Abstract
Background
Gait impairments are well documented in those with PD. Prior studies suggest that gait impairments may be worse and ongoing in those with PD who demonstrate FOG compared to those with PD who do not.
Purpose
Our aim was to determine the effects of manipulating step length and cadence individually, and together, on gait coordination in those with PD who experience FOG, those with PD who do not experience FOG, healthy older adults, and healthy young adults.
Methods
Eleven participants with PD and FOG, 16 with PD and no FOG, 18 healthy older, and 19 healthy young adults walked across a GAITRite walkway under four conditions: Natural, Fast (+50% of preferred cadence), Small (−50% of preferred step length), and SmallFast (+50% cadence and −50% step length). Coordination (i.e. phase coordination index) was measured for each participant during each condition and analyzed using mixed model repeated measure ANOVAs.
Results
FOG was not elicited. Decreasing step length or decreasing step length and increasing cadence together affected coordination. Small steps combined with fast cadence resulted in poorer coordination in both groups with PD compared to healthy young adults and in those with PD and FOG compared to healthy older adults.
Conclusions
Coordination deficits can be identified in those with PD by having them walk with small steps combined with fast cadence. Short steps produced at high rate elicit worse coordination than short steps or fast steps alone.
Keywords: Parkinson disease, freezing of gait, coordination
Introduction
Gait impairments are well documented in those with Parkinson disease (PD).(1–6) Decreased stride length, decreased velocity, and poor coordination are some impairments that can be become debilitating for an individual with PD. Freezing of gait (FOG),a particularly troubling symptom characterized by a spontaneous increase in cadence and decrease in step length with an accompanying inability to produce effective stepping, (3, 7) is associated with falls and reduced quality of life in this population.(8, 9) It has been suggested that gait impairments in people with PD are worse in those who experience FOG (PD+FOG) than those who do not experience FOG (PD−FOG). For example, those with PD+FOG exhibit increased step-time asymmetry, step length variability, and cadence compared to PD−FOG.(5, 10, 11)
Coordination of steps has also been shown to be dysfunctional in those with PD during gait, particularly in PD+FOG. Plotnik et al. demonstrated that those with PD+FOG exhibit poorer coordination during forward walking than those with PD−FOG, even when FOG is not elicited.(5) Danoudis et al. (12) also showed those with PD+FOG have poorer coordination during preferred gait, as well as gait with an imposed decrease in step length. Nanhoe-Mahabier et al. (6) showed those with PD have worse coordination while walking on a treadmill compared to healthy controls, but no differences exist between groups during forward walking overground. These studies demonstrate that gait coordination is affected in those with PD. However, it remains unclear how coordination is affected during gait conditions that are characteristic of FOG (i.e. increased cadence with progressively decreasing step length (3, 13, 14)) and differences in coordination between those with PD+FOG and PD−FOG require further examination. Identifying differences in coordination during conditions characteristic of FOG by manipulating cadence and step length independently of one another and also concurrently may have potential implications for understanding the underlying mechanisms of FOG.
In this study, we used PCI, a measure of gait coordination, to study the effects of manipulating step length and cadence independently and in combination on gait coordination in healthy controls (young and old) and those with PD+FOG and PD−FOG. Healthy old individuals were included to determine how those with PD differ from individuals of the same age without PD. Healthy young were included to examine differential effects of aging and PD on coordination. To this end, coordination was measured during: 1) step length manipulation, while holding cadence fixed; 2) cadence manipulation, while holding step length fixed; and 3) combined step length and cadence manipulation. We hypothesized that decreasing step length or increasing cadence would decrease coordination in people with PD compared to healthy controls. We expected these effects to be additive, i.e. coordination would be poorest when step length was reduced and cadence was concomitantly increased. Finally, we hypothesized that the PD+FOG group would demonstrate the poorest coordination and be more affected by gait manipulations compared to PD−FOG and healthy controls. We expected healthy young and healthy older adults to be similarly affected by gait manipulations, and less affected than those with PD.
Methods
Participants
Individuals with PD, healthy older, and healthy young adults participated. Subjects with PD were divided into PD+FOG and PD−FOG based upon a score of ≥2 on item 3 of the Freezing of Gait Questionnaire (FOG-Q)(15), indicating freezing episodes occurring at least once per week. Participants with PD were recruited from the XXXX Movement Disorders Center database. All participants with PD had a diagnosis of idiopathic PD according to established criteria (16) and were asked to come in “OFF” medication (≥12 hour overnight withdrawal of anti-parkinson medication). Healthy older adults (>30 years old) were often the spouses of those with PD and were age-matched with the PD group. Healthy young adults (<30 years old) were doctoral students at XXXX. Participants were excluded if they were unable to follow multiple step commands or unable to walk independently without the use of an assistive device. All participants gave informed consent as approved by the XXXX Human Research Protection Office.
Outcome Variables
Phase coordination index (PCI) was the primary outcome measure. PCI quantifies gait coordination by taking into account the accuracy and consistency of the timing of stepping phases and was calculated as defined in previous literature.(17) Higher PCI values indicate poorer coordination.(5, 17) Correlation between PCI and total FOG-Q score was included as a secondary outcome measure. Prior work has only correlated FOG-Q and PCI during natural gait. Therefore, we sought to correlate PCI during gait conditions characteristic of FOG with the FOG-Q.
Data sources/Measurement: Gait
Participants with PD completed the freezing of gait questionnaire (FOG-Q)(15) and were assessed using the Movement Disorder Society Unified Parkinson Disease Rating Scale Motor Subscale III (MDS-UPDRS-3) (18) to quantify disease severity. All participants walked across a 4.9 m GAITRite (CIR Systems, Inc., Sparta, NJ) walkway, on level ground in an open room, under four conditions: Natural (preferred cadence and step length), Fast (50% above preferred cadence), Small (50% below preferred step length), and SmallFast (50% above preferred cadence and 50% below preferred step length). The Natural condition was completed first to establish criteria for the three subsequent conditions, which were randomized. Ten trials were performed for each condition. Once a participant completed 10 trials of the Natural gait condition, his/her average natural cadence was calculated from trials 4, 5, and 6, as these trials were near the middle of the 10 trial block and could be analyzed quickly enough to inform settings for the subsequent conditions. For the Fast condition, a metronome was set to +50% of the individual’s natural cadence with instructions to “keep as close to your normal step length as possible.” For the Small condition, a metronome was set to each participant’s natural cadence and the participant was instructed to take small steps (approximately 50% of natural step length) “no bigger than where your heel comes to your big toe” to the metronome. The SmallFast condition was a combination of the Small and Fast conditions in which the metronome was set to +50% of natural cadence and the same instructions for small steps were given as previously described.
All participants practiced the Fast, Small, and SmallFast conditions one to two times with the metronome. After practicing, the participant began the recorded trials. The metronome was on at the start of each trial in order to remind the participant of the cadence to keep, but was turned off during data collection as to not provide an external auditory cue during recorded walking. Auditory cues are known to enhance performance in people with PD and the purpose of this study was to observe the effects of cadence and step length manipulation during a natural, uncued state.(19–21)
Data Processing
Individual footfall data such as heel on/off, toe on/off, swing time, and stride time were collected within GAITRite. Footfall data were used to calculate PCI as previously defined using custom written Matlab software (MathWorks, Natick, MA).(17) Cadence and step length were also collected and averaged per condition within GAITRite. Average cadence and step length were analyzed to determine if participants were able to perform each condition as instructed.
Statistical Approach
Mixed model repeated measures ANOVA with an unstructured covariance structure was implemented using SAS v 9.3 (SAS Institute, Inc., Cary, NC, USA). Group (PD+FOG, PD−FOG, healthy old, healthy young) was used as the between subject factor and gait condition (Natural, Fast, Small, SmallFast) as the within subject factor. We corrected for multiple comparisons by dividing alpha 0.05 by the number of comparisons made (Bonferroni correction); a post-hoc p-value of 0.0025 was considered significant for evaluating interactions, while a post-hoc p-value of 0.0033 was considered significant for evaluating between-condition differences. Spearman’s correlation was used to determine relationships between FOG-Q scores and PCI. A p-value of <0.05 was considered significant. All measures are reported as mean ± standard deviation, unless otherwise noted.
Results
Twenty-eight participants with idiopathic PD (16 PD−FOG, 12 PD+FOG), 19 healthy older adults, and 19 healthy young adults participated. One participant with PD was excluded due to the inability to walk independently in all conditions. One healthy older adult was excluded due to the inability to follow directions adequately. Sex, age, and disease severity characteristics are included in Table 1.
Table 1.
Final Sample Characteristics
| Characteristic | Healthy Young N=19 | Healthy Old N=18 | PD−FOG N=16 | PD+FOG N=11 |
|---|---|---|---|---|
| Sex (M/F)*† | 9/10 | 6/12 | 5/11 | 10/1 |
| Age (yrs) £‡ | 25.3±2.9 | 68.4±7.5 | 67.6±9.5 | 70.8 ± 6.9 |
| Average Leg Length (cm) | 89.6±7.4 | 88.3±5.7 | 85.5±6.1 | 93.0±6.1 |
| Hoehn & Yahr OFF¥ | 2.2±.44 | 2.2 ±.26 | ||
| MDS-UPDRS-3 OFF*§ | 26.1±9.4 | 44.8 ± 11.8 | ||
| FOG-Q Score*¥ | 2.8±1.8 | 11.3±2.2 |
all group(s) significantly different; p<0.05
Young significantly different from all other groups; p<0.05
Chi square analysis;
One way ANOVA;
Mann-Whitney U Test;
Independent samples t-test
Abbreviations:
M, Male;
F, Female
Yrs, years;
MDS-UPDRS-3, Movement Disorder Society Unified Parkinson Disease Rating Scale Motor Subscale 3;
FOG-Q, Freezing of Gait Questionnaire.
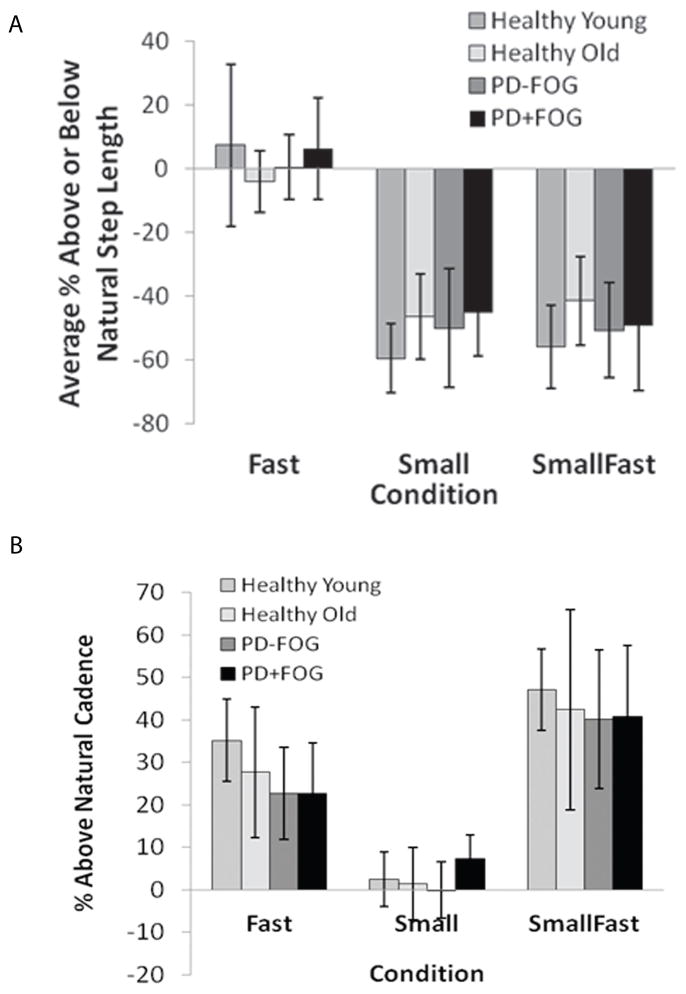
Mean performance of each group during each condition is shown in Figure 1. All groups were able to decrease step length (Fig. 1A) and increase cadence (Fig. 1B) as instructed. There were no between-group differences in percent change from Natural in step length (p=0.37) or in cadence (p=0.18) for any condition. There were also no between-group differences in percent change in velocity for any condition (p=0.62).
Figure 1.
Mean performance (% of Natural) for step length (A) and cadence (B) in each group during Fast, Small, and SmallFast conditions. There were no significant differences between groups within each condition.
Phase Coordination Index (PCI)
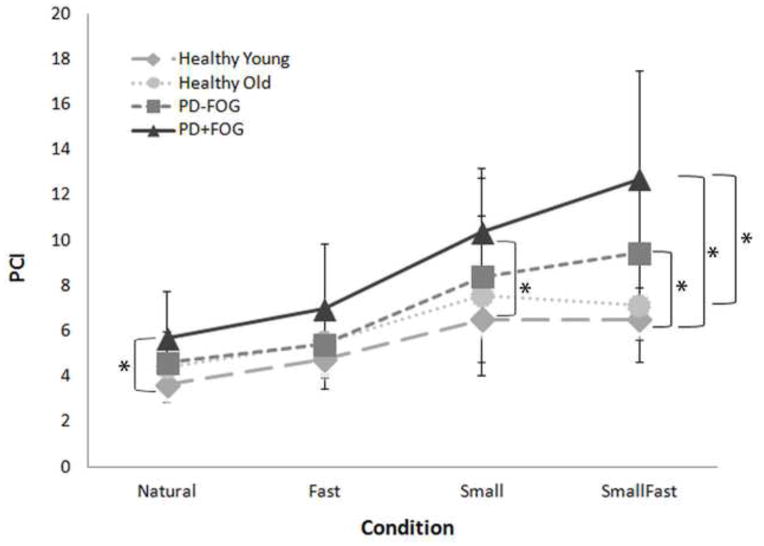
No FOG episodes occurred during this study. Average PCI values across all gait conditions combined were 5.4±1.8 (healthy young), 6.1±2.6 (healthy old), 6.9±3.5 (PD−FOG), and 8.9±4.2 (PD+FOG). Overall, PCI values were significantly different between groups (p<0.001; DF=3; F=9.4) and conditions (p<0.001; DF=3; F=47.43). A significant condition x group interaction effect was also observed (p=0.005; DF=9; F=2.75) (Figure 2). Specifically, the SmallFast condition had a more pronounced effect on coordination in the PD group compared to healthy young (p<0.0025). Additionally, those with PD+FOG had poorer coordination during the SmallFast condition compared to healthy old (p=0.0005) and poorer coordination during the Natural and Small conditions compared to healthy young (p<0.0025). Those with PD+FOG also had worse coordination during SmallFast compared to their coordination during Natural walking (p<0.0033).
Figure 2.
PCI Values of Young, Old, PD−FOG, and PD+FOG in the four different walking conditions. Values are means +/− standard deviations.
Outcome Data/FOG-Q Correlation
Among those with PD, there was a significant but moderate correlation between FOG-Q score and PCI in the Small condition (rs=0.48; p=0.01) and a trend toward significance between FOG-Q and PCI in the SmallFast condition (rs=0.36; p=0.06). FOG-Q score was not correlated with PCI in the Natural (rs=0.27; p=0.17) or Fast (rs=0.17; p=0.38) conditions.
Discussion
The results from this study demonstrate that coordination is somewhat affected in all groups when walking with decreased step length, increased cadence, or both. Post-hoc analyses revealed that when taking short, fast steps, coordination of those with PD is significantly worse than healthy young, and those with PD+FOG exhibit worse coordination during this condition than healthy older adults. Further, coordination during natural gait or gait with short steps is worse in the PD+FOG group compared to healthy young adults. Importantly, these differences are not due to performance differences, as all groups similarly modified their step length and cadence as instructed during each task. Lastly, no FOG episodes occurred during this study.
Our results support previous work that demonstrated coordination of steps to be dysfunctional in people with PD. (6, 12, 17) Prior work also linked coordination to FOG(5), though no differences were observed between the PD−FOG and PD+FOG groups in the present study. Additionally, it has been shown that imposing a high cadence or decreased step length on those with PD+FOG can elicit FOG, (10, 13) although no FOG episodes were observed during this study.
Plotnik et al. suggest that though FOG is a transient event, there is ongoing gait impairment in those who experience FOG compared to those who do not. (5, 11) Additionally, Danoudis et al. (12) demonstrated those with PD who experience FOG have decreased coordination compared to those who do not experience FOG during preferred gait and gait with decreased step length. Our results agree with this, as the PD+FOG group had on average higher PCI values than the other three groups across all four conditions.
Specifically, Danoudis et al. (12) demonstrated that PCI was significantly worse in those with PD+FOG compared to those with PD−FOG during preferred gait and when asked to walk at 50% and 75% of normalized step length. At 25% of normalized step length there was no difference in PCI between groups, though there was an increased incidence of FOG in this condition. As such, the authors suggest that coordination may be associated with step length, but may not explain FOG. However, they acknowledge that poor coordination cannot be ruled out as being associated with FOG. We agree that poor coordination may be associated with decreased step length, but may or may not explain FOG. Our data show that conditions with small step lengths resulted in poorer coordination in PD+FOG compared to healthy controls and that coordination in the Small condition was correlated with FOG-Q. Nonetheless, FOG was not elicited by any manipulations.
Chee et al.(13) demonstrated that when those with PD+FOG were asked to walk at 25% of normalized step length an increased number of FOG episodes were observed and suggested that decreased step length in combination with the sequence effect (consecutive short steps become even shorter) causes FOG. Likewise, Moreau et al. (10) manipulated cadence and velocity above and below preferred levels in ten individuals with FOG. They concluded that a high cadence or velocity can induce FOG. However, there was a significant decrease in stride length during the imposed fast cadence and an inability to increase step length during fast velocity conditions. Unlike the results from Chee et al.(13) and Moreau et al.(10), those with PD in the current study did not demonstrate reduced stride length during Small or Fast conditions with respect to controls. Our study differs from Chee et al.(13) and Moreau et al.(10) in that participants were instructed to take short steps at their natural cadence and to maintain normal step length during the Fast condition. These instructions may have increased the volitional control of each participant’s gait pattern and reduced the chances of eliciting a freezing event.
All participants manipulated step length and cadence as instructed in this study. This retained ability to adjust walking patterns as instructed is in keeping with prior work showing that people with PD have the ability to control stepping rate(2) and provision of auditory cues, such as a metronome, can enhance gait performance.(19–21) Though the metronome for this study was turned off during recorded trials, all participants were allowed to practice with it on.
Those with PD were able to increase their cadence during the Fast and SmallFast conditions and were able to keep close to their determined natural cadence during the Small condition. However, these complex gait manipulations did not elicit FOG. This suggests that there may be a difference between the volitional reproduction of the gait characteristics associated with FOG and the spontaneously occurring increase in cadence and decreasing step length that occur prior to a freezing event. It remains unclear whether increased cadence compensates for the decreased stride length prior to a freeze(1, 22) or if increased cadence during “pre-freezing” strides is a response that indicates a system out of control, with freezing occurring due to the combination of gait hypokinesia and hastening steps. (3, 14)
Several limitations to this study must be acknowledged. Though some findings were statistically significant, we were unable to detect a difference between PD−FOG and PD+FOG. This may be due to a relatively small sample size and the large variation within each condition per group. Further, participants were not matched on sex and the PD−FOG and PD+FOG were not matched on disease severity. This makes it difficult to conclude definitively whether our measures of coordination are attributable to disease severity, FOG status, or both. In addition, we only increased cadence and decreased step length. Though small steps and fast cadence precede FOG and PCI was the worst during the SmallFast condition, we cannot say whether PCI would be different during conditions with slow cadence and/or large steps. PCI was also evaluated while individuals were OFF anti-parkinson medication. The factors contributing to ON medication FOG may be different from OFF medication FOG, therefore PCI values may differ for those who experience FOG while ON medication.
Coordination deficits can be identified in those with PD by having them walk with small, fast steps. Future research is needed to determine if PCI is an appropriate measure to investigate whether there is a threshold value of PCI that may distinguish people with PD+FOG from those with PD−FOG. Further, volitional reproduction of gait characteristics associated with FOG does not elicit FOG. Future research may be aimed at identifying the involuntary mechanisms that contribute to the increased cadence and decreasing step length prior to a freezing event.
Research Highlights.
Small, fast steps are known to precede freezing of gait in Parkinson disease (PD).
We asked how step length and/or cadence manipulation impacts gait coordination.
We compared four groups: PD with and without freezing, and old and young controls.
Taking small or small, fast steps worsened coordination in PD, particularly those with freezing.
Taking small or small, fast steps did not elicit any freezing episodes.
Acknowledgments
Sources of Funding:
This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grants TL1RR024995, UL1RR024992 and by the National Institute of Neurological Disorders and Stroke grant R01NS077959. Additional support was provided by a grant from the University Research Strategic Alliance at Washington University in St. Louis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank Ryan Duncan, PT, DPT, and Laura Pilgram for assistance with data collection. Thanks to Karen-Steger May for statistical support. This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grants TL1RR024995 and UL1RR024992, and a grant from the University Research Strategic Alliance at Washington University in St. Louis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement:
April J. Williams: Grants TL1RR024995 and UL1RR024992. No other conflicts of interest to disclose.
Daniel S. Peterson: Grants TL1RR024995 and UL1RR024992. No other conflicts of interest to disclose.
Gammon M. Earhart: Grants UL1RR024992, R01NS077959, R01NS041509, R01 HD070855. No other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996 Apr;119( Pt 2):551–68. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Iansek R, Matyas T, Summers J. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord. 1998 Jan;13(1):61–9. doi: 10.1002/mds.870130115. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001 Nov;16(6):1066–75. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]
- 4.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003 Aug 15;212(1–2):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 5.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci. 2008 Apr;27(8):1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, et al. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience. 2011 May 19;182:217–24. doi: 10.1016/j.neuroscience.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 7.Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23( Suppl 2):S423–5. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- 8.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004 Aug;19(8):871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 9.de Boer AG, Wijker W, Speelman JD, de Haes JC. Quality of life in patients with Parkinson’s disease: development of a questionnaire. J Neurol Neurosurg Psychiatry. 1996 Jul;61(1):70–4. doi: 10.1136/jnnp.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreau C, Defebvre L, Bleuse S, Blatt JL, Duhamel A, Bloem BR, et al. Externally provoked freezing of gait in open runways in advanced Parkinson’s disease results from motor and mental collapse. J Neural Transm. 2008 Oct;115(10):1431–6. doi: 10.1007/s00702-008-0099-3. [DOI] [PubMed] [Google Scholar]
- 11.Plotnik M, Giladi N, Hausdorff JM. Is freezing of gait in Parkinson’s disease a result of multiple gait impairments? Implications for treatment. Parkinsons Dis. 2012;2012:459321. doi: 10.1155/2012/459321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danoudis M, Iansek R, Simpson P. Freezing of gait in Parkinson’s disease: Further insights into pathophysiological mechanisms. Parkinsonism Relat Disord. 2012 Mar 5; doi: 10.1016/j.parkreldis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain. 2009 Aug;132(Pt 8):2151–60. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- 14.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord. 2006 Sep;21(9):1419–24. doi: 10.1002/mds.20998. [DOI] [PubMed] [Google Scholar]
- 15.Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009 Apr 15;24(5):655–61. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 16.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999 Oct 15;88(5):539–43. [PubMed] [Google Scholar]
- 17.Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp Brain Res. 2007 Aug;181(4):561–70. doi: 10.1007/s00221-007-0955-7. [DOI] [PubMed] [Google Scholar]
- 18.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008 Nov 15;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 19.Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, et al. The effect of external rhythmic cues (auditory and visual) on walking during a functional task in homes of people with Parkinson’s disease. Arch Phys Med Rehabil. 2005 May;86(5):999–1006. doi: 10.1016/j.apmr.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Yoo JY, Ryu JS, Park HK, Chung SJ. The effects of visual and auditory cues on freezing of gait in patients with Parkinson disease. Am J Phys Med Rehabil. 2012 Jan;91(1):2–11. doi: 10.1097/PHM.0b013e31823c7507. [DOI] [PubMed] [Google Scholar]
- 21.Bryant MS, Rintala DH, Lai EC, Protas EJ. An evaluation of self-administration of auditory cueing to improve gait in people with Parkinson’s disease. Clin Rehabil. 2009 Dec;23(12):1078–85. doi: 10.1177/0269215509337465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zijlstra W, Rutgers AW, Van Weerden TW. Voluntary and involuntary adaptation of gait in Parkinson’s disease. Gait Posture. 1998 Jan 1;7(1):53–63. doi: 10.1016/s0966-6362(97)00037-4. [DOI] [PubMed] [Google Scholar]