). Current recorded at each CFM was converted to concentration using in vitro calibration immediately following experiments (Logman et al. 2000). The purpose of recording with FSCV at a CFM in this experiment was to identify DA neurons ascending through the MFB for stimulation with phasic- and tonic-like pulse trains, and to ensure optimal stimulating electrode placement. Non-stimulated control rats were anesthetized with urethane, but did not undergo surgical manipulations or any stimulation.
Following optimization of the stimulating electrode placement, no stimulation was given for 1 h to reduce optimization- and handling-induced striatal gene expression (Daberkow et al. 2007). The experimental stimulation protocol was then begun. Electrical stimulation was optically isolated (NL 800, Neurolog, Medical Systems, Great Neck, NY, USA) and synchronized with FSCV recordings. The stimulation protocol was chosen based on previous work noting enhanced expression of mRNA for the IEG zif268 following phasic-like (“burst”), but not tonic-like (“regular”), stimulation (Chergui et al. 1997). Stimulation was comprised of constant-current, biphasic pulses (2 ms and 300 µA each phase) and was delivered in either a phasic-like (5 pulses at 30 Hz repeated every 1 s for 60 s; n=5 METH-pretreated; n=5 saline-pretreated) or tonic-like (300 pulses at 5 Hz; n=5 METH-pretreated; n=5 saline-pretreated) pattern. Stimulation trains were 60 s in duration and were repeated a total of 15 times, with 2 min separating each stimulation train, for a total stimulation session time of 45 min. Immediately following stimulation sessions, animals were euthanized and brains were rapidly extracted. Non-stimulated control rats were sacrificed 180 min after being anesthetized with urethane (n=5 METH-pretreated; n=5 saline-pretreated). Brains were flash frozen in 2-methylbutane (EMD Millipore, Billerica, MA, USA) on dry ice and stored at −80°C.
Dopamine transporter (DAT) autoradiography
To determine the extent of METH-induced DA depletions, frozen brains were sectioned (12-µm coronal sections) using a cryostat (Cryocut 1800; Leica, Wetzlar, Germany). Sections were thaw-mounted onto Superfrost Plus (VWR, Aurora, CO, USA) slides. Slides were stored at −20°C until needed. DA transporter (DAT) autoradiography was performed as detailed previously by others and us (Boja et al. 1992; O'Dell et al. 2011; Pastuzyn et al. 2012). Briefly, slides were incubated in buffer containing fluoxetine to block radioligand binding to the serotonin transporter in striatum. Slides then were incubated in the continued presence of fluoxetine with [125I]RTI-55 (PerkinElmer, Waltham, MA, USA). Slides were then rinsed, dried under a stream of warm air, and exposed to film (Kodak Biomax MR; Eastman Kodak, Rochester, NY, USA) for 24 h before being developed.
Radioactive in situ hybridization
To assess striatal IEG expression, frozen slides containing striatal sections were post-fixed and delipidated as previously described (Ganguly and Keefe 2001). As detailed previously, detection of ppt (Chapman et al. 2001; Johnson-Davis et al. 2002; Horner et al. 2005), ppe (Ganguly and Keefe 2000; Chapman et al. 2001; Ganguly and Keefe 2001; Daberkow et al. 2007), zif268 (Keefe and Ganguly 1998; Keefe and Adams 1998), and Arc (Daberkow et al. 2007; Daberkow et al. 2008; Barker-Haliski et al. 2012a) mRNAs was accomplished using ribonucleotide probes. Antisense ribonucleotide probes were transcribed from linearized plasmids using 35S-UTP (PerkinElmer) and SP6 (ppe and ppt) or T7 (zif268 and Arc) RNA polymerases (Roche, Indianapolis, IN, USA). The radioactive in situ hybridization was performed as previously described (Ganguly and Keefe 2001) with slight modifications to final washing procedures. The last four washes on the second day were either at 55°C (Arc and ppt) or room temperature (ppe and zif268). Slides were dipped in ddH2O, air-dried, and exposed to film. Exposure times were: ppt, zif268, and Arc, two weeks; ppe, three days.
Fluorescent in situ hybridization
To assess pathway-specific expression of Arc mRNA, slides were post-fixed and delipidated as for radioactive in situ hybridization above. As previously described (Daberkow et al. 2007; Barker-Haliski et al. 2012a), expression of Arc and ppe mRNAs in striatum was determined by performing double-label fluorescent in situ hybridization (FISH) using probes directed against Arc and ppe mRNAs. Arc and ppe antisense ribonucleotide probes were synthesized using digoxigenin-UTP (DIG-UTP) and fluorescein-UTP (FITC-UTP) with T7 and SP6 RNA polymerases using DIG or FITC labeling kits (Roche), respectively. Slides were hybridized and detected as previously described (Daberkow et al. 2007; Daberkow et al. 2008; Barker-Haliski et al. 2012b), except that a 1:50,000 solution of SYTOX Green (Molecular Probes; Life Technologies, Grand Island, NY, USA) was used as a nuclear stain instead of DAPI. As controls, a set of slides was run in parallel either without ribonucleotide probes or without antibodies. Lack of signal on these slides was taken as evidence of the specificity of the in situ hybridization histochemical labeling.
Image analysis
Films from DAT autoradiography and radioactive in situ hybridization histochemistry were developed, and images were digitized using a video camera (CCD72S; Dage-MTI, Michigan City, IN, USA) and fiber optic light box. The intensity of the light was adjusted so that it fell within the linear range of the camera, as determined by a photographic step tablet (Eastman Kodak Co.). Densitometric analysis of the digitized images was then accomplished using NIH ImageJ software, yielding average gray values in DM and DL striatum for both hemispheres. Two rostral (+1.6 mm from Bregma) and two middle striatal sections (+0.7 mm from Bregma) were analyzed per rat. For each hemisphere, the average gray value of the corpus callosum was subtracted from the average gray value of DM and DL striatum to correct for background staining. Decreases in DAT in METH-pretreated rats were calculated as a percent of DAT levels in saline-pretreated rats.
FISH images were collected using a Leica DM4000B automated upright microscope (63X oil immersion objective) connected to a Leica EL6000 external light source with a mercury metal halide bulb and a Leica DFC300 FX digital color camera. Surveyor computer software (Objective Imaging Ltd.; Cambridge, UK) was used to control the automated stage, perform multichannel scanning, and capture the fluorescent images. A 2 × 2 montage (0.38 mm2) was captured in the area of striatum with the most Arc expression, as identified from the film autoradiograms of Arc mRNA expression. In the case of rats with no apparent stimulation-induced Arc mRNA expression on the film autoradiograms, montages of FISH staining were captured in DL striatum, as DL striatum was the location where the majority of rats stimulated in a phasic-like manner showed Arc expression. The total numbers of Arc-positive/ppe-negative (i.e., presumed striatonigral neurons) and Arc-positive/ppe-positive (i.e., striatopallidal neurons) neurons in each image were counted by an experimenter blinded to the treatment groups. Furthermore, the average signal intensity of Arc expression in each individual neuronal population (striatonigral and striatopallidal) was measured in ImageJ by first individually outlining all Arc-positive/ppe-positive neurons in an image and measuring signal intensity in those cells. Then, the Arc-positive/ppe-negative neurons in the image were outlined and the average Arc signal intensity of those neurons was measured.
Statistical analysis
Rectal temperatures were compared using repeated measures MANOVA with pretreatment as a factor and time as a repeated measure. Post hoc one-way ANOVAs with Tukey-Kramer HSD tests were used to further interrogate the significant pretreatment × time interaction for the body-temperature data. For radioactive in situ hybridization and DAT autoradiography, average gray values were compared between pretreatment (METH or saline) and stimulation (no stimulation, tonic, or phasic) groups by two-way ANOVA. For FISH, the numbers of Arc-positive/ppe-negative and Arc-positive/ppe-positive cells were compared between pretreatment and stimulation groups by two-way ANOVA. The relative amount of Arc signal in the striatal neuron populations was also examined by calculating a “Difference Score”, which was the average gray value of the Arc-positive/ppe-negative population minus the average gray value of the Arc-positive/ppe-positive population for each animal. This “Difference Score” was compared between pretreatment and stimulation groups by two-way ANOVA. Post hoc Tukey-Kramer HSD tests were performed when ANOVA revealed significant interactions or main effects. Statistical tests were performed using JMP (v. 9.0) or SAS (v. 9.3) software.
Results
METH pretreatment and DAT autoradiography
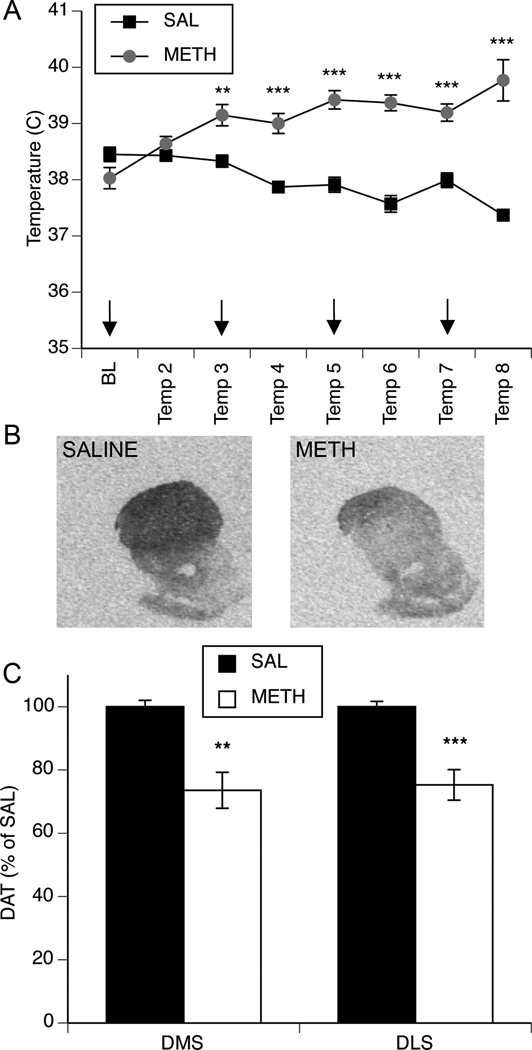
A treatment × time repeated measures MANOVA revealed that the METH “binge” pretreatment paradigm differentially altered body temperature across METH- and saline-treated rats (Fig. 1a; significant effect of time, F(7,30)=4.45, p=0.0032; significant effect of treatment F(1,30)=53.43, p<0.0001; significant time × treatment interaction, F(7,30)=35.20, p<0.0001). Post hoc analysis revealed that METH-treated animals were significantly hyperthermic relative to saline-treated animals two hours after METH injection and every hour thereafter (p<0.001 at 120–420 min after METH). METH pretreatment resulted in significant decreases in striatal DAT three to five weeks later, as assessed by [125I]RTI-55 autoradiography (Fig. 1b–c). A two-way ANOVA revealed a main effect of pretreatment (DM striatum, F(1,24)=17.1, p<0.001; DL striatum, F(1,24)=20.3, p=0.0001), but no significant main effect of stimulation and no significant pretreatment × stimulation interaction (p>0.05).
Fig. 1. Effect of methamphetamine pretreatment on body temperature and dopamine innervation in the dorsal striatum.
(A) METH treatment resulted in significantly increased body temperatures relative saline-treated controls. Arrows indicate time of METH injections. Data are average rectal temperatures (°C; mean±SEM, n=15 for saline-treated and n=15 for METH-treated). *Significantly different from saline-treated rats at the same time point. BL, baseline; **p<0.001, ***p<0.0001. (B) Representative images of [125I]RTI-55 striatal DAT binding in a saline- and METH-pretreated rat 3–5 weeks after METH pretreatment. (C) METH pretreatment resulted in partial dopamine denervation in both the dorsomedial (DMS) and dorsolateral (DLS) striatum 3–5 weeks after METH pretreatment as assessed by [125I]RTI-55 binding to dopamine transporters. Data are presented as percent of saline-pretreated values (±SEM, n=15 for saline-pretreated and n=15 for METH-pretreated). *Significant effect of METH pretreatment. **p<0.001, ***p<0.0001.
Effect of phasic- and tonic-like stimulation of the MFB on Arc expression
Stimulation of the MFB resulted in changes in striatal Arc expression in both saline- and METH-pretreated rats (Fig. 2a). A two-way ANOVA (pretreatment × stimulation) for Arc mRNA expression in DM striatum revealed a main effect of stimulation (Fig. 2b; F(2,24)=5.29, p<0.05), but no significant effect of pretreatment (p>0.05) and no significant stimulation × pretreatment interaction (p>0.05). Post hoc analysis of the main effect of stimulation revealed that phasic-like stimulation increased Arc mRNA expression in the DM striatum both relative to non-stimulated controls (p=0.03) and rats receiving tonic-like stimulation (p<0.05). However, Arc mRNA expression in rats receiving tonic-like stimulation was not different from the non-stimulated control group (p>0.05).
Fig. 2. Effect of MFB stimulation on expression of Arc and zif268 in striatum of METH- and saline-pretreated rats.
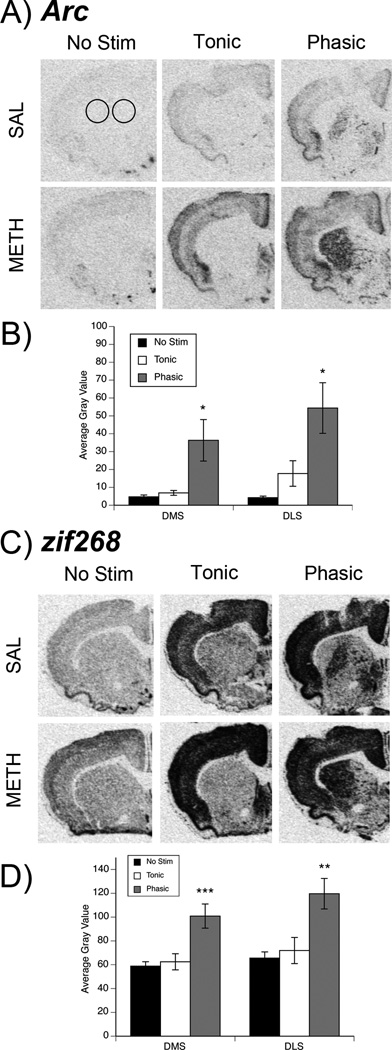
(A, C) Representative images of striatal Arc (A) or zif268 (C) mRNA expression in saline- (top row) and METH- (bottom row) pretreated rats. Circles on the Arc/saline-pretreated/no stimulation image represent the regions of interest (ROIs) measured in both dorsomedial (DMS) and dorsolateral (DLS) striatum for all genes in this study. (B, D) Quantification of Arc (B) and zif268 (D) expression in stimulation groups collapsed across pretreatment group. Data are average gray values (arbitrary units; mean±SEM; n=10 for each stimulation group per subregion, per gene product). *Significantly different from both no stimulation (No Stim) and Tonic groups, p<0.05. **Significantly different from both No Stim and Tonic groups, **p<0.01. ***Significantly different from both No Stim and Tonic groups, p<0.001.
Similarly, in DL striatum, there was a main effect of stimulation (Fig. 2b; F(2,24)=7.24, p<0.01), but no main effect of pretreatment (p>0.05) and no significant stimulation × pretreatment interaction (p>0.05). Post hoc analysis again revealed that phasic-like stimulation increased Arc mRNA expression relative to that seen in non-stimulated controls (p<0.01) and rats receiving tonic-like stimulation (p=0.03). As in DM striatum, there was no significant difference in Arc mRNA expression in rats receiving tonic-like stimulation relative to non-stimulated controls (p>0.05). Thus, regardless of whether rats had partial DA loss induced by METH pretreatment, phasic-like activation increased the expression of Arc mRNA in DM and DL striatum.
Effect of phasic- and tonic-like stimulation of the MFB on zif268 expression
Stimulation of the MFB resulted in changes in striatal zif268 expression in both saline- and METH-pretreated rats that were similar to those observed for Arc (Fig. 2c). A two-way ANOVA (pretreatment × stimulation) in DM striatum revealed a main effect of stimulation (Fig. 2d; F(2,24)=9.46, p<0.001), but no effect of pretreatment (p>0.05) and no significant interaction (p>0.05). Post hoc analysis of the main effect of stimulation revealed that zif268 expression was significantly greater in rats receiving phasic-like stimulation relative to both rats receiving tonic-like stimulation (p<0.001) or no stimulation (p<0.001). The expression of zif268, however, was not different between rats receiving tonic-like stimulation and rats receiving no stimulation (p>0.05).
Likewise, in DL striatum, there was a significant main effect of stimulation (Fig. 2d; F(2,24)=8.12, p<0.01), but no main effect of pretreatment (p>0.05) and no significant interaction (p>0.05). Again, post hoc analysis revealed that zif268 expression in rats receiving phasic-like stimulation was significantly greater than that in rats receiving tonic-like stimulation (p<0.01) or no stimulation (p<0.01). The expression of zif268 was not significantly different between rats receiving tonic-like stimulation and non-stimulated controls (p>0.05). Thus, as was the case for Arc, phasic- but not tonic-like stimulation of the MFB increased zif268 expression in both METH- and saline-pretreated rats.
Effects of phasic- and tonic-like stimulation of the MFB on ppt expression
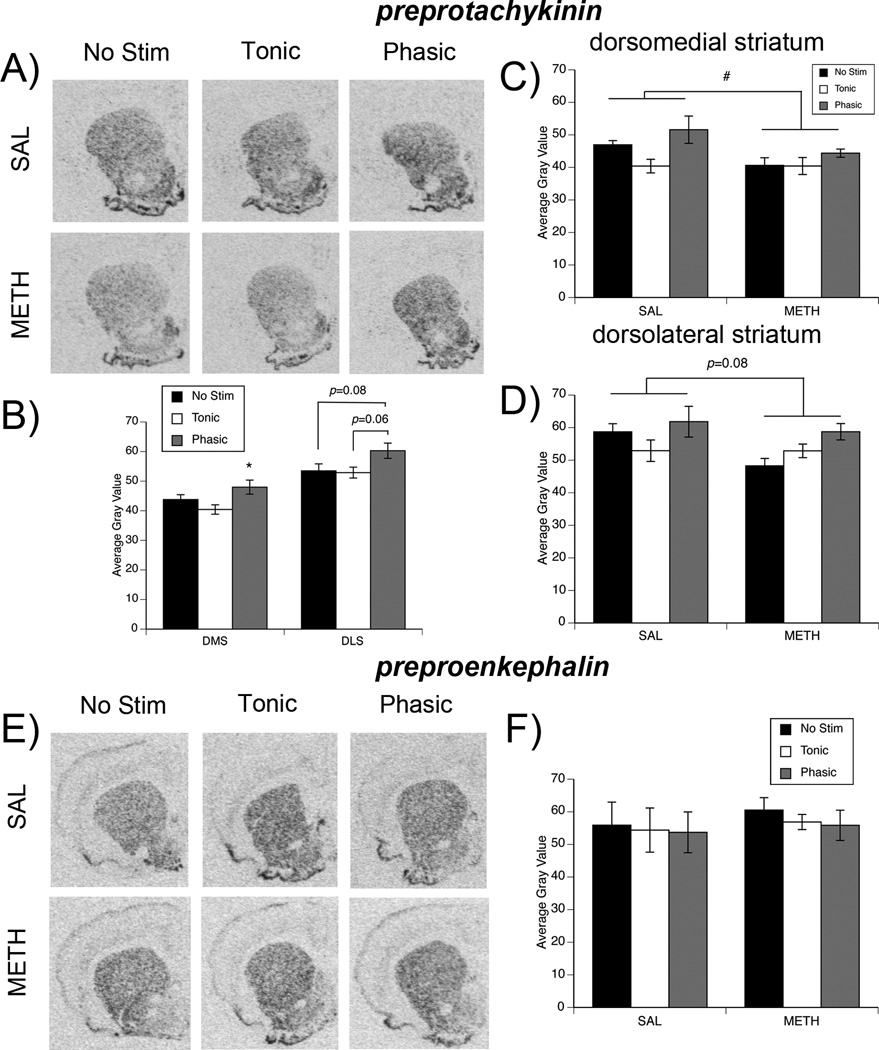
We and others (Nisenbaum et al. 1996; Chapman et al. 2001; Johnson-Davis et al. 2002) have previously reported that partial DA loss, such as that induced by METH pretreatment, results in a long-term decrease in ppt expression. Consistent with this prior work, in the present study, METH-pretreatment was associated with a decrease in ppt mRNA expression (Fig. 3a, c, d). Two-way ANOVA of ppt mRNA expression in DM striatum revealed a main effect of pretreatment (Fig. 3c; F(1,24)=4.87, p<0.05), with ppt expression being lower overall in METH-pretreated rats. The ANOVA also revealed a main effect of stimulation (Fig. 3b; F(2,24)=4.6, p<0.05), but no significant interaction (p>0.05). Post hoc analysis of the main effect of stimulation revealed that phasic-like stimulation significantly elevated ppt mRNA expression in DM striatum relative to rats receiving tonic-like stimulation of MFB (p<0.02).
Fig. 3. Effect of MFB stimulation on expression of preprotachykinin and preproenkephalin in striatum of METH- and saline-pretreated rats.
(A) Representative images of striatal ppt mRNA expression in saline- (top row) and METH- (bottom row) pretreated rats. (B) Quantification of ppt expression in stimulation groups collapsed across pretreatment groups. Data are average gray values (arbitrary units; mean±SEM, n=10 for each stimulation group per subregion). *Significantly different from Tonic group, p<0.05. (C, D) Quantification of ppt expression separated by pretreatment group to demonstrate the effect of METH pretreatment on ppt expression. Data are average gray values (arbitrary units; mean±SEM, n=5 for saline- and METH-pretreated animals per stimulation group for both DMS and DLS). #Main effect of pretreatment, p <0.05. (E) Representative images of striatal ppe mRNA expression in saline- (top row) and METH- (bottom row) pretreated rats. (F) Quantification of ppe expression in dorsomedial striatum. Data are average gray values (arbitrary units; mean±SEM, n=5 for saline- and METH-pretreated animals per stimulation group). Data from dorsolateral striatum are not shown, as there was no subregion difference.
In DL striatum, there was a trend towards an effect of pretreatment (Fig. 3d; F(1,24)=3.34; p=0.08) and a main effect of stimulation (Fig. 3b; F(2,24)=3.67, p<0.05), but no significant interaction (p>0.05). Post hoc analysis revealed strong trends indicating that phasic-like stimulation increased ppt expression in DL striatum relative to both that seen in rats receiving tonic-like stimulation (p=0.057) and control rats that did not receive stimulation (p=0.084). As with the other genes, ppt mRNA expression was not different between rats receiving tonic-like stimulation vs. no stimulation controls (p=0.98).
Effects of phasic- and tonic-like stimulation of the MFB on ppe expression
Neither METH pretreatment nor stimulation of the MFB in either a phasic-like or tonic-like pattern resulted in any significant changes in striatal ppe mRNA expression in saline- or METH-pretreated rats (Fig. 3e–f). Thus, in both DM and DL striatum, there were no significant main effects of pretreatment or stimulation and no significant interactions (all p>0.05).
Effects of phasic- and tonic-like stimulation of the MFB on Arc expression in subpopulations of striatal neurons
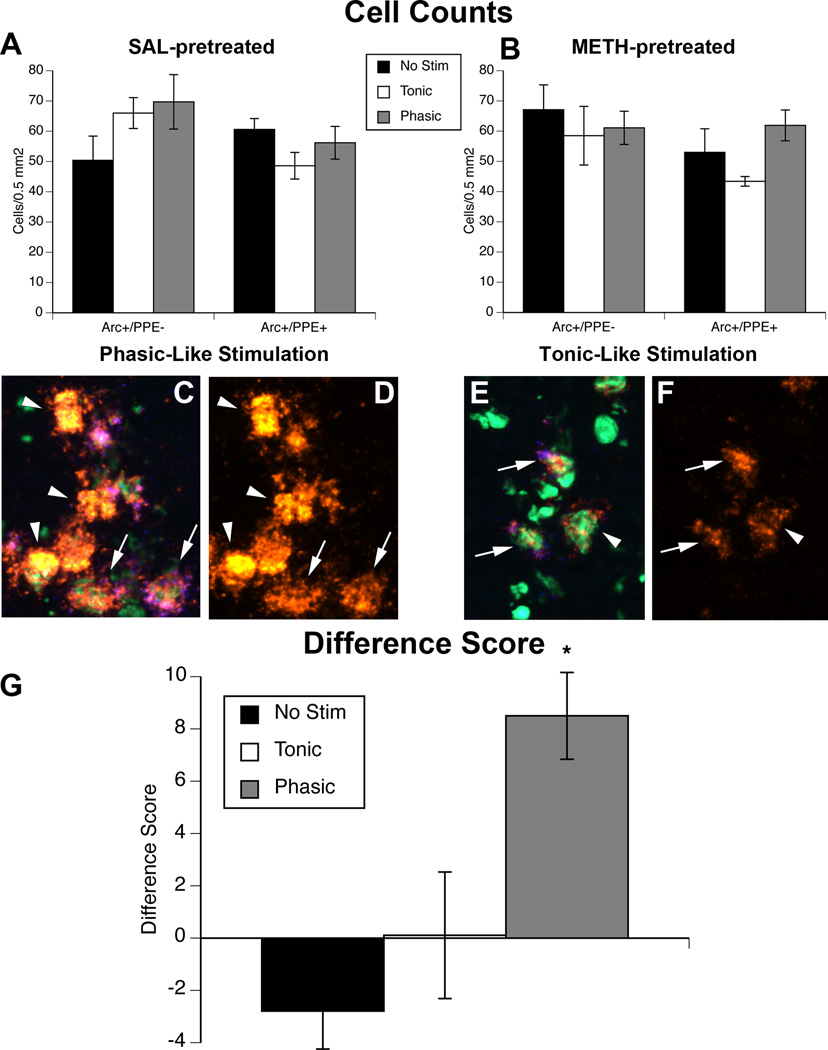
Stimulation of the MFB did not cause any changes in the numbers of ppe-negative (presumed striatonigral) and ppe-positive (striatopallidal) neurons positive for Arc mRNA expression (p>0.05; Fig. 4a–b). However, it was clear during blinded image analysis that the relative amount of signal for Arc mRNA was different between striatonigral and striatopallidal neurons (Fig. 4c–f). Thus, we measured the relative average gray value of Arc expression in the two efferent neuron populations separately and calculated a “Difference Score” (see Methods) reflecting the difference in the average gray value of the Arc signal in ppe-negative vs. ppe-positive neurons. Two-way ANOVA of this “Difference Score” revealed a significant main effect of stimulation (F(2,24)=9.3, p=0.001), but no main effect of pretreatment (p>0.05) and no significant interaction (p>0.05). Post hoc analysis of the main effect of stimulation revealed that greater Arc mRNA expression in ppe-negative neurons was induced by phasic-like stimulation relative to either tonic-like stimulation (p<0.001) or no stimulation (p<0.02).
Fig. 4. Phasic-like stimulation of dopamine neurons increases Arc expression in striatonigral neurons.
(A–B) Numbers of Arc-positive/ppe-negative and Arc-positive/ppe-positive cells/0.5 mm2 in the striatum of A) saline (SAL)- and B) METH-pretreated rats receiving no stimulation (No Stim) or tonic-like or phasic-like stimulation of the MFB. (C–F) Representative fluorescent in situ hybridization images of Arc mRNA expression in presumed striatonigral neurons (Arc-positive/ppe-negative; arrowheads) and striatopallidal neurons (Arc-positive/ppe-positive; arrows) in rats that received phasic-like (C–D) or tonic-like (E–F) stimulation. Arc is orange, ppe is blue, SYTOX nuclear stain is green. Images in C and E show all three channels, while images in D and F show only the Arc channel. Scale bar=10 µm. (G) Graph showing the “Difference Score” collapsed across pretreatment (p>0.05), defined as the difference between the average gray value of Arc-positive/ppe-negative and Arc-positive/ppe-positive neurons. *Significantly different from Tonic and No Stim groups, p<0.05, p<0.001, respectively.
Discussion
Consistent with prior reports in the literature, the present results show that partial striatal DA loss, such as that induced by a neurotoxic regimen of METH, is associated with decreased ppt mRNA expression in the striatum (Nisenbaum et al. 1996; Chapman et al. 2001; Johnson-Davis et al. 2002). Furthermore, the present results confirm prior work (Chergui et al. 1997) that phasic-like stimulation of the MFB increases zif268 expression in striatum. Additionally, the present work extends these previous studies by showing that phasic-like stimulation also increases Arc and ppt mRNA expression in striatonigral neurons and, importantly, that this effect of phasic-like stimulation is effective in driving gene expression in presumed striatonigral efferent neurons even in the setting of partial DA loss induced by METH. Thus, the present data reinforce the idea that phasic-like DA neuron activity appears to selectively affect the function of striatonigral efferent neurons and suggest that sufficient circuitry remains in rats with partial striatal DA loss to restore striatal function by enhancing phasic DA neurotransmission
The present data suggest that augmentation of phasic-like DA neurotransmission in animals with partial striatal DA loss may generally restore the ability of residual DA neurons to regulate striatonigral efferent neuron gene expression. As noted above, partial DA loss, such as that induced by 6-OHDA or a neurotoxic regimen of METH, is associated with an impairment of phasic-like, but not tonic-like, DA signals (Bergstrom and Garris 2003; Howard et al. 2011). It has been proposed, based on electrophysiological (Onn et al. 2000) and computer modeling (Dreyer et al. 2010) studies, that D1 DA receptors, and thus striatonigral efferent neurons (Gerfen and Surmeier 2011), are most sensitive to the higher levels of extracellular DA resulting from phasic DA activity. Alternatively, it has been suggested that D2 DA receptors, which are selectively expressed by striatopallidal efferent neurons, are largely saturated by DA under tonic extracellular DA levels and thus are largely insensitive to phasic increases in extracellular DA levels (Dreyer et al. 2010). Taken together, these data suggest that the decrease in phasic DA neurotransmission associated with partial DA loss selectively impairs D1 receptor activation and, therefore, normal gene expression in striatonigral neurons. Our present results further suggest that augmenting phasic-like DA neurotransmission can restore the degree of D1 dopamine receptor activation and, thus, striatonigral neuron function.
Consistent with this model, expression of ppe mRNA in D2 DA receptor-expressing striatopallidal neurons was not altered either by METH-induced neurotoxicity or by phasic- or tonic-like stimulation of the MFB in this study. Previous studies from our lab and others that found a decrease in ppt expression in the setting of partial striatal DA loss also reported no change in ppe mRNA expression (Nisenbaum et al. 1996; Chapman et al. 2001; Johnson-Davis et al. 2002). Interestingly, expression of ppe mRNA in striatopallidal neurons only changes (increases) in the setting of extensive (~80–90%) denervation of the striatum (Gerfen et al. 1991; Nisenbaum et al. 1996), and this same degree of striatal DA denervation is necessary before a decrease in tonic, extracellular levels of DA occurs (Abercrombie et al. 1990; Castaneda et al. 1990). Given that METH neurotoxicity resulted in an ~25% striatal DA denervation (Fig. 1c), METH-induced neurotoxicity is not associated with changes in tonic extracellular levels of DA (Robinson et al. 1990; Cass and Manning 1999). It is therefore not surprising that the METH-pretreated rats in this study, as in previous reports, did not have changes in ppe expression.
One confound of the current study is non-selective stimulation of axons in the MFB. The MFB is highly heterogeneous and contains non-dopaminergic neurons that project to both striatum and the cortex (Nieuwenhuys et al. 1982). Additionally, the striatum receives glutamatergic afferents from various cortical areas (McGeorge and Faull 1989; Ramanathan et al. 2002), and electrical stimulation of the cortex augments striatal IEG expression in monkey (Parthasarathy and Graybiel 1997) and rat (Fu and Beckstead 1992; Liste et al. 1995). Therefore, it is possible that the gene expression measured here was partially induced through a non-dopaminergic pathway, potentially relayed through the cortex. However, electrical stimulation of MFB released DA in the striatum as measured by FSCV (data not shown), the stimulating electrode was optimized within the MFB to elicit this DA release, and previous work has demonstrated that the D1 antagonist SCH23390 impairs expression of zif268 (NGFI-A) caused by MFB stimulation (Chergui et al. 1997). Furthermore, as is apparent in Fig. 2 and Fig. 3, cortical activation of Arc and zif268 expression is notable in animals that received phasic- or tonic-like stimulation, whereas only animals receiving phasic-like stimulation showed increased striatal IEG expression. We noted no correlation between gene expression in striatum and that in the cortex directly overlying striatum in either stimulation group (data not shown), suggesting that the cortical and striatal gene expression are not linked. Thus, these data suggest that the striatal gene expression is likely induced by electrical stimulation of DA neurons ascending through the MFB, although we cannot definitively rule out a contribution of other circuits to the effects at present.
Somewhat analogous to METH-induced neurotoxicity, Parkinson’s disease (PD) is characterized in part by loss of striatal DA nerve terminals (Hornykiewicz and Kish 1987) and dysfunction in striatal gene products (Nisbet et al. 1995). Importantly, cognitive impairments have been recognized in PD patients during the preclinical stage (Abbruzzese et al. 2009). While the pathology underlying these cognitive impairments is not understood, theoretical models indicate that deficits in D1 receptor signaling may play a role (Frank et al. 2004; Guthrie et al. 2009; Wiecki and Frank 2010). It is thus informative that decreases in indices of D1 DA receptor-expressing striatonigral neuron function, including transcriptional activation of IEGs essential for consolidation of long-term memories (Barker-Haliski et al. 2012a), and deficits in basal ganglia-mediated learning and memory functions are apparent in rats with partial DA depletions (less than 80%), such as those induced by METH (Chapman et al. 2001; Daberkow et al. 2005; Daberkow et al. 2008; Son et al. 2011; Pastuzyn et al. 2012). Taken together, these data suggest that deficits in phasic DA signaling and downstream striatonigral gene expression alterations may be involved in the cognitive disabilities apparent in both the setting of METH-induced neurotoxicity and the preclinical stages of PD. The present data further suggest that approaches to augment residual phasic DA signaling in the context of partial DA denervation or to replicate such signaling in the setting of more extensive DA denervation may prove fruitful in managing cognitive deficits associated with deficits in DA signaling, such as those observed in individuals with a history of METH abuse or PD.
Our findings demonstrate that augmenting phasic DA signaling in the partially DA denervated striatum enhances striatal gene expression to the same extent as in the intact striatum. Deficits in striatonigral neuron gene expression induced by large DA-depleting brain lesions are reversed with administration of levodopa (L-DOPA) (Zeng et al. 1995; Westin et al. 2001). Given that L-DOPA increases vesicular content of DA (Pothos et al. 1996) and electrically evoked DA release in intact rats (Garris et al. 1994; Rodriguez et al. 2007), it seems likely that it will augment phasic DA signaling in the context of partial striatal DA loss. Whether the post-synaptic consequences of partial DA denervation can be reversed with L-DOPA treatment or administration of other agents that enhance phasic DA signaling, such as amphetamine (Ramsson et al. 2011; Daberkow et al. 2013), which is used as a cognitive enhancer in treating attention-deficit hyperactivity disorder and drug addiction (Brady et al. 2011; Steiner and Van Waes 2013), is currently being examined.
Acknowledgements
This work was supported by NIH Grants DA024036 (KAK, CDH, MBH, and PAG) and DA032502 (EDP).
Text abbreviations
- Arc
activity-regulated cytoskeleton-associated gene
- CFM
carbon fiber microelectrode
- DA
dopamine
- DAT
dopamine transporter
- DIG
digoxigenin
- DL
dorsolateral
- DM
dorsomedial
- FISH
fluorescent in situ hybridization
- FITC
fluorescein
- FSCV
fast-scan cyclic voltammetry
- IEG
immediate-early gene
- MFB
medial forebrain bundle
- METH
methamphetamine
- ppe
preproenkephalin
- ppt
preprotachykinin
Footnotes
The authors declare no conflicts of interest.
References
- Abbruzzese G, Trompetto C, Marinelli L. The rationale for motor learning in Parkinson's disease. Eur. J. Phys. Rehabil. Med. 2009;45:209–214. [PubMed] [Google Scholar]
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Barker-Haliski ML, Oldenburger K, Keefe KA. Disruption of subcellular Arc/Arg 3.1 mRNA expression in striatal efferent neurons following partial monoamine loss induced by methamphetamine. J. Neurochem. 2012a;123:845–855. doi: 10.1111/jnc.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Haliski ML, Pastuzyn ED, Keefe KA. Expression of the core exon-junction complex factor eukaryotic initiation factor 4A3 is increased during spatial exploration and striatally-mediated learning. Neuroscience. 2012b;226:51–61. doi: 10.1016/j.neuroscience.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom BP, Garris PA. “Passive stabilization” of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson's disease: a voltammetric study in the 6-OHDA-lesioned rat. J. Neurochem. 2003;87:1224–1236. doi: 10.1046/j.1471-4159.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse. 1992;12:27–36. doi: 10.1002/syn.890120104. [DOI] [PubMed] [Google Scholar]
- Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacol. Biochem. Behav. 2011;99:285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr. Opin. Neurobiol. 1997;7:157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal. Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J. Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda E, Whishaw IQ, Robinson TE. Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J. Neurosci. 1990;10:1847–1854. doi: 10.1523/JNEUROSCI.10-06-01847.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J. Pharmacol. Exp. Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Nomikos GG, Gonon F, Fredholm BB, Svennson TH. Increased expression of NGFI-A mRNA in the rat striatum following burst stimulation of the medial forebrain bundle. Eur. J. Neurosci. 1997;9:2370–2382. doi: 10.1111/j.1460-9568.1997.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J. Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol. Biochem. Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Arc mRNA induction in striatal efferent neurons associated with response learning. Eur. J. Neurosci. 2007;26:228–241. doi: 10.1111/j.1460-9568.2007.05630.x. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox. Res. 2008;14:307–315. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38:259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J. Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Fu L, Beckstead RM. Cortical stimulation induces fos expression in striatal neurons. Neuroscience. 1992;46:329–334. doi: 10.1016/0306-4522(92)90055-7. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Keefe KA. Effects of MK-801 on D1 dopamine receptor-mediated immediate early gene expression in the dopamine-depleted striatum. Brain Res. 2000;871:156–159. doi: 10.1016/s0006-8993(00)02435-5. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Keefe KA. Unilateral dopamine depletion increases expression of the 2A subunit of the N-methyl-D-aspartate receptor in enkephalin-positive and enkephalin-negative neurons. Neuroscience. 2001;103:405–412. doi: 10.1016/s0306-4522(01)00005-7. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J. Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, McGinty JF, Young WS., III Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J. Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J. Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie M, Myers CE, Gluck MA. A neurocomputational model of tonic and phasic dopamine in action selection: a comparison with cognitive deficits in Parkinson's disease. Behav. Brain Res. 2009;200:48–59. doi: 10.1016/j.bbr.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv. Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J. Pharmacol. Exp. Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Howard CD, Keefe KA, Garris PA, Daberkow DP. Methamphetamine neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J. Neurochem. 2011;118:668–676. doi: 10.1111/j.1471-4159.2011.07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O'Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Davis KL, Hanson GR, Keefe KA. Long-term post-synaptic consequences of methamphetamine on preprotachykinin mRNA expression. J. Neurochem. 2002;82:1472–1479. doi: 10.1046/j.1471-4159.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Adams AC. Differential effects of N-methyl-D-aspartate receptor blockade on eticlopride-induced immediate early gene expression in the medial and lateral striatum. J. Pharmacol. Exp. Ther. 1998;287:1076–1083. [PubMed] [Google Scholar]
- Keefe KA, Ganguly A. Effects of NMDA receptor antagonists on D1 dopamine receptor-mediated changes in striatal immediate early gene expression: evidence for involvement of pharmacologically distinct NMDA receptors? Dev. Neurosci. 1998;20:216–228. doi: 10.1159/000017315. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Guitteny AF, Fouque B, Teoule R, Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc. Natl. Acad. Sci. U. S. A. 1990;87:230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liste I, Rozas G, Guerra MJ, Labandeira-Garcia JL. Cortical stimulation induces Fos expression in striatal neurons via NMDA glutamate and dopamine receptors. Brain Res. 1995;700:1–12. doi: 10.1016/0006-8993(95)00958-s. [DOI] [PubMed] [Google Scholar]
- Logman MJ, Budygin EA, Gainetdinov RR, Wightman RM. Quantitation of in vivo measurements with carbon fiber microelectrodes. J. Neurosci. Methods. 2000;95:95–102. doi: 10.1016/s0165-0270(99)00155-7. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Belcher AM, Feinstein EM, O'Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102(Suppl 1):61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J. Comp Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Nisbet AP, Foster OJ, Kingsbury A, Eve DJ, Daniel SE, Marsden CD, Lees AJ. Preproenkephalin and preprotachykinin messenger RNA expression in normal human basal ganglia and in Parkinson's disease. Neuroscience. 1995;66:361–376. doi: 10.1016/0306-4522(94)00606-6. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, Crowley WR, Kitai ST. Partial striatal dopamine depletion differentially affects striatal substance P and enkephalin messenger RNA expression. Brain Res. Mol. Brain Res. 1996;37:209–216. doi: 10.1016/0169-328x(95)00317-l. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav. Brain Res. 2011;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48–S56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J. Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuzyn ED, Chapman DE, Wilcox KS, Keefe KA. Altered learning and Arc-regulated consolidation of learning in striatum by methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2012;37:885–895. doi: 10.1038/npp.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Pothos E, Desmond M, Sulzer D. L-3,4-dihydroxyphenylalanine increases the quantal size of exocytotic dopamine release in vitro. J. Neurochem. 1996;66:629–636. doi: 10.1046/j.1471-4159.1996.66020629.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J. Neurosci. 2002;22:8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsson ES, Howard CD, Covey DP, Garris PA. High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. J. Neurochem. 2011;119:1162–1172. doi: 10.1111/j.1471-4159.2011.07407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Yew J, Paulson PE, Camp DM. The long-term effects of neurotoxic doses of methamphetamine on the extracellular concentration of dopamine measured with microdialysis in striatum. Neurosci. Lett. 1990;110:193–198. doi: 10.1016/0304-3940(90)90810-v. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Morales I, Gonzalez-Mora JL, Gomez I, Sabate M, Dopico JG, Rodriguez-Oroz MC, Obeso JA. Different levodopa actions on the extracellular dopamine pools in the rat striatum. Synapse. 2007;61:61–71. doi: 10.1002/syn.20342. [DOI] [PubMed] [Google Scholar]
- Schroder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Son JH, Latimer C, Keefe KA. Impaired formation of stimulus-response, but not action-outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2011;36:2441–2451. doi: 10.1038/npp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Van Waes V. Addiction-related gene regulation: Risks of exposure to cognitive enhancers vs. other psychostimulants. Prog. Neurobiol. 2013;100:60–80. doi: 10.1016/j.pneurobio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Westin JE, Andersson M, Lundblad M, Cenci MA. Persistent changes in striatal gene expression induced by long-term L-DOPA treatment in a rat model of Parkinson's disease. Eur. J. Neurosci. 2001;14:1171–1176. doi: 10.1046/j.0953-816x.2001.01743.x. [DOI] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson's disease. Prog. Brain Res. 2010;183:275–297. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Jolkkonen J, Jenner P, Marsden CD. Chronic L-DOPA treatment differentially regulates gene expression of glutamate decarboxylase, preproenkephalin and preprotachykinin in the striatum of 6-hydroxydopamine-lesioned rat. Neuroscience. 1995;66:19–28. doi: 10.1016/0306-4522(94)00574-o. [DOI] [PubMed] [Google Scholar]