Abstract
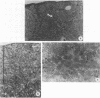
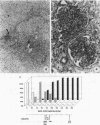
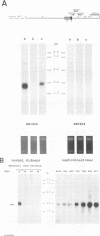
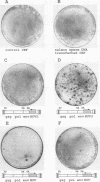
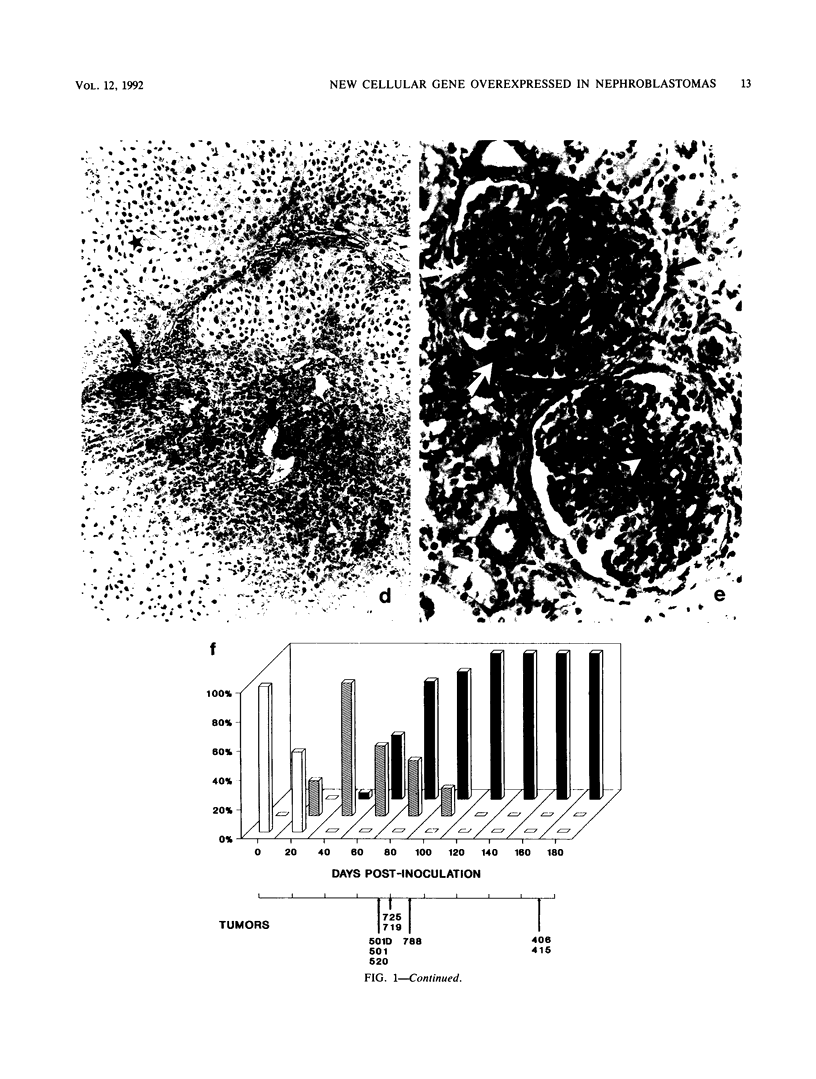
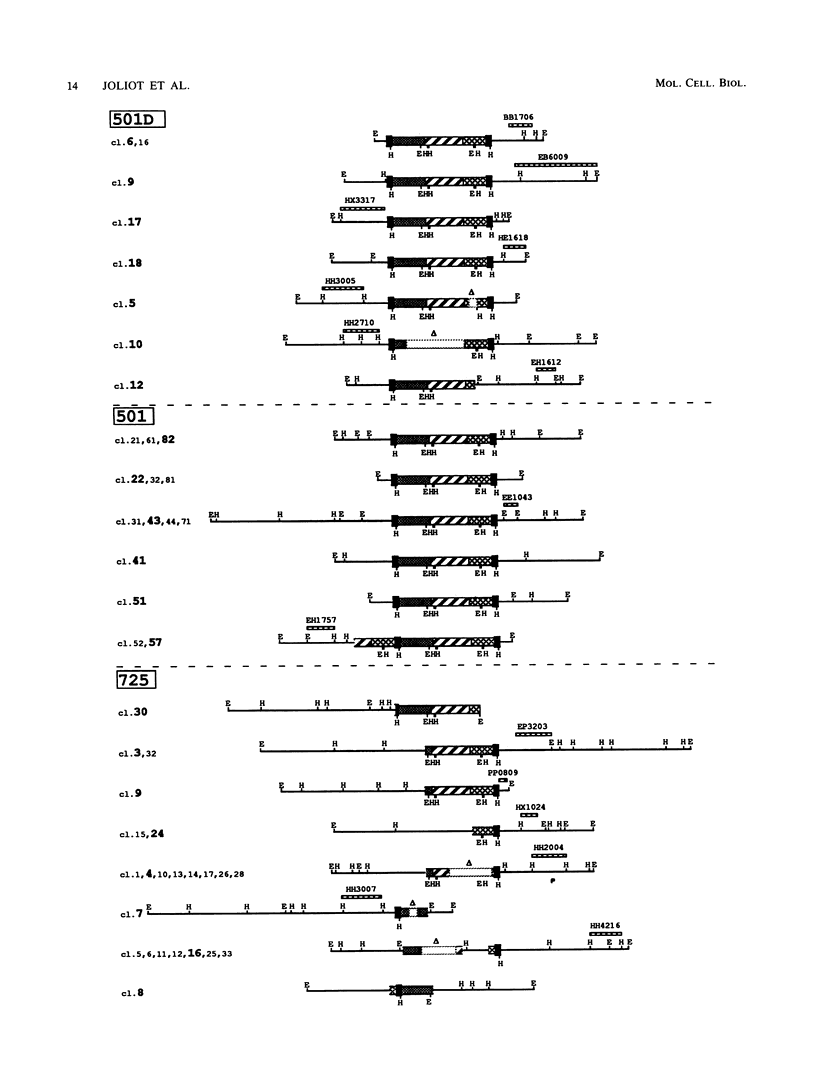
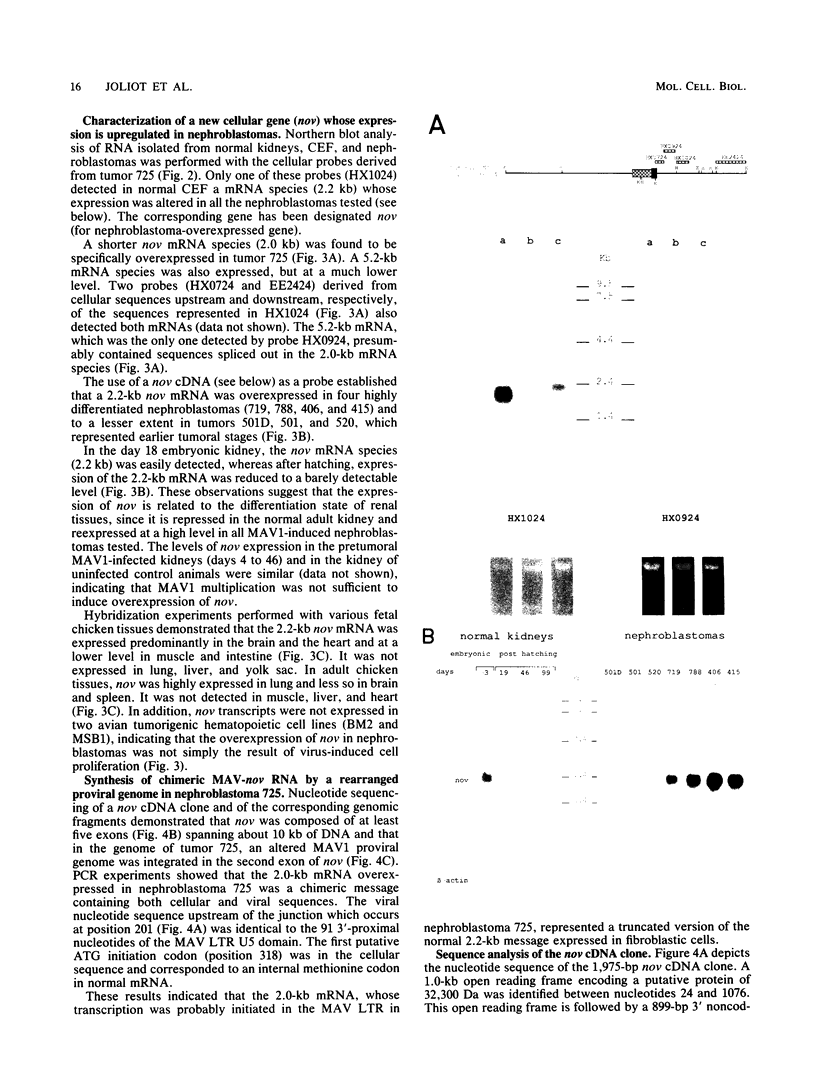
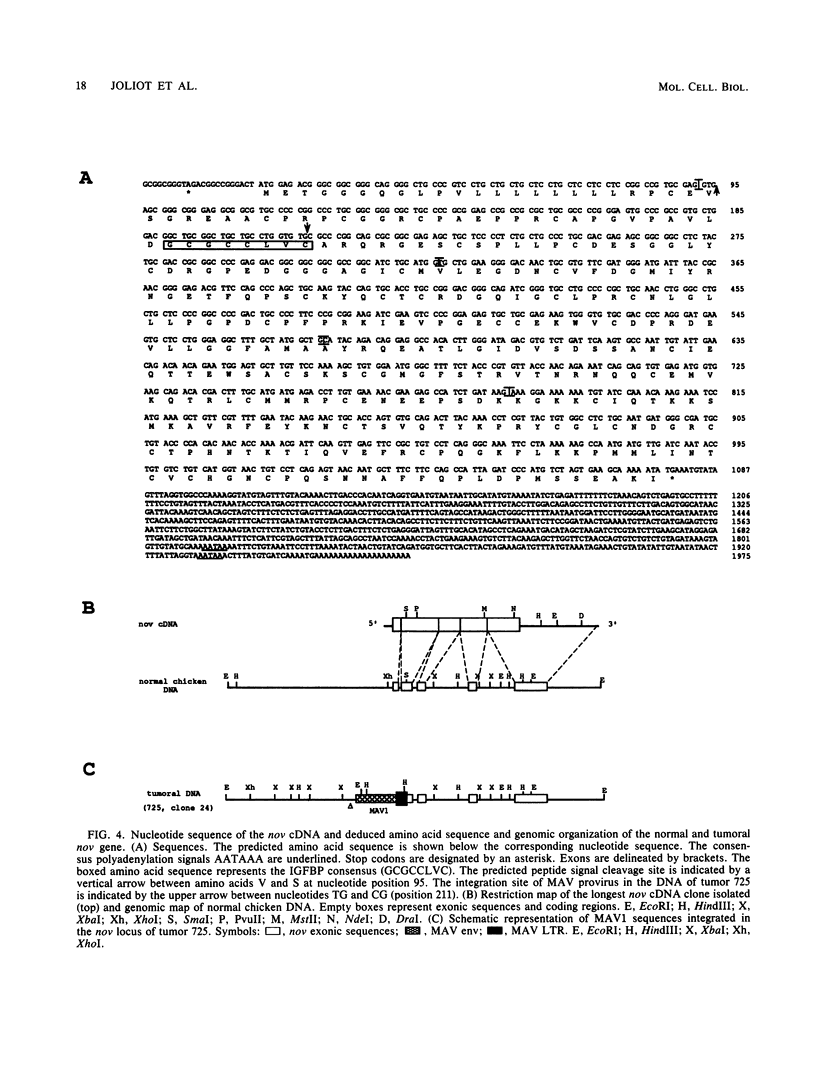
Histological and anatomopathological studies performed on 152 independent myeloblastosis-associated virus type 1 (MAV1)-induced nephroblastomas allowed us to precisely define the chronology of tumor development in chickens. Three tumors representing increasing developmental stages were used to construct genomic libraries and to study both the state of proviral genomes and the sites of MAV1 integration in genomic DNA. We established that increasing levels of proviral rearrangement, eventually leading to the elimination of infectious MAV genomes, were associated with tumor progression and that 22 individual tumors, representative of different developmental stages, did not contain any common MAV1 integration site. Cloning of cellular fragments flanking the MAV1-related proviruses in tumor DNA showed that each one of eight nephroblastomas tested expressed a high level of an as yet unidentified cellular gene (nov) whose transcription is normally arrested in adult kidney cells. Cloning of the normal nov gene established that in one tumor, fused long terminal repeat-truncated nov mRNA species were expressed, indicating that at least in that case, the high level of nov expression was under the control of the MAV long terminal repeat promoter. The normal nov gene encodes a putative 32-kDa secreted polypeptide, which is a member of a new family of proteins likely to be involved in cell growth regulation. We also showed that the expression of an amino-terminal-truncated nov product in chicken embryo fibroblasts was sufficient to induce their transformation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albiston A. L., Herington A. C. Cloning and characterization of the growth hormone-dependent insulin-like growth factor binding protein (IGFBP-3) in the rat. Biochem Biophys Res Commun. 1990 Jan 30;166(2):892–897. doi: 10.1016/0006-291x(90)90894-s. [DOI] [PubMed] [Google Scholar]
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Beard J. W., Chabot J. F., Beard D., Heine U., Houts G. E. Renal neoplastic response to leukosis virus strains BAI A (avian myeloblastosis virus) and MC29. Cancer Res. 1976 Feb;36(2 Pt 1):339–353. [PubMed] [Google Scholar]
- Binkert C., Landwehr J., Mary J. L., Schwander J., Heinrich G. Cloning, sequence analysis and expression of a cDNA encoding a novel insulin-like growth factor binding protein (IGFBP-2). EMBO J. 1989 Sep;8(9):2497–2502. doi: 10.1002/j.1460-2075.1989.tb08386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham D. M., Igarashi A., Potter R. L., Grotendorst G. R. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991 Sep;114(6):1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman A., Groffen C., Kortleve D. J., Geurts van Kessel A., Drop S. L. Isolation and characterization of a cDNA encoding the low molecular weight insulin-like growth factor binding protein (IBP-1). EMBO J. 1988 Aug;7(8):2417–2423. doi: 10.1002/j.1460-2075.1988.tb03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Böni J., Ferdinand F. J., Franklin R. M. Developmental and molecular aspects of nephroblastomas induced by avian myeloblastosis-associated virus 2-O. J Virol. 1985 Jul;55(1):213–222. doi: 10.1128/jvi.55.1.213-222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Collart K. L., Aurigemma R., Smith R. E., Kawai S., Robinson H. L. Infrequent involvement of c-fos in avian leukosis virus-induced nephroblastoma. J Virol. 1990 Jul;64(7):3541–3544. doi: 10.1128/jvi.64.7.3541-3544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Cytotoxic T lymphocyte recognition of transfected cells expressing a cloned retroviral gene. 1983 Oct 27-Nov 2Nature. 305(5937):815–818. doi: 10.1038/305815a0. [DOI] [PubMed] [Google Scholar]
- GUILLON J. C., CHOUROULINKOV I., RENAULT L. LES TUMEURS R'ENALES SPONTAN'EES DES GALLINAC'ES. A PROPOS DE 23 OBSERVATIONS. Bull Assoc Fr Etud Cancer. 1963 Oct-Dec;50:593–620. [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Goodenow M. M., Hayward W. S. 5' long terminal repeats of myc-associated proviruses appear structurally intact but are functionally impaired in tumors induced by avian leukosis viruses. J Virol. 1987 Aug;61(8):2489–2498. doi: 10.1128/jvi.61.8.2489-2498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberger D. Minipreps of DNA from bacteriophage lambda. Nucleic Acids Res. 1987 Aug 25;15(16):6737–6737. doi: 10.1093/nar/15.16.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy P., Koufos A., Morgan K., Li F. P., Meadows A. T., Cavenee W. K. Familial predisposition to Wilms' tumour does not map to the short arm of chromosome 11. Nature. 1988 Nov 24;336(6197):374–376. doi: 10.1038/336374a0. [DOI] [PubMed] [Google Scholar]
- HEINE U., DE THE G., ISHIGURO H., SOMMER J. R., BEARD D., BEARD J. W. Multiplicity of cell response to the BAI strain A (myeloblastosis) avian tumor virus. II. Nephroblastoma (Wilms' tumor): ultrastructure. J Natl Cancer Inst. 1962 Jul;29:41–105. [PubMed] [Google Scholar]
- Helmboldt C. F., Jortner B. S. Histologic patterns of the avian embryonal nephroma. Avian Dis. 1966 Nov;10(4):452–462. [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff V., Compton D. A., Chao L. Y., Strong L. C., Geiser C. F., Saunders G. F. Lack of linkage of familial Wilms' tumour to chromosomal band 11p13. Nature. 1988 Nov 24;336(6197):377–378. doi: 10.1038/336377a0. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES D. B. Nephrotic glomerulonephritis. Am J Pathol. 1957 Mar-Apr;33(2):313–329. [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Ioh R. S., Bauer D. M., Zapf J. Molecular cloning of a new human insulin-like growth factor binding protein. Biochem Biophys Res Commun. 1991 Apr 15;176(1):219–225. doi: 10.1016/0006-291x(91)90912-q. [DOI] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Martinerie C., Perbal B. Expression of a gene encoding a novel potential IGF binding protein in human tissues. C R Acad Sci III. 1991;313(8):345–351. [PubMed] [Google Scholar]
- Nishizawa M., Goto N., Kawai S. An avian transforming retrovirus isolated from a nephroblastoma that carries the fos gene as the oncogene. J Virol. 1987 Dec;61(12):3733–3740. doi: 10.1128/jvi.61.12.3733-3740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. P., Yang G. P., Sanders L., Lau L. F. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990 Jul;10(7):3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Perbal B., Lipsick J. S., Svoboda J., Silva R. F., Baluda M. A. Biologically active proviral clone of myeloblastosis-associated virus type 1: implications for the genesis of avian myeloblastosis virus. J Virol. 1985 Oct;56(1):240–244. doi: 10.1128/jvi.56.1.240-244.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. Oncogenes at viral integration sites. Cell Growth Differ. 1990 Oct;1(10):503–510. [PubMed] [Google Scholar]
- Ping A. J., Reeve A. E., Law D. J., Young M. R., Boehnke M., Feinberg A. P. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989 May;44(5):720–723. [PMC free article] [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell. 1987 Jan 30;48(2):231–240. doi: 10.1016/0092-8674(87)90426-0. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Eccles M. R., Wilkins R. J., Bell G. I., Millow L. J. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985 Sep 19;317(6034):258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Macdonald-Bravo H., Mattéi M. G., Bravo R. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ. 1991 May;2(5):225–233. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. E., Haber D. A., Stanton V. P., Strong L. C., Skolnick M. H., Housman D. E. Familial predisposition to Wilms tumor does not segregate with the WT1 gene. Genomics. 1991 Aug;10(4):927–930. doi: 10.1016/0888-7543(91)90181-d. [DOI] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Siller W. G. Renal pathology of the fowl--a review. Avian Pathol. 1981 Jul;10(3):187–262. doi: 10.1080/03079458108418474. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Soret J., Dambrine G., Perbal B. Induction of nephroblastoma by myeloblastosis-associated virus type 1: state of proviral DNAs in tumor cells. J Virol. 1989 Apr;63(4):1803–1807. doi: 10.1128/jvi.63.4.1803-1807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret J., Kryceve-Martinerie C., Crochet J., Perbal B. Transformation of Brown Leghorn chicken embryo fibroblasts by avian myeloblastosis virus proviral DNA. J Virol. 1985 Jul;55(1):193–205. doi: 10.1128/jvi.55.1.193-205.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S. L., Smith R. E., Faras A. J. Avian nephroblastoma virus MAV-2(N) and avian osteopetrosis virus MAV-2(O) are genetically distinct. J Gen Virol. 1982 May;60(Pt 1):185–189. doi: 10.1099/0022-1317-60-1-185. [DOI] [PubMed] [Google Scholar]
- Watts S. L., Smith R. E. Pathology of chickens infected with avian nephoblastoma virus MAV-2(N). Infect Immun. 1980 Feb;27(2):501–512. doi: 10.1128/iai.27.2.501-512.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Papkoff J., Moscovici C., Varmus H. E. Identification of a provirally activated c-Ha-ras oncogene in an avian nephroblastoma via a novel procedure: cDNA cloning of a chimaeric viral-host transcript. EMBO J. 1986 Feb;5(2):301–309. doi: 10.1002/j.1460-2075.1986.tb04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]